Abstract
Background
Hospitalizations are common among patients with idiopathic pulmonary fibrosis (IPF). We investigated the impact of hospitalizations on outcomes in patients with IPF.
Methods
The IPF-PRO Registry is an observational US registry that enrolled patients with IPF that was diagnosed or confirmed at the enrolling center in the previous 6 months. Associations between patient characteristics and hospitalization, and between hospitalization and mortality, were analyzed using Cox regression models.
Results
A total of 1002 patients with IPF were enrolled into the IPF-PRO Registry. Over a median follow-up time of 23.7 months (maximum: 67.0 months), 568 patients (56.7%) had at least one hospitalization. Of these patients, 319 (56.2%) had at least one respiratory-related hospitalization and 120 (21.1%) had at least one hospitalization with ventilatory support. Younger age (HR 0.68 [95% CI 0.55, 0.84] per 5-year increase for patients < 62 years), lower BMI (0.96 [0.93, 0.98] per 1-point increase), lower FVC % predicted (0.90 [0.83, 0.97] per 10% increase), oxygen use at rest (2.85 [2.18, 3.72]) and history of pulmonary hypertension (2.02 [1.37, 2.96]) at enrollment were associated with an increased risk of respiratory-related hospitalization during follow-up. In a multivariable model, there was an eightfold increase in the risk of mortality during hospitalization or within 90 days of discharge compared with outside of this period. The risk of mortality associated with a respiratory hospitalization or a hospitalization with ventilatory support was even greater.
Conclusions
Data from the IPF-PRO Registry demonstrate that hospitalizations are common among patients with IPF. The risk of mortality during hospitalization or within 90 days of discharge was high, particularly among patients who were hospitalized for a respiratory cause or received ventilatory support.
Trial registration ClinicalTrials.gov, NCT01915511. Registered 5 August 2013, https://clinicaltrials.gov/ct2/show/NCT01915511
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-021-01851-4.
Keywords: Interstitial lung disease, Mechanical ventilation, Mortality, Pulmonary fibrosis, Respiratory function tests
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrosing interstitial lung disease associated with progressive decline in lung function and a poor prognosis [1]. Hospitalizations are common among patients with IPF and are associated with high mortality, particularly in patients who require intensive care or mechanical ventilation [2–6].
The Idiopathic Pulmonary Fibrosis Prospective Outcomes (IPF-PRO) Registry (NCT01915511) is a multicenter, observational registry of patients with IPF in the US that aims to improve understanding of the clinical course and impact of IPF and current practices in its diagnosis and care [7]. Most previous studies assessing hospitalizations in patients with IPF have been based on databases of insurance claims or electronic medical records. Observational registries provide the opportunity to investigate the risk and impact of hospitalizations in clinical practice. We used data from the IPF-PRO Registry to evaluate the risk of hospitalization, the characteristics of patients who were hospitalized, the procedures and medications received during hospitalization, and post-hospitalization mortality in a large cohort of patients with IPF.
Methods
The design of the IPF-PRO Registry has been published [7]. Briefly, 1002 patients with IPF that was diagnosed or confirmed at the enrolling center in the previous 6 months were enrolled between June 2014 and October 2018. Retrospective data were collected from patients’ medical records. Patients were then followed prospectively, with follow-up data collected approximately every 6 months until death, lung transplant, or withdrawal. Data for this analysis were extracted from the database in June 2020. The study was approved by the Duke University Institutional Review Board (Pro00046131). The protocol was approved by the relevant Institutional Review Boards and/or local Independent Ethics Committees prior to patient enrollment at each site listed in the Acknowledgments. All patients provided consent prior to entering the registry.
The Kaplan–Meier method was used to describe the frequency and timing of hospitalizations during the follow-up period. In descriptive analyses, we compared the demographic and clinical characteristics at enrollment into the registry of the patients who were and were not hospitalized during follow-up. Hospitalizations were categorized as having a respiratory or a non-respiratory cause (according to the investigator) and as with or without ventilatory support. Ventilatory support was designated if the investigator reported either “invasive ventilation” or “non-invasive ventilation”. Diagnostic tests, procedures and medications received during hospitalization, and discharge information for the first, second and third hospitalization, were assessed descriptively among all patients and in subgroups based on whether the hospitalization had a respiratory cause and whether the patient received ventilatory support.
Associations between patient characteristics at enrollment and the time to first hospitalization, first respiratory-related hospitalization, and first hospitalization with ventilatory support were analyzed using univariable, multivariable and parsimonious Cox regression models. The multivariable model included all the covariates. The parsimonious model included covariates identified following backward stepwise selection with an alpha-to-stay criterion of 0.05.
Associations between hospitalization (all, respiratory-related, with ventilatory support) and mortality during hospitalization or within 90, 180 and 360 days of discharge were analyzed using univariable and multivariable Cox regression models. In the analysis assessing the associations between hospitalizations and mortality, models were fit using the entire analysis cohort; in the analyses assessing the associations between respiratory-related hospitalizations and hospitalizations with ventilatory support, models were fit using the subset of patients with at least one hospitalization. The univariable model included hospitalization as a binary time-dependent covariate, the value of which switched from 0 to 1 at the time of hospitalization through the 90, 180, or 360 days following a hospitalization. Each threshold of time was examined in a separate model. All hospitalizations were considered while the patient remained in the risk set. The multivariable model included age, body mass index (BMI), forced vital capacity (FVC) % predicted, diffusing capacity of the lungs for carbon monoxide (DLco) % predicted, oxygen at rest, and history of coronary artery disease or heart failure at enrollment in addition to the time-dependent hospitalization covariate described above. These variables were identified through modeling of data from all patients in the IPF-PRO Registry (see Additional file 1: Appendix S1 for details).
In the Cox regression models, missing data were handled using multiple imputation. The multiple imputation was performed assuming that the data were missing at random with an arbitrary missing pattern. Using the Full Conditional Specification method, missing data were filled in five times to produce five data sets that were complete. Each complete data set was then analyzed using standard statistical analyses, the results of which were averaged to generate the final inferential results.
Results
Hospitalizations
A total of 1002 patients were enrolled into the IPF-PRO Registry at 46 sites. Data from one patient who was in hospital when enrolled, and who died during that hospitalization, were excluded from this analysis. The maximum follow-up time in the registry was 67.0 months and the median was 23.7 months. Over the follow-up period, 568 patients (56.7%) had at least one hospitalization (Additional file 1: Figure S1). Of these patients, 319 (56.2%) had at least one respiratory-related hospitalization (Additional file 1: Figure S2) and 120 (21.1%) had at least one hospitalization with ventilatory support (Additional file 1: Figure S3). Most patients who were hospitalized had one (54.0%) or two (21.3%) hospitalizations during follow-up. Among patients with at least one hospitalization, the first hospitalization had a respiratory cause in 250 patients (44.0%) and included provision of ventilatory support in 69 patients (12.1%). Of the 69 patients who received ventilatory support during their first hospitalization, 25 (36.2%) received invasive ventilation.
Characteristics of hospitalized and non-hospitalized patients
Compared with those who were not hospitalized, a greater proportion of the patients who were hospitalized during follow-up were former smokers (68.8% vs 60.0%), used oxygen at rest (22.9% vs 15.9%) and used oxygen with activity (38.9% vs 28.4%) at enrollment (Table 1). Lung function at enrollment was similar in patients who were and were not hospitalized during follow-up (Table 1). Among those who were and were not hospitalized during follow-up, 27.1% and 19.4% of patients, respectively, had been hospitalized in the 12 months prior to enrollment.
Table 1.
Demographic and clinical characteristics at enrollment of patients who were and were not hospitalized during follow-up in the IPF-PRO Registry
| Hospitalized during follow-up (n = 568) | Not hospitalized during follow-up (n = 433) | |||
|---|---|---|---|---|
| Measure | Missing data | Measure | Missing data | |
| Male | 419 (73.8) | 0 | 328 (75.8) | 0 |
| Age, years | 71 (66, 75) | 0 | 70 (66, 76) | 0 |
| White | 528 (93.0) | 13 (2.3) | 400 (92.4) | 10 (2.3) |
| Body mass index, kg/m2 | 29.0 (25.9, 32.6) | 21 (3.7) | 28.8 (26.0, 31.8) | 20 (4.6) |
| Smoking status | 1 (0.2) | 0 | ||
| Current | 10 (1.8) | 8 (1.8) | ||
| Former | 391 (68.8) | 260 (60.0) | ||
| Never | 166 (29.2) | 165 (38.1) | ||
| Private insurance | 336 (59.2) | 25 (4.4) | 268 (61.9) | 9 (2.1) |
| Diagnostic criteria for IPFa | 2 (0.4) | 2 (0.5) | ||
| Definite | 376 (66.2) | 278 (64.2) | ||
| Possible/probable | 190 (33.5) | 153 (35.3) | ||
| FVC % predicted | 69.2 (58.5, 79.3) | 35 (6.2) | 71.4 (60.8, 84.7) | 33 (7.6) |
| DLco % predicted | 40.8 (30.9, 49.8) | 58 (10.2) | 44.5 (35.5, 54.3) | 58 (13.4) |
| Oxygen use at rest | 130 (22.9) | 1 (0.2) | 69 (15.9) | 4 (0.9) |
| Oxygen use with activity | 221 (38.9) | 2 (0.4) | 123 (28.4) | 5 (1.2) |
| History of coronary artery disease or congestive heart failure | 191 (33.6) | 3 (0.5) | 125 (28.9) | 5 (1.2) |
| History of pulmonary hypertension | 42 (7.4) | 3 (0.5) | 29 (6.7) | 4 (0.9) |
| History of emphysema | 72 (12.7) | 5 (0.9) | 54 (12.5) | 4 (0.9) |
| History of sleep apnea | 158 (27.8) | 3 (0.5) | 119 (27.5) | 3 (0.7) |
Data are n (%) or median (Q1, Q3)
aAccording to 2011 ATS/ERS/JRS/ALAT diagnostic guidelines [24]
First hospitalizations during follow-up in the registry
The median (Q1, Q3) time from enrollment to the first hospital admission was 9.9 (4.2, 17.0) months. The median duration of the first hospitalization was 4 (2, 8) days. Among patients whose first hospitalization had a respiratory cause, the median (Q1, Q3) duration of hospitalization was 6 (3, 12) days. Among patients who received ventilatory support during their first hospitalization, the median (Q1, Q3) duration of hospitalization was 10 (6, 18) days. Median (Q1, Q3) duration of hospitalization was 18 (13, 28) days in patients who received invasive ventilation and 8 (3, 12) days in patients who received non-invasive ventilation.
Among patients whose first hospitalization had a respiratory cause, and who had data available on diagnostic tests and procedures, 35.6% had a chest CT, 28.0% had respiratory cultures performed, 25.6% had an echocardiogram, and 13.2% had a bronchoscopy during the hospitalization, while 10.0% and 17.6% of patients, respectively, received invasive and non-invasive ventilation (Table 2). Data on the outcome of hospitalization and on discharge destination were missing for 36.1% and 46.0% of patients, respectively. Among the 167 patients whose first hospitalization had a respiratory cause and who had data available on the outcome of hospitalization, 136 (81.4%) were discharged and, of those, 114 (83.8%) were discharged to home (Table 2).
Table 2.
Characteristics of the first hospitalization
| Total (n = 568) | Respiratory-related hospitalization (n = 250) | Non-respiratory related hospitalization (n = 318) | Hospitalized with ventilatory support (n = 69) | Hospitalized without ventilatory support (n = 499) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Missing data | Measure | Missing data | Measure | Missing data | Measure | Missing data | Measure | Missing data | |
| Hospitalized at enrolling center | 208 (36.6) | 21 (3.7) | 122 (48.8) | 1 (0.4) | 86 (27.0) | 20 (6.3) | 43 (62.3) | 0 | 165 (33.1) | 21 (4.2) |
| Ventilator use | 499 (87.9) | 181 (72.4) | 318 (100) | 0 | 499 (100) | |||||
| Invasive | 25 (4.4) | 25 (10.0) | 0 | 25 (36.2) | 0 | |||||
| Non-invasive | 44 (7.7) | 44 (17.6) | 0 | 44 (63.8) | 0 | |||||
| Diagnostic tests and procedures | ||||||||||
| Chest CT | 89 (15.7) | 328 (57.7) | 89 (35.6) | 10 (4.0) | 0 | 318 (100) | 32 (46.4) | 0 | 57 (11.4) | 328 (65.7) |
| Bronchoscopy | 33 (5.8) | 329 (57.9) | 33 (13.2) | 11 (4.4) | 0 | 318 (100) | 22 (31.9) | 0 | 11 (2.2) | 329 (65.9) |
| Echocardiogram | 64 (11.3) | 329 (57.9) | 64 (25.6) | 11 (4.4) | 0 | 318 (100) | 25 (36.2) | 0 | 39 (7.8) | 329 (65.9) |
| Respiratory culture | 498 (87.7) | 180 (72.0) | 318 (100) | 34 (49.3) | 464 (93.0) | |||||
| Positive | 18 (3.2) | 18 (7.2) | 0 | 9 (13.0) | 9 (1.8) | |||||
| Negative | 52 (9.2) | 52 (20.8) | 0 | 26 (37.7) | 26 (5.2) | |||||
| Medications | ||||||||||
| Antibiotics | 327 (57.6) | 9 (3.6) | 318 (100) | 0 | 327 (65.5) | |||||
| Yes | 149 (26.2) | 149 (59.6) | 0 | 47 (68.1) | 102 (20.4) | |||||
| No | 92 (16.2) | 92 (36.8) | 0 | 22 (31.9) | 70 (14.0) | |||||
| Steroids | 328 (57.7) | 10 (4.0) | 318 (100) | 0 | 328 (65.7) | |||||
| Yes | 120 (21.1) | 120 (48.0) | 0 | 46 (66.7) | 74 (14.8) | |||||
| No | 120 (21.1) | 120 (48.0) | 0 | 23 (33.3) | 97 (19.4) | |||||
| Anticoagulants | 328 (57.7) | 10 (4.0) | 318 (100) | 0 | 328 (65.7) | |||||
| Yes | 55 (9.7) | 55 (22.0) | 0 | 33 (47.8) | 22 (4.4) | |||||
| No | 185 (32.6) | 185 (74.0) | 0 | 36 (52.2) | 149 (29.9) | |||||
| Outcome | 205 (36.1) | 83 (33.2) | 122 (38.4) | 10 (14.5) | 195 (39.1) | |||||
| Discharged | 305 (53.7) | 136 (54.4) | 169 (53.1) | 41 (59.4) | 264 (52.9) | |||||
| Died | 46 (8.1) | 22 (8.8) | 24 (7.5) | 11 (15.9) | 35 (7.0) | |||||
| Remained inpatient | 12 (2.1) | 9 (3.6) | 3 (0.9) | 7 (10.1) | 5 (1.0) | |||||
| Discharge destination | 261 (46.0) | 112 (44.8) | 149 (46.9) | 28 (40.6) | 233 (46.7) | |||||
| Home | 270 (47.5) | 114 (45.6) | 156 (49.1) | 31 (44.9) | 239 (47.9) | |||||
| Rehabilitation center | 10 (1.8) | 7 (2.8) | 3 (0.9) | 4 (5.8) | 6 (1.2) | |||||
| Assisted living/nursing facility | 5 (0.9) | 2 (0.8) | 3 (0.9) | 1 (1.4) | 4 (0.8) | |||||
| Another hospital | 3 (0.5) | 2 (0.8) | 1 (0.3) | 1 (1.4) | 2 (0.4) | |||||
| Inpatient hospice | 2 (0.4) | 2 (0.8) | 0 | 1 (1.4) | 1 (0.2) | |||||
| Other | 2 (0.4) | 2 (0.8) | 0 | 0 | 2 (0.4) | |||||
| Unknown | 15 (2.6) | 9 (3.6) | 6 (1.9) | 3 (4.3) | 12 (2.4) | |||||
Data are n (%)
Second and third hospitalizations during follow-up in the registry
A total of 261 and 140 patients had a second and third hospitalization during the follow-up period, respectively. The median (Q1, Q3) times from enrollment to the second and third hospital admission were 15.5 (8.6, 25.1) and 19.3 (10.7, 28.1) months, respectively. The median duration of the second hospitalization was 4 days; the median duration of the third hospitalization was also 4 days. The diagnostic tests, procedures and medications received, and the proportions of patients discharged, for the second and third hospitalizations during the follow-up period are presented in Additional file 1: Tables S1 and S2.
Association between patient characteristics and hospitalization
The results of the univariable and multivariable models are shown in Tables 3 and 4, and Additional file 1: Table S4. In the parsimonious models, not having private health insurance, lower DLco % predicted, and oxygen use at rest at enrollment, and hospitalization in the 12 months prior to enrollment, were associated with an increased risk of hospitalization during follow-up (Table 3). Younger age, lower BMI, lower FVC % predicted, oxygen use at rest and history of pulmonary hypertension at enrollment were associated with an increased risk of respiratory-related hospitalization during follow-up (Table 4). Younger age, lower FVC % predicted, lower DLco % predicted, and oxygen use at rest or with activity at enrollment were associated with an increased risk of hospitalization with ventilatory support (Additional file 1: Table S3).
Table 3.
Association between patient characteristics at enrollment and hospitalization
| Univariable model | Multivariable model | Parsimonious model | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Female | 1.02 (0.85, 1.24) | 0.804 | 1.08 (0.89, 1.32) | 0.424 | ||
| Age | 0.017 | 0.217 | ||||
| < 62 years, per 5-year increase | 0.79 (0.65, 0.95) | 0.014 | 0.84 (0.68, 1.02) | 0.079 | ||
| ≥ 62 years, per 5-year increase | 1.09 (1.02, 1.17) | 0.018 | 1.02 (0.95, 1.10) | 0.568 | ||
| Hispanic/Latino ethnicity | 1.23 (0.78, 1.92) | 0.372 | 1.16 (0.72, 1.86) | 0.541 | ||
| Body mass index, per 1-point increase | 0.99 (0.97, 1.01) | 0.287 | 0.99 (0.97, 1.01) | 0.180 | ||
| Current/former smoker | 1.27 (1.06, 1.52) | 0.010 | 1.23 (1.02, 1.49) | 0.033 | ||
| Private insurance | 0.82 (0.69, 0.98) | 0.029 | 0.84 (0.70, 1.02) | 0.072 | 0.84 (0.70, 1.00) | 0.046 |
| Diagnostic criteria of definite IPFa | 0.96 (0.80, 1.14) | 0.630 | 0.96 (0.80, 1.14) | 0.624 | ||
| FVC % predicted, per absolute 10% increase | 0.89 (0.84, 0.94) | < 0.001 | 0.95 (0.89, 1.01) | 0.101 | ||
| DLco % predicted, per absolute 10% increase | 0.83 (0.77, 0.88) | < 0.001 | 0.92 (0.85, 1.00) | 0.056 | 0.87 (0.81, 0.93) | < 0.001 |
| Oxygen use at rest | 1.99 (1.63, 2.44) | < 0.001 | 1.46 (1.12, 1.91) | 0.005 | 1.63 (1.31, 2.03) | < 0.001 |
| Oxygen use with activity | 1.69 (1.42, 2.01) | < 0.001 | 1.21 (0.95, 1.53) | 0.122 | ||
| History of coronary artery disease or congestive heart failure | 1.19 (1.00, 1.42) | 0.046 | 1.14 (0.95, 1.38) | 0.161 | ||
| History of pulmonary hypertension | 1.21 (0.88, 1.66) | 0.239 | 0.87 (0.62, 1.22) | 0.429 | ||
| History of emphysema | 1.09 (0.85, 1.40) | 0.476 | 0.95 (0.73, 1.23) | 0.686 | ||
| History of sleep apnea | 0.97 (0.81, 1.17) | 0.751 | 0.96 (0.79, 1.18) | 0.722 | ||
| Hospitalization in 12 months prior to enrollment | 1.51 (1.25, 1.83) | < 0.001 | 1.30 (1.06, 1.58) | 0.011 | 1.30 (1.08, 1.58) | 0.007 |
Multivariable model included all the covariates listed. Parsimonious model included covariates selected after performing backwards selection on the multivariable model
aAccording to 2011 ATS/ERS/JRS/ALAT diagnostic guidelines [24]
Table 4.
Association between patient characteristics at enrollment and respiratory-related hospitalization
| Univariable model | Multivariable model | Parsimonious model | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Female | 1.12 (0.87, 1.44) | 0.374 | 1.24 (0.95, 1.61) | 0.124 | ||
| Age | < 0.001 | < 0.001 | < 0.001 | |||
| < 62 years, per 5-year increase | 0.62 (0.50, 0.77) | < 0.001 | 0.73 (0.58, 0.91) | 0.005 | 0.68 (0.55, 0.84) | < 0.001 |
| ≥ 62 years, per 5-year increase | 0.99 (0.90, 1.09) | 0.810 | 0.91 (0.82, 1.01) | 0.068 | 0.92 (0.83, 1.01) | 0.085 |
| Hispanic/Latino ethnicity | 2.21 (1.34, 3.66) | 0.002 | 1.43 (0.84, 2.45) | 0.189 | ||
| Body mass index, per 1-point increase | 0.98 (0.96, 1.00) | 0.070 | 0.95 (0.93, 0.98) | < 0.001 | 0.96 (0.93, 0.98) | < 0.001 |
| Current/former smoker | 1.01 (0.79, 1.29) | 0.927 | 1.04 (0.80, 1.36) | 0.763 | ||
| Private insurance | 1.00 (0.79, 1.27) | 0.987 | 0.95 (0.72, 1.24) | 0.689 | ||
| Diagnostic criteria of definite IPFa | 1.06 (0.83, 1.35) | 0.645 | 1.10 (0.86, 1.42) | 0.440 | ||
| FVC % predicted, per absolute 10% increase | 0.82 (0.76, 0.89) | < 0.001 | 0.93 (0.85, 1.01) | 0.080 | 0.90 (0.83, 0.97) | 0.005 |
| DLco % predicted, per absolute 10% increase | 0.75 (0.69, 0.83) | < 0.001 | 0.90 (0.80, 1.00) | 0.057 | ||
| Oxygen use at rest | 2.88 (2.24, 3.70) | < 0.001 | 2.32 (1.65, 3.25) | < 0.001 | 2.85 (2.18, 3.72) | < 0.001 |
| Oxygen use with activity | 2.31 (1.84, 2.91) | < 0.001 | 1.32 (0.96, 1.80) | 0.087 | ||
| History of coronary artery disease or congestive heart failure | 0.83 (0.66, 1.06) | 0.133 | 0.87 (0.67, 1.13) | 0.300 | ||
| History of pulmonary hypertension | 2.37 (1.63, 3.43) | < 0.001 | 1.69 (1.10, 2.60) | 0.016 | 2.02 (1.37, 2.96) | < 0.001 |
| History of emphysema | 1.11 (0.80, 1.55) | 0.530 | 0.79 (0.54, 1.15) | 0.216 | ||
| History of sleep apnea | 1.00 (0.78, 1.28) | 0.989 | 1.19 (0.90, 1.57) | 0.221 | ||
| Hospitalization in 12 months prior to enrollment | 1.17 (0.91, 1.50) | 0.221 | 0.91 (0.69, 1.20) | 0.501 | ||
Multivariable model included all the covariates listed. Parsimonious model included covariates selected after performing backwards selection on the multivariable model
aAccording to 2011 ATS/ERS/JRS/ALAT diagnostic guidelines [24]
Mortality
The Kaplan–Meier estimated rates of death at month 60 were 30.8% among patients with at least one hospitalization and 18.0% among non-hospitalized patients.
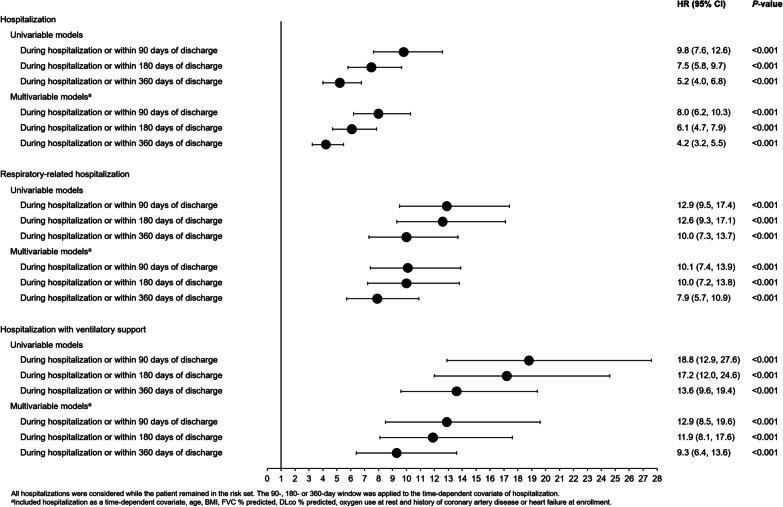
In both univariable and multivariable models, there were significant associations between hospitalization, respiratory-related hospitalization and hospitalization with ventilatory support and risk of mortality during the hospitalization or within 90, 180 and 360 days of discharge (Fig. 1). In multivariable models, there was an eightfold increase in the risk of mortality during hospitalization or within 90 days of discharge compared with the risk of mortality outside this period. There was a tenfold increase in the risk of mortality during a respiratory-related hospitalization or within 90 days of discharge compared with the risk of mortality outside this period among patients with at least one hospitalization. There was a 13-fold increase in the risk of mortality during hospitalization with ventilatory support or within 90 days of discharge compared with the risk of mortality outside this period among patients with at least one hospitalization.
Fig. 1.
Associations between hospitalization and mortality
Discussion
We used data from the IPF-PRO Registry to evaluate the risk and impact of hospitalizations in patients with IPF. To our knowledge, the data from the IPF-PRO Registry are the first to assess associations between patient characteristics and risk of hospitalization, and outcomes following hospitalization, among patients with IPF in a registry setting. Hospitalizations were common in patients enrolled in this registry, with 24% of patients hospitalized in the year prior to enrollment, and 57% of patients hospitalized at least once over a median follow-up of 23.7 months. These data are consistent with analyses of insurance claims databases, which have reported rates of hospitalization among patients with IPF ranging from 38 to 49% over a 1-year period [8–10].
In our analyses, younger age, lower BMI, lower FVC, oxygen use at rest, and a history of pulmonary hypertension at enrollment were associated with an increased risk of respiratory-related hospitalization. As would be expected, more severe lung function impairment at enrollment, as shown by lower FVC, lower DLco, or oxygen use, was associated with a greater risk of hospitalization with ventilatory support. Younger age was also associated with a greater risk of hospitalization with ventilatory support. This is consistent with data from the US Nationwide Inpatient Sample, which demonstrated a greater likelihood of mechanical ventilation in patients with IPF who were younger [3]. Our observation of a greater risk of hospitalization in the youngest patients is consistent with a previous analysis of data from the IPF-PRO Registry in which there was an increase in the risk of mortality in patients aged under 60 years [11]. We may speculate that the youngest patients represent a different cohort to the elderly patients, with disease that is more often familial than a disease of aging and that is associated with worse outcomes.
In the IPF-PRO Registry, the median length of stay in hospital was 4 days. Median length of stay was longer among patients who were hospitalized for a respiratory cause or who received ventilatory support (6 and 10 days, respectively). These findings are similar to the length of hospital stay reported in other observational studies in patients with IPF [4, 5, 12–14]. Analyses of the Premier Healthcare Database and the Nationwide Inpatient Sample, two broadly representative datasets of hospitalized patients in the US, found that the median/mean length of hospital stay among patients with IPF was 5 and 7.4 days, respectively [5, 14].
The most common procedures undertaken during respiratory-related hospitalizations in the IPF-PRO Registry were chest CT, respiratory cultures and echocardiogram. While it may seem surprising that almost two-thirds of patients with IPF who were hospitalized for a respiratory cause and had available data on diagnostic tests and procedures did not receive a CT, similarly low rates of chest CT in hospitalized patients with IPF have been observed in other studies [5, 15]. This may indicate that many clinicians do not request a CT scan if they do not think the results are necessary to inform patient management.
Among patients with data available, 54% of the hospitalized patients in our study were discharged, with most discharged to their home. Data from the US Nationwide Inpatient Sample found that 46% of patients with IPF who were hospitalized were routinely discharged and that 18% required home healthcare after discharge [14]. Our analyses showed a high risk of mortality in the year following hospital discharge, particularly among patients who were hospitalized for a respiratory cause or who received ventilatory support. Patients who were hospitalized had an eightfold increase in the risk of mortality during hospitalization or within 90 days of discharge compared with patients who were not hospitalized. Other studies have also found high post-hospitalization mortality in patients with IPF. Data from a single-center study showed that among 134 patients with fibrotic interstitial lung diseases including IPF who were hospitalized following acute respiratory worsening and survived to discharge, median time to death post-discharge was 9.4 months [16]. An analysis of data from 150 patients with IPF hospitalized at a tertiary referral center demonstrated much shorter survival among patients who survived a respiratory than a non-respiratory admission (median 9.1 versus 43.5 months) [12]. In-hospital mortality rates among patients with IPF have been reported to be approximately 13% to 15% [4, 5, 13–15, 17] and to be particularly high in patients who receive mechanical ventilation [3, 6, 12, 18]. Based on data from the Premier Healthcare Database, mechanical ventilation was associated with, on average, a more than fivefold increase in the risk of in-hospital mortality [4, 5].
Our analyses have several limitations. Due to the challenges inherent in real-world studies, there were substantial missing data on in-hospital procedures, medications and discharges. The definition of a respiratory versus non-respiratory related hospitalization was based solely on investigator report. We are unable to determine which hospitalizations were due to acute exacerbations of IPF or respiratory infections. Associations between patient characteristics and risk of hospitalization were based on data collected at enrollment and the impact of changes in functional parameters were not assessed. The impact of antifibrotic therapy, which has been shown to reduce the risk of acute exacerbations and respiratory-related hospitalizations [19–21] and mortality [21–23], on mortality during and following hospitalization was not assessed.
Conclusions
Data from the IPF-PRO Registry demonstrate that hospitalizations are common among patients with IPF. Younger age, lower BMI, lower FVC, oxygen use at rest, and a history of pulmonary hypertension at enrollment were associated with an increased risk of respiratory hospitalization. The risk of mortality during hospitalization or within 90 days following discharge was high, particularly among patients who were hospitalized for a respiratory cause or who received ventilatory support. A podcast of Dr. Hyun Kim discussing these data is available at: https://www.usscicomms.com/respiratory/kim/IPF-PROhospitalizations.
Supplementary Information
Additional file 1. Appendix S1. Identification of variables for inclusion in the multivariable Cox regression model assessing associations between hospitalization and mortality. Table S1. Characteristics of the second hospitalization. Table S2. Characteristics of the third hospitalization. Table S3. Association between patient characteristics at enrollment and hospitalization with ventilatory support. Figure S1. Time to first hospitalization. Figure S2. Time to first respiratory-related hospitalization. Figure S3. Time to first hospitalization with ventilatory support.
Acknowledgements
We thank the principal investigators and enrolling centers in the IPF-PRO Registry: Albert Baker, Lynchburg Pulmonary Associates, Lynchburg, VA; Scott Beegle, Albany Medical Center, Albany, NY; John A. Belperio, University of California Los Angeles, Los Angeles, CA; Rany Condos, NYU Medical Center, New York, NY; Francis Cordova, Temple University, Philadelphia, PA; Daniel A. Culver, Cleveland Clinic, Cleveland, OH; Daniel Dilling, Loyola University Health System, Maywood, IL; John Fitzgerald (formerly Leann Silhan), UT Southwestern Medical Center, Dallas, TX; Kevin R. Flaherty, University of Michigan, Ann Arbor, MI; Kevin Gibson, University of Pittsburgh, Pittsburgh, PA; Mridu Gulati, Yale School of Medicine, New Haven, CT; Kalpalatha Guntupalli, Baylor College of Medicine, Houston, TX; Nishant Gupta, University of Cincinnati Medical Center, Cincinnati, OH; Amy Hajari Case, Piedmont Healthcare, Atlanta, GA; David Hotchkin, The Oregon Clinic, Portland, OR; Tristan J. Huie, National Jewish Health, Denver, CO; Robert J. Kaner, Weill Cornell Medical College, New York, NY; Hyun J. Kim, University of Minnesota, Minneapolis, MN; Lisa H. Lancaster (formerly Mark Steele), Vanderbilt University Medical Center, Nashville, TN; Joseph A. Lasky, Tulane University, New Orleans, LA; Doug Lee, Wilmington Health and PMG Research, Wilmington, NC; Timothy Liesching, Lahey Clinic, Burlington, MA; Randolph Lipchik, Froedtert & The Medical College of Wisconsin Community Physicians, Milwaukee, WI; Jason Lobo, UNC Chapel Hill, Chapel Hill, NC; Tracy R. Luckhardt (formerly Joao A. de Andrade), University of Alabama at Birmingham, Birmingham, AL; Yolanda Mageto (formerly Howard Huang), Baylor University Medical Center at Dallas, Dallas, TX; Prema Menon (formerly Yolanda Mageto), Vermont Lung Center, Colchester, VT; Lake Morrison, Duke University Medical Center, Durham, NC; Andrew Namen, Wake Forest University, Winston Salem, NC; Justin M. Oldham, University of California, Davis, Sacramento, CA; Tessy Paul, University of Virginia, Charlottesville, VA; David Zhang (formerly Anna Podolanczuk, David Lederer, Nina M. Patel), Columbia University Medical Center/New York Presbyterian Hospital, New York, NY; Mary Porteous (formerly Maryl Kreider), University of Pennsylvania, Philadelphia, PA; Rishi Raj (formerly Paul Mohabir), Stanford University, Stanford, CA; Murali Ramaswamy, PulmonIx LLC, Greensboro, NC; Tonya Russell, Washington University, St. Louis, MO; Paul Sachs, Pulmonary Associates of Stamford, Stamford, CT; Zeenat Safdar, Houston Methodist Lung Center, Houston, TX; Shirin Shafazand (formerly Marilyn Glassberg), University of Miami, Miami, FL; Ather Siddiqi (formerly Wael Asi), Renovatio Clinical, The Woodlands, TX; Barry Sigal, Salem Chest and Southeastern Clinical Research Center, Winston Salem, NC; Mary E. Strek (formerly Imre Noth), University of Chicago, Chicago, IL; Sally Suliman, (formerly Jesse Roman), University of Louisville, Louisville, KY; Jeremy Tabak, South Miami Hospital, South Miami, FL; Rajat Walia, St. Joseph’s Hospital, Phoenix, AZ; Timothy P. M. Whelan, Medical University of South Carolina, Charleston, SC.
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment for development of this article. Writing support was provided by Julie Fleming and Wendy Morris of Fleishman-Hillard, London, UK, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. Boehringer Ingelheim was given the opportunity to review the article for medical and scientific accuracy as well as intellectual property considerations.
Abbreviations
- BMI
Body mass index
- DLco
Diffusing capacity of the lungs for carbon monoxide
- FVC
Forced vital capacity
- HR
Hazard ratio
- IPF
Idiopathic pulmonary fibrosis
Authors’ contributions
HJK, LDS, SB and CSC were involved in the design of the study. HJK and MES were involved in data acquisition. SB was involved in data analysis. All authors were involved in the interpretation of the data and in the writing and critical review of the manuscript. All authors read and approved the final manuscript.
Funding
The IPF-PRO™ Registry is funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and co-ordinated by the Duke Clinical Research Institute (DCRI).
Availability of data and materials
The datasets analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Duke University Institutional Review Board (Pro00046131). The protocol was also approved by the relevant Institutional Review Boards and/or local Independent Ethics Committees prior to patient enrolment at each site listed in the Acknowledgments. All patients provided informed consent.
Consent for publication
Not applicable.
Competing interests
HJK is a member of the Publication Committee for the IPF-PRO/ILD-PRO Registry. LDS and MLN are faculty members in the Duke Clinical Research Institute (DCRI), which receives funding support from Boehringer Ingelheim Pharmaceuticals, Inc to co-ordinate the IPF-PRO/ILD-PRO Registry. AA reports grants and personal fees from Boehringer Ingelheim, personal fees from Genentech and grants from the Pulmonary Fibrosis Foundation and the National Institutes of Health. SB, ESW and CSC are employees of Boehringer Ingelheim Pharmaceuticals, Inc. MES reports grants, personal fees and non-financial support from Boehringer Ingelheim; grants from Galapagos; and personal fees from FibroGen.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyun J. Kim, Email: kimxx015@umn.edu
The IPF-PRO Registry Investigators:
Albert Baker, Scott Beegle, John A. Belperio, Rany Condos, Francis Cordova, Daniel A. Culver, Daniel Dilling, John Fitzgerald, Leann Silhan, Kevin R. Flaherty, Kevin Gibson, Mridu Gulati, Kalpalatha Guntupalli, Nishant Gupta, Amy Hajari Case, David Hotchkin, Tristan J. Huie, Robert J. Kaner, Hyun J. Kim, Lisa H. Lancaster, Mark Steele, Joseph A. Lasky, Doug Lee, Timothy Liesching, Randolph Lipchik, Jason Lobo, Tracy R. Luckhardt, Joao A. Andrade, Yolanda Mageto, Howard Huang, Prema Menon, Yolanda Mageto, Lake Morrison, Andrew Namen, Justin M. Oldham, Tessy Paul, David Zhang, Anna Podolanczuk, David Lederer, Nina M. Patel, Mary Porteous, Maryl Kreider, Rishi Raj, Paul Mohabir, Murali Ramaswamy, Tonya Russell, Paul Sachs, Zeenat Safdar, Shirin Shafazand, Marilyn Glassberg, Ather Siddiqi, Wael Asi, Barry Sigal, Mary E. Strek, Imre Noth, Sally Suliman, Jesse Roman, Jeremy Tabak, Rajat Walia, and Timothy P. M. Whelan
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2.Durheim MT, Collard HR, Roberts RS, et al. Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials. Lancet Respir Med. 2015;3(5):388–396. doi: 10.1016/S2213-2600(15)00093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mooney JJ, Raimundo K, Chang E, Broder MS. Mechanical ventilation in idiopathic pulmonary fibrosis: a nationwide analysis of ventilator use, outcomes, and resource burden. BMC Pulm Med. 2017;17(1):84. doi: 10.1186/s12890-017-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durheim MT, Judy J, Bender S, et al. In-hospital mortality in patients with idiopathic pulmonary fibrosis: a US cohort study. Lung. 2019;197(6):699–707. doi: 10.1007/s00408-019-00270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durheim MT, Judy J, Bender S, et al. A retrospective study of in-hospital mortality in patients with idiopathic pulmonary fibrosis between 2015 and 2018. Medicine (Baltimore) 2020;99(47):e23143. doi: 10.1097/MD.0000000000023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alqalyoobi S, Fernández Pérez ER, Oldham JM. In-hospital mortality trends among patients with idiopathic pulmonary fibrosis in the United States between 2013–2017: a comparison of academic and non-academic programs. BMC Pulm Med. 2020;20(1):289. doi: 10.1186/s12890-020-01328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien EC, Durheim MT, Gamerman V, et al. Rationale for and design of the Idiopathic Pulmonary Fibrosis Prospective Outcomes (IPF-PRO) Registry. BMJ Open Respir Res. 2016;3(1):e000108. doi: 10.1136/bmjresp-2015-000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collard HR, Chen SY, Yeh WS, et al. Health care utilization and costs of idiopathic pulmonary fibrosis in U.S. Medicare beneficiaries aged 65 years and older. Ann Am Thorac Soc. 2015;12(7):981–987. doi: 10.1513/AnnalsATS.201412-553OC. [DOI] [PubMed] [Google Scholar]
- 9.Raimundo K, Chang E, Broder MS, et al. Clinical and economic burden of idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med. 2016;16:2. doi: 10.1186/s12890-015-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu YF, Wu N, Chuang CC, et al. Patterns and economic burden of hospitalizations and exacerbations among patients diagnosed with idiopathic pulmonary fibrosis. J Manag Care Spec Pharm. 2016;22(4):414–423. doi: 10.18553/jmcp.2016.22.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder L, Neely ML, Hellkamp AS, et al. Predictors of death or lung transplant after a diagnosis of idiopathic pulmonary fibrosis: insights from the IPF-PRO Registry. Respir Res. 2019;20(1):105. doi: 10.1186/s12931-019-1043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown AW, Fischer CP, Shlobin OA, et al. Outcomes after hospitalization in idiopathic pulmonary fibrosis: a cohort study. Chest. 2015;147(1):173–179. doi: 10.1378/chest.13-2424. [DOI] [PubMed] [Google Scholar]
- 13.Cottin V, Schmidt A, Catella L, et al. Burden of idiopathic pulmonary fibrosis progression: a 5-year longitudinal follow-up study. PLoS ONE. 2017;12(1):e0166462. doi: 10.1371/journal.pone.0166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooney JJ, Raimundo K, Chang E, Broder MS. Hospital cost and length of stay in idiopathic pulmonary fibrosis. J Med Econ. 2017;20(5):518–524. doi: 10.1080/13696998.2017.1282864. [DOI] [PubMed] [Google Scholar]
- 15.Pedraza-Serrano F, Jiménez-García R, López-de-Andrés A, et al. Characteristics and outcomes of patients hospitalized with interstitial lung diseases in Spain, 2014 to 2015. Medicine (Baltimore) 2019;98(21):e15779. doi: 10.1097/MD.0000000000015779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moua T, Westerly BD, Dulohery MM, et al. Patients with fibrotic interstitial lung disease hospitalized for acute respiratory worsening: a large cohort analysis. Chest. 2016;149(5):1205–1214. doi: 10.1016/j.chest.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Pedraza-Serrano F, López de Andrés A, Jiménez-García R, et al. Retrospective observational study of trends in hospital admissions for idiopathic pulmonary fibrosis in Spain (2004–2013) using administrative data. BMJ Open. 2017;7(2):e013156. doi: 10.1136/bmjopen-2016-013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallick S. Outcome of patients with idiopathic pulmonary fibrosis (IPF) ventilated in intensive care unit. Respir Med. 2008;102(10):1355–1359. doi: 10.1016/j.rmed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Collard HR, Richeldi L, Kim DS, et al. Acute exacerbations in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Eur Respir J. 2017;49(5):1601339. doi: 10.1183/13993003.01339-2016. [DOI] [PubMed] [Google Scholar]
- 20.Ley B, Swigris J, Day BM, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196(6):756–761. doi: 10.1164/rccm.201701-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempsey TM, Sangaralingham LR, Yao X, et al. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200(2):168–174. doi: 10.1164/rccm.201902-0456OC. [DOI] [PubMed] [Google Scholar]
- 22.Behr J, Prasse A, Wirtz H, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J. 2020;56(2):1902279. doi: 10.1183/13993003.02279-2019. [DOI] [PubMed] [Google Scholar]
- 23.Kang J, Han M, Song JW. Antifibrotic treatment improves clinical outcomes in patients with idiopathic pulmonary fibrosis: a propensity score matching analysis. Sci Rep. 2020;10(1):15620. doi: 10.1038/s41598-020-72607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Appendix S1. Identification of variables for inclusion in the multivariable Cox regression model assessing associations between hospitalization and mortality. Table S1. Characteristics of the second hospitalization. Table S2. Characteristics of the third hospitalization. Table S3. Association between patient characteristics at enrollment and hospitalization with ventilatory support. Figure S1. Time to first hospitalization. Figure S2. Time to first respiratory-related hospitalization. Figure S3. Time to first hospitalization with ventilatory support.
Data Availability Statement
The datasets analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.