Abstract
Urine samples from children with human immunodeficiency virus (HIV) infection and healthy controls were examined for mycoplasmas by culture. Standard biochemical assays, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and PCR (16S and 16S-23S spacer rRNA region) were used for identification of isolates. Mycoplasmas were identified from 13 (87%) of 15 HIV-positive patients and 3 (20%) of 15 HIV-negative control patients. The frequency and type of mycoplasma varied with the severity of HIV infection. Mycoplasma penetrans, Mycoplasma pirum, Mycoplasma fermentans, and Mycoplasma genitalium were isolated from patients with severe immunodeficiency. Mycoplasma hominis and Ureaplasma urealyticum were isolated more frequently from children in the early stages of HIV infection and from HIV-negative patients. Mycoplasma penetrans was isolated from one (50%) of two patients in Centers for Disease Control and Prevention (CDC) group B and from five (55.5%) of nine pediatric patients with AIDS (CDC group C). This is the first report that indicates that “AIDS-associated” mycoplasmas are more common in HIV-infected children than in HIV-negative controls.
Mycoplasmas are associated with a variety of diseases in plants, animals, and humans, including diseases involving the immune system. The recent isolation of these organisms from adults with AIDS suggests that mycoplasmas might be cofactors in patients with AIDS (2, 4, 8, 18, 22, 25, 26, 34, 44). The four species of mycoplasmas identified as being associated with AIDS (“AIDS-associated” mycoplasmas) include Mycoplasma fermentans, Mycoplasma pirum, Mycoplasma genitalium, and Mycoplasma penetrans (26). This study was designed to determine if mycoplasmas are present in the urine of children who test positive for human immunodeficiency virus (HIV) and whether the presence of specific mycoplasmas is correlated with age or degree of immunosuppression.
MATERIALS AND METHODS
Patient information.
Fifteen children who tested positive for HIV were identified at the pediatric HIV clinics in Greenville and Columbia, S.C., and 15 HIV-negative patients were identified at the pediatric nephrology clinic in Greenville (Table 1). HIV status was determined by PCR for proviral DNA and immune complex-dissociated p24 antigen assay (enzyme immunoassay with neutralization) (30). All HIV-positive children were presumed to have acquired HIV from their mother during pregnancy, delivery, or breast-feeding. The HIV-positive patients were classified according to the classification system of the Centers for Disease Control and Prevention (CDC) for HIV infection in children (5). By that system, infected children are separated into mutually exclusive categories according to infectious, clinical, and immunologic status. The immunologic classification is determined by the age-specific CD4 T-lymphocyte count or the percentage of total lymphocytes that are CD4 lymphocytes. Categories N, A, B, and C refer to HIV-positive children with no, mild, moderate, or severe symptoms and signs, respectively. Children who tested positive for HIV but who were classified in category E (which includes children who test positive for HIV due to transplacental antibodies but in whom infection with HIV is not confirmed) were not included in this study. After obtaining informed consent, urine specimens were collected from each HIV-positive child and HIV-negative control. A urine specimen was obtained from some patients at more than one clinic visit. When more than one urine specimen was collected, the clinical and laboratory data at the time of collection of the last urine specimen were used to classify the child according to the CDC classification system. The patient information obtained included age, sex, and CDC classification. The laboratory data included the CD4 count.
TABLE 1.
Sexes and ages of HIV-positive children and HIV-negative controls
| Parameter | HIV-positive children | HIV-negative controls |
|---|---|---|
| No. of children | 15 | 15 |
| No. of males:no. of females | 8:7 | 13:2 |
| Median (range) age (yr) | 5.6 (0.6–13.3) | 9 (4.0–16.0) |
Preparation and inoculation of urine sediments for culture.
Urine was collected in a sterile urine container and was transferred to a 15-ml centrifuge tube and centrifuged at 500 × g for 10 min at 4°C. The pellet was suspended in 1 ml of Friis broth medium, and 100 μl was used to inoculate four broth media (2 ml each of Friis, SP4, PH, and Hayflicks [HF] broth media) and four agar plates (Friis, SP4, PH, and HF agar plates) designed to support the primary isolation of mycoplasmas (13, 43). The supernatant obtained was centrifuged again in a 15-ml centrifuge tube at 2,600 × g for 10 min at 4°C, and the pellet was resuspended and inoculated into another set of broth and agar plates as described above. This isolation procedure resulted in the use of 16 separate isolation media for each urine specimen. A urine sample was defined as the portion of urine used in each of the 16 isolation steps. The procedures for the processing of the urine specimens and methods for the isolation of mycoplasmas were designed by Christian T. K. H. Stadtlander.
Isolation of mycoplasmas from culture.
Inoculated broth and agar plates were incubated at 37°C in the presence of 5% CO2 (35, 41, 42, 46) for as long as 6 weeks. The broth cultures were examined frequently for thread-like sediments and pH change. The agar surfaces were observed with an inverted microscope for the presence of colonies with a fried-egg appearance. Sham-inoculated controls were maintained for broth and agar plates for each batch of media. One milliliter of culture-positive broth was passed through a 0.2-μm-pore-size Acrodisc filter (Gelman Sciences). The filtrate (approximately 1 ml) was inoculated into 20 ml of the same broth and also onto an agar medium. Pieces of 1 cm2 were cut with a sterile scalpel from agar plates containing individual mycoplasma colonies, transferred first into a 2-ml and then into a 25-ml tissue culture flask containing 20 ml of broth, and incubated in the presence of 5% CO2. Cultures were observed daily for a color change. Flasks showing a change in color were subcultured in a petri dish containing solid medium for observation for colony morphologies with a typical fried-egg appearance. The contents of the flask containing the agar blocks that showed a color change were filtered through a 0.2-μm-pore-size filter and aliquoted into samples of 1 ml, and the samples were placed in sterile vials and stored at −80°C. All stocks were passed through the filter by a standard cloning and filtration procedure. Briefly, the isolates obtained were filtered through a 0.2-μm-pore-size filter by using a 3-ml syringe and were cultured on SP4 solid medium, and the isolated colonies were picked and again inoculated into a broth culture. To obtain the mycoplasma isolates in pure form for further analysis, the isolates were subjected to a cloning and filtration procedure as described previously (41).
Preliminary identification.
Cloned isolates were grown on SP4 agar medium, and an isolated colony was picked with a sterile Pasteur pipette. The agar plugs were transferred to 2 ml of SP4 broth, and the colonies were disrupted by pressing the plug against the wall of the test tube to release the organisms. The isolates were incubated for 3 to 5 days at 37°C and were used as stock for all identification procedures. The biochemical identification methods used included glucose utilization, arginine hydrolysis, and urease hydrolysis (1). The test media used were modified versions of those described previously (43). The test media and appropriate controls (Mycoplasma arginini ATCC 23714, Mycoplasma buccale ATCC 23636, M. genitalium ATCC 33530, Mycoplasma hominis ATCC 23114, Mycoplasma lipophilum ATCC 27104, Mycoplasma orale ATCC 23714, M. pirum ATCC 25960, Mycoplasma pneumoniae ATCC 15531, Mycoplasma primatum ATCC 15497, Mycoplasma salivarium ATCC 23064, Acholeplasma laidlawii ATCC 23206, and Ureaplasma urealyticum ATCC 27168) were inoculated with 1 ml of a 24-h broth culture containing approximately 108 CFU/ml and were incubated at 37°C in the presence of 5% CO2 for at least 2 weeks.
SDS-PAGE.
Analysis of the protein profile was performed by using one-dimensional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (14). The Mycoplasma pellets were washed three times in 0.25 M NaCl and were resuspended in phosphate-buffered saline (PBS; pH 7.5), and the protein content was estimated and adjusted to 1 mg/ml (Bio-Rad, Richmond, Calif.). A 100-μl sample was heated for 3 min with 20 μl of SDS–2-mercaptoethanol buffer (1 ml of 10% SDS with 20 μl of 2-mercaptoethanol), and 20 μl of the adjusted protein preparation was electrophoresed in a 10% polyacrylamide gel (Dc protein assay kit; Bio-Rad) at 200 V for 60 min or until the bromophenol blue dye front reached the bottom of the gel. The gels were stained with Coomassie blue or were blotted onto nitrocellulose membranes for Western blot analysis.
Western blot analysis.
The electrophoresed proteins were transferred from the gel to a nitrocellulose membrane (presoaked in distilled water) with the Bio-Rad transfer cell at 100 V for 1 h. The nitrocellulose membranes were removed and blocked with 3% bovine serum albumin in PBS (pH 7.5) on a shaker overnight. After washing three times in PBS-N (PBS with 0.05% [vol/vol] Nonidet P-40) the membrane was incubated with primary antibody (see Acknowledgments) at 37°C for 1 h (10, 14). After washing three times in PBS-N, a horseradish peroxidase-linked secondary antibody (Sigma Chemical Co., St. Louis, Mo.) was added and the washing and blocking steps were repeated. The nitrocellulose membrane was treated with solution A (50 ml of PBS [pH 7.5] and 300 μl of hydrogen peroxide) and solution B (10 ml of ice-cold methanol and 0.3 g of horseradish peroxidase color developing reagent [DAB]) for the detection of proteins.
PCR-based confirmation of mycoplasma species.
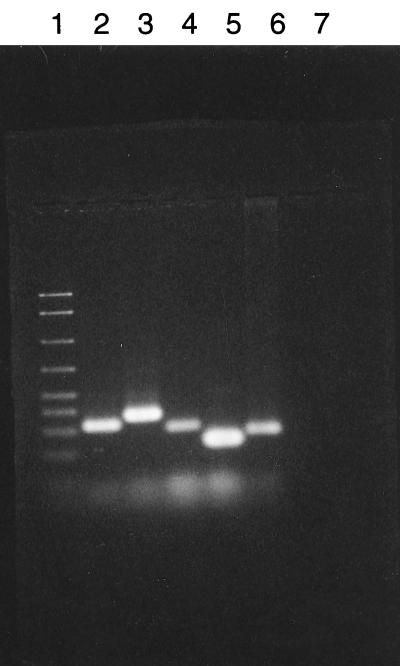
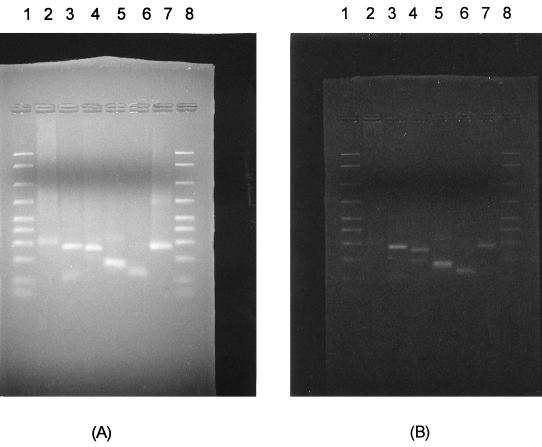
One-milliliter samples of 24-h broth cultures were centrifuged at 15,000 × g for 30 min at 4°C. The pellet was washed three times with PBS (pH 7.3) and was centrifuged again as described above. The sediment was suspended in 100 μl of lysis buffer (1 mM EDTA [pH 8.0], 10 mM Tris-HCl [pH 8.0], 0.1% Triton X-100, 200 μg of proteinase K per ml). The mixture was incubated at 55°C overnight. The samples were heated for 5 min at 95°C to inactivate the proteinase K. Ten microliters of the mixture was used for analysis. Two different PCR procedures, a two-step PCR used to amplify the intergenic rRNA spacer regions and the 16S rRNA gene (19, 44) PCR, were used for confirmation of species. All reactions were performed in 50-μl volumes. Ten microliters of the second-step PCR product and 15 μl of the 16S rRNA PCR product were electrophoresed in a 2.0% agarose gel for 45 to 60 min at 60 V in a Tris-borate buffer (pH 8.0). The gel was stained with ethidium bromide and the products were visualized with a UV transilluminator and photographed with a Polaroid camera (Fig. 1 and 2). Controls consisted of known mycoplasmas (M. salivarium ATCC 23064) obtained from the American Type Culture Collection (ATCC).
FIG. 1.
Agarose gel electrophoresis of the second-step PCR products. 16S-23S rRNA PCR of intergenic spacer regions of Mycoplasma species isolated from urine sediments. Ten microliters of the second-step PCR products was subjected to electrophoresis in a 2% agarose gel. Lane 1, 50- to 2,000-bp DNA marker; lane 2, M. pirum; lane 3, M. fermentans; lane 4, M. penetrans; lane 5, M. hominis; lane 6, M. salivarium ATCC 23064; lane 7, negative control.
FIG. 2.
Restriction enzyme digestion pattern of the second-step PCR products. Five microliters of the second-step products was digested with 1 U of HindIII or VspI, electrophoresed in a 2% agarose gel, and stained with ethidium bromide. Lanes 1 and 8, 2,000- to 50-bp DNA marker; lanes 2, M. penetrans; lanes 3, M. fermentans; lanes 4, M. genitalium; lanes 5, M. pirum; lanes 6, M. hominis; lanes 7, M. salivarium ATCC 23064, used as a positive control. (A) HindIII-cleaved PCR products; (B) VspI-cleaved PCR products.
Sequence analysis.
The amplified products were analyzed by cycle sequencing with dye-labeled terminators (ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kits; Perkin-Elmer). The nucleotide sequences obtained were compared with known mycoplasmal DNA sequences by searching the databases (GenBank, EMBL, DDBJ, and PDB) by using a basic and a local alignment search tool (BLAST) and an SRS-Fasta sequence subset search with default parameters. The database accession numbers of the known mycoplasmas used for comparison are gb/L10839 for M. penetrans, gb/M24289 for M. fermentans, D14526 for M. genitalium, dbj/D14527 for M. pirum, and emb/x58559 for M. hominis. The 16S-23S intergenic spacer rRNA regions and the sequence for the 16S rRNA gene were used for confirmation of mycoplasma identification (3, 16, 17, 19, 44).
Statistical methods.
Statistical analysis was performed by using the statistical software package Breeze/STAT. The P value used was the corrected chi-square test value.
RESULTS
Preliminary isolation of mycoplasmas was successful with both centrifugation speeds used for the processing of the urine samples. However, centrifugation at 500 × g yielded more frequent isolation (61.1%) than centrifugation at 2,600 × g (38.8%). The summary of the isolation yields by different criteria is shown in Table 2. Among the four different growth media used for isolation, SP4, HF, Friis, and PH media accounted for 37.0, 19.2, 19.2, and 24.6% isolation, respectively. The percent isolation frequency of mycoplasmas from broth medium (51.4%) and solid medium (48.6%) were not much different. Use of methods such as blind passages and plate washes increased the chances of isolation of mycoplasmas from cultures negative for mycoplasmas in the first round of isolation attempts.
TABLE 2.
Isolation of mycoplasmas from urine sediments
| Characteristic | % Isolation |
|---|---|
| Centrifugation speed | |
| 500 × g | 61.1 |
| 2,600 × g | 33.9 |
| Growth medium | |
| Broth | 51.4 |
| Agar | 48.6 |
| Type of growth medium | |
| SP4 | 37.0 |
| HF | 19.2 |
| Friis | 19.2 |
| PH | 24.6 |
| Other methods | |
| Blind passagea | 26.4 |
| Plate washb | 8.3 |
| Colony picksc | 65.3 |
At least two blind passages were done with samples which showed no signs of growth before a sample was considered negative for mycoplasmas.
When organisms could not be grown from agar blocks, liquid medium was transferred to those plates, and after 24 h of incubation, the liquid medium was aspirated from the agar surface and transferred into broth cultures to obtain stocks.
Agar surfaces showing a colony morphology with a typical fried-egg appearance were cut and transferred into broth cultures.
More than one urine specimen was collected from four HIV-positive children. Five urine specimens were obtained from one child, four urine specimens were obtained from one child, and two urine specimens were obtained from each of the three children. Table 3 presents the age and sex of the HIV-positive patients in each CDC classification, the mean age of the patients, and the median CD4 cell count. Glucose utilization, arginine hydrolysis, and urease hydrolysis test results confirmed the patterns consistent with the genus Mycoplasma. SDS-PAGE, Western blotting, and PCR confirmed the presence of individual Mycoplasma species. Identification of individual mycoplasma species was consistent between methods (Table 4).
TABLE 3.
Number of patients, mean age of patients, and median CD4 count according to CDC classification
| CDC classification | No. of patients | Mean (range) age (yr) | Median CD4 count (no. of cells/mm3) |
|---|---|---|---|
| N | 1 | 11.5 | 744 |
| A | 3 | 6.0 (2.5–6.0) | 300 |
| B | 2 | 2.7 (0.6–4.10) | 654 |
| C | 9 | 5.0 (1.4–13.3) | 280 |
TABLE 4.
Preliminary identification of mycoplasmas isolated from pediatric patients
| Mycoplasma species | Biochemical test result
|
PCR result
|
||||
|---|---|---|---|---|---|---|
| Glucose utiliza-tion | Arginine hydro-lysis | Urease hydro-lysis | Size (bp) of 16S-23S rRNA product | HindIIIb | VspIb | |
| M. penetrans | + | + | − | 295 | − | − |
| M. fermentans | + | + | − | 364 | + | + |
| M. pirum | + | + | − | 323 | + | + |
| M. genitalium | + | − | − | 253 | − | + |
| M. hominis | − | + | − | 236 | − | + |
Second PCR products.
Digestion with restriction enzymes HindIII and VspI.
Table 4 presents the data obtained from biochemical tests and DNA analysis by PCR that was used to identify the species of mycoplasmas isolated from the HIV-positive pediatric patients. By use of the results from the preliminary genus identification procedures recommended by the Subcommittee for the Taxonomy of Mollicutes, namely, (i) colony appearance (typical fried-egg appearance), (ii) filterability (isolates can be passed through a 0.2-μm-pore-size filter), (iii) absence of reversion in antibiotic-free growth medium, and (iv) biochemical characteristics, and by use of DNA analysis, the identities of the isolates were confirmed.
Mycoplasmas were isolated from 13 (87%) of 15 children who tested positive for HIV and from 3 (20%) of 15 HIV-negative controls. The difference was statistically significant (P < 0.001). Table 5 presents the age, CDC classification, median CD4 count, and Mycoplasma species for patients from whom more than one mycoplasma was isolated. The isolation of multiple mycoplasma species from 5 (33%) of 15 HIV-positive patients ranging from ages 2.3 to 11.0 years was random and did not correlate with the median CD4 counts (Table 5). However, four (80%) of these five patients had been classified in CDC class C and, accordingly, showed severe degrees of immunologic suppression. The distribution of HIV-positive patients according to the degree of immunologic suppression and CDC classification is presented in Table 6. Five (33%) of the 15 patients were severely immunosuppressed, 7 (47%) of the 15 patients were classified as moderately immunosuppressed, and 3 (20%) of the 15 patients showed no signs of immunosuppression. Table 6 also presents the distribution of patients according to age and immunologic suppression: 8 (53%), 6 (40%), and 1 (7%) of the 15 patients belonged to the age groups of >6, 1 to 5, and <1 year, respectively. In two urine specimens from HIV-positive children, mycoplasma-like organisms were observed, but identification was not possible because repeated attempts at the isolation of pure colonies on agar plates were hindered due to contamination with other organisms.
TABLE 5.
Age, CDC classification, and median CD4 count for patients from whom more than one Mycoplasma species was isolated
| Age (yr) | CDC classi-fication | Median CD4 count (no. of cells/mm3) | Mycoplasma species isolated |
|---|---|---|---|
| 2.3 | C2 | 696 | M. hominis, M. penetrans |
| 6.0 | A3 | 90 | M. hominis, U. urealyticum |
| 9.8 | C2 | 330 | M. hominis, U. urealyticum |
| 9.8 | C2 | 280 | M. hominis, M. penetrans |
| 11.0 | C3 | 30 | M. hominis, M. penetrans |
TABLE 6.
Distribution of patients according to degree of immunologic suppression, age, and CDC classification
| Degree of immunologic suppression | No. of patients with the following CDC classification:
|
No. of patients of the following age (median CD4 count [no. of cells/mm3]):
|
|||||
|---|---|---|---|---|---|---|---|
| N | A | B | C | <12 mo | 1–5 yr | >6 yr | |
| None | 1 | 1 | 1 | 2 (1,425) | 1 | ||
| Moderate | 1 | 2 | 4 | 1 (859) | 2 (572) | 4 | |
| Severe | 1 | 4 | 2 (98) | 3 | |||
M. hominis and U. urealyticum.
M. hominis and U. urealyticum were identified in the urine from both HIV-negative controls and HIV-positive children. The difference in the rate of isolation of M. hominis and U. urealyticum from HIV-positive and HIV-negative controls was not statistically significant. The average ages of the controls and the HIV-positive patients from whom M. hominis was isolated were 7.0 and 9.0 years, respectively. The average ages of the controls and the HIV-positive patients from whom U. urealyticum was isolated were 8.0 and 8.4 years, respectively.
AIDS-associated mycoplasmas.
Table 7 presents the frequency of isolation of Mycoplasma species from controls and from HIV-positive children according to CDC classification, and Table 8 presents the frequency of isolation of Mycoplasma species by age. M. penetrans, M. fermentans, M. genitalium, and M. pirum were isolated only from children with a CDC classification of B or C.
TABLE 7.
Mycoplasma species isolated from HIV-negative controls and HIV-positive children according to CDC classification
| CDC classification | No. of patients | No. (%) of each species isolated
|
|||||
|---|---|---|---|---|---|---|---|
| M. hominis | U. urealyticum | M. genitalium | M. pirum | M. fermentans | M. penetrans | ||
| Control | 15 | 3 (20) | 3 (20) | ||||
| N | 1 | 1 (100) | |||||
| A | 3 | 2 (67) | 2 (67) | ||||
| B | 2 | 1 (50) | |||||
| C | 9 | 4 (44) | 1 (11) | 1 (11) | 1 (11) | 1 (11) | 5 (55.5) |
TABLE 8.
Mycoplasma species isolated from HIV-positive children according to age
| Age (yr) | No. of patients | Median CD4 count (no. of cells/mm3) | No. (%) of each species isolated
|
|||||
|---|---|---|---|---|---|---|---|---|
| M. hominis | U. urealyticum | M. genitalium | M. pirum | M. fermentans | M. penetrans | |||
| <1 | 1 | 859 | ||||||
| 1–5 | 6 | 572 | 1 (17) | 1 (17) | 1 (17) | 2 (33) | ||
| >6 | 8 | 290 | 6 (75) | 3 (38) | 1 (13) | 4 (50) | ||
DISCUSSION
We identified AIDS-associated mycoplasmas more frequently from the urine of HIV-positive children than from the urine of HIV-negative controls (P < 0.001). To our knowledge, this study is the first to report the identification of AIDS-associated mycoplasmas in the urine of HIV-positive children. Mycoplasma isolation (culture) is notoriously difficult and requires screening of multiple urine samples in a variety of media. Although difficult and laborious, direct isolation is considered the “gold standard” for the detection of these organisms. This is in contrast to techniques such as PCR, which provide only indirect evidence (19).
The species distribution of mycoplasmas isolated from HIV-positive and HIV-negative children was different. None of the AIDS-associated mycoplasmas were isolated from HIV-negative children. Only M. hominis and U. urealyticum were isolated from these children. These mycoplasmas are commensal hosts of the urogenital tracts of women (15). As many as 80% of sexually active asymptomatic women are colonized with U. urealyticum (6, 7, 38). Younger age, lower socioeconomic status, sexual activity with multiple partners, and oral contraceptive use are associated with increased rates of colonization (6, 7). Vertical transmission of mycoplasmas from the genital tract of the mother can occur either in utero or at delivery (7, 38, 45). The rate of transmission to full-term infants born to colonized mothers is 45 to 66% (7, 38). Colonization of infants is reported to be transient, with isolation rates decreasing after 3 months of age, although preterm infants might carry these organisms in the respiratory tract for several months (45). The ages of the three HIV-negative controls from whom M. hominis or U. urealyticum was isolated were 8.0, 10.0, and 11.0 years, respectively. It is possible that the M. hominis and U. urealyticum organisms identified in the HIV-negative children in our study reflect the persistence of these organisms from the newborn period. Several of the HIV-negative controls were adolescents, and it is possible that they were sexually active, which might provide an alternate explanation for the presence of these organisms. M. hominis and U. urealyticum were isolated more commonly from HIV-positive children, although the result was not statistically significant. As with infection due to M. hominis and U. urealyticum, HIV infection is more frequent in mothers of younger age and lower socioeconomic status and in those who are sexually active with multiple partners. Vertical transmission of HIV to the child of an HIV-positive mother is possible in utero, during delivery, and with breast-feeding (39). It is possible that HIV and these mycoplasmas are vertically transmitted at similar times during gestation or delivery.
M. penetrans and other AIDS-associated mycoplasmas were identified only from older HIV-positive children with a CDC classification of B or C. AIDS-associated mycoplasmas were not identified in the urine of the single HIV-positive child who was under the age of 1 year or from the four HIV-positive children with a CDC classification of N or A. It is possible that we did not identify AIDS-associated mycoplasmas in HIV-positive children under the age of 1 year or in those with a CDC classification of N or A because of the small number of patients in our study.
M. penetrans was isolated from 6 (54.5%) of the 11 children with a CDC classification of B or C and 5 (55.5%) of the 9 children with a CDC classification of C. M. penetrans was isolated from more than one urine specimen from two of the HIV-positive patients. Both patients were over 1 year of age and had CDC classifications of B and C, respectively. M. penetrans was isolated in the last two of four urine specimens from one patient and the last four of the five urine specimens from the other patient. The ages of the first patient at the times of collection of the four urine specimens were 2.0, 2.1, 2.2, and 2.3 years, respectively. The ages of the second patient were 8.7, 8.8, 8.9, 9.0, and 9.8 years, respectively. Little is known about the temporal relationship of AIDS-associated mycoplasmas to infection with HIV. The data for these two patients suggest that M. penetrans is acquired after birth by children who acquire HIV perinatally and persists in the urine for as long as 1.0 year.
Unidentifiable mycoplasmas were found in the urine of two of the HIV-positive children. This raises the possibility of as yet undiscovered Mycoplasma species in patients with AIDS. It is also possible that the unidentified mycoplasmas were known species not considered to be human pathogens.
There are numerous reports of the isolation of AIDS-associated mycoplasmas from HIV-positive adults (8, 18, 20, 21). In most studies, these species are isolated more frequently from HIV-positive adults than from controls (8, 18). These observations suggest that mycoplasmas might be a cofactor or a copathogen in the pathogenesis of AIDS. The term “cofactor” implies that an AIDS-associated mycoplasma acts synergistically with HIV in the pathogenesis of AIDS, whereas the term “copathogen” implies that infection with an AIDS-associated mycoplasma requires both HIV infection and associated immunodeficiency. Our study confirms that AIDS-associated mycoplasmas are more common in HIV-positive children than in HIV-negative controls and therefore provides modest support for the hypothesis that these mycoplasmas might be cofactors or copathogens in children with AIDS. Numerous reports support this possibility (4, 27–29, 31, 33, 34, 37, 40). The variation in the time between the acquisition of HIV infection and the development of symptoms and signs of disease and the low frequency of HIV-infected lymphocytes in the peripheral blood of infected individuals suggest the possibility of alternate factors. Mycoplasmas might act as a cofactor by enhancing virus replication or accelerating disease progression (9, 22). The idea is supported by the observation that treatment of HIV-infected cell cultures with tetracycline analogues active against mycoplasmas inhibits cell killing without affecting virus replication and that certain mycoplasmas enhance the cytopathic changes brought about by HIV (9, 23, 24, 27). As well, M. fermentans is reported to enhance the in vitro cytopathic effect in CEM and U937 cells (24). Additionally, mycoplasmas have been shown in vitro to be powerful immunomodulators that induce the activation of B or T lymphocytes, to stimulate cytokine secretion by several cell types, and to produce superantigens (4, 12, 25, 32, 37). Furthermore, mycoplasma adhesion peptide has biologic similarities to CD4 and class II major histocompatibility complex proteins (36).
In conclusion, Mycoplasma species were identified in the urine of HIV-positive children. AIDS-associated mycoplasmas were found statistically more frequently in the urine of HIV-positive children than in the urine of HIV-negative controls. M. penetrans was the most frequently isolated AIDS-associated mycoplasma and was found in 45% of the children with a CDC classification of B or C and 55.5% of the children with a CDC classification of C.
ACKNOWLEDGMENTS
This work was supported by a grant from the Greenville Hospital System/Clemson University Biomedical Cooperative. Reagents were obtained through the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. The M. hominis monoclonal antibody (monoclonal antibody 10A4.11) was obtained from H. Watson and G. Cassell; M. penetrans, M. pirum, and M. genitalium antisera were obtained from Ricardo Rosenbusch, and M. fermentans antiserum was obtained from J. G. Tully.
We thank Missy Hathcox for assistance in preparation of the manuscript.
REFERENCES
- 1.Aluotto B B, Wittler R G, Williams C O, Faber J E. Standardized bacteriologic techniques for the characterization of Mycoplasma species. Int J Syst Bacteriol. 1970;20:35–58. [Google Scholar]
- 2.Bauer F A, Wear D J, Angritt P, Lo S-C. Mycoplasma fermentans (incognitus strain) infection in the kidneys of patients with aquired immunodeficiency syndrome and associated nephropathy: a light microscopic, immunohistochemical, and ultrastructural study. Hum Pathol. 1991;22:63–69. doi: 10.1016/0046-8177(91)90063-u. [DOI] [PubMed] [Google Scholar]
- 3.Behbahani N, Blanchard A, Cassell G H, Montagnier L. Phylogenetic analysis of Mycoplasma penetrans, isolated from HIV-infected patients. FEMS Microbiol Lett. 1993;109:63–66. doi: 10.1111/j.1574-6968.1993.tb06144.x. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard A, Montagnier L. AIDS-associated mycoplasmas. Annu Rev Microbiol. 1994;48:687–712. doi: 10.1146/annurev.mi.48.100194.003351. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell M B, Oxtoby M J, Simonds R J, Lindegren M L, Rogers M F the Centers for Disease Control and Prevention. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morbid Mortal Weekly Rep. 1994;43(No. RR-12):1–10. [Google Scholar]
- 6.Carey J C, Blackwelder W C, Nugent R P, Matteson M A, Rao A V, Eschenbach D A, Lee M L F, Rettig P J, Regan J A, Geromanos K L, Martin D H, Pastorek J G, Gibbs R S, Lipscomb K A the Vaginal Infections and Prematurity Study Group. Antepartum cultures for Ureaplasma urealyticum are not useful in predicting pregnancy outcome. Am J Obstet Gynecol. 1991;164:728–733. doi: 10.1016/0002-9378(91)90505-l. [DOI] [PubMed] [Google Scholar]
- 7.Cassell G H, Waites K B, Watson H L, Crouse D T, Harasawa R. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clin Microbiol Rev. 1993;6:69–87. doi: 10.1128/cmr.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirgwin K D, Cummings M C, DeMeo L R, Murphy M, McCormack W M. Identification of mycoplasmas in urine from persons infected with human immunodeficiency virus. Clin Infect Dis. 1993;17(Suppl.):S264–S266. doi: 10.1093/clinids/17.supplement_1.s264. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury M I H, Munakata T, Koyanagi Y, Kobayashi S, Arai S, Yamamoto N. Mycoplasma can enhance HIV replication in vitro: a possible cofactor responsible for the progression of AIDS. Biochem Biophys Res Commun. 1990;170:1365–1370. doi: 10.1016/0006-291x(90)90545-x. [DOI] [PubMed] [Google Scholar]
- 10.Clyde W A., Jr Growth inhibition tests. Methods Mycoplasmol. 1983;1:405–410. [Google Scholar]
- 11.Cole B C, Atkin C L. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol Today. 1991;12:271–276. doi: 10.1016/0167-5699(91)90125-D. [DOI] [PubMed] [Google Scholar]
- 12.Feng S-H, Lo S-C. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans. Infect Immun. 1994;62:3916–3921. doi: 10.1128/iai.62.9.3916-3921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freundt E A. Culture media for classic mycoplasmas. Methods Mycoplasmol. 1983;1:127–135. [Google Scholar]
- 14.Gallagher S, Smith J A. One-dimensional polyacrylamide gel electrophoresis of proteins. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 15.Grattard F, Soleihac B, de Barbeyrac B, Bebear C, Seffert P, Pozzetto B. Epidemiologic and molecular investigations of genital mycoplasmas from women and neonates at delivery. Pediatr Infect Dis J. 1995;14:853–858. doi: 10.1097/00006454-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Grau O, Kovacic R, Griffais R, Launay V, Montagnier L. Development of PCR-based assays for the detection of two human mollicute species, Mycoplasma penetrans and M. hominis. Mol Cell Probes. 1994;8:139–148. doi: 10.1006/mcpr.1994.1019. [DOI] [PubMed] [Google Scholar]
- 17.Grau O, Kovacic R, Griffais R, Montagnier L. Development of a selective and sensitive polymerase chain reaction assay for the detection of Mycoplasma pirum. FEMS Microbiol Lett. 1993;106:327–334. doi: 10.1111/j.1574-6968.1993.tb05984.x. [DOI] [PubMed] [Google Scholar]
- 18.Grau O, Slizewicz B, Tuppin P, Launay V, Bourgeois E, Sagot N, Moynier M, Lafeuillade A, Bachelez H, Clauvel J-P, Blanchard A, Bahraoui E, Montagnier L. Association of Mycoplasma penetrans with human immunodeficiency virus infection. J Infect Dis. 1995;172:672–681. doi: 10.1093/infdis/172.3.672. [DOI] [PubMed] [Google Scholar]
- 19.Harasawa R, Mizusawa H, Nozawa K, Nakagawa T, Asada K, Kato I. Detection and tentative identification of dominant mycoplasma species in cell cultures by restriction analysis of the 16S-23S rRNA intergenic spacer regions. Res Microbiol. 1993;144:489–493. doi: 10.1016/0923-2508(93)90057-9. [DOI] [PubMed] [Google Scholar]
- 20.Hayes M M, Foo H-H, Timenetsky J, Lo S-C. In vitro antibiotic susceptibility testing of clinical isolates of Mycoplasma penetrans from patients with AIDS. Antimicrob Agents Chemother. 1995;39:1386–1387. doi: 10.1128/aac.39.6.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katseni V L, Gilroy C B, Ryait B K, Ariyoshi K, Bieniasz P D, Weber J N, Taylor-Robinson D. Mycoplasma fermentans in individuals seropositive and seronegative for HIV-1. Lancet. 1993;341:271–273. doi: 10.1016/0140-6736(93)92617-3. [DOI] [PubMed] [Google Scholar]
- 22.Lancet Mycoplasma and AIDS—what connection? Lancet. 1991;337:20–22. . (Editorial.) [PubMed] [Google Scholar]
- 23.Lemaître M, Guétard D, Hénin Y, Montagnier L, Zerial A. Protective activity of tetracycline analogs against the cytopathic effect of the human immunodeficiency viruses in CEM cells. Res Virol. 1990;141:5–16. doi: 10.1016/0923-2516(90)90052-k. [DOI] [PubMed] [Google Scholar]
- 24.Lemaitre M, Henin Y, Destouesse F, Ferrieux C, Montagnier L, Blanchard A. Role of mycoplasma infection in the cytopathic effect induced by human immunodeficiency virus type 1 in infected cell lines. Infect Immun. 1992;60:742–748. doi: 10.1128/iai.60.3.742-748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo S-C. Mycoplasmas and AIDS. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 525–545. [Google Scholar]
- 26.Lo S-C, Dawson M S, Newton III P B, Sonoda M A, Shih J W-K, Engler W F, Wang R Y-H, Wear D J. Association of the virus-like infectious agent originally reported in patients with AIDS with acute fatal disease in previously healthy non-AIDS patients. Am J Trop Med Hyg. 1989;41:364–376. [PubMed] [Google Scholar]
- 27.Lo S-C, Tsai S, Benish J R, Shih J W, Wear D J, Wong D M. Enhancement of HIV-1 cytocidal effects in CD4+ lymphocytes by the AIDS-associated mycoplasma. Science. 1991;251:1074–1076. doi: 10.1126/science.1705362. [DOI] [PubMed] [Google Scholar]
- 28.Lo S-C, Wear D J, Shih J W-K, Wang R Y-H, Newton III P B, Rodriguez J F. Fatal systemic infections of nonhuman primates by Mycoplasma fermentans (incognitus strain) Clin Infect Dis. 1993;17(Suppl. 1):S283–S288. doi: 10.1093/clinids/17.supplement_1.s283. [DOI] [PubMed] [Google Scholar]
- 29.Lo S-C, Wang R Y-H, Newton III P B, Yang N-Y, Sonoda M A, Shih J W-K. Fatal infection of silvered leaf monkeys with a virus-like infectious agent (VLIA) derived from a patient with AIDS. Am J Trop Med Hyg. 1989;40:399–409. doi: 10.4269/ajtmh.1989.40.399. [DOI] [PubMed] [Google Scholar]
- 30.Miles S A, Balden E, Magpantay L, Wei L, Leiblein A, Hofheinz D, Toedter G, Stiehm E R, Bryson Y the Southern California Pediatric AIDS Consortium. Rapid serologic testing with immune-complex-dissociated HIV p24 antigen for early detection of HIV infection in neonates. N Engl J Med. 1993;328:297–302. doi: 10.1056/NEJM199302043280501. [DOI] [PubMed] [Google Scholar]
- 31.Minion F C, Jarvill-Taylor K. Membrane-associated hemolysin activities in mycoplasmas. FEMS Microbiol Lett. 1994;116:101–106. doi: 10.1111/j.1574-6968.1994.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 32.Montagnier L, Blanchard A. Mycoplasmas as co-factors in infection due to the human immunodeficiency virus. Clin Infect Dis. 1993;17(Suppl. 1):S309–S315. [PubMed] [Google Scholar]
- 33.Nir-Paz R, Israel S, Honigman A, Kahane I. Mycoplasmas regulate HIV-LTR-dependent gene expression. FEMS Microbiol Lett. 1995;128:63–68. doi: 10.1111/j.1574-6968.1995.tb07501.x. [DOI] [PubMed] [Google Scholar]
- 34.Poulin S A, Perkins R E, Kundsin R B. Antibiotic susceptibilities of AIDS-associated mycoplasmas. J Clin Microbiol. 1994;32:1101–1103. doi: 10.1128/jcm.32.4.1101-1103.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razin S, Freundt E A. The mycoplasmas. In: Bryant M P, Holt J G, Pfennig N, Staley J T, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 740–789. [Google Scholar]
- 36.Root-Bernstein R S, Hobbs S H. Homologies between mycoplasma adhesion peptide, CD4 and class II MHC proteins: a possible mechanism for HIV-mycoplasma synergism in AIDS. Res Immunol. 1991;142:519–523. doi: 10.1016/0923-2494(91)90096-2. [DOI] [PubMed] [Google Scholar]
- 37.Ruuth E, Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez P J, Regan J A. Vertical transmission of Ureaplasma urealyticum in full term infants. Pediatr Infect Dis J. 1987;6:825–828. doi: 10.1097/00006454-198709000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Scarlatti G. Paediatric HIV infection. Lancet. 1996;348:863–868. doi: 10.1016/S0140-6736(95)11030-5. [DOI] [PubMed] [Google Scholar]
- 40.Shibata K-I, Sasaki T, Watanabe T. AIDS-associated mycoplasmas possess phospholipases C in the membrane. Infect Immun. 1995;63:4174–4177. doi: 10.1128/iai.63.10.4174-4177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subcommittee on the Taxonomy of Mollicutes. Proposal of minimal standards for descriptions of new species of the class Mollicutes. Int J Syst Bacteriol. 1979;29:172–180. [Google Scholar]
- 42.Tully J G. Cultivation and morphology. In: Tully J G, editor. Molecular and diagnostic procedures in mycoplasmology. Vol. 1. San Diego, Calif: Academic Press, Inc.; 1995. pp. 29–33. [Google Scholar]
- 43.Tully J G. General cultivation techniques for mycoplasmas and spiroplasmas. Methods Mycoplasmol. 1993;1:99–103. [Google Scholar]
- 44.van Kuppeveld F J M, van der Logt J T M, Angulo A F, van Zoest M J, Quint W G V, Niesters H G M, Galama J M D, Melchers W L G. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol. 1992;58:2606–2615. doi: 10.1128/aem.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waites K B, Crouse D T, Cassell G H. Systemic neonatal infection due to Ureaplasma urealyticum. Clin Infect Dis. 1993;17(Suppl. 1):S131–S135. doi: 10.1093/clinids/17.supplement_1.s131. [DOI] [PubMed] [Google Scholar]
- 46.Whitcomb R F, Tully J G, Bové J M, Bradbury J M, Christiansen G, Kahane I, Kirkpatrick B C, Laigret F, Leach R H, Neimark H C, Pollack J D, Razin S, Sears B B, Taylor-Robinson D. Revised minimum standards for description of new species of the class Mollicutes (division Tenericutes) Int J Syst Bacteriol. 1995;45:605–612. [Google Scholar]