Abstract
Distal aortic compression of the lower esophagus with consecutive dilatation of the proximal esophagus was first described in the literature in 1932. Here, the authors describe the case of a 66 year-old male complaining of gastroesophageal reflux. Due to a positive family history of carcinoma of the esophagus and compression of the dorsal esophagus during an esophageal barium swallow test, further tests were performed. Endoscopy and CT exam revealed a dilated esophagus due to compression of a crossing aorta. Because of mild symptoms and the absence of dysphagia, no further treatment was necessary except for the use of a proton pump inhibitor and recommended follow-ups every one to two years.
Keywords: Aortic compression of the esophagus, barium swallow, dysphagia aortica, achalasia
Introduction
Aortic compression of the esophagus was first mentioned in the literature in 1932 by Pape 1 as the cause of “dysphagia aortica,” described as a difficulty in swallowing caused by external compression due to age-related elongation of the aorta.
In 1969, McMillan and Hyde2 discussed how compression of the lower esophagus that caused dysphagia was often misdiagnosed in elderly women. The causes of the compression were said to be, on the one hand, the elongation and dilation of the aorta and, on the other hand, the spine becoming kyphotic in elderly individuals. The authors presented seven cases of female patients aged 63–85 years, all complaining of dysphagia and retrosternal pain. In four cases, a conservative procedure was initiated, and two of the patients were treated using endoscopic bougie dilatation. The other patient underwent surgical intervention. The authors classified four severity levels of dysphagia and stated that only moderate or severe dysphagia would require endoscopic or surgical intervention.
Other authors also established this phenomenon among patients, mostly elderly women with a history of hypertensive or arteriosclerotic heart failure, in many cases with kyphosis.3–6
Almost all patients had gone to the doctor because of dysphagia, while complaints of heartburn or abdominal or retrosternal pain were less common. In general, the greater the number of symptoms, especially dysphagia, the more explicit the findings on CT images and barium swallow were. Manometric findings provide additional important information.4 Esophagoduodenoscopy with histology can exclude important differential diagnoses such as achalasia and Barrett’s esophagus.
Case report
Due to a positive family history of esophageal cancer and episodes of heartburn, a 66-year-old male underwent an endoscopic gastric examination in 2013, where gastritis was suspected. Furthermore, a small mucous tongue was revealed at the level of the diaphragmatic opening suspicious for Barrett’s esophagus. The histological examinations revealed type C gastritis, so no Helicobacter pylori eradication treatment was necessary. Due to intermittent heartburn and regurgitation, treatment with a proton pump inhibitor (PPI) was implemented.
On a chest CT, which was conducted in November 2015 to investigate a chronic cough, a long segment widening of the esophagus was described as an incidental finding without evidence of tumor. On endoscopy, once again, there was no evidence of dysplasia. Following esophageal manometry, achalasia was suspected. With long-term treatment in the form of a PPI, the patient was free of complaints. Therefore, the patient had GERD-like symptoms (gastroesophageal reflux disease), and he also suffered from a hiatus herniation with moderate reflux disease, which was effectively better treated with PPI. These circumstances made it hard to establish the diagnosis. A follow-up examination with endoscopy was conducted in 2016. At this point, the patient did not complain of dysphagia and had hardly any other symptoms or findings.
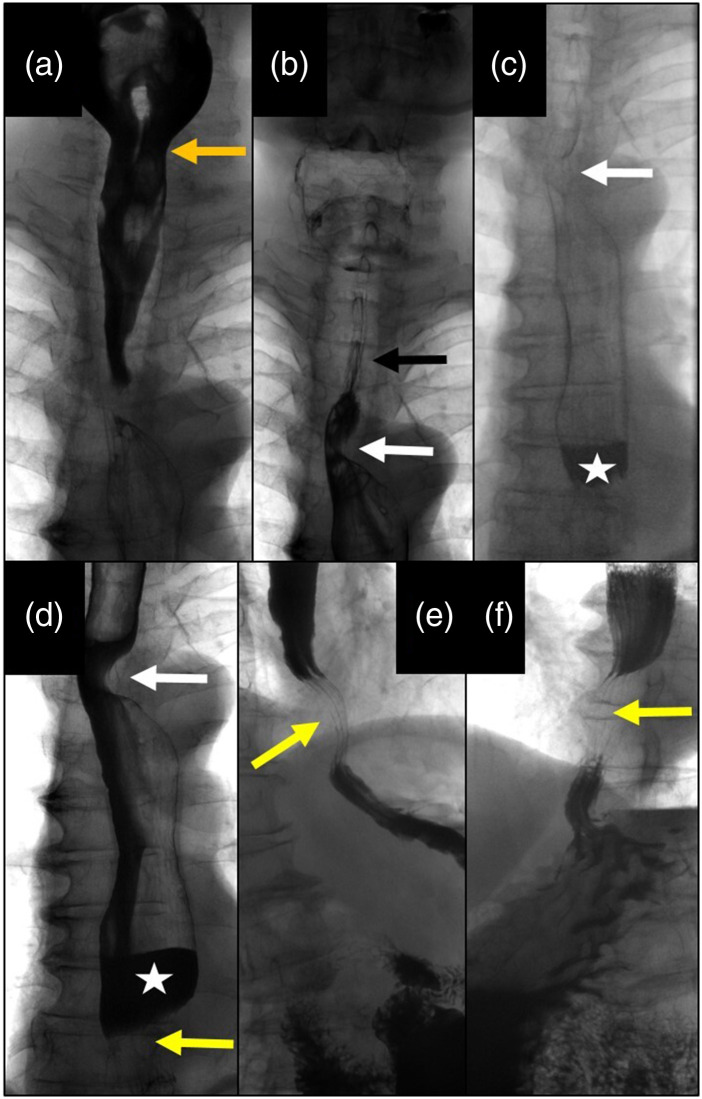
In August 2018, a barium swallow examination was performed as a follow-up exam. This showed regular oral and pharyngeal phases of deglutition. The upper esophagus was not distended and exhibited a proper mucosal surface (Fig. 1(a)). A harmonic dorsal impression of the esophagus due to prominent Arteria lusoria was demonstrated at the level of the aortic arch. (Fig. 1(b)). During deglutition, the retrocardiac esophagus was dilated by 4 cm. In addition, an air-fluid level, delayed peristalsis, and tertiary contractions could be seen (Fig. 1(c)). Three centimeters above the diaphragm, a harmonic dorsal impression with ventral dislocation of the esophagus could be demonstrated. The length of compression of the esophagus measured 2.5 cm, with a prominent contrast filling defect (Figs. 1(d)–(f)). Distally, the esophagus presented a regular diameter, and the cardia showed a regular opening up to 2 cm with a regular mucosal surface. There was normal mucous folding of the stomach and rapid gastric passage into the duodenum. There was no constant sphincter opening typically seen in patients with scleroderma and no signs of achalasia; the mucosal surface was normal with no signs of cancer.
Fig. 1.
Barium swallow images: (a) Normal pharyngeal phase with regular opening of the upper sphincter. (b) Normal barium passage through the upper esophagus with a regular mucosal surface (black arrow): lateral impression due to an accessory arteria lusoria (white arrow). (c) Dilation of the distal esophagus with slow passage of barium and formation of an air-barium level (white asterisk). (d) Distal esophagus dilated with air-barium level (asterisk) due to aortic compression (lower arrow); note the impression of the arteria lusoria (upper arrow). (e) Right lateral view of the aortic impression of the distal esophagus (arrow). (f) Left lateral view of the esophageal compression 3 cm above the diaphragm due to the crossing aorta (arrow) with upstream barium congestion.
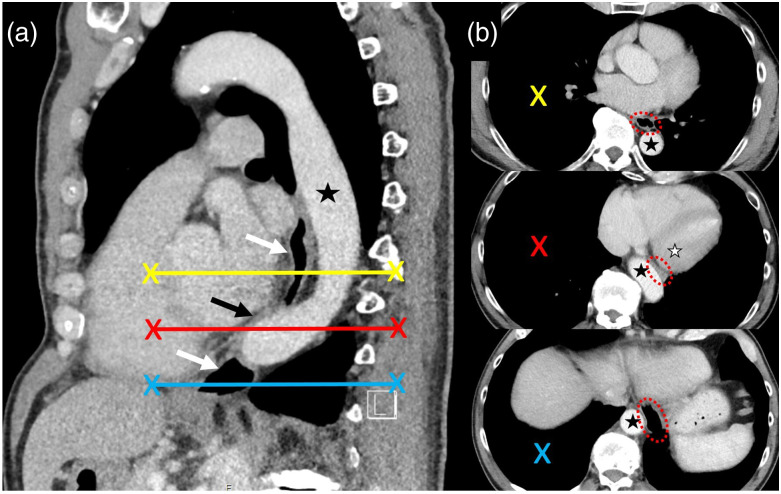
The chest CT images from 2015 retrospectively revealed the cause of the esophageal impression: the esophagus was pinched between the left ventricle and aorta. The aorta crossed the spine right at that level, which caused compression of the esophagus (Fig. 2).
Fig. 2.(.
a) Sagittal CT reformation through the aorta (black asterisk) and esophagus (white arrows). Aortic kinking with compression of the esophagus (black arrow). (b) Corresponding axial CT slices with highlighted esophagus (red circle), which is compressed between the aorta (black asterisk) and left heart (white asterisk).
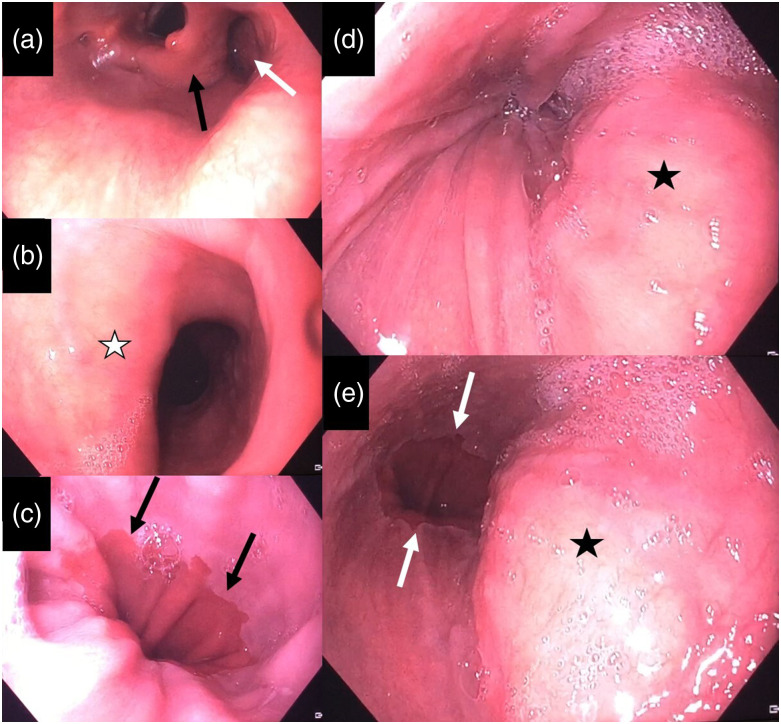
For further clarification, another esophagogastroduodenoscopy was conducted. This examination showed a normal z-line and no signs of reflux esophagitis (Figs. 3(a)–(c)). Furthermore, a small axial hiatal hernia was detected. The dilation of the esophagus could be confirmed, matching the barium swallow. The distal esophagus demonstrated dorsal bulging with arterial pulsation without mucosal damage. The endoscope easily passed this aortic narrowing of the esophagus into the normal stomach (Figs. 3(d) and (e)).
Fig. 3.
Endoscopic images of the esophagus. (a) Normal hypopharyngeal region with the larynx (black arrow) and adjacent piriform sinus (white arrow). (b) Upper esophagus with a profound fold on the left side (white asterisk) due to accessory arteria lusoria. (c) Regular Z-line of the distal esophagus (black arrows). (d) Pulsating mass in the distal esophagus (black asterisk), corresponding to the crossing aorta with compression of the esophagus. (e) Endoscopic passage is possible with view of the Z-line.
Due to good esophageal patency, no further treatment was necessary. With regard to the positive family anamnesis of esophageal cancer, a follow-up of alternating barium swallow and esophagogastroduodenoscopy are recommended at intervals of one to two years.
Discussion
The esophagus usually begins its descent into the mediastinum to the right of the aorta. It crosses the aorta at the aorta–esophageal decussation site in the lower third of the posterior mediastinum before penetrating the diaphragm through the diaphragmatic hiatus.7 Compression of the lower esophagus is not a rare condition, but it is often misdiagnosed or left undetected.2 There are risk factors in favor of the diagnosis, namely, being female, older, and having a history of heart disease and kyphosis.2,5,6 With age, the aorta becomes sclerotic and elongated. Kyphosis allows the aorta to elongate dorsally.
Bar-On et al.8 described a severe case of a patient with acute scoliosis whose esophagus was pinched between the aorta and the spine.
When metaplasia, such as Barrett’s esophagus or malignancy, is suspected, diagnostic examinations using barium swallow, manometry, and esophagogastroduodenoscopy with histopathological examination are necessary. One should also exclude achalasia to avoid a pointless intervention such as the Heller procedure.
Our patient had GERD-like symptoms, he also suffered from a hiatus herniation with moderate reflux disease, which was effectively better treated with PPI. These circumstances made it hard to establish the diagnosis. With mild symptoms or only heartburn but no dysphagia, as in our case, treatment with PPI with follow-ups and periodic barium swallows and esophagogastroduodenoscopy every two years are usually sufficient.
In more severe cases, an endoscopic bougie procedure or a surgical intervention to implement anterolateral transposition of the esophagus should be considered. In our case, neither dilatation nor other opening therapy was effective, because of the compression effect caused by the pulsating aorta. At that time, the initial endoscopist did not realize the functional compression.
Treatment options are endoscopic dilation with bougies or, more invasively, a thoracic endovascular aortic repair procedure.9 However, a conservative approach is usually sufficient, together with a PPI and follow-up examinations.
Other masses in the middle mediastinum need to be considered in the differential diagnosis of esophageal compression from outside, such as lymphoma; metastases; multiple myeloma; extramedullary hematopoiesis; sarcoid, bronchogenic, or pericardial cyst; and intrathoracic goiter.10 Chest CT helps tremendously in further investigating these entities.
Our case demonstrates a rare differential diagnosis of achalasia, which needs to be considered to avoid dangerous diagnostic or therapeutic procedures.
Footnotes
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data availability statement: Data is available upon special request.
ORCID iDs
Manuela Tomforde https://orcid.org/0000-0002-4758-4109
Mathias Sturzenegger https://orcid.org/0000-0001-5565-2530
References
- 1.Pape R. Uber einen abnormen verlauf (‘tiefe Rechtslage’) der mesa aotitischen aorta descendens. Fortschr Roetgenstr 1932;46:257–269. [Google Scholar]
- 2.McMillan IKR, Hyde I. Compression of the oesophagus by the aorta. Thorax 1969;24:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keates PG, Magidson O. Dysphagia associated with sclerosis of the aorta. Br J Radiol 1955;28:184–190. [DOI] [PubMed] [Google Scholar]
- 4.Mittal RK, Siskind BN, Hongo M, et al. Dysphagia aortica. Dig Dis Sci 1986;31:379–384. [DOI] [PubMed] [Google Scholar]
- 5.Kim J-H, Jang S-W, Kim D-B, et al. A patient with dysphagia due to an aortic aneurysm. Korean Circ J 2009;39:258–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouawad NJ, Ahluwalia GS. Dysphagia in the aging cardiovascular patient. J Thorac Dis 2017;9:E1005–E1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snell RS. Clinicalanatomy for medical student. 6th ed.Philadelphia, PA: Lippincott Williams & Wilkins, 2000. [Google Scholar]
- 8.Bar-On E, Harari M, Floman Y, et al. Compression of the esophagus by the spine and the aorta in untreated scoliosis. Arch Orthop Trauma Surg 1998;117:405–407. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui J, Hughes F. Dysphagia due to thoracic aortic aneurysm, relieved by thoracic endovascular aneurysm repair: a case report and review of the literature. Case Rep 2011;2011:bcr0920114793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juanpere S, Cañete N, Ortuño P, et al. A diagnostic approach to the mediastinal masses. Insights Imaging 2013;4:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]