Abstract
Aging is associated with the highest risk for morbidity and mortality to chronic or metabolic diseases, which are present in fifty percent of the elderly. Improving metabolic and immune function of the elderly would improve quality of life and reduce risk for all other diseases. Tissue resident macrophages and the NLRP3 inflammasome are established drivers of inflammaging and metabolic dysfunction. Energy sensing-signaling pathways connect sterile and metabolic inflammation with cellular senescence and tissue dysfunction. We discuss recent advances in the immunometabolism field. Common themes revealed by recent publications include the alterations in metabolic signaling (SIRTUIN, AMPK or mTOR pathways) in aged immune cells, the impact of senescence on inflammaging and tissue dysfunction and the age-related changes in metabolic tissues, especially adipose tissue, as an immunological organ. Promising gerotherapeutics are candidates to broadly target nutrient and energy sensing, inflammatory and senescence pathways, and have potential to improve healthspan and treat age-related diseases.
Introduction
Age is the greatest risk factor for chronic diseases such as metabolic disease, cancer and neurodegenerative diseases [1]. The pillars of aging, including inflammation and metabolism, define key mechanistic areas of research that are critical to the aging process and age-related diseases [2]. Inflammaging, described as the accumulating chronic low-grade inflammation with age, drives age-related pathology including metabolic disease, autoimmunity and frailty [3]. In metabolic tissues, inflammaging leads to excess adiposity, impaired metabolism, impaired lipolytic signaling and reduced metabolic flexibility. Recent work has identified direct links of inflammaging and metabolic dysfunction to cellular senescence. Although further work is still needed to clarify how immunosenescence is a part of cellular senescence, this research identifies intersecting points of immunometabolism, immunosenescence and inflammaging at the molecular or cellular level and within critical tissues for a greater understanding of age-related diseases.
Cellular senescence is a cell state where irreversible cell cycle arrest is elicited via various cellular stresses or macromolecular damage [4]. Senescent cells accumulate with age, especially in metabolic tissues such as white adipose tissue (AT) and liver, and this accumulation inhibits lifespan and healthspan [5,6]. The characteristics of senescent cells include an inability to proliferate, with activation of p53/p21 or pRb/p16 pathway, and production of a high level of senescence associated secretory products (SASPs), such as IL-1β, IL-6 and MCP-1 [4,7,8]. Their accumulation can be driven by increased expression of inhibitory molecules permitting escape from immunosurveillance and suboptimal cytotoxicity from aged immune cells [9,10]. The SASPs not only mediates activation of tissue resident cells, immune cell infiltration, but also alters nicotinamide adenine dinucleotide (NAD) metabolism, a central metabolite in energy metabolism [4,11]. SASP factors stimulate expression of CD38, a NADase on the surface of cells including immune cells, which are responsible for the decline of NAD in aging [11-13]. Interestingly, cellular senescence can be modulated by differential NAD metabolic changes. High NAD+/NADH ratio mediated by the high mobility group A proteins (HMGA) - nicotinamide phophoribosyltransferase (NAMPT) axis increases glycolysis, mitochondrial respiration and SASP in oncogene-induced senescence [14]. In contrast, mitochondrial dysfunction-mediated senescence is partly attributed to lower NAD+/NADH levels and sustained activation of the NAD-AMPK signaling pathway [15]. The family of SIRTUIN proteins, NAD+ dependent deacetylases, are downregulated in senescent non-immune cells and aged immune cells and also have been identified to drive cellular senescence [13,16].
The SASP, SIRTUINS and NAD metabolism also provide new links to inflammaging, especially those induced by the NLRP3 inflammasome, a canonical sensor of a wide-range of damage signals (damageassociated molecular patterns: DAMPs) that accumulate with aging (Figure 1). SASP production by senescent cells is regulated by the inflammasome and IL-1 signaling; furthermore, certain SASP factors themselves may act as DAMPs [12]. The NLRP3 inflammasome, is highly expressed in tissue resident macrophages, and it’s activation by DAMPs results in NF-κB activation, NLRP3 inflammasome complex formation, and caspase 1-dependent release of IL-1β and IL-18. The role for the NLRP3 inflammasome is demonstrated in aged Nlrp3-deficient mice, which have restored glucose metabolism, catecholamine-induced lipolysis, bone density, and cognition, among other improvements in healthspan [17-20]. Aged knockout mice also show increased autophagy, NAD+ levels and SIRT1 protein [17]. Furthermore, small molecule inhibition of the NLRP3 inflammasome in animal models of Alzheimer’s Disease, atherosclerosis and myocardial infarction inhibit inflammation and have beneficial effects in preventing disease or preserving tissue function [21-24]. The hyperactivation of inflammasome and SASP-mediated inflammation are likely to be promoted by declining autophagy in aging [12,25]. The full extent of autophagy or other pathways as connecting cell-autonomous or non-cell-autonomous factors between inflammasome activation and senescence accumulation during aging is unknown.
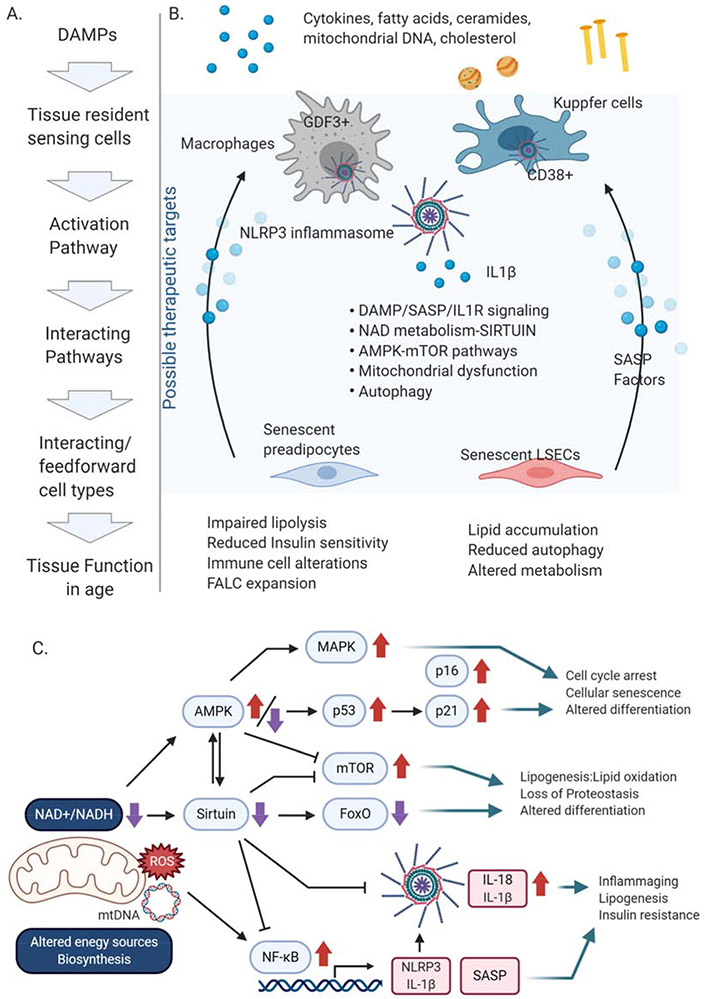
Figure 1. Immunometabolism during inflammaging.
A. Sequence of events leading to inflammaging and metabolic dysfunction in tissues. B. Schematic to describe the triggers of inflammation, the responding tissue resident myeloid cells in AT and liver, the pathways that are activated within those myeloid cells or in neighboring cells, the interacting cell types and the resulting metabolic and tissue dysfunction. C. Pathways of energy or nutrient sensing and NLRP3 inflammasome activation. Alterations in energy sources, including (1) sensing of DAMPs to activate the NLRP3 inflammasome pathway or (2) changes in ATP/ADP and NAD+/NADH to drive activation of the sirtuin or AMPK pathway. Mitochondrial dysfunction that results in elevated reactive oxygen species (ROS), mitochondrial (mt) DNA or altered NAD+/NADH levels are common themes that activate and alter these pathways in aging.
Immunosenescence is a component of inflammaging and refers to the dysregulation of the aged immune system, indicating both the inappropriate dysfunctional cells and the hyperactive cells, but not the cellular senescence of immune cells. Immune cells may express commonly used senescence markers, p16INK4a and p21CIP1; however, it is controversial as to whether the cells are truly senescent, as these kinase inhibitors are also required for immune cell differentiation. In this review, we focus on the recently identified changes in metabolic pathways of aged immune cells to better understand immunosenescence (Figure 1&2).
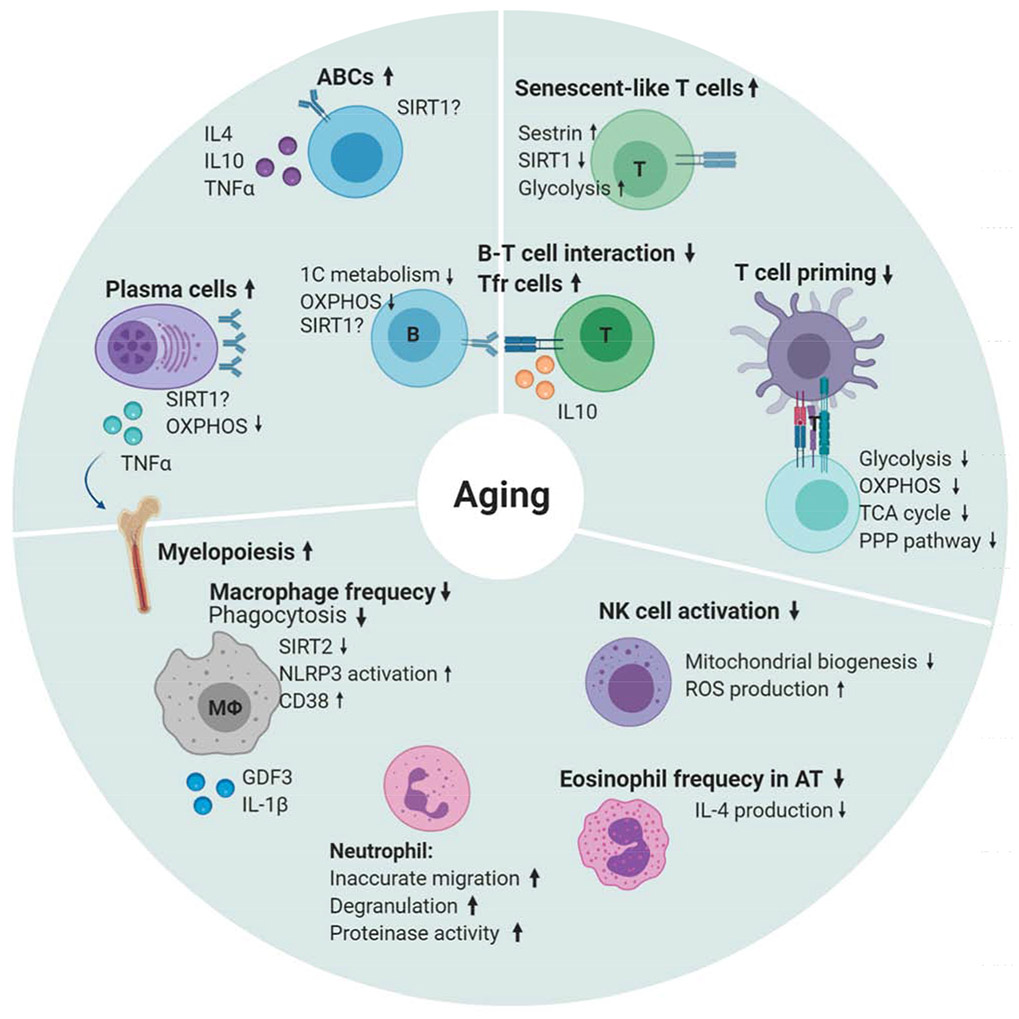
Figure 2. Immunosenescence in aging.
Immunosenescence is a concept focused on the alterations in the aged immune system. Aged immune cells may be dysfunctional and inactive or hyper-inflammatory. Adaptive immunity shows increases in TNFα+ plasma cells producing autoantibodies and inflammatory, aged B cells (ABCs) that are unresponsive through their B cell receptor. Antigen-specific antibody production, one carbon metabolism and oxidative phosphorylation are decreased with age. There is an increased frequency of IL10+ regulatory T follicular helper cells that fail to interact with B cells effectively, ultimately results in reduced T cell priming. T cells also show impaired metabolism, reduced SIRT1, but increased senescent features. Innate Immunity shows increases in myelopoiesis, elevated basal NLRP3 activation, decreased phagocytosis and reduced NAD metabolism. Other innate cells, including natural killer cells, eosinophils and neutrophils are altered in number and function.
Dysregulated metabolic pathways within aged immune cells
Innate Immunity:
Hematopoietic cells are predisposed towards myelopoiesis during aging. Myeloid-derived suppressor cells, a heterogenous population of myeloid cells accumulate in the marrow in a NF-κB-dependent mechanism [26], produce IL-1β, and have greater suppressive activity on T cell proliferation in aged mouse models [27]. Their IL-1β production also inhibits B cell lymphopoiesis [27], but promotes plasma cell expansion [28]. The precise contribution of myelopoiesis and recruitment versus the proliferative capacity of tissue resident macrophages and whether each is regulated by the NLRP3 inflammasome during aging is unclear.
Aged macrophages have heightened inflammatory pathways, including the NLRP3 inflammasome activation, but reduced phagocytic ability, antigen presentation, mitochondrial dysfunction and impaired cellular metabolism [29-33] (Figure 2). Macrophages from aged Nlrp3-deficient mice have reduced inflammation, in part, mediated through increased reactive oxygen levels and expression of growth differentiation factor (GDF)-3 [29,31]. Recent work highlighted the role for the de novo production of NAD via the kynurenine pathway metabolism of tryptophan, which regulates mitochondrial function, inflammation and macrophage phenotype [33]. Declines in that cell-autonomous production of NAD in aged macrophages increases inflammation and impairs oxidative metabolism [33]. Additional research shows SIRTUIN 2 (SIRT2) inhibits inflammasome activation through deacetylation [34]. These results suggest that reduced NAD metabolism also impairs SIRT2 activity leading to the NLRP3 inflammasome activation during aging. Interestingly NAD supplementation, via nicotinamide mononucleotide, reduces reactive oxygen levels, NLRP3 inflammasome activation and bone loss, suggesting that metabolic defects in aged macrophages may be overcome [35]. Further links between the de novo NAD pathway, NAD+/NADH levels, the sirtuin pathway and inflammasome activation in aged macrophages remain to be explored.
Other innate immune cells, including natural killer (NK) cells and eosinophils are also altered with age (see Figure 2), but which metabolic pathways are altered within these cell types remain unclear.
Adaptive Immunity:
B cell lymphopoiesis moderately declines with age. However, increased numbers of plasma cells, with elevated levels of IL-1 and TNFα, promote myelopoiesis in aging bone marrow [28]. These changes, along with altered memory B cell subsets [36], and increased accumulation of aged B cells (ABCs), contribute to impaired aged humoral responses [37]. ABCs secrete TNFα, IL4 and IL10, and respond to TLR agonists, but not B cell receptor signaling and therefore are directly linked to the reduced lymphopoiesis and reduced responses to antigen challenge during aging [37] (Figure 2).
B cells rely on glycolytic metabolism for activation and differentiation [38]. With aging there are mild decreases in glycolysis and pronounced defects in mitochondrial energy production [39]. There are also alterations in expression of genes involved in metabolism, with decreases in expression level of sirtuin (Sirt)1, FOXO1 and carnitine palmitoyl transferase in antibody secreting cells from human individuals [39]. SIRT1 is highly expressed in resting B cells and downregulated by stimuli inducing activation-induced cytidine deaminase (AID) which is required for immunoglobulin class switch DNA recombination and somatic hypermutation [40], therefore SIRTUIN signaling may also drive reduced somatic hypermutation and class-switching. These results are described in circulating B cells, but a major finding over the last few years, is the recognition of the accumulation of tissue resident B cells, especially in the white AT and liver [41,42].
Aged T cells are characterized by reduced thymic output, oligoclonality of T cell repertoire, and accumulation of highly differentiated T cells, exhausted T cells and activated regulatory T cells, possibly leading to a higher risk of autoimmunity and infection [43]. Aging causes suboptimal induction of mitochondrial biogenesis and metabolism. Upon activation with reduced levels of metabolites in glycolysis, the pentose phosphate pathway and the TCA cycle, which impairs activation [44]. Increased mTOR signaling in aged T cells via miR-21 also entails impaired tissue homing and memory cell differentiation of T cells [45,46]. Supplementation with a TCA cycle metabolite, alpha-ketoglutarate extends lifespan and, interestingly, also induces IL-10 production in T cells [47]. IL-10, traditionally an anti-inflammatory cytokine, may play a complicated role in aging. There is an accumulation of IL-10 producing T follicular helper cells with age, which ultimately curtails T cell-dependent antibody response to vaccination [48].
During aging there is an accumulation of senescent-like T cells, highly differentiated functional memory T cells that exhibit low proliferation capacity. These cells express sestrins, stress-inducible metabolic regulators known to inhibit mTORC1 signaling. Sestrins are responsible for the low responsiveness via hyperactivation of AMPK-MAPK pathway and acquisition of NK receptor and dependent cytotoxicity [49,50]. Defective SIRT1-FOXO1 axis regulates their glycolytic capacity and granzyme B production, although the specific role of these memory T cells in aging needs to be investigated [51]. T cells with dysfunctional mitochondria by deficient mitochondrial transcription factor A (TFAM), a key regulator of mitochondria DNA, have impaired activation and induce inflammaging, as well as premature aging in mice [52]. These results demonstrate the importance of T cell-intrinsic metabolism on age-related pathology.
Association of aged tissue resident immune cells with metabolic dysfunction
Adipose tissue (AT):
AT is a critical organ in energy balance and nutritional homeostasis. Increased adiposity and metabolic dysfunction during aging is highly dependent upon the NLRP3 inflammasome-induced inflammaging [29,42,53]. White adipocytes traditionally store lipid and use discrete mechanisms for release of the stored triglyceride. Lipolysis drives the release of free fatty acids, glycerol, and proteins to be used as metabolic and signaling substrates. AT shows reduced lipolytic signaling and reduced NAD levels, driven by accumulation of CD38 [13]. Changes in AT cellular composition including the altered immune cells and the accumulating senescent burden impairs both NAD and lipid metabolism (Figure 3). The immune cell alteration and lipolytic impairment may also be linked to impaired thermogenesis and reduced responses to infection during aging.
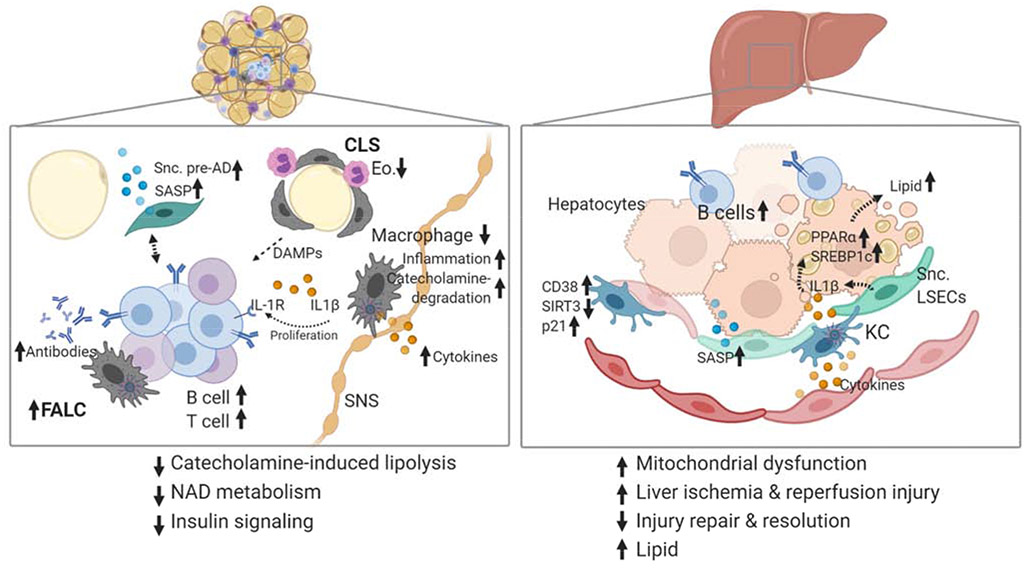
Figure 3. Aged tissue contains inflammatory and senescent cells contributing to metabolic dysfunction.
Schematic to highlight aged tissue. Liver and adipose tissue are two critical metabolic tissues that show alterations in immune cells, metabolic signaling and senescence. (Left) White visceral adipose tissue is the first to show elevated NLRP3 inflammasome activation, accumulating B and T cells in fat-associated lymphoid clusters and elevated levels of senescent cell burden. NLRP3-expressing macrophages are found in crown-like-structures (CLS), near sympathetic nerves (SNS), and fat-associated lymphoid structures (FALCs). CLS also have eosinophils (Eo.) in close contact, which support tissue resident macrophages. Dying adipocytes in CLS provide a source of damageassociated molecular patterns that activate macrophages and drive FALC accumulation. B cells in FALCs secrete antibodies, express the IL-1 receptor (IL-1R) and respond to NLRP3-induced IL1β by proliferation. Macrophages near SNS upregulate catecholamine degradation and inflammatory genes driving impairing of lipolysis. Senescent preadipocytes (Snc. Pre-AD) secrete SASP factors and interact with immune cells in ways that are not yet clear. (Right) B cells are increased in aged liver. Aged Kupffer cells (KC) upregulate the NADase, CD38, and senescence marker p21, but have lower levels of SIRT3, contributing to impaired NAD levels in liver. Mitochondrial DNA Cytokines from immune cells and SASP factors from senescent cells are increased in aged tissues. IL1β is a SASP factor secreted from KCs and Senescent liver sinusoidal endothelial cells (Snc. LSECs) which increases lipid accumulation via PPARα and SREBP1c in hepatocytes.
Resident visceral AT F4/80+ CD11b+ SiglecF− macrophages are numerically decreased, although they express an elevated inflammatory profile, with increased NLRP3 inflammasome activation that is at least partially regulated by GDF3 [29,30]. F4/80+ CD11b+ SiglecF+ eosinophils, critical regulators of insulin sensitivity and inflammation during obesity, are also decreased with age. Eosinophil production of IL-4 recruits alternatively activated macrophages and improves insulin sensitivity [53]. Aged-Nlrp3 deficient mice have increased (restored) AT macrophages. Along with decreased myeloid cells, there are expansions in adaptive immune system, include T and B lymphocytes [42,54]. Aged adipose B cells are a subset of the ABCs, with a few distinct differences. They express the IL-1 receptor, and their proliferative capacity requires the NLRP3 inflammasome and IL-1 signaling [29,42]. A subset of AT B cells are plasma cells, expressing IgM antibodies, and may contribute to the global increase in autoantibodies [41]. Functionally, aged murine AT macrophages upregulate catecholamine-degradation machinery, reducing lipolytic signaling in adipocytes [29]. Aged AT B cells also mediate lipolytic impairment although the precise mechanisms are unclear [42]. Overall, these data point towards a sequential series of events, likely connected by specific inflammatory signals such as senescent cell accumulation leading to metabolic dysfunction (Figure 3).
AT microenvironment niches are altered with age. Macrophages are abundant throughout the parenchyma, surrounding crown-like-structures and lining both sympathetic nerves and blood vessels, whereas eosinophils are found surrounding crown-like-structures [53]. In the aged visceral AT, but not other depots, lymphoid clusters, termed fat-associated lymphoid clusters (FALCs), which harbor the expansion of T and B lymphocytes, are increased [42,55]. Lymphoid clusters develop in response to acute or chronic inflammation and are an essential component of infection clearance [56]. Given the increase in Tfh cells and B cells during aging, but with impaired adaptive and humoral responses, it remains to be seen whether aged FALCs are immunologically responsive. Interestingly, the accumulation of lymphoid clusters does occur in other aged tissues, including the bladder [57], but direct links between metabolic signaling, senescence and FALC formation need to be dissected out (Figure 3).
Liver:
Aging reduces liver volume, disrupts blood flow and increases susceptibility to liver fibrosis, non-alcoholic fatty liver disease, and liver injury [58]. AT proximity and anatomical location to the liver, as well as its propensity for secreting various factors, makes the AT a prime suspect for cross-tissue regulation of inflammation and metabolic dysfunction in the liver with age. Similar to AT, liver shows elevated senescence impaired metabolism and an expansion of B cells [13,41]. Kupffer cells, liver resident macrophages, are important in liver homeostasis, but there is limited data regarding their roles in aging. Aging upregulates CD38 expression in Kupffer cells, resulting in NAD decline and SIRT3-dependent mitochondrial dysfunction [13,59]. The increase in CD38 expression on Kupffer cells was paralleled by increased senescent cell marker, p21CIP1, and increased signature of pro-inflammatory M1-like cells. Cellular senescence is likely to participate in some areas of age-related liver pathology, as liver fat accumulation may be accelerated by the increase in senescent p16INK4A+ LSEC and Kupffer cells [60] (Figure 3).
Mechanistically, the NLRP3 inflammasome and IL-1β drive the pathology of aged liver, as Nlrp3-deficiency rescued age-related glucose intolerance and high cholesterol [61]. In liver injury, aging enhances cGAS/STING-mediated NLRP3 activation by mitochondrial DNA in macrophages, worsening liver ischemia and reperfusion injury [62]. Similarly, endotoxin-induced liver inflammation is also slowly resolved during aging, resulting in chronic activation of NLRP3 and IL-1β production [63]. The increased IL-1β induces lipogenic machineries via PPARα and SREBP1c to drive lipid accumulation in aged hepatocytes, indicating a direct link between NLRP3 inflammasome and liver tissue lipid accumulation [63] (Figure 3).
Senolytic therapy as gerotherapeutics to target immunometabolism
With aging, two immunological features, inflammaging and immunosenescence are interconnected through cell-specific metabolic changes. There are many gerotherapeutics targeting the pillars of aging, aimed at improving healthspan and which have beneficial effects on AMPK-mTOR (e.g., metformin, rapamycin) and SIRTUIN signaling (e.g., resveratrol), autophagy or reducing inflammation [4]. (See Table 1 for a list of recent publications using gerotherapeutics). Based on the research revealing the interactions of these pillars of aging, gerotherapeutics that directly target a single pillar may also indirectly affect all pillars. Senolytics, agents that induce the death of senescent cells specifically are currently in clinical trials as a new type of gerotherapeutic for improving age-related diseases [4,11,64,65]. Both natural senolytics, such as fisetin, and FDA approved compounds, such as dasatinib in combination with quercetin, selectively eliminate senescent cells resulting in improved immune cell frequencies and reduced inflammation in addition to the improved health span benefits [4,64]. Senolytics may also remove p16Ink4a+ Kupffer cells, prevent DC activation and Th1/Th17 immunogenicity by limiting mtDNA from senescent cells, which in turn improve tissue homeostasis [60,65]. In the future, additional research is required to reveal the impact of senolytics on aged immune system and the affected immune cell types to improving health span during aging.
Table 1.
Effects of gerotherapeutics on immune cells. IRI: Ischemia-reperfusion injury; S.pnm.: Streptococcus pneumoniae
| Drug | Model | Effects | Reference |
|---|---|---|---|
| Rapamycin | • C57BL/6 mice • 22–24m • S. pnm. |
• Conferred modest protection against mortality. • Diminished lung damage rather than reduced bacterial burden. • No effect on levels of SASPs in whole lung homogenates. |
[66] |
| • C57BL/6 mice • 16–18m • West Nile virus |
• Reduced thymic cellularity. • Reduced the mortality, but not significant. |
[67] | |
| mTOR inhibitor (RAD001, BEZ235) | • Human subjects • ≥ 65 years • Influenza vaccine |
• Induced higher antibody titers. • Improved protection against influenza infection. |
[68] |
| Dasatinib+Quercetin (D+Q) | • C57BL/6 mice • 18m • IRI |
• Reduced systemic levels of CD8+ IFN-γ+, CD4+IFN-γ+, and CD4+IL-17+ cells after renal IRI. | [65] |
| • p16-Cre/R26-mTmG mice • 10m |
• Removed p16High F4/80+ cells in liver. | [69] | |
| Metformin | • Human PMBC • Avg 62 years |
• Ameliorated the Th17 profile by increasing autophagy and improving mitochondrial bioenergetics. | [70] |
Acknowledgements
This work was supported by NIH grants RO1 AG063543 (PDR), RO1 AG063543-02S1 (PDR, CDC), P01 AG043376 (PDR), U19 AG056278 (PDR), P01 AG062413 (PDR), R01 AG069819 (PDR, CDC), R00 AG058800 (CDC), T32 AG029796 (KL), the Michael J. Fox Foundation for Parkinson’s Research (PDR), the University of Minnesota Clinical and Translational Science Institute (PDR, CDC) and the Medical Discovery Team on the Biology of Aging (CDC, PDR). CDC also is supported by the Fesler-Lampert Chair in Aging Studies and the Glenn Foundation for Medical Research and AFAR Grants for Junior Faculty. These figures were created with BioRender.com. We would like to thank Stephanie Cholensky for her comments and edits on the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References
- 1.Vetrano DL, Calderon-Larranaga A, Marengoni A, Onder G, Bauer JM, Cesari M, Ferrucci L, Fratiglioni L: An international perspective on chronic multimorbidity: Approaching the elephant in the room. The journals of gerontology Series A, Biological sciences and medical sciences (2018) 73(10):1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA et al. : Geroscience: Linking aging to chronic disease. Cell (2014) 159(4):709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A: Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol (2018) 14(10):576–590. [DOI] [PubMed] [Google Scholar]
- 4.Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, Passos JF, Pignolo RJ, Tchkonia T, Niedernhofer LJ: Senolytic drugs: Reducing senescent cell viability to extend health span. Annual review of pharmacology and toxicology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi HS, Kim SY, Kim JT, Lee YS, Moon JS, Kim M, Kang YE, Joung KH, Lee JH, Kim HJ, Chun K et al. : T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell death & disease (2019) 10(3):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL: Fat tissue, aging, and cellular senescence. Aging cell (2010) 9(5):667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X, Unger MA, Vijg J, Melov S, Campisi J: Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging cell (2017) 16(5):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, Mazula DL, Brown AK, Berning M, Aversa Z, Kotajarvi B et al. : The senescence-associated secretome as an indicator of age and medical risk. JCI Insight (2020) 5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, Virasami A, Sebire NJ, Kinsler V, Valdovinos A, LeSaux CJ et al. : Senescent cells evade immune clearance via hla-e-mediated nk and cd8(+) t cell inhibition. Nature communications (2019) 10(1):2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, Windheim J et al. : Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nature communications (2018) 9(1):5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chini C, Hogan KA, Warner GM, Tarrago MG, Peclat TR, Tchkonia T, Kirkland JL, Chini E: The nadase cd38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular nad(+) decline. Biochemical and biophysical research communications (2019) 513(2):486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G et al. : A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol (2013) 15(8):978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Camacho-Pereira J, Tarrago MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN: Cd38 dictates age-related nad decline and mitochondrial dysfunction through an sirt3-dependent mechanism. Cell metabolism (2016) 23(6):1127–1139. These authors show that CD38, a hydrolase, is required for the age-related NAD decline and mitochondrial dysfunction, at least partially through SIRT3 activity. CD38 is elevated in all metabolic tissues, including liver and adipose, during age and contributes to dysfunctional tissue metabolism.
- 14. Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, Noma KI et al. : Nad(+) metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol (2019) 21(3):397–407. The authors show the rate limiting enzyme of the NAD salvage pathway (NAMPT), via high mobility group A, promotes SASP production by enhancing glycolysis and mitochondrial respiration. These results indicate that any anti-aging therapeutics by NAD supplementation should be carefully considered.
- 15. Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA et al. : Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell metabolism (2016) 23(2):303–314. The authors examined mitochondrial dysfunction and its role in inducing senescence. They identified a unique SASP profile associated with this type of senescence, including the lack of the IL-1 production. This publication shos a direct link between mitochondrial dysfucntion and aging phenotypes in progeroid mice.
- 16.Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J, Lu C, Nicastri M, Bretz C, Winkler JD, Amaravadi R et al. : Sirt1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol (2020) 22(10):1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marin-Aguilar F, Lechuga-Vieco AV, Alcocer-Gomez E, Castejon-Vega B, Lucas J, Garrido C, Peralta-Garcia A, Perez-Pulido AJ, Varela-Lopez A, Quiles JL, Ryffel B et al. : Nlrp3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging cell (2020) 19(1):e13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, Owen JS, Thomas MJ, Francis J, Parks JS, Dixit VD: The nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell reports (2012) 1(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Munzberg H et al. : Canonical nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell metabolism (2013) 18(4):519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauernfeind F, Niepmann S, Knolle PA, Hornung V: Aging-associated tnf production primes inflammasome activation and nlrp3-related metabolic disturbances. Journal of immunology (2016) 197(7):2900–2908. [DOI] [PubMed] [Google Scholar]
- 21.Marin-Aguilar F, Castejon-Vega B, Alcocer-Gomez E, Lendines-Cordero D, Cooper MA, de la Cruz P, Andujar-Pulido E, Perez-Alegre M, Muntane J, Perez-Pulido AJ, Ryffel B et al. : Nlrp3 inflammasome inhibition by mcc950 in aged mice improves health via enhanced autophagy and pparalpha activity. The journals of gerontology Series A, Biological sciences and medical sciences (2020) 75(8):1457–1464. [DOI] [PubMed] [Google Scholar]
- 22.van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slutter B, Foks AC, Bot I, Kuiper J: Nlrp3 inflammasome inhibition by mcc950 reduces atherosclerotic lesion development in apolipoprotein e-deficient mice-brief report. Arteriosclerosis, thrombosis, and vascular biology (2017) 37(8):1457–1461. [DOI] [PubMed] [Google Scholar]
- 23.van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC, Robertson AA, Pasterkamp G, Hoefer IE: The selective nlrp3-inflammasome inhibitor mcc950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. European heart journal (2017) 38(11):828–836. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O'Neill LAJ, Lynch MA: Inhibiting the nlrp3 inflammasome with mcc950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in app/ps1 mice. Brain, behavior, and immunity (2017) 61(306–316. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy LD, Young ARJ, Young CNJ, Soilleux EJ, Fielder E, Weigand BM, Lagnado A, Brais R, Ktistakis NT, Wiggins KA, Pyrillou K et al. : Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nature communications (2020) 11(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores RR, Clauson CL, Cho J, Lee BC, McGowan SJ, Baker DJ, Niedernhofer LJ, Robbins PD: Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a nf-kappab-dependent mechanism. Aging cell (2017) 16(3):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy DE, Knight KL: Inhibition of b lymphopoiesis by adipocytes and il-1-producing myeloid-derived suppressor cells. Journal of immunology (2015) 195(6):2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pioli PD, Casero D, Montecino-Rodriguez E, Morrison SL, Dorshkind K: Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity (2019) 51(2):351–366 e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, Goldberg EL, Youm YH, Brown CW, Elsworth J, Rodeheffer MS et al. : Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature (2017) 550(7674):119–123. This publication examined impaired lipolytic signaling in aged visceral adipose tissue and described a new role for AT macrophages in degrading catecholamines to drive that impairment. This is one of the first publications to provide bulk RNA sequencing of macrophages from aged adipose tissue.
- 30. Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL: Aging is associated with an increase in t cells and inflammatory macrophages in visceral adipose tissue. Journal of immunology (2011) 187(12):6208–6216. This publication is the first to describe the changes in adipose tissue immune cells (macrophages, CD4 T regulatory cells and CD4 T helper cells) with age. They show the aged adipose tissue macrophages are inflammatory.
- 31.Stout-Delgado HW, Cho SJ, Chu SG, Mitzel DN, Villalba J, El-Chemaly S, Ryter SW, Choi AM, Rosas IO: Age-dependent susceptibility to pulmonary fibrosis is associated with nlrp3 inflammasome activation. American journal of respiratory cell and molecular biology (2016) 55(2):252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pence BD, Yarbro JR: Aging impairs mitochondrial respiratory capacity in classical monocytes. Experimental gerontology (2018) 108(112–117. [DOI] [PubMed] [Google Scholar]
- 33. Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, Contrepois K, Wang Q, Lee BA, Coronado M, Bernstein D et al. : Macrophage de novo nad(+) synthesis specifies immune function in aging and inflammation. Nature immunology (2019) 20(1):50–63. This publication shows that tryptophan metabolism and kynurenine pathway regulate de novo NAD synthesis, which is required for macrophage immune response and differentiation. Aged macrophages fail to upregulate the KP resulting in impaired NAD synthesis and macrophages responses.
- 34. He M, Chiang HH, Luo H, Zheng Z, Qiao Q, Wang L, Tan M, Ohkubo R, Mu WC, Zhao S, Wu H et al. : An acetylation switch of the nlrp3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell metabolism (2020) 31(3):580–591 e585. These authors have identified new regulation of the NLRP3 inflammasome. It's activation requires acetylation and can be deacytelated by SIRT2. Their results suggest that dysregulation of the acetylation switch, by age-related loss of SIRT2 or NAD metabolism, could be an origin of inlammation during aging.
- 35.Liang H, Gao J, Zhang C, Li C, Wang Q, Fan J, Wu Z, Wang Q: Nicotinamide mononucleotide alleviates aluminum induced bone loss by inhibiting the txnip-nlrp3 inflammasome. Toxicology and applied pharmacology (2019) 362(20–27. [DOI] [PubMed] [Google Scholar]
- 36.Frasca D, Diaz A, Romero M, Blomberg BB: Human peripheral late/exhausted memory b cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Experimental gerontology (2017) 87(Pt A):113–120. [DOI] [PubMed] [Google Scholar]
- 37.Cancro MP: Age-associated b cells. Annual review of immunology (2020) 38(315–340. [DOI] [PubMed] [Google Scholar]
- 38.Waters LR, Ahsan FM, Ten Hoeve J, Hong JS, Kim DNH, Minasyan A, Braas D, Graeber TG, Zangle TA, Teitell MA: Ampk regulates igd expression but not energy stress with b cell activation. Scientific reports (2019) 9(1):8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurupati RK, Haut LH, Schmader KE, Ertl HC: Age-related changes in b cell metabolism. Aging (2019) 11(13):4367–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gan H, Shen T, Chupp DP, Taylor JR, Sanchez HN, Li X, Xu Z, Zan H, Casali P: B cell sirt1 deacetylates histone and non-histone proteins for epigenetic modulation of aid expression and the antibody response. Sci Adv (2020) 6(14):eaay2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schaum N, Lehallier B, Hahn O, Palovics R, Hosseinzadeh S, Lee SE, Sit R, Lee DP, Losada PM, Zardeneta ME, Fehlmann T et al. : Ageing hallmarks exhibit organ-specific temporal signatures. Nature (2020) 583(7817):596–602. This publication performed a comprehensive, whole-organism analysis of ageing dynamics using bulk and single cell RNA sequencing. They found linear and nonlinear shifts in gene expression with associated genes clustered in biological groups such as extracellular matrix regulation, unfolded protein protein binding, mitochondrial function and inflammatory and immune response. They find widespread activation of immune cells that is first detectable in white adipose depots during middle age. Gene expression changes are highly correlated with corresponding protein levels in plasma.
- 42. Camell CD, Gunther P, Lee A, Goldberg EL, Spadaro O, Youm YH, Bartke A, Hubbard GB, Ikeno Y, Ruddle NH, Schultze J et al. : Aging induces an nlrp3 inflammasome-dependent expansion of adipose b cells that impairs metabolic homeostasis. Cell metabolism (2019) 30(6): 1024–1039 e1026. This publication examines the age-related expansion of adipose tissue B cells in fat-associated lymphoid clusters and describes a unique role for the NLRP3 inflammasome and IL1b in driving that expansion and AT B cell ability to impair adipose tissue metabolism.
- 43.Elyahu Y, Hekselman I, Eizenberg-Magar I, Berner O, Strominger I, Schiller M, Mittal K, Nemirovsky A, Eremenko E, Vital A, Simonovsky E et al. : Aging promotes reorganization of the cd4 t cell landscape toward extreme regulatory and effector phenotypes. Sci Adv (2019) 5(8):eaaw8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ron-Harel N, Notarangelo G, Ghergurovich JM, Paulo JA, Sage PT, Santos D, Satterstrom FK, Gygi SP, Rabinowitz JD, Sharpe AH, Haigis MC: Defective respiration and one-carbon metabolism contribute to impaired naïve t cell activation in aged mice. Proceedings of the National Academy of Sciences of the United States of America (2018) 115(52):13347–13352. This is a systemic analysis of proteins and metabolites in young versus aged T cells. They show that metabolic rewiring, including mitochondrial biogenesis and respiration, occurs in young naive T cells following stimulation, but is dampened in the aged naive T cells. Addition of metabolites, formate and glycine, was able to enhance aged T cell activation.
- 45.Kim C, Hu B, Jadhav RR, Jin J, Zhang H, Cavanagh MM, Akondy RS, Ahmed R, Weyand CM, Goronzy JJ: Activation of mir-21-regulated pathways in immune aging selects against signatures characteristic of memory t cells. Cell reports (2018) 25(8):2148–2162 e2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davenport B, Eberlein J, van der Heide V, Jhun K, Nguyen TT, Victorino F, Trotta A, Chipuk J, Yi Z, Zhang W, Clambey ET et al. : Aging of antiviral cd8(+) memory t cells fosters increased survival, metabolic adaptations, and lymphoid tissue homing. Journal of immunology (2019) 202(2):460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asadi Shahmirzadi A, Edgar D, Liao CY, Hsu YM, Lucanic M, Asadi Shahmirzadi A, Wiley CD, Gan G, Kim DE, Kasler HG, Kuehnemann C et al. : Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell metabolism (2020) 32(3):447–456 e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almanan M, Raynor J, Ogunsulire I, Malyshkina A, Mukherjee S, Hummel SA, Ingram JT, Saini A, Xie MM, Alenghat T, Way SS et al. : Il-10-producing tfh cells accumulate with age and link inflammation with age-related immune suppression. Sci Adv (2020) 6(31):eabb0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanna A, Gomes DC, Muller-Durovic B, McDonnell T, Escors D, Gilroy DW, Lee JH, Karin M, Akbar AN: A sestrin-dependent erk-jnk-p38 mapk activation complex inhibits immunity during aging. Nature immunology (2017) 18(3):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira BI, De Maeyer RPH, Covre LP, Nehar-Belaid D, Lanna A, Ward S, Marches R, Chambers ES, Gomes DCO, Riddell NE, Maini MK et al. : Sestrins induce natural killer function in senescent-like cd8(+) t cells. Nature immunology (2020) 21(6):684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeng MY, Hull PA, Fei M, Kwon HS, Tsou CL, Kasler H, Ng CP, Gordon DE, Johnson J, Krogan N, Verdin E et al. : Metabolic reprogramming of human cd8(+) memory t cells through loss of sirt1. The Journal of experimental medicine (2018) 215(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Desdin-Mico G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabande-Rodriguez E, Blanco EM, Alfranca A, Cusso L, Desco M, Ibanez B et al. : T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science (2020) 368(6497):1371–1376. These authors examined the effects of TFAM-deficiency in T cells and found T cell metabolic failure, leading to the accumulation of circulating cytokines, and premature aging.
- 53.Brigger D, Riether C, van Brummelen R, Mosher KI, Shiu A, Ding Z, Zbaren N, Gasser P, Guntern P, Yousef H, Castellano JM et al. : Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat Metab (2020) 2(8):688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frasca D, Blomberg BB: Adipose tissue inflammation induces b cell inflammation and decreases b cell function in aging. Frontiers in immunology (2017) 8(1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benezech C, Luu NT, Walker JA, Kruglov AA, Loo Y, Nakamura K, Zhang Y, Nayar S, Jones LH, Flores-Langarica A, McIntosh A et al. : Inflammation-induced formation of fat-associated lymphoid clusters. Nature immunology (2015) 16(8):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, Morais da Fonseca D, Harrison OJ, Tamoutounour S, Byrd AL, Smelkinson M et al. : White adipose tissue is a reservoir for memory t cells and promotes protective memory responses to infection. Immunity (2017) 47(6):1154–1168 e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ligon MM, Wang C, DeJong EN, Schulz C, Bowdish DME, Mysorekar IU: Single cell and tissue-transcriptomic analysis of murine bladders reveals age- and tnfalpha-dependent but microbiota-independent tertiary lymphoid tissue formation. Mucosal immunology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allaire M, Gilgenkrantz H: The aged liver: Beyond cellular senescence. Clin Res Hepatol Gastroenterol (2020) 44(1):6–11. [DOI] [PubMed] [Google Scholar]
- 59. Tarrago MG, Chini CCS, Kanamori KS, Warner GM, Caride A, de Oliveira GC, Rud M, Samani A, Hein KZ, Huang R, Jurk D et al. : A potent and specific cd38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue nad(+) decline. Cell metabolism (2018) 27(5):1081–1095 e1010. 78c is a CD38 inhibitor that reverses age-related NAD decline to improve glucose tolerance, muscle function, exercise capacity and cardiac function. NAD levels were increased in adipose tissue of aged treated mice. Aged mice also showed inhibition of mTOR and attenuation of telomere-associated DNA damage.
- 60. Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN et al. : Cellular senescence drives age-dependent hepatic steatosis. Nature communications (2017) 8(15691. Senescent cell accumulation promotes hepatic fat accumulation and steatosis. Senescent cell elimination using the INK-ATTAC mice or senolytic drugs reduces hepatic steatosis. Mechanistically, mitochondria have impaired fatty acid oxidation leading to senescence in hepatocytes.
- 61.Gallego P, Castejon-Vega B, Del Campo JA, Cordero MD: The absence of nlrp3-inflammasome modulates hepatic fibrosis progression, lipid metabolism, and inflammation in ko nlrp3 mice during aging. Cells (2020) 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong W, Rao Z, Rao J, Han G, Wang P, Jiang T, Pan X, Zhou S, Zhou H, Wang X: Aging aggravated liver ischemia and reperfusion injury by promoting sting-mediated nlrp3 activation in macrophages. Aging cell (2020) 19(8):e13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung KW, Lee EK, Kim DH, An HJ, Kim ND, Im DS, Lee J, Yu BP, Chung HY: Age-related sensitivity to endotoxin-induced liver inflammation: Implication of inflammasome/il-1beta for steatohepatitis. Aging cell (2015) 14(4):524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, McGuckian C et al. : Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine (2018) 36(18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iske J, Seyda M, Heinbokel T, Maenosono R, Minami K, Nian Y, Quante M, Falk CS, Azuma H, Martin F, Passos JF et al. : Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nature communications (2020) 11(1):4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ: Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Experimental gerontology (2012) 47(12):958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldberg EL, Romero-Aleshire MJ, Renkema KR, Ventevogel MS, Chew WM, Uhrlaub JL, Smithey MJ, Limesand KH, Sempowski GD, Brooks HL, Nikolich-Zugich J: Lifespan-extending caloric restriction or mtor inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging cell (2015) 14(1):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mannick JB, Morris M, Hockey HP, Roma G, Beibel M, Kulmatycki K, Watkins M, Shavlakadze T, Zhou W, Quinn D, Glass DJ et al. : Torc1 inhibition enhances immune function and reduces infections in the elderly. Science translational medicine (2018) 10(449). [DOI] [PubMed] [Google Scholar]
- 69.Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner KD, Bulavin DV: Defined p16(high) senescent cell types are indispensable for mouse healthspan. Cell metabolism (2020) 32(1):87–99 e86. [DOI] [PubMed] [Google Scholar]
- 70.Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, Apovian CM et al. : Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell metabolism (2020) 32(1):44–55 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]