Abstract
There is a significant interest in novel waste management solutions to treat wastewater from swine operations. Anaerobic digestion is a rising and prominent solution, but this technology still generates highly concentrated effluent that requires further remediation. Therefore, the aim of this study was to explore the feasibility of cultivating the cyanobacterium Spirulina platensis in swine effluent for future applications in biological waste treatment and value-added fermentation. To accomplish this goal, growth of S. platensis was characterized in varying proportions of ideal, synthetic Zarrouk medium and anaerobically digested pig effluent (ADPE) to obtain growth rate models. Results yielded a positive correlation between S. platensis growth rate and Zarrouk medium proportion, with the highest growth rate in 100% Zarrouk media but comparable growth in the 50/50% Zarrouk/ADPE mixture. This study demonstrates the potential for S. platensis to further improve the treatment efficacy of anaerobic digestion systems, and the exploratory analysis also highlights that further testing is required to investigate possible carbon availability, chemical inhibition, and overall nutrient reduction in ADPE. This research contributes important data toward the feasibility of producing value-added cyanobacterial biomass while simultaneously consuming excess nutrients to aid in agricultural wastewater management efforts and generate cost-effective products in a more sustainable manner.
Keywords: Spirulina, Cyanobacteria, Swine effluent, Anaerobic digestion, Biomass production
Spirulina; Cyanobacteria; Swine effluent; Anaerobic digestion, Biomass production.
1. Introduction
In response to rising production and demand of swine operations globally, new and effective strategies for handling swine waste are needed. Currently, anaerobic digestion has emerged as the leading solution for waste-to-energy bioconversion and wastewater treatment (Tsui and Wong, 2019; Hussain et al., 2020). Although anaerobic digestion succeeds at reducing nutrient levels within animal waste, the effluent from digesters generally contains nitrate and phosphate concentrations in excess of environmental regulations for discharge into the environment. Consequently, these excess nutrients can be used to support growth of other microorganisms for value-added biomass production; cyanobacteria and algae are key candidates, as these photoautotrophs use sunlight as their energy source. In the last decade, several studies have examined the ability of cyanobacteria and microalgae to thrive in different types of wastewater (Singh et al., 2019; Zhang et al., 2018, 2020). Photoautotrophic biomass can have a multitude of downstream applications, including biofuel/biodiesel, biofertilizers, nutrient supplements, and other secondary metabolites (Singh et al., 2019). Spirulina platensis has been found to be one of the most versatile cyanobacterial species and has been cultivated in a variety of types of wastewater and is known to have a relatively fast growth rate (Olguin et al., 2003; Saranraj and Sivasakthi, 2014), serving as a promising candidate for remediation applications.
The ability for microorganisms to tolerate the chemical composition of anaerobically digested pig effluent (ADPE) is critical to bioremediation success, where the ultimate goal is to both (a) cultivate biomass to create a value-added byproduct, and (b) reduce overall nutrient concentrations in the ADPE effluent below safe discharge levels. Previous studies have begun to examine the capacity of cyanobacteria and algae for growth in ADPE: for example, some studies have explored cultivating S. platensis in ADPE diluted with seawater, observing up to 96% reduction in nitrogen compounds and 87% reduction in phosphorous compounds (Olguin et al., 2003; Mezzomo et al., 2010). However, ADPE compositions described in literature vary substantially due to wide variability in the system design, temperature, climate, and influent composition among anaerobic digesters (Borges et al., 2013; Canizares-Villanueva et al., 1994; Moheimani et al., 2018; Nwoba et al., 2017; Olguin et al., 2003; see variables and literature data compiled in Table 1). Different combinations of nutrient concentrations and other chemical parameters can affect the ability of different microbial species to produce biomass or other desirable secondary metabolites in ADPE. Thus, there is a significant need to determine the ability of cyanobacteria and microalgae to tolerate different ADPE compositions.
Table 1.
ADPE compositions as described in literature along with the ADPE source used in the current study.
| Ayre et al. from Moheimani et al. (2018) | Nwoba et al. (2017) | Olguin et al. (2003) | Borges et al. (2013) | Canizares-Villanueva et al. (1994) | Boland et al. (this work) | |
|---|---|---|---|---|---|---|
| Total Ammonia (mg/L) | 960–1000 | 1315.17 ± 40.48 | 1209–1370 | 361.35 ± 61.01 | 203.2 | 144–167 |
| Total Phosphate (mg/L) | 25.0–26.5 | 34.55 ± 3.75 | 164–620 | 86.01 ± 3.17 | 95.6 | 92.25–94.5 |
| Nitrite (ug/L) | 8.0–8.5 | 10.53 ± 2.15 | - | - | - | - |
| Magnesium (mg/L) | 165–175 | 224 | - | - | - | - |
| Potassium (mg/L) | 530–545 | 799 | - | - | - | 237–240 |
| Total Iron (mg/L) | 8.5–9.5 | 12.4 | - | - | - | - |
| Total alkalinity (mmol/L) | - | 129 | 3099–5450 | 1,427.13 ± 176.59 | - | - |
| Nitrate (mg/L) | 14.0–14.5 | 18.70 ± 2.96 | - | - | 4.3 | - |
| COD (mg/L) | 1200–1350 | 1585.50 ± 122.50 | 2746–4157 | - | 546.3 | 1057–1777 |
| Total nitrogen (mg/L) | 1050–1101 | 1430 | 1405–1519 | - | - | - |
| pH | - | 8.2 ± 0.09 | 7.76–8.56 | 7.8 ± 0.19 | - | 6.92–7.23 |
Abbreviations used: COD, chemical oxygen demand.
In this research, S. platensis was selected as the cyanobacterial species of interest to explore its versatility in varying concentrations of ADPE as compared to performance in an ideal growth environment. Zarrouk medium is an established medium for cultivating cyanobacteria and has been in use since 1966 (Zarrouk, 1966). A modified version of Zarrouk medium has been formulated specifically for the production of S. platensis that is more cost-efficient and shows no significant differences in growth rates (Raoof et al., 2006). Park et al. (2013) reported that this low-cost version of Zarrouk medium was capable of culturing S. platensis when combined with municipal wastewater. Thus, this low-cost version of medium was selected for the similar application of growing S. platensis in combination with ADPE. This study explored the robustness of S. platensis to tolerate and thrive in varying concentrations of ADPE from a locally operated thermophilic anaerobic digester, providing insight into how environmental suitability may impact the efficacy of biomass production.
2. Materials and methods
2.1. Media formulations
Spirulina Medium: Pre-mixed 1X Spirulina medium was purchased from UTEX Culture Collection of Algae at The University of Texas at Austin (product number Spir).
Zarrouk Medium: Zarrouk medium contains three separate solutions and was not autoclaved after mixing to prevent precipitation of metals. Solutions 1 and 2 were autoclaved separately and Solution A5 was sterilely filtered; all three solutions were then combined. Zarrouk medium composition was as follows: 500 mL/L Solution 1 (16.8 g NaHCO3, 0.5 g K2HPO4 (per L)), 500 mL/L Solution 2 (2.5 g NaNO3, 1 g K2SO4, 1 g NaCl, 0.2 g MgSO4·7H2O, 0.04 g CaCl2, 0.01 g FeSO4·7H2O, 0.08 g NaEDTA (per L)), 1 mL/L Solution A5 (2.86 g H3BO3, 1.81 g MnCl2·4H2O, 0.222 g ZnSO4·7H2O, 0.079 g CuSO4·4H2O, 0.015 g MoO3 (per L)).
Zarrouk and ADPE Mixtures: ADPE was sourced from a semi-continuous, 12-L working volume (14-L capacity) thermophilic anaerobic digester operating at 50 °C. The reactor was fed with 1.2 L of swine wastewater per day, producing 1 L of ADPE per day with a hydraulic residence time of 10 days total. 10 L of ADPE were collected in total and stored at 4 °C in a sealed container. The ADPE was not autoclaved to avoid altering the chemical composition. To sterilize it, the ADPE was first centrifuged at 3260 x g for 10 min, separating the solids, and the liquid was filtered through a 0.2 μm filter into a sterile flask. Compositional analyses of the effluent were conducted to identify the major components and available nutrients of the effluent (Table 1), tested by the NC State Biological and Agricultural Engineering Environmental Analysis laboratory according to Environmental Protection Agency standard methods (Table 2).
Table 2.
ADPE conditions, measurements, and methods.
| Analyte | Value | Method and Equipment |
|---|---|---|
| pH | 7.02 | Electrode-pH meter. Standard Methods 4500-H + B or EPA Method 150.1. |
| COD (mg/L) | 1366.66 | Potassium dichromate/sulfuric acid digestion-colorimetric analysis. EPA approved method. EPA Method 410.4. |
| TS (mg/L) | 2048 | Gravimetric method. Standard Methods 2540B. |
| VS (mg/L) | 880 | Ignition-Gravimetric method. Standard Methods 2540E. |
| TKN (mg/L) | 237 | K2SO4–CuSO4 digestion, ammonia-salicylate-nitroprusside-hypochlorite colorimetry on a Autoanalyzer System. Standard Methods 4500Norg B or EPA Method 351.2. |
| NH4–N (mg/L) | 144 | Ammonia-salicylate-nitroprusside-hypochlorite colorimetry on a Autoanalyzer System. Standard Methods 4500-NH3 G. |
| TP (mg/L) | 94.5 | K2SO4–CuSO4 digestion. Ortho phosphate-molybdate-antimony-ascorbic acid colorimetry on a Autoanalyzer System. Standard Method 4500-P F. |
Abbreviations used: COD, chemical oxygen demand; TS, total solids; VS, volatile solids; TKN, total Kjeldahl nitrogen; NH4–N, ammonia nitrogen; TP, total phosphorus.
2.2. Spirulina stock culturing
Spirulina platensis UTEX LB 2340 was acquired from UTEX Culture Collection of Algae. Upon strain arrival, the 10-mL culture was opened to allow atmospheric gas transfer, and 1:10 dilutions were transferred into 10 mL of fresh Spirulina medium. Transfers were propagated in the same manner every two weeks for stock culture maintenance. Stock cultures were manually agitated once a day during cultivation by sealing caps, inverting until well mixed and then vortexing (Fisherbrand Digital Vortex Mixer) for 30 s at 342 x g. White fluorescent light bulbs (double Philips F32T8/TL841 800 Series 32 Watt) were used. For the stock cultures, the bulbs were plugged in through a ceiling hanger laid on its side. Stock cultures were kept under 12/12 h light/dark cycles with no temperature adjustment (constant ∼27 °C, monitored by ambient room thermometer). The samples were placed ∼20 cm from the light source. The light intensity was reduced by placing one layer of white printer paper over the light source. Cultures and light sources were covered with large sheets of aluminum foil to minimize the impact of ambient light fluctuations.
2.3. Inoculum Preparation
Cultures used to inoculate experiments were pre-adapted in Zarrouk medium by 1:10 transfers from Spirulina medium stock cultures into 10 mL fresh Zarrouk medium, then cultivated for two weeks. Inoculum cultures were agitated continuously at 120 rpm inside a Fisher Scientific Isobath SWB 27 incubator (no water was used). Temperature of the incubator was controlled at 30–31 °C, and cultures were subjected to 24-hour light. Light was applied through a ceiling hanger that rested on top of two wooden 2 × 4s on top of the incubator, spaced so that light could penetrate between them. White printer paper was placed between the spacing to reduce the light intensity of the fluorescent bulbs.
2.4. Media composition experiments
Four medium formulations were tested in triplicate: 100% Zarrouk, 50% Zarrouk/50% ADPE, 25% Zarrouk/75% ADPE, and 100% ADPE. Experiments were completed in serum bottles with 50 mL working volume. Empty serum bottles were sealed with rubber stoppers and crimp-capped shut, followed by autoclaving on a 20-minute dry cycle at 121 °C. ADPE and Zarrouk media were then added to the serum bottles (as per treatment) via sterile needle and 30-mL syringe. Serum bottles were inoculated from S. platensis cultures pre-adapted in Zarrouk medium (see Inoculum Preparation section above). Each serum bottle was inoculated from a separate inoculum culture tube to more clearly capture representative biological variability. The cultures were spun down at 3260 x g for 10 min and then resuspended in their respective media compositions (so that media mixtures were not diluted). Optical density of the exponentially growing inoculum cultures (OD730) was 0.5 or greater. All serum bottles were inoculated to a uniform initial optical density (0.01–0.05). Experimental serum bottles were agitated continuously at 120 rpm inside a Fisher Scientific Isobath SWB 27 incubator (no water was used) controlled at 30–31 °C. These cultures were secured in the incubator by attaching a tube rack to the shaker with zip ties. Lighting was the same as for the inoculum cultures (see Inoculum Preparation section above).
The applied light intensity (luminous flux, lux) for each sample was estimated as follows. The fluorescent bulbs produce 2100 lumens each (4200 lumens total). The incubator had an area of 1.5 ft2 and each serum bottle occupied ∼1/12 of the area illuminated by the bulb, which resulted in 30,138 lux (in total) or ∼2,511.6 lux per serum bottle. The maximum intensity that S. platensis can tolerate according to UTEX is 3,200 lux. The estimated light supplied was therefore not anticipated to be overly excessive, causing photorespiration, but also would not limit cyanobacterial growth (Wang et al., 2006).
Daily optical density (OD730) and pH measurements were taken for the duration of the experiment using a spectrophotometer (Genesys 10S UV-Vis) and pH probe (Fisherbrand Accumet AB150 pH Benchtop Meter). Sample volumes of 1 mL were aseptically extracted from serum bottles with 2-mL syringes by luer lok connection to a needle inserted through the rubber stopper permitting gas transfer. The sterile filter on the end of the needle was removed before sampling and replaced after sampling. The serum bottles were sprayed with 70% ethanol before and after sampling. If the value of the OD730 reading was greater than 0.3, then samples were diluted with water (having the same optical density as the blank) to ensure that the measurement was within the linear range of the spectrophotometer. The experiment was terminated after 14 days.
2.5. Biomass vs. OD curve generation
The correlation between OD730 and biomass was quantified using cultures subjected to the same conditions as the media composition experiments. Eight serum bottles filled with 50 mL of Zarrouk medium were used to culture 400 mL total of S. platensis biomass. Serum bottles were prepared and inoculated as described in Section 2.4 and cultivated for 14 days with no intermittent sampling. Contents of the serum bottles were harvested by centrifugation in 50-mL Falcon tubes (3260 x g for 40 min at 4 °C). The supernatant was decanted and pellets were resuspended in tap water (to prevent cell lysis) and combined into a concentrated suspension. A series of 12 dilutions was prepared with dilutions spaced by ∼0.1 OD unit. The OD730 of each dilution was measured and recorded, and duplicate 15-mL aliquots of each dilution were distributed into 50-mL Falcon tubes. Three aliquots of tap water were also distributed as blank controls. Falcon tubes were dried in a drying oven at 80 °C for 35 h ensuring evaporation of all water. Both the initial and final mass of the Falcon tubes were recorded.
2.6. Statistical analysis
Excel analysis was used to establish a linear regression between biomass concentration and optical density, thus determining an equation for conversion between the two variables. Linear regression models were also performed for each media composition treatment. Optical density values were converted to biomass concentration, underwent a natural log transformation, were averaged by treatment, and then were plotted against time (t, hours) using JMP Pro 14. Error was estimated as the 95% confidence interval for the mean value of ln (Biomass). In order to determine the significance of the relationship between media composition and S. platensis growth, the cor.test function in R was used to calculate the Pearson's correlation coefficient between the percentage of Zarrouk medium in each treatment and the growth rates generated by the models.
3. Results and discussion
Table 1 compiles data regarding several variable parameters of ADPE chemical composition collected from the literature alongside the parameters measured for the ADPE source used in the presented experiment. When comparing the ADPE used in this study to other sources, it can be seen that ammonia levels were the lowest of the compiled studies, and phosphorus was at a moderate to high range. Potassium and pH were also lower than other reported studies, and chemical oxygen demand was in the moderate range. While it is difficult to isolate a single variable without performing an extensive array of tests analyze individual components, it is important to test different sources of ADPE, as they may have any combination of parameter values.
3.1. Biomass vs. optical density correlation results
The biomass dry weight vs. optical density provided empirical data relating actual biomass concentration to optical density as a proxy measurement specifically for S. platensis in the experimental conditions used in this study, allowing growth curves to be more easily and systematically quantified. Using the collected biomass and optical density data, the following relationship was quantified by linear regression (Eq. 1; see Figure 1):
| OD730·1.368 + 0.0691 = Biomass (g/L) | (1) |
Figure 1.
Biomass versus optical density at 730 nm (absorbance) of S. platensis LB2340 cultivated in 100% Zarrouk medium.
The regression analysis had an R-squared value of 0.8615 and indicated moderate variability, which is likely attributed to the clumping of S. platensis cells. Clumping increases heterogeneity within samples and can prevent stability in optical density readings, which subsequently increases variability. Nonetheless, establishing this correlation allowed a representative quantification of biomass in the media composition experiments.
3.2. Cultivation of S. platensis in varied proportions of Zarrouk medium and ADPE
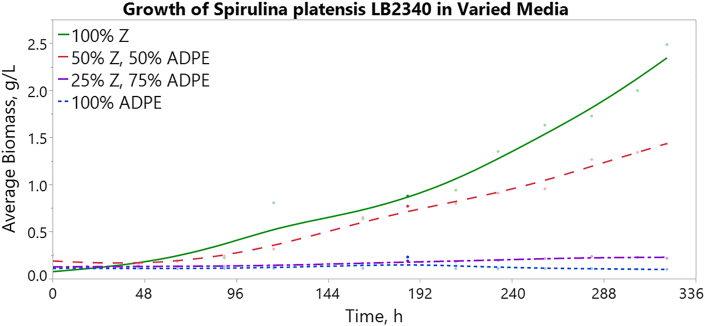
Varying the Zarrouk and ADPE proportions in the medium composition demonstrated the ability of S. platensis to tolerate and/or thrive on ADPE as a substrate. Figure 2 shows the growth curve for each treatment using the OD730 readings over time and the biomass vs. OD relationship described in the previous section. The 100% Zarrouk and 50/50% Zarrouk/ADPE media were observed to have higher cell density than the 25/75% Zarrouk/ADPE and 100% ADPE media over the course of the experiment (Figure 2).
Figure 2.
Average biomass of S. platensis grown over time in varied media: 100% Zarrouk, 50/50% Zarrouk/ADPE, 25/75% Zarrouk/ADPE, and 100% ADPE.
Ordered from highest to lowest biomass accumulation, the treatments follow as: 100% Zarrouk, 50/50% Zarrouk/ADPE, 25/75% Zarrouk/ADPE, and 100% ADPE. Growth rate was also assessed for each condition (Figure 3, with the shaded region representing a 95% confidence interval of the mean). The resulting regression models were obtained for each treatment (Eqs. (2), (3), (4), and (5), with time measured in hours):
| 100% Zarrouk: ln(Biomass) = 0.01002t - 2.246; R2 = 0.92 | (2) |
| 50/50% Zarrouk/ADPE: ln(Biomass) = 0.007589t - 2.073; R2 = 0.91 | (3) |
| 25/75% Zarrouk/ADPE: ln(Biomass) = 0.002081t - 2.166; R2 = 0.74 | (4) |
| 100% ADPE: ln(Biomass) = -0.00009896t - 2.184; R2 = 0.00 | (5) |
Figure 3.
Natural log of average biomass of S. platensis grown over time in varied media: 100% Zarrouk, 50/50% Zarrouk/ADPE, 25/75% Zarrouk/ADPE, and 100% ADPE. Shaded region indicates 95% confidence interval.
The linear regression models show comparable growth between the 100% Zarrouk and 50/50% Zarrouk/ADPE treatments for approximately the first 192 h (8 days) before a sharp rise in growth in the 100% Zarrouk medium (Figure 3). In comparison, the 25/75% Zarrouk/ADPE and 100% ADPE treatments experienced a slower rate of growth and no growth, respectively. Further analysis of the relationship between Zarrouk medium proportion and the growth rate produced a Pearson's correlation coefficient of 0.958 and a corresponding p-value of 0.042, indicating a statistically significant positive correlation between proportion of Zarrouk medium and growth rate.
Culture pH remained constant for approximately the first 10 days of the experiment before rising across all media types (Figure 4). Variation in initial pH of the media treatments was due to different pHs and buffers within ADPE and Zarrouk medium. The pH profiles over time of both the 100% Zarrouk and 50/50% Zarrouk/ADPE were similar: 100% Zarrouk and 50/50% Zarrouk/ADPE began at a pH of 9.07 and 9.00, respectively, before rising to a final pH of 10.14 and 10.23, respectively. In contrast, the pH of the 25/75% Zarrouk/ADPE and 100% ADPE media began at lower initial pHs and experienced smaller rises of 0.54 and 0.37, respectively. The lower pH changes may be attributed to lower biomass concentration within the media, as biomass is key to driving the pH increase through the consumption of acidic dissolved carbon dioxide during photosynthesis.
Figure 4.
pH over time of S. platensis grown over time in varied media: 100% Zarrouk, 50/50% Zarrouk/ADPE, 25/75% Zarrouk/ADPE, and 100% ADPE. Shaded region indicates 95% confidence interval.
The results of the media composition test demonstrated a positive correlation between Zarrouk medium proportion and growth rate, with substantial biomass accumulation still occurring at lower (50%) ADPE concentrations, which is more than five times higher than ADPE concentrations reported in previous studies (Olguin et al., 2003; Mezzomo et al., 2010). The variation in growth between the four media composition treatments tested could potentially be explained by a variety of factors. Carbon availability within each medium is a key variable influencing cyanobacterial growth. Zarrouk medium is fortified with sodium bicarbonate, providing an alternative carbon source to dissolved atmospheric carbon dioxide. Consequently, growth rate may be a function of carbonate ion concentration within each medium. This hypothesis may be tested in future experimentation by supplementing ADPE with sodium bicarbonate to better control carbonate ion concentration across all media. Additional possible explanations for the slower growth rates observed with ADPE could also include inhibitory compounds in the ADPE medium (i.e., salts, heavy metals), an inhibitory pH, or lack of available nutrients (i.e., macro/micronutrients). For any future research, detailed composition analyses of growth media throughout the course of the experiment may more accurately explain differences in growth.
The presented study demonstrates that S. platensis shows a differential growth response with respect to the concentration of ADPE, with enhanced growth positively correlated with the proportion of Zarrouk medium. As Zarrouk medium is ideally designed to support cyanobacterial growth, this outcome is not necessarily surprising. While the current results did not show growth on pure ADPE, it is possible that pure ADPE could still serve as a feasible substrate upon further investigation of bicarbonate availability, other possible nutrient inhibitions, further supplementation of ADPE, or even dilution with tap water to improve growth results. Future work should also focus on monitoring nitrogen and phosphorus levels throughout growth to determine rates and amounts of nutrient reduction. These exploratory results presented here provide a step forward in utilizing ADPE for cyanobacterial cultivation to combine value-added biomass production with nutrient reduction, with the goal of improving the economic feasibility and sustainability of anaerobic digestion.
4. Conclusions
Based on the presented study results, given the appropriate conditions, utilization of ADPE as a growth medium for S. platensis is plausible only if diluted and mixed with an ideal synthetic medium.
Declarations
Author contribution statement
Matthew Baker; Sam Blackman; Erin Cooper; Kevin Smartt; David Walser: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Megan Boland: Contributed reagents, materials, analysis tools or data.
Praveen Kolar; Ashley E. Beck: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mari S. Chinn: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by USDA National Institute of Food and Agriculture, Hatch 1018813 and NC State University Biological and Agricultural Engineering partially.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Ben Cauthen and colleagues from Cavanaugh Solutions, Dr. Mike Boyette, Dr. Rachel Slivka, and Dr. Mahmoud Sharara for technical help.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Borges J.A., Rosa G.M., Meza L.H.R., Henrard A.A., Souza M.R.A.Z., Costa J.A.V. Spirulina sp. LEB-18 culture using effluent from the anaerobic digestion. Braz. J. Chem. Eng. 2013;30:2. [Google Scholar]
- Canizares-Villanueva R.O., Ramos A., Corona A.I., Monroy O., de la Torre M., Gomez-Lorejo C., Travies L. Phormidium treatment of anaerobically treated swine wastewater. Water Res. 1994;28(9):1891–1895. [Google Scholar]
- Hussain Z., Mishra J., Vanacore E. Waste to energy and circular economy: the case of anaerobic digestion. J. Enterprise Inf. Manag. 2020;33:4. [Google Scholar]
- Mezzomo N., Saggiorato A.G., Siebert R., Tatsch P.O., Lago M.C., Hemkemeier M., Costa J.A.V., Bertolin T.E., Colla L.M. Cultivation of microalgae Spirulina platensis (Arthrospira platensis) from biological treatment of swine wastewater. Food Sci. (N. Y.) 2010;30(1):173–178. [Google Scholar]
- Moheimani N.R., Vadiveloo A., Ayre J.M., Pluske J.R. Nutritional profile and in vitro digestibility of microalgae grown in anaerobically digested piggery effluent. Algal Res. 2018;35:362–369. [Google Scholar]
- Nwoba E.G., Moheimani N.R., Ubi B.E., Ogbonna J.C., Vadiveloo A., Pluske J.R., Huisman J.R. Macroalgae culture to treat anaerobic digestion piggery effluent (ADPE) Bioresour. Technol. 2017;15:15–23. doi: 10.1016/j.biortech.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Olguin E., Galicia S., Mercado G., Perez T. Annual Production of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003;15(2):249–257. [Google Scholar]
- Park Y.-I., Labrecque M., Lavoie J.-M. Influence of elevated CO2 and municipal wastewater feed on the productivity, morphology, and chemical composition of Arthrospira (Spirulina) platensis. ACS Sustain. Chem. Eng. 2013;1:1348–1356. [Google Scholar]
- Raoof B., Kaushik B.D., Prasanna R. Formulation of a low-cost medium for mass production of Spirulina. Biomass Bioenergy. 2006;30(6):537–542. [Google Scholar]
- Saranraj P., Sivasakthi S. Spirulina platensis–food for future: a review. Asian J. Pharmaceut. Sci. Technol. 2014;4(1):26–33. [Google Scholar]
- Singh J.S., Kumar A., Singh M. Cyanobacteria: a sustainable and commercial bio-resource in production of bio-fertilizer and bio-fuel from waste waters. Environ. Sustain. Indicat. 2019;3:100008. [Google Scholar]
- Tsui T.H., Wong J.W. A critical review: emerging bioeconomy and waste-to-energy technologies for sustainable municipal solid waste management. Waste Dispos. Sustain. Energy. 2019;1:151–167. [Google Scholar]
- Wang C.-Y., Fu C.-C., Liu Y.-C. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 2006;37:21–25. [Google Scholar]
- Zarrouk C. University of Paris; France: 1966. Contribution a l’edtude d’une cyanobacterie: Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima (Setchell et Gardner) Geitler. PhD thesis. [Google Scholar]
- Zhang Q., Yu Z., Jin S., Liu C., Li Y., Guo D., Hu M., Ruan R., Liu Y. Role of surface roughness in the algal short-term cell adhesion and long-term biofilm cultivation under dynamic flow condition. Algal Res. 2020;46:101787. [Google Scholar]
- Zhang Q., Yu Z., Zhu L., Ye T., Zuo J., Li X., Xiao B., Jin S. Vertical-algal-biofilm enhanced raceway pond for cost-effective wastewater treatment and value-added products production. Water Res. 2018;139:144–157. doi: 10.1016/j.watres.2018.03.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.