Abstract
Opuntia ficus-indica L. Mill cladodes are considered to be a source of an abundance of bioactive compounds. To identify a natural product that can be used in the chemoprevention and treatment of cancer, this study was conducted to produce an anticancer agent extracted from in vitro-derived cladodes of prickly pear cactus. Toward this goal, assays of seed germination and micropropagation revealed that the highest seed germination rate was 66% and that the highest shoot number per explant was obtained with benzyl adenine (BA) (2 mg/l) and kinetin (Kin) (1 mg/l) within 2 months, at 22.6. In addition, the maximum length of shoots was obtained with BA (3 mg/l) and Kin (0.5 mg/l), at 7.44 cm. The in vitro-derived cladode extract showed higher total phenolic and kaempferol contents than the in vivo-derived cladode extract (total phenolics 156.5 mg/g and 86 mg/g DW; kaempferol 2.807 mg/g and 1.304 mg/g DW, respectively). These remarkable results reflected the anticancer activity on the viability and proliferation/migration of PC3 prostate and mammary Mcf7-7 cells. In terms of cytotoxicity, the IC50 values on PC3 and Mcf7 cells were 5775.7 and 6311.3 μg/ml, respectively, showing dose-dependent increases. Meanwhile, from in vivo analyses of the plants, the IC50 values were 5927.93 and 6825.6 μg/ml, respectively, again showing dose-dependent increases.
Keywords: Opuntia ficus-indica L. Mill, Cladode extract, Micropropagtion, Anticancer activity, kaempferol, PC3, Mcf7
Opuntia ficus-indica L. Mill; Cladode extract; Micropropagtion, Anticancer activity; kaempferol; PC3; Mcf7.
1. Introduction
Cancer chemoprevention targets both normal and high-threat individuals via treatment using drugs and other chemical agents in order to inhibit, delay, or reverse cancer development (Kelloff et al., 1999a,b). In the last decade, hundreds of cancer chemopreventive agents have been developed in the United States alone, but only a few new drugs have been approved (Steele et al., 1996; Kelloff et al., 2000). There is thus a need for more effective and less toxic agents, especially those from natural products, to successfully prevent and treat cancer (Darwesh et al., 2018). Extracts of cactus (Opuntia ficus-indica L. Mill) have been reported to show various pharmacological activities through antioxidant activity, decreasing the risk of diseases such as cancer, diabetes, and cardiovascular and neurodegenerative disorders. In previous research investigating the antioxidant agents contained in extracts of pulp, peel, seeds, and cladodes of various cacti, it was proven that the specific antioxidant contents were related to the color of the fruit, while the cladodes of all cultivars were shown to contain similar and highly effective antioxidants (Maryna et al., 2019). Opuntia ficus-indica L. Mill contains different flavonoids including kaempferol, which is one of the flavonoids with the most efficient anticancer effects (Muhammad et al., 2019). Compared with other flavonoids, it was found that kaempferol exhibits different biological properties via its antioxidant and antineoplastic activities (Sultan et al., 2016). This unique flavonoid may reduce the proliferation of ovarian cancer cells and remarkably decrease the expression of vascular endothelial growth factor (VEGF) which is a marker of angiogenesis in these cells (Collazo-Siques et al., 2003). In a cancer cell model, kaempferol was shown to be involved in inhibiting angiogenesis through suppressing the extracellular signal-regulated kinase (ERK)-NFκB-cMycp21-VEGF pathway (Li et al., 2003). It has been shown that kaempferol inhibited cell proliferation in a dose-dependent manner by two processes; (i) regulating cyclin-dependent kinase 1 (CDK1) associated with the transition from G2 to M phase (cyclin B), as well as (ii) regulating a tumor suppressor gene that plays a key role in cell cycle arrest, p53 or PLK-1, in MCF-7 breast cancer and HeLa cervical cancer cells, respectively (Shin et al., 2004; Hansen et al., 2010).
A valuable alternative method for plant micropropagation and the production of biologically active substances under controed conditions is tissue culture, which can be applied regardless of the season via various strategies to develop and increase the accumulation of biomass and biosynthesis of secondary metabolites in plant cell and organ cultures (El-Baz et al., 2015; Mohamed et al., 2015; Sugandh, 2017; Darwesh and Elshahawy 2021). The present study focused on the micropropagation of Opuntia ficus-indica L. Mill from seeds and determined the capacity of in vitro-derived plants to produce phenolic compounds exhibiting anticancer activity. Another goal here was to establish an efficient method for the sterilization, germination, and micropropagation of Opuntia ficus-indica from seeds. Finally, this study estimated the anticancer activity of in vitro-derived cladode extract on the viability and proliferation/migration of PC3 prostate and mammary Mcf7 cells.
2. Material and methods
2.1. Seed sterilization and germination
Cactus fruit (Opuntia ficus-indica L. Mill) seeds were obtained from the Agriculture Research Center, Egypt. For sterilization, the seeds were cleaned thoroughly under running tap water and then sterilized using 20% sodium hypochlorite and Tween solution by shaking for 5–10 min, followed by washing three times using sterilized distilled water (Hussein et al., 2019a). Different treatments were carried out to break the dormancy and promote the germination of the seeds (Table 1).
Table 1.
Treatments used in assessing germination in Egyptian Opuntia ficus-indica L. Mill.
| Immersion in different H2O2 concentrations | ||
|---|---|---|
| Treatments | Immersed in | Duration of treatment |
| Control (T1) | Sterilized distilled water without H2O2 | 24 h |
| T2 | 5% H2O2 | 24 h |
| T3 | 10% H2O2 | 24 h |
| T4 | 15% H2O2 | 24 h |
| T5 |
30% H2O2 |
24 h |
| Scarifying with sand followed by immersion in different concentrations of H2O2 | ||
| T6 | 3% H2O2 | 24 h |
| T7 | 5% H2O2 | 24 h |
| T8 | 7% H2O2 | 24 h |
| T9 |
10%H2O2 |
24 h |
| Scarifying with sand followed by immersion in 5% H2O2 for 24 h and then immersion in different concentrations of gibberellic acid (GA3) solution for 48 h | ||
| T10 | GA3 solution (0.5 mg/L) | |
| T11 | GA3 solution (1.0 mg/L) | |
| Seeds cultured on half-strength Murashige & Skoog (MS) basal medium containing different concentrations of GA3 under aseptic conditions | ||
| Control (C1) | ½ -strength MS | |
| C2 | ½ -strength MS+ 0.5 mg/L gibberellic acid | |
| C3 | ½ -strength MS +1.0 mg/L gibberellic acid | |
| C4 | ½-strength MS+1.5 mg/L gibberellic acid | |
At the end of the incubation period, all seeds were cultured on half-strength MS basal medium (Snedecor and Cochran, 1994) supplemented with 3 % (w/v) sucrose, solidified with 0.8 % (w/v) agar. The pH was adjusted to 5.8, followed by incubation at 25 °C ± 2 °C; after 8 weeks, aseptic plantlets were obtained. The effects of treatments on germination regarding the composition of the media were compared to the control (C1) when seeds were cultured on half-strength MS basal medium without GA3. Three replicates of each treatment, with 30 seeds per treatment, were performed and assayed. The number of germinated seeds was recorded at the maximum time taken to stop germination (60 days). The percentages were arcsine-transformed and subjected to analysis of variance (ANOVA) (Silva and Azevedo, 2009).
2.2. Micropropagation technology
For the multiplication of Opuntia ficus-indica L. Mill, the germinated shoots were obtained from seedlings and cultured on MS basal medium containing different concentrations of benzyl adenine (BA) (1.0, 2.0, and 3.0 mg/L) in combination with kinetin (Kin) (0.5, 1.0, 1.5, and 2.0 mg/L) (Table 2). All media were prepared by standard procedures and the cultures were incubated for 2 months in a growth chamber with a photoperiod of 16 h light and 8 h dark at 25 °C ± 2 °C.
Table 2.
Composition of media used for multiplication of Egyptian Opuntia ficus-indica.
| Treatments | Growth regulators (cytokinins) |
|
|---|---|---|
| BA (mg/L) | Kin (mg/L) | |
| S0 | 0.0 | 0.0 |
| S1 | 1.0 | 0.5 |
| S2 | 1.0 | 1.0 |
| S3 | 1.0 | 1.5 |
| S4 | 1.0 | 2.0 |
| S5 | 2.0 | 0.5 |
| S6 | 2.0 | 1.0 |
| S7 | 2.0 | 1.5 |
| S8 | 2.0 | 2.0 |
| S9 | 3.0 | 0.5 |
| S10 | 3.0 | 1.0 |
| S11 | 3.0 | 1.5 |
| S12 | 3.0 | 2.0 |
2.3. Rooting of in vitro induced shoots
After three subculture cycles of micropropagation, the small cladodes (3.5–4 cm) derived from shoot bunches were excised and rooted on MS basal medium supplemented with indole butyric acid at 0.5 mg/l. This medium was selected based on the results of previous experiments by García-Saucedo et al. (2005) and El Finti et al. (2013).
2.4. Preparation of samples and determination of total phenolics
Opuntia ficus-indica L. Mill cladodes of in vitro-derived plants from tissue culture and in vivo-derived plants after culturing for 4 months under greenhouse conditions were carefully cut into small pieces and dried in a ventilated oven at 40 °C for 3 days. After drying, the cladode fragments were ground for a few minutes in a domestic coffee grinder and sieved. The crushed samples were subjected to extraction and determination of bioactive compounds.
For the determination of total phenolics, the folin polyphenolic method was conducted on both extracts from in vivo- and in vitro-derived plantlets in accordance with the work of Agbor et al. (2014) and Hussein et al. (2019b).
2.5. HPLC determination of kaempferol
HPLC determination of kaempferol was carried out on samples from both in vivo- and in vitro-derived plantlets, in accordance with the work of, Kelly et al. (1995) using a thermo system (Ultimate 3000). A thermohypersil reverse phase C18 column (2.5 × 30 cm) was operated at 25 °C with a mobile phase consisting of linear gradient elution from water-ACN (90:10) to water-ACN (55:45) over 8 min. The UV absorption spectra of the standards as well as the samples were recorded in the range of 220–400 nm. Samples and standard solutions as well as the mobile phase were degassed and filtered through a 0.45 μm membrane filter (Millipore) before injection. Identification of the compounds was performed by comparison of their retention time and UV absorption spectrum with those of the standards.
2.6. Determination of sample cytotoxicity on cells using the MTT protocol
The ethanol extract (containing phenolic and bioactive substances) was used to determine the anticancer activity. Two models of cancer cells, PC3 and Mcf7, were applied to evaluate the anticancer activity of the extracted agents using the MTT protocol, in accordance with the work of Mosmann (1983) and Abd El-Hady et al. (2017). Each experiment on the anticancer activity was performed in triplicate.
2.7. Statistical analysis
The experimental design used was randomized complete blocks with three replications. Statistical analyses were carried out using IBM® SPSS® (SPSS Inc; IBM Corporation, NY, USA) Statistics Version 25 (2017) for Windows. Data were tested for a normal distribution by Shapiro-Wilk's test (Shapiro and Wilk, 1965; Razali and Wah, 2011). Data were subjected to ANOVA with a P-value of <0.05 being considered statistically significant. The treatment means were compared by least significant difference post-hoc test as reported by Snedecor and Cochran (1994), with a P-value of <0.05 being considered statistically significant (Darwesh et al., 2020).
3. Results and discussion
3.1. Effects of different treatments on seed germination
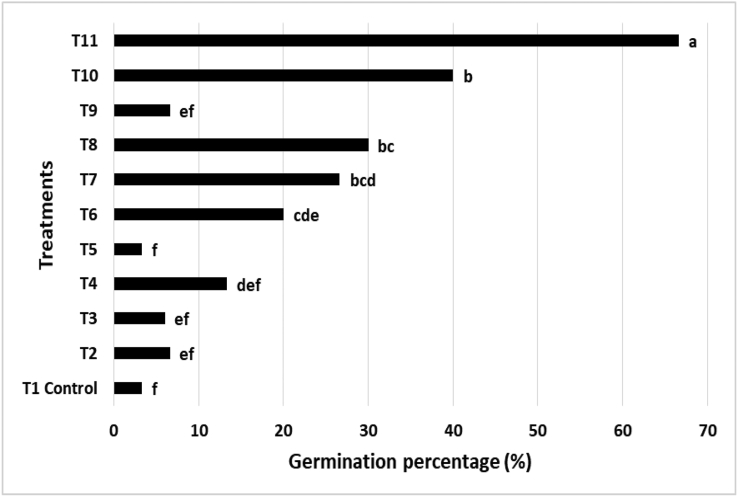
Opuntia ficus-indica L. Mill seeds have innate and enforced dormancy (Rojas-Aréchiga and Vázquez-Yanes, 2000), which has led most protocols to rely on vegetative micropropagation as an uncomplicated and rapid approach. In contrast, micropropagation using seeds facilitates the selection of desirable genotypes that can tolerate various stresses, promote biomass production, and ensure optimal fruit quality, among others, which may be utilized for breeding purposes. Furthermore, micropropagation by seeds results in variability, allowing the genetic variety of populations and species to be preserved (Altare et al., 2006). In addition, the micropropagation of Opuntia ficus-indica L. Mill in vitro is an appropriate method for obtaining these seasonal plants all year round for purposes such as studying their levels of active compounds and biological activity. Against this background, different treatments were examined to determine an efficient germination protocol (Table 1) for these plants under a daily photoperiod of 16/8 h light/dark. The results showed an increase in the germination rate when the seeds of Opuntia were immersed in H2O2 solution (Figure 1). The best treatment for germination was scarification with sand, followed by immersion in 5% H2O2 for 24 h and then immersion in 1.0 mg/L gibberelic acid (GA3) solution for 48 h, leading to a germination rate of 66.6%. The methods involving chemical scarification also increased the germination rate (Figure 1), as also reported by Altare et al. (2006). The combination of mechanical scarification (sanded seeds) with chemical scarification (immersion in H2O2 for 24 h) resulted in a germination rate of Opuntia spp. of 67.5% (Areli et al., 2018); although the chemical scarification by itself exhibited significant results, the germination rates were lower than 60 %. The seeds took nearly 40–60 days to emerge after treatment (Altare et al., 2006).
Figure 1.
Germination%rates of Opuntia ficus-indica L. Mill seeds germinated with different treatments.
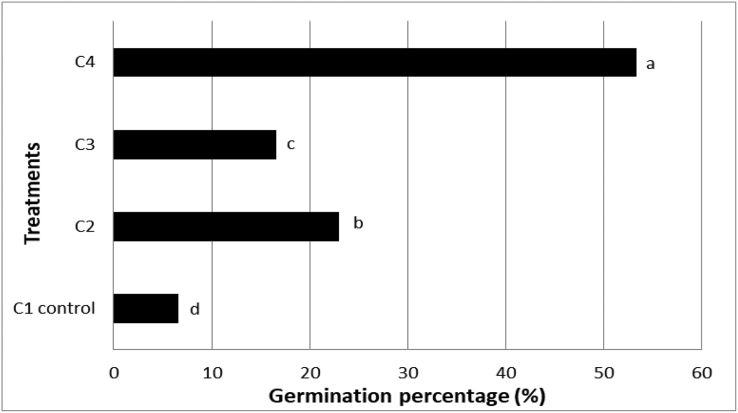
P-value <0.05 and method of comparison showed significance with 1.5 mg/L concentration of GA3 in MS basal media as shown in C4 treatment with 53.3% (Figure 2). The seeds took more than 60 days to germinate (60–90 days) and the germination rate was low compared with that upon scarifying the seeds with sand followed by immersion in H2O2 at 5% for 24 h, and then immersion in 1.0 mg/L GA3 solution for 48 h, as shown in Figure (2). In this respect, Areli et al. (2018) obtained an in-vitro germination rate of Opuntia microdasys of 60% by applying half -strength MS with GA3 at a concentration of 0.5 mg/L. The same trend was observed in Opuntia engelmannii, with a germination rate greater than 80%.
Figure 2.
Germination % of Opuntia ficus-indica L. Mill seeds germinated on media containing different concentrations of GA3.
3.2. Multiplication rate and shoot length of Opuntia ficus-indica L. Mill
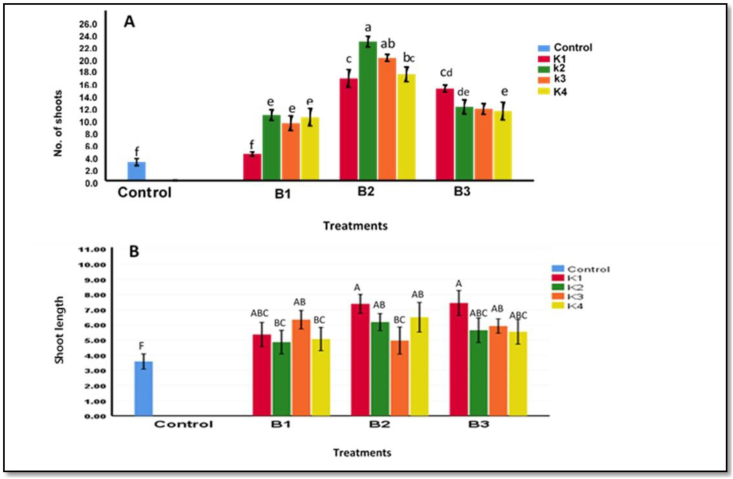
The germinated shoots obtained from seedlings using the efficient germination treatment (T11) and after growth to a length of about 2–3 cm were used as secondary explants for multiplication experiments. There were notable effects of adding different concentrations of cytokinins (N6-benzyl-adenine) in combination with kinetin on the multiplication rates of Opuntia ficus-indica L. Mill, as presented in Table 3. In this regard, several cytokinins have been used for both prickly pear initiation and shoot proliferation (Juárez and Passera, 2002; Khalafalla et al., 2007). The treatments differed in their effects and the greatest number of shoots (22.6). formed upon S6 treatment on MS medium containing 2 mg/L BA + 1 mg/L Kin. The responses of plant shoot proliferation are known to vary according to the type of cytokinin and its concentration (Estrada-Luna et al., 2008; Ali et al., 2016).
Table 3.
Effect of BA and Kin concentrations on in vitro shoot induction of Opuntia ficus-indica L. Mill.
| Treatments | Shoot number per explant (mean ± SE) | Length of shoots (cm) (mean ± SE |
|---|---|---|
| S0 | 3.0f ± 0.57 | 3.58c ± 0.5 |
| S1 | 4.3f ± 0.33 | 5.36abc ±0.80 |
| S2 | 10.6e ± 1.88 | 4.86bc ±0.77 |
| S3 | 9.33e ± 1.20 | 6.34ab ± 0.60 |
| S4 | 10.3e ± 1.45 | 5.06bc ±0.76 |
| S5 | 16.6c ± 1.45 | 7.38a ± 0.62 |
| S6 | 22.6a ± 0.88 | 6.18ab ± 0.56 |
| S7 | 20.0ab ± 1.57 | 4.96bc ±0.89 |
| S8 | 17.3bc ±1.20 | 6.50ab ± 0.98 |
| S9 | 15.0cd ± 0.57 | 7.44a ± 0.82 |
| S10 | 12.0de ± 1.15 | 5.64abc ±0.81 |
| S11 | 11.66e ± 0.88 | 5.92ab ± 0.47 |
| S12 | 11.33e ± 1.45 | 5.54abc ±0.81 |
SE = standard error; S = treatment number; means with different superscripts in the same column differ significantly.
Comparison with a previous study by El Finti et al. (2012) on Moroccan cultivars revealed that adding 5 mg/L BA alone was optimal for inducing a large number of shoots. Our results on Egyptian cultivar confirmed that adding a high concentration of BA of 5 mg/L alone was not the most efficient method for multiplication; adding the combination of the two cytokinins BA and Kin was more effective for our Egyptian cultivar and the number of shoots formed per explant was shown to be dependent on the concentrations of both cytokinins (Figure 3A). This confirms that each species of cactus, even within the same genus, responds differently to growth regulators; for this reason, in vitro propagation systems have to be established and developed specifically for each species (Hubstenberger et al., 1992). Notably, the response regarding shoot length varied depending on the concentrations of both cytokinins and the greatest shoot length was observed upon S9 treatment, at 7.44 cm (Figure 3B), on MS medium supplemented with 3.0 mg/L BA + 0.5 mg/L Kin. The results indicated. The results indicated that increasing the concentration of BA while decreasing the concentration of Kin when using both cytokinins in combination significantly increased the length of shoots (Figure 4). In conclusion, this study shows that the interaction between these two hormones is important for multiplication of the Egyptian cultivar (Figure 4).
Figure 3.
Effects of different treatments of BA (B1: 1 mg/L, B2: 2 mg/L, B3: 3 mg/L) and Kin (K1: 0.5 mg/L, K2: 1 mg/L, K3 1.5 mg/L, K4: 2 mg/L) on (A) multiplication rate (number of shoots) and (B) shoot length of Opuntia ficus-indica L. Mill explanted on MS medium.
Figure 4.
Different stages of in vitro micropropagation of Egyptian Opuntia ficus-indica. A, seedling under culture conditions; B, germinated shoots used as secondary explants for multiplication; C, micropropagation of explants on MS medium with 5 mg/L BA alone; D, maximum number of shoots of micropropagated explants on MS medium with 2 mg/L BA and 1 mg/L Kin; E, maximum number of shoots on MS medium with 2 mg/L BA and 1 mg/L Kin after 2 months; F, maximum length of shoots on MS medium with 3 mg/L BA and 0.5 mg/L Kin.
3.3. Determination of total phenolics and kaempferol
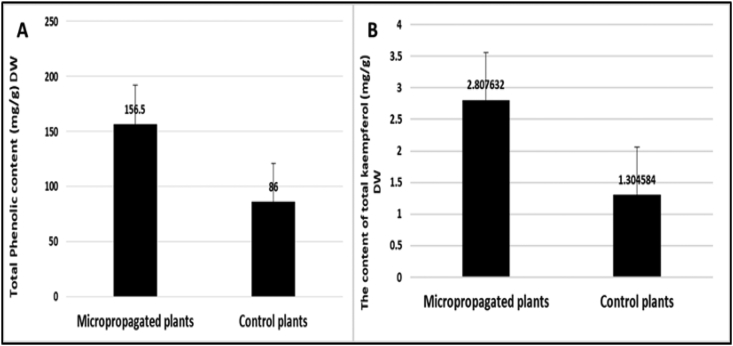
Figure 5A presents the values of total phenolic content in micropropagated in vitro- and in vivo-derived cladode extracts from a greenhouse, used as a control in this study. The micropropagated plants (in vitro) were harvested from the medium conferring the best multiplication (S6) in order to determine total phenolics using Folin Ciocalteu reagent (Agbor et al., 2014). The highest value of total phenolic was recorded for the in vitro-derived cladode extract (156.5 mg/g dry weigh), while the corresponding value for the in vivo-derived cladode extract was 86 mg/g DW. In general, the addition of both cytokinins BA (2 mg/L) and Kin (1 mg/L) enhanced the accumulation of phenolics more than the finding in the control harvested from a greenhouse.
Figure 5.
Total phenolic content (A) and kaempferol content (B) in micropropagated plants compared with those of control plants.
With regard to the flavonoid (kaempferol), it was detected in micropropagated in vitro- and in vivo-derived cladode extracts using HPLC. The highest kaempferol content was observed in the in vitro-derived cladode extract (2.807 mg/g DW), whereas the value of kaempferol in the in vivo-derived cladode extract was 1.304 mg/g DW, as shown in Figure (5B).
Notably, some polyphenols were produced only by cladodes of certain varieties of cactus. Snowshoeing cactus presents high levels of unusual flavonoid-like compounds such as kaempferol-3- rutinoside (1.46 mg/g) and isorhamnetin-3-O-rutinoside (1.37 mg/g DW). Other studies confirmed that changes in the type and concentration of cytokinin applied can markedly affect product accumulation. For instance, cytokinins increased the production of alkaloids in cell cultures of Catharanthus roseus (Decendi et al., 1992), lignans in Phyllanthus amarus shoots (Nitnaware et al., 2011), and anthocyanins in Oxalis linearis callus (Meyer and Van Staden, 1995). Our findings in this study confirmed that kaempferol content in in vitro Egyptian Opuntia ficus-indica L. Mill cladodes was 2.807 mg/g DW, which was much higher under optimal cytokinin conditions than in cytokinin-free control in vivo-drived plants from a greenhouse (1.304 mg/g DW). This difference is a result of the positive effect of suitable concentrations of cytokinins on the accumulation of kaempferol.
3.4. Effect of in vitro and in vivo-derived cladode extracts on viability and proliferation/migration of PC3 cells
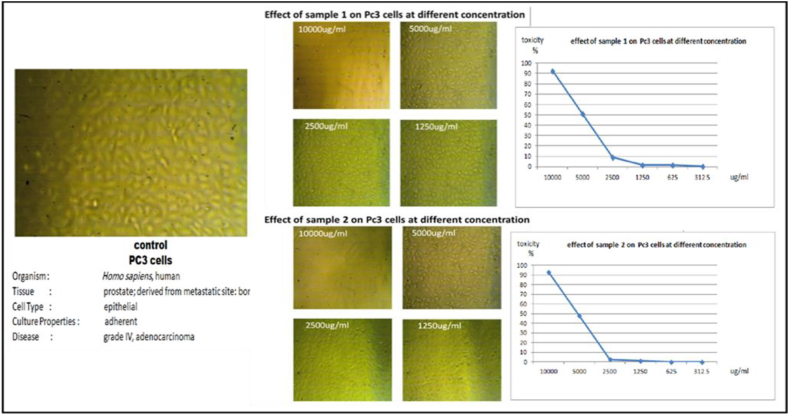
PC3 cells were used as a model for evaluating the produced bioactive compounds as anticancer agents. To achieve this, the proliferative effect of the crude extract of in vitro-derived cladodes (sample 1) on PC3 cells was analyzed using the MTT assay (Table 4). The PC3 cells were treated with various concentrations of the crude extract of in vitro-derived cladodes (312.5, 625, 1250, 2500, 5000, and 10.000 μg/ml). The results showed that the growth inhibition of PC3 was dose-dependent, with an IC50 of 5775.7 μg/ml (Figure 6).
Table 4.
Effects of various concentrations of in vitro Opuntia ficus-indica L. Mill cladode extracts on viability and proliferation/migration of PC3 cells.
| ID | Conc. μg/ml |
OD | Mean OD | ST.E | Viability % | Toxicity % | IC50 | ||
|---|---|---|---|---|---|---|---|---|---|
| Pc3 | 1:2 | 0.324 | 0.295 | 0.311 | 0.31 | 0.0084 | 100 | 0 | μg/ml |
| 1 | 10000 | 0.023 | 0.024 | 0.022 | 0.023 | 0.0006 | 7.42 | 92.58 | 5775.7 |
| 5000 | 0.142 | 0.155 | 0.157 | 0.151 | 0.0047 | 48.82 | 51.18 | ||
| 2500 | 0.284 | 0.286 | 0.277 | 0.282 | 0.0027 | 91.08 | 8.92 | ||
| 1250 | 0.305 | 0.311 | 0.299 | 0.305 | 0.0035 | 98.39 | 1.61 | ||
| 625 | 0.293 | 0.321 | 0.301 | 0.305 | 0.0083 | 98.39 | 1.61 | ||
| 312.5 | 0.303 | 0.3 | 0.321 | 0.308 | 0.0066 | 99.35 | 0.65 | ||
| 2 | 10000 | 0.019 | 0.025 | 0.023 | 0.022 | 0.0018 | 7.20 | 92.80 | 5927.93 |
| 5000 | 0.168 | 0.172 | 0.144 | 0.161 | 0.0087 | 52.04 | 47.96 | ||
| 2500 | 0.304 | 0.299 | 0.303 | 0.302 | 0.0015 | 97.42 | 2.58 | ||
| 1250 | 0.311 | 0.298 | 0.309 | 0.306 | 0.0040 | 98.71 | 1.29 | ||
| 625 | 0.309 | 0.315 | 0.313 | 0.312 | 0.0018 | 100.75 | 0 | ||
| 312.5 | 0.302 | 0.307 | 0.326 | 0.312 | 0.0073 | 100.54 | 0 | ||
Figure 6.
Effect of various concentrations of in vitro (sample 1) and in vivo (sample 2) Opuntia ficus-indica L. Mill cladode extracts on viability and proliferation/migration of PC3 cells.
The proliferative effect of the crude extract of in vivo-derived cladodes on PC3 cells was estimated using the MTT assay at various concentrations (312.5, 625, 1250, 2500, 5000, and 10,000 μg/ml). The results indicated that the growth inhibition of PC3 was dose-dependent, with an IC50 of 5927.93 μg/ml (Figure 6).
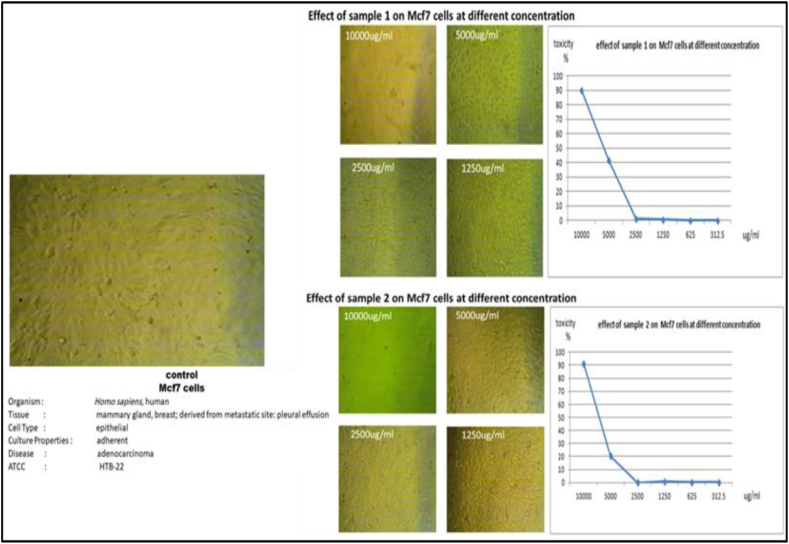
3.5. Effect of in vitro and in vivo cladode extracts on viability and proliferation/migration of Mcf7 cells
Mcf7 cells were also used in the evaluation of the produced bioactive compounds as anticancer agents. The effects of the in vitro micropropagated cladodes (sample 1) on Mcf7 cells were estimated using the MTT assay (Table 5). The Mcf7 cells were treated with different concentrations (312.5, 625, 1250, 2500, 5000, and 10.000 μg/ml); the results showed that the growth inhibition of Mcf7 was dose-dependent, with an IC50 of 6311.3 μg/ml (Figure 7). For in vivo extracts, the proliferative effect of the crude extract of in vivo-derived cladodes on Mcf7 cells was estimated using the MTT assay at various concentrations (312.5, 625, 1250, 2500, 5000, and 10.000 μg/ml). The results indicated that the growth inhibition of Mcf7 was dose-dependent, with an IC50 of 6825.6 μg/ml (Figure 7).
Table 5.
Effects of various concentrations of in vitro Opuntia ficus-indica L. Mill cladode extracts on viability and proliferation/migration Mcf7 cells.
| ID | Conc. μg/ml | OD | Mean OD | ST.E | Viability % | Toxicity % | IC50 | ||
|---|---|---|---|---|---|---|---|---|---|
| Mcf7 | 1:02 | 0.362 | 0.379 | 0.366 | 0.369 | 0.005 | 100 | 0 | μg/ml |
| 1 | 10000 | 0.042 | 0.036 | 0.031 | 0.036 | 0.003 | 9.846 | 90.154 | 6311.3 |
| 5000 | 0.219 | 0.204 | 0.220 | 0.214 | 0.005 | 58.085 | 41.916 | ||
| 2500 | 0.354 | 0.374 | 0.362 | 0.363 | 0.006 | 98.464 | 1.536 | ||
| 1250 | 0.369 | 0.352 | 0.378 | 0.366 | 0.008 | 99.277 | 0.723 | ||
| 625 | 0.371 | 0.359 | 0.375 | 0.368 | 0.005 | 99.819 | 0.181 | ||
| 312.5 | 0.363 | 0.379 | 0.370 | 0.371 | 0.005 | 100.452 | 0 | ||
| 2 | 10000 | 0.026 | 0.033 | 0.037 | 0.032 | 0.003 | 8.672 | 91.328 | 6825.6 |
| 5000 | 0.284 | 0.301 | 0.295 | 0.293 | 0.005 | 79.494 | 20.506 | ||
| 2500 | 0.384 | 0.353 | 0.369 | 0.369 | 0.009 | 99.910 | 0.090 | ||
| 1250 | 0.374 | 0.353 | 0.368 | 0.365 | 0.006 | 98.916 | 1.084 | ||
| 625 | 0.369 | 0.371 | 0.362 | 0.367 | 0.003 | 99.548 | 0.452 | ||
| 312.5 | 0.378 | 0.364 | 0.359 | 0.367 | 0.006 | 99.458 | 0.542 | ||
Figure 7.
Effect of various concentrations of in vitro (sample 1) and in vivo (sample 2) Opuntia ficus-indica L. Mill cladode extracts on viability and proliferation/migration of Mcf7 cells.
This study is considered to be the first to compare the extracts of in vitro- and in vivo-derived cladodes of Opuntia ficus-indica L. Mill plants in terms of their anticancer activity. The PC3 and Mcf7 cells were treated with both in vitro-extracts of cladodes from both in vitro- (sample 1) and in vivo-derived cacti (sample 2). The in vivo-derived cladode extract exhibited greater effects on the proliferation of PC3 and Mcf7 cells than the in vitro one with the highest total phenolic and kaempferol contents. Notably, in the previous studies kaempferol, -3-O-rhamnoside dose-dependently reduced the proliferation of prostate cancer cells by the expression of caspase-8, -9, and -3, and induced the release of GM-CSF in PC3 cells. This. in turn, elevated the chemotaxis of DC through the activation of phospholipase C and protein kinase C (PKC) (Abou-Elella and Ali, 2014).
Moreover, kaempferol effectively suppressed the growth of breast cancer cell lines (VM7Luc4E2, MDA- MB-231, and Mcf7) (Bandyopadhyay et al., 2008) through cell arrest at the G2/M stage, and DNA fragmentation at the sub-G0 phase (Azevedo et al., 2015). Furthermore, kaempferol was reported to elevate the levels of proapoptotic enzymes such as cleaved caspase-9, -7, -3, p21, p53, and Bax (Zhu and Xue, 2018). In contrast, O. humifusa extracts were shown to induce apoptosis of Mcf7 cells and colon SW-480 cells and suppress the growth of U87MG glioblastoma cells through the production of ROS within the cells (Hahm et al., 2010; Diantini et al., 2012; Kim et al., 2014). As mentioned previously by Serra et al. (2013), the polyphenol-rich juice of various Opuntia affected HT-29 colon cancer cell lines, as it provoked cell cycle arrest. Interestingly, this effect occurred via an increase of ROS leading to cell death; this effect was also reported in ovarian cancer cells (Feugang et al., 2010). A previous study by Marissa et al. (2015) that estimated the accumulation of total phenolic acids and flavonoids in (in vitro cultures) of callus and cell suspensions of three Opuntia species (O. streptacantha, O. megacantha, and O. ficus-indica) under controlled conditions indicated that the levels of phenolic compounds, flavonoids, and antioxidant activity were similar in the callus and suspension systems, whereas 1.5–1.9 fold higher levels of antioxidant compounds accumulated in comparison to the findings in in vivo cladodes. This emphasizes that Opuntia in vitro cultures are an efficient alternative system to obtain metabolites of these Opuntia species under controlled conditions (in vitro). This is compatible with the results in the current study that confirmed that the highest value of total phenolics was recorded for the in vitro– derived cladode extract (under controlled conditions) at 156.5 mg/g dry weight, while the value of total phenolics in the in vivo-derived cladode extract was 86 mg/g DW. Meanwhile, the highest kaempferol content was observed in the in vitro cladode extract 2.807 mg/g DW, whereas the value of kaempferol in the in vivo-derived cladode extract was 1.304 mg/g DW. These values reflect the level of the anticancer activity.
4. Conclusion
This is the first study to focus on the possibility of using the in vitro extract of Egyptian Prickly pear cladodes as an anticancer agent. The highest seed germination rate of 66% was recorded upon applying an efficient protocol of seed germination. In micropropagation, the highest shoot number per explant and maximum length of shoots were obtained. The extract from cladodes from in vitro-derived plants contained the highest contents of total phenolics (156.5 mg/g) and kaempferol (2.807 mg/g). The in vitro-derived cladode extract had high activity against both cancer PC3 and Mcf7 cell lines compared with the extract from cladodes from in vivo derived plants, with probable safety toward normal cell lines. Therefore, this extract can be applied as useful and highly efficient anticancer agent.
Declarations
Author contribution statement
Alaa Heikal, Marwa E. Abd Elsadek, Abeer Salama, Hussein S. Taha: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abd El-Hady F.K., Fayad W., Iodice C., El-Shahid Z.A., Abdel-Aziz M.S., Crudele E., Tommonaro G. Investigating on the correlation between some biological activities of marine sponge-associated bacteria extracts and isolated diketopiperazines. Curr. Microbiol. 2017;74:6–13. doi: 10.1007/s00284-016-1144-3. [DOI] [PubMed] [Google Scholar]
- Abou-Elella F.M., Ali R.F.M. Antioxidant and anticancer activities of different constituents extracted from Egyptian prickly pear cactus (Opuntia ficus-indica) peel. Biochem. Anal. Biochem. 2014;3:158. [Google Scholar]
- Agbor G.A., Vinson J.A., Donnelly P.E. Folin-ciocalteau reagent for polyphenolic assay. Int. J. Food Sci. Nutr. Diet. 2014;3(801):2326–3350. [Google Scholar]
- Ali S.I., Mohamed A.A., Sameeh M.Y., Darwesh O.M., Abd El-Razik T.M. Gamma-irradiation affects volatile oil constituents, fatty acid composition and antimicrobial activity of fennel (Foeniculum vulgare) seeds extract. Res. J. Pharmaceut. Biol. Chem. Sci. 2016;7(1):524–532. [Google Scholar]
- Altare M., Trione S., Guevara J.C., Cony M. Stimulation and promotion of germination in Opuntia ficusindica seeds. J. Profess. Assoc. Cactus Devel. 2006;8:91–100. [Google Scholar]
- Areli G.C.M., Humberto R.V., Valentín R.T., José A.V., Francisca R.G. Pre-germination treatments in four prickly pear cactus (Opuntia sp.) species from Northeastern Mexico. Aust. J. Crop. Sci. 2018;12(10):1676–1684. [Google Scholar]
- Azevedo C., Correia-Branco A., Araujo J.R., Guimaraes J.T., Keating E., Martel F. The chemopreve ntiveeffect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer. 2015;67:504–513. doi: 10.1080/01635581.2015.1002625. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S., Romero J.R., Chattopadhyay N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol. Cell. Endocrinol. 2008;287:57–64. doi: 10.1016/j.mce.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Collazo-Siques P., Valverde M.E., Paredes-Lopez O., Guevara-Lara F. Expression of ripening-related genes in prickly pear (Opuntia sp.) fruits. Plant Foods Hum., Nutr. 2003;58:317–326. doi: 10.1023/b:qual.0000040286.28359.76. [DOI] [PubMed] [Google Scholar]
- Darwesh O.M., Eida M.F., Matter I.A. Isolation, screening and optimization of L-asparaginase producing bacterial strains inhabiting agricultural soils. Biosci. Res. 2018;15(3):2802–2812. [Google Scholar]
- Darwesh O.M., El-Maraghy S.H., Abdel-Rahman H.M., Zaghloul R.A. Improvement of paper wastes conversion to bioethanol using novel cellulose degrading fungal isolate. Fuel. 2020;262:116518. [Google Scholar]
- Darwesh O.M., Elshahawy I.E. Silver nanoparticles inactivate sclerotial formation in controlling white rot disease in onion and garlic caused by the soil borne fungus Stromatinia cepivora. Eur. J. Plant Pathol. 2021;2021 [Google Scholar]
- Decendi T.A., Liu D., Ouelhazi L., Doireau P., Me´rillon J.M., Rideau M. Cytokinin-enhanced accumulation of indole alkaloids in Catharanthus roseus cell cultures-the factors affecting the cytokinin response. Plant Cell Rep. 1992;11:400–403. doi: 10.1007/BF00234369. [DOI] [PubMed] [Google Scholar]
- Diantini A., Subarnas A., Lestari K., Halimah E., Susilawati Y., Supriyatna S. Kaempferol-3O-rhamnoside isolated from the leaves of Schima wallichii Korth. Inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol. Lett. 2012;3:1069–1072. doi: 10.3892/ol.2012.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Finti A., El Boullani R., Ait Aabd N., Msanda F., Serghini M.A., El Mousadik A.B. In vitro propagation of three Moroccan prickly pear cactus Opuntia and plant establishment in soil. Not. Sci. Biol. 2013;5(1):39–44. [Google Scholar]
- El Finti A., El Boullani R., El Ayadi F., Ait Aabd N., El Mousadik A. Micropropagation in vitro of Opuntia ficus-indica in south of Morocco. Intern. J. Chem. Biochem. Sci. 2012;1:6–10. [Google Scholar]
- El-Baz F.K., Mahmoud K., El-Senousy W.M., Darwesh O.M., El Gohary A.E. Antiviral– antimicrobial and schistosomicidal activities of Eucalyptus camaldulensis essential oils. Int. J. Pharmaceut. Sci. Rev. Res. 2015;31(1):262–268. [Google Scholar]
- Estrada-Luna A., Martínez-Hernández J.J., Torres-Torres M.E., ChabléMoreno F. In vitro micropropagation of the ornamental prickly pear cactus Opuntia lanigera Salm-Dyck and effects of sprayed GA3 after transplantation to ex-vitro conditions. Sci. Hortic. 2008;117:378–385. [Google Scholar]
- Feugang J.M., Ye F., Zhang D.Y. Cactus pear extracts induce reactive oxygen species production and apoptosis in ovarian cancer cells. Nutr. Cancer. 2010;62(5):692–699. doi: 10.1080/01635581003605508. [DOI] [PubMed] [Google Scholar]
- García-Saucedo P., Valdez-Morales M., Valverde M.E., Cruz-Hernández A., Paredes-López O. Plant regeneration of three Opuntia genotypes used as human food. Plant Cell Tissue Organ Cult. 2005;80:215–219. [Google Scholar]
- Hahm S.W., Park J., Son Y.S. Opuntia humifusa partitioned extracts inhibit the growth of U87MG human glioblastoma cells. Plant Foods Hum. Nutr. 2010;65(3):247–252. doi: 10.1007/s11130-010-0188-y. [DOI] [PubMed] [Google Scholar]
- Hansen R.K., Oesterreich S., Lemieux P., Sarge D.K., Fuqua S.A.W. Quercetin Inhibits heat shock protein induction but not heat shock factor DNA binding in human breast carcinoma cells. Biochem. Biophys. Res. Commun. 2010;239:851856. doi: 10.1006/bbrc.1997.7572. [DOI] [PubMed] [Google Scholar]
- Hubstenberger J.F., Clayton P.W., Phillips G.C. Vol. 20. 1992. Micropropagation of cacti (cactaceae) pp. 49–68. (Biotechnology in Agriculture and Forestry. High Tech and Micropropagation. Berlin Int. Hort. Congr.). [Google Scholar]
- Hussein H.A., Darwesh O.M., Mekki B.B., El-Hallouty S.M. Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol. Rep. 2019;24 doi: 10.1016/j.btre.2019.e00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein H.A., Darwesh O.M., Mekki B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019;18:101080. [Google Scholar]
- Juárez M.A., Passera C.B. In vitro propagation of Opuntia ellisiana Griff. And acclimatization to field conditions. Biocell. 2002;26(3):319–324. [PubMed] [Google Scholar]
- Kelloff G.J., Crowell J.A., Steel V., Lubet R.A., Boone C.W., Malone W.A., Hawk E.T., Lieberma R., Lawrence J.A., Sigman C.C. Progress in cancer chemoprevention. Ann. Acad. Sci. 1999;889:1–13. doi: 10.1111/j.1749-6632.1999.tb08718.x. [DOI] [PubMed] [Google Scholar]
- Kelloff G.J., Crowell J.A., Steele V.E., Lubet R.A., Malone W.A., Boone C.W. Progressin cancer chemoprevention: development of diet-derived chemopreventive agents. J. Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- Kelloff G.J., Sigman C.C., Greenwald P. Cancer chemoprevention: progress and promise. Eur. J. Cancer. 1999;35:2031–2038. doi: 10.1016/s0959-8049(99)00299-3. [DOI] [PubMed] [Google Scholar]
- Kelly K.L., Kimball B.A., Johnston J.J. Quantitation of digitoxin, digoxin, and their metabolites by high-performance liquid chromatography using pulsed amperometric detection. J. Chromatogr. A. 1995;711:289–295. [Google Scholar]
- Khalafalla M.M., Abdellatef E., Mohameed A.M., Osman M.G. Micropropagation of cactus (Opuntia ficus-indica) as strategic tool to combat desertification in arid and semi-arid regions. Int. J. Sustain. Crop Prod. 2007;2(4):1–8. [Google Scholar]
- Kim J., Soh S.Y., Shin J., Cho C.W., Choi Y.H., Nam S.Y. Bioactives in cactus (Opuntia ficus-indica) stems possess potent antioxidant and pro-apoptotic activities through COX-2 involvement. J. Sci. Food Agric. 2014;95(13):2601–2606. doi: 10.1002/jsfa.6968. [DOI] [PubMed] [Google Scholar]
- Li B., Dedman J.R., Kaetzel M.A. Intron disruption of the annexin IV gene reveals novel transcripts. J. Biol. Chem. 2003;278(44):43276–44383. doi: 10.1074/jbc.M306361200. [DOI] [PubMed] [Google Scholar]
- Marissa R.M., Ana Paulina B., Francoise Gue´raud R., Anne N.S. Establishment of callus and cell suspensions of wild and domesticated Opuntia species: study on their potential as a source of metabolite production. Plant Cell Tissue Organ Cult. 2015;124:181–189. [Google Scholar]
- Maryna W., Alba D.T., Gernot O., Hugo A. Cactus pear antioxidants: a comparison between fruit pulp, fruit peel, fruit seeds and cladodes of eight different cactus pear cultivars (Opuntia fcus-indica and Opuntia robusta) J. Food Meas. Characteriz. 2019;13:2347–2356. [Google Scholar]
- Meyer H.J., Van Staden J. The in vitro production of an anthocyanin from callus cultures of Oxalis linearis. Plant Cell Tissue Organ Cult. 1995;40:55–58. [Google Scholar]
- Mohamed A.A., Ali S.I., Darwesh O.M., El-Hallouty S.M., Sameeh M.Y. Chemical compositions, potential cytotoxic and antimicrobial activities of nitraria retusa methanolic extract sub-fractions. Internat. J Toxicol. Pharmacol. Res. 2015;7(4):204–212. [Google Scholar]
- Mosmann T. Rapid colorimetric assays for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muhammad I., Bahare S., Javad S., Tanweer A.G., Farhan S., Ali I. Kaempferol: a key emph. Anticancer Pot. Mol. 2019;24:2277. [Google Scholar]
- Nitnaware K.M., Naik D.G., Nikam T.D. Thidiazuron-induced shoot organogenesis and production of hepatoprotective lignin phyllanthin and hypophyllanthin in Phyllanthus amarus. Plant Cell Tissue Organ Cult. 2011;104:101–110. [Google Scholar]
- Razali N.M., Wah Y.B. Power comparisons of Shapiro –Wilk, Kolmogorov–smirnov, lilliefors and anderson–darling tests. J. Stat. Model Anal. 2011;2(1):21–33. [Google Scholar]
- Rojas-Aréchiga M., Vázquez-Yanes C. Cactus seed germination: a review. J. Arid Environ. 2000;44:85–104. [Google Scholar]
- Serra A.T., Poejo J., Matias A.A., Bronze M.R., Duarte C.M. Evaluation of Opuntia spp. derived products as anti-proliferative agents in human colon cancer cell line (HT29) Food Res. Int. 2013;54(1):892–901. [Google Scholar]
- Shapiro S.S., Wilk M.B. An analysis of variance test for normality (complete samples) Biometrika. 1965;52(3/4):591–611. [Google Scholar]
- Shin K.S., Sullenger B.A., Lee S.W. Ribozyme-mediated induction of apoptosis in human cancer cells by targeted repair of mutant p53 RNA. Mol. Ther. 2004;10(2):365–372. doi: 10.1016/j.ymthe.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Silva F., Azevedo C.A. Vol. 7. American Society of Agricultural and Biological Engineers; Reno-Nv-Usa: 2009. Principal components analysis in the software assistat-statistical attendance. (World Congress on Computers in Agriculture). [Google Scholar]
- Snedecor G.W., Cochran W.G. ninth ed. Iowa State Univ. Press; Ames, Iowa, USA: 1994. Statistical Methods. [Google Scholar]
- Steele V.E., Sharma S., Mehta R., Elmore E., Redpath L., Rudd C., Bagheri D., Sigman C.C., Kelloff G.J. Use of in vitro assays to predict the efficacy of chemopreventive agents in whole animals. J. Cell. Biochem. 1996;26:29–53. doi: 10.1002/jcb.240630704. [DOI] [PubMed] [Google Scholar]
- Sugandh S. Plant tissue culture: a promising t ool of quality material production with special reference to micropropagation of banana. Biochem. Cell. Arch. 2017;17(1):1–26. [Google Scholar]
- Sultan Y.Y., Ali M.A., Darwesh O.M., Embaby M.A., Marrez D.A. Influence of nitrogen source in culture media on antimicrobial activity of Microcoleus lacustris and Oscillatoria rubescens. Res. J. Pharmaceut. Biol. Chem. Sci. 2016;7(2):1444–1452. [Google Scholar]
- Zhu L., Xue L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2018 doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.