Summary
The fascinating functions of proteins and peptides in biological systems have attracted intense interest to explore their mimics using polymers, including polypeptides synthesized from polymerization. The folding, structures and functions of proteins and polypeptides are largely dependent on their sequence. However, sequence-tunable polymerization for polypeptide synthesis is a long-lasting challenge. The application of polypeptides is also greatly hindered by their susceptibility to enzymatic degradation. Although poly-α/β-peptide has proven to be an effective strategy to address the stability issue, the synthesis of poly-α/β-peptide from polymerization is not available yet. Hereby, we demonstrate a living and controlled copolymerization on α-NCA and β-NTA to prepare sequence-tunable poly-α/β-peptides. This polymerization strategy shows a prominent solvent-driven characteristic, providing random-like copolymers of poly-α/β-peptides in THF and block-like copolymers of poly-α/β-peptides in a mixed solvent of CHCl3/H2O (95/5, v/v), and opens new avenues for sequence-tunable polymerization and enables facile synthesis of proteolysis tunable poly-α/β-peptides for diverse applications.
Subject areas: Chemistry, Organic chemistry, Polymer chemistry
Graphical abstract

Highlights
-
•
Realizing controlled synthesis of poly-α/β-peptides via one-pot polymerization
-
•
Sequence-tunable copolymerization via solvent-dependent polymerization kinetics
-
•
Adjustable proteolytic stability and antibacterial activity of poly-α/β-peptides
-
•
Tunable self-assembly behavior of poly-α/β-peptides via one-pot polymerization
Chemistry; Organic chemistry; Polymer chemistry
Introduction
Proteins and peptides play important roles in biological systems and have been actively explored for both fundamental and applicational studies (Hancock and Sahl, 2006; Liu and Yin, 2020; Schmidt et al., 2015). However, the application of proteins and peptides is hindered by their susceptibility to enzymatic degradation. In addition, it is important to have a deep understanding on folding of proteins and peptides, and to explore new folding designs and structures (Hamuro et al., 1999; Horne and Gellman, 2008; Sang et al., 2020). These drive the development of α/β-peptides, such as α/β-peptide foldamers, for exploration and application in diverse fields such as drug discovery, protein mimicking and antimicrobial study (Girvin et al., 2019; Girvin and Gellman, 2018; Goodman et al., 2007; Pilsl and Reiser, 2011). α/β-peptides have demonstrated great potential in biological functions owing to their increased stability upon enzymatic degradation and controlled sequence in designing folding and functions (Schmitt et al., 2004, 2007).
Currently, α/β-peptides can only be synthesized via the solid-phase peptide synthesis that is time consuming and difficult for large-scale synthesis, especially for long polypeptides and incorporation of unnatural amino acids (Figure 1A) (Chan-Seng and Lutz, 2014). Although poly-α-peptides can be synthesized via the ring-opening polymerization of α-amino acid N-carboxyanhydrides (α-NCAs) (Cheng and Deming, 2012; Deming, 1997; Kricheldorf, 2006; Rasines Mazo et al., 2020; Song et al., 2019; Wu et al., 2018, 2020), no reliable polymerization strategy is available for synthesis of poly-α/β-peptides. In addition, the folded, self-assembled structures and functions of proteins and polypeptides are largely dependent on their defined sequence along the backbone (Girvin et al., 2019; Goodman et al., 2007; Schmitt et al., 2007). However, the synthesis of sequence-controlled polypeptides via polymerization is a long-lasting challenge (Jones, 2008; Lutz, 2010; Lutz et al., 2013). Therefore, a breakthrough in sequence-controlled poly-α/β-peptide synthesis is highly desired, though sequence-controlled polymerizations are reported recently on the synthesis of polyacrylates, polynorbornene, poly(styrene-b-maleimide) and polyesters (Jia et al., 2021; McGraw et al., 2020; Pfeifer and Lutz, 2007; Yasir et al., 2020).
Figure 1.
Strategies to prepare poly-α/β-peptides
(A) Solid-phase synthesis to prepare α/β-peptide oligomers.
(B) The solvent-tuning copolymerization on α-NCAs and β-NTAs to prepare random-like poly-α/β-peptides in THF and block-like poly-α/β-peptides in CHCl3/H2O (95/5, v/v).
(C) Sequence-tunable polymerization on α-NCAs and β-NTAs to prepare sequence-tunable poly-α/β-peptides in CHCl3/H2O (95/5, v/v).
Hereby, we report a facile and controlled synthesis of sequence-tunable poly-α/β-peptides from the copolymerization on α-NCAs and β-amino acid thiocarboxyanhydrides (β-NTAs) under mild conditions using primary amine as the initiator (Figure 1B). The reaction rate of α-NCAs and β-NTAs in the copolymerization on a mixture of α-NCAs/β-NTAs can be tuned with solvents to give either poly-α/β-peptides with random-like distribution of two types of amino acid subunits or poly-α/β-peptides with block-like sequence (Figure 1B). Moreover, this α-NCA/β-NTA copolymerization strategy enables the sequence-tunable synthesis of poly-α/β-peptides for functional studies (Figure 1C).
Results and discussion
Using Nε-tert-butyloxycarbonyl-L-lysine NCA (Boc-α-L-Lys NCA) and β3-benzyl-L-carboxyhomoglycine NTA (Bn-β3-LCHG NTA) as representative monomers (Figures S1–S15), we found that 4-tert-butylbenzylamine (tBuBnNH2) can initiate the copolymerization on a mixture of α-NCA and β-NTA at room temperature in variable solvents (DMF, CHCl3, DMSO, Toluene, CH3CN, DCM, and THF) to give expected poly-α/β-peptides, poly-(Boc-α-L-Lys/Bn-β3-LCHG), with narrow dispersities (Đ = 1.13–1.23) (Table 1, entry 1–7). We performed the following polymerization studies in THF that is a widely used solvent for α-NCA polymerization. The polymerization on the mixture of Boc-α-L-Lys NCA and Bn-β3-LCHG NTA at variable monomer:initiator ratios preceeded successfully using tBuBnNH2 as the initiator to give correponding poly-α/β-peptides with expected chain lengths and narrow dispersities (Đ = 1.19–1.31) (Table 1, entry 7–11). The structural diversity of poly-α/β-peptides can be obtained easily via the copolymerization on a mixture of α-NCA and β-NTA bearing different sidechain substitution groups, as demonstrated by the copolymerizaiton on a mixture of Boc-α-L-Lys NCA/β3-HPhG NTA, Boc-α-L-Lys NCA/β-HG NTA, and Boc-α-L-Lys NCA/β2,3-CH(±) NTA to give corresponding poly-α/β-peptides with narrow dispersities (Đ = 1.12–1.19) (Table 1, entry 12–14).
Table 1.
Copolymerization on α-NCAs and β-NTAsa
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Monomers | [Mα]0:[Mβ]0:[I]0 | Solvent | Mn, th (kDa) | Mn, GPC (kDa) | Đ |
| 1 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 15:15:1 | DMF | 6.7 | 6.1 | 1.17 |
| 2 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 15:15:1 | CHCl3 | 6.7 | 5.7 | 1.16 |
| 3 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 15:15:1 | DMSO | 6.7 | 4.4 | 1.17 |
| 4 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 15:15:1 | Toluene | 6.7 | 6.3 | 1.23 |
| 5 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 15:15:1 | MeCN | 6.7 | 4.8 | 1.13 |
| 6 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 15:15:1 | DCM | 6.7 | 5.5 | 1.16 |
| 7 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 15:15:1 | THF | 6.7 | 7.2 | 1.19 |
| 8 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 30:15:1 | THF | 10.1 | 9.1 | 1.19 |
| 9 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 45:15:1 | THF | 13.5 | 13.6 | 1.25 |
| 10 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 60:15:1 | THF | 16.9 | 16.1 | 1.26 |
| 11 | Boc-α-L-Lys NCA, Bn-β3-LCHG NTA | 60:30:1 | THF | 20.1 | 19.0 | 1.31 |
| 12 | Boc-α-L-Lys NCA, β3-HPhG NTA | 15:15:1 | THF | 5.8 | 5.5 | 1.13 |
| 13 | Boc-α-L-Lys NCA, β-HG NTA | 15:15:1 | THF | 4.6 | 5.2 | 1.12 |
| 14 | Boc-α-L-Lys NCA, β2,3-CH(±) NTA | 15:15:1 | THF | 5.5 | 4.7 | 1.14 |
Copolymerizations on Boc-α-L-Lys NCA and Bn-β3-LCHG NTA were operated in the nitrogen-filled glove box at room temperature to prepare poly-α/β-peptides using different solvents (entry 1–7), and with variable chain lengths (entry 7–11, using THF as the solvent); Copolymerizations on α-NCAs and β-NTAs with different combinations at room temperature (entry 12–14, using THF as the solvent). Mn,GPC is the obtained number of average molecular weight from GPC; Đ means the dispersity index; DP is the obtained degree of copolymerization.
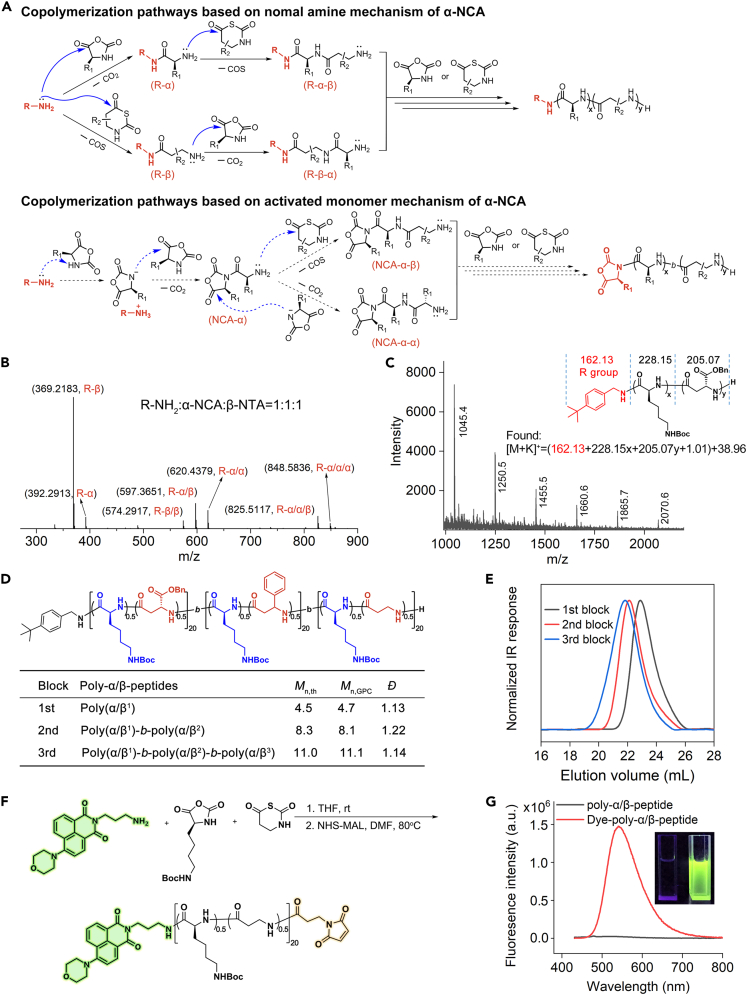
Previous study in our group indicated that primary amine-initiated ring-opening polymerization on β-NTAs follows a normal amine mechanism (NAM), utilizing terminal amine as the reactive center for chain propagation (Zhou et al., 2020). The ring-opening polymerization on α-NCAs is proposed to follow either a NAM or an activated monomer mechanism (AMM) (Cheng and Deming, 2012; Hadjichristidis et al., 2009). Therefore, primary amine-initiated ring-opening polymerization on a mixture of α-NCA/β-NTA could follow either a NAM or an AMM mechanism (Figure 2A). To figure out the mechanism we performed ESI-MS characterization on a 1:1:1 mixture (molar ratio) of tBuBnNH2, Boc-α-L-Lys NCA and Bn-β3-LCHG NTA and found multiple peaks, bearing the terminal group from the initiator tBuBnNH2, corresponding to possible compounds in the NAM pathway (Figures 2A and 2B); in sharp contrast, we found no observable ESI-MS peaks, bearing a terminal NCA ring, corresponding to any possible compound in the AMM pathway (Figures 2A and 2B). The MALDI-TOF-MS characterization on tBuBnNH2-initiated copolymerization of Boc-α-L-Lys NCA/Bn-β3-LCHG NTA revealed the existence of expected poly-α/β-peptides all with an C-terminal tBuBnNH group, indicating the NAM route of copolymerization on α-NCA/β-NTA (Figure 2C).
Figure 2.
Mechanism study of primary amine-initiated copolymerization on α-NCA and β-NTA
(A) Two possible initiation and chain propagation pathways based on normal amine mechanism and activated monomer mechanism of α-NCA.
(B) HRESI-MS analysis on mixing tBuBnNH2, Boc-α-L-lys NCA and Bn-β3-LCHG NTA at 1:1:1 molar ratio for polymerization initiation study (using THF as the solvent at 25°C, [M]0 = 0.2 M).
(C) MALDI-TOF-MS characterization of tBuBnNH2-initiated copolymerization on Boc-α-L-lys NCA and Bn-β3-LCHG NTA ([Mα]0:[Mβ]0:[I]0 = 15:15:1, using THF as the solvent at 25°C, [M]0 = 0.2 M).
(D) tBuBnNH2-initiated copolymerization on α-NCA and β-NTA to prepare poly-α/β-peptide triblock copolymer with each block having different α/β-amino acid composition. GPC characterization was conducted using DMF as the mobile phase at a flow rate of 1 mL/min.
(E) GPC traces of the poly-α/β-peptide triblock copolymer.
(F) Synthesis of dual functionalized poly-α/β-peptide with a C-terminal morpholino-naphthalimide dye and an N-terminal maleimide and under reaction conditions: (1) Dye-NH2, Boc-α-L-lys NCA and β-HG NTA at a molar ratio of 1:10:10 in THF at rt. (2) 5 equiv 3-Maleimidopropionic acid N-hydroxysuccinimide ester (NHS-MAL) relative to Dye-NH2 in DMF at 80°C.
(G) Fluorescent spectra of Dye-poly-α/β-peptide at 0.1 mg/mL in DMSO and the image of this solution under UV light at 365 nm.
The NAM mechanism indicates that primary amine-initiated polymerization on α-NCA/β-NTA is a living polymerization that is capable to prepare poly-α/β-peptides with multiple blocks and with defined C-terminal groups from the primary amine initiators. Using a 1:1 molar ratio mixture of an α-NCA and a β-NTA, we successfully synthesized the triblock copolymer poly(Boc-α-L-Lys0.5/Bn-β3-LCHG0.5)20-b-poly(Boc-α-L-Lys0.5/β3-HPhG0.5)20-b-poly(Boc-α-L-Lys0.5/β2,3-CH0.5)20 with a narrow dispersity of 1.14 (Figures 2D and 2E). A primary amine with a morpholino-naphthalimide fluorophore was able to initiate the copolymerization on a mixture of Boc-α-L-Lys NCA and β-HG NTA to give a C-terminal fluorophore-labelled poly-α/β-peptide that displays strong fluorescence (Figures 2F and 2G). This poly-α/β-peptide can be further functionalized with an N-terminal maleimide to give a terminal dual-functionalized poly-α/β-peptide that has broad application in protein conjugation, imaging and mechanism study (Figures S16–S18) (Baumann et al., 2020; Feng et al., 2010). These demonstrations on block copolymer synthesis and C-terminal functionalization confirmed living polymerization property and polymerization initiation via the nucleophilic addition of primary amine initiator.
When THF was used as the solvent for copolymerization on a mixture of α-NCA and β-NTA, using Boc-α-L-Lys NCA and Bn-β3-LCHG NTA as the model, we found no significant gradient separation in compositional profile of two amino acid subunits within the poly-α/β-peptide chain, though Boc-α-L-Lys NCA have a slightly higher reactivity than Bn-β3-LCHG NTA according to the kinetic plot of residual monomer (Figures 3A–3C) and instantaneous copolymer composition Fα-NCA (Figures 3C and S19). (Gleede et al., 2019) This copolymerization proceeded close to “ideal copolymerization” (rα-NCA × rβ-NTA = 0.93, rα-NCA × rβ-NTA = 1 means ideal copolymerization) (Yasir et al., 2020), which in combination with the instantaneous copolymer composition Fα-NCA revealed that the resulting poly-α/β-peptide was a random-like copolymer and the β-amino acid residue has a nearly even distribution along poly-α/β-peptide chain (Figure 3C) (Yasir et al., 2020). The obtained number average molecular weight (Mn) of poly-α/β-peptide from different feeding ratio of monomer/initiator [Mα + Mβ]0/[I]0 matched the theoretical values of Mn well and displayed a linear correlation with [Mα + Mβ]0/[I]0, which in combination with the low dispersity (Đ = 1.19–1.26) indicating a controllable polymerization (Figure 3D).
Figure 3.
Primary amine-initiated polymerization on α-NCA and β-NTA in THF
(A) tBuBnNH2-initiated copolymerization on Boc-α-L-Lys NCA and Bn-β3-LCHG NTA in THF gives poly-α/β-peptides as random-like polymers.
(B) Conversion of Boc-α-L-Lys NCA and Bn-β3-LCHG NTA in tBuBnNH2-initiated copolymerization using THF as the solvent ([Mα]0:[Mβ]0:[I]0 = 15:15:1, rt, [M]0 = 0.2 M).
(C) The instantaneous copolymer composition of α-amino acid in the poly-α/β-peptides chains (F) versus total conversion of monomers based on the reactivity ratios of Boc-α-L-Lys NCA (blue) and Bn-β3-LCHG NTA (red).
(D) The relationship between Mn or dispersity (Đ) value (Table 1, entry 7–10) of poly-α/β-peptide prepared via α-NCA and β-NTA copolymerization and the initial molar ratio of the α- and β-amino acid monomers:initiator ([Mα + Mβ]0/[I]0) ratio.
(E) tBuBnNH2-initiated copolymerization on Boc-α-L-Lys NCA and β2,3-CH(±) NTA for synthesis of HDP-mimicking amphiphilic poly-α/β-peptides.
(F) Antimicrobial activities of poly-α/β-peptides against multiple clinically isolated drug-resistant bacteria, evaluating with the minimum inhibitory concentration (MIC). The poly-α/β-peptides have incrementally increased the ratio of hydrophobic β2,3-CH subunits from 30% to 70%.
(G) The half-life of poly(α-L-Lys)20 and Poly(α-L-Lys0.5β2,3-CH0.5)20 against trypsin (trypsin/polymer = 1/40, w/w) at 25°C in PBS buffer.
(H) The half-life of poly(α-L-Lys)20 and Poly(α-L-Lys0.5β2,3-CH0.5)20 against proteinase K23 (proteinase K23/polymer = 1/10, w/w) at 25°C in PBS buffer.
Poly-α/β-peptides have proven to be important mimics and modifications of nature polypeptides, such as host defense peptide (HDP), to effectively improve the stability upon enzymatic degradation and therapeutic potential (Konai et al., 2018; Schmitt et al., 2004, 2007). The copolymerization on a mixture of α-NCA and β-NTA enabled facile synthesis of poly-α/β-peptides for functional studies, as our proof-of-concept demonstration to mimic HDP using Boc-α-L-Lys NCA and β2,3-CH(±) NTA (Figures 3E and 3F, S22–S25). The obtained cationic/hydrophobic amphiphilic poly-α/β-peptides had 30%, 50% or 70% of the β-amino acid residues and displayed potent and broad-spectrum activities against a series of multi-drug resistant Gram positive and Gram negative bacteria, including nine clinically isolated pathogens of Escherichia coli, Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus (Figure 3F). It's noteworthy that incorporation of β-amino acid residues substantially increased polypeptides' stability against enzymatic degradation, with the observation that poly(α-L-Lys0.5/β2,3-CH0.5)20 displayed two-fold and three-fold increase of the half-life than poly(α-L-Lys)20 in the presence of trypsin and proteinase K23, respectively (Figures 3G and 3H, S26–S29).
Surprisingly, the copolymerization on α-NCA and β-NTA displayed a solvent-dependent tuning of the reaction and resulting poly-α/β-peptides, giving random-like copolymers of poly-α/β-peptides using THF as the polymerization solvent as demonstrated above, and block-like copolymers of poly-α/β-peptides using a mixed solvent of CHCl3/H2O (95/5, v/v) as demonstrated by the PEG-NH2 initiated copolymerization on a mixture of Boc-α-L-Lys NCA and Bn-β3-LCHG NTA (Figure 4A). Meanwhile, MALDI-TOF-MS analysis on PEG-NH2 initiated copolymerization of Boc-α-L-Lys NCA/Bn-β3-LCHG NTA in the mixed solvent of CHCl3/H2O showed the molecular weight of copolymerization product matching theoretical values of poly-α/β-peptides with an C-terminal PEG-NH group, indicating NAM route of the copolymerization as well in the mixed solvent of CHCl3/H2O (Figures 2A and S30). The block-like copolymerization in CHCl3/H2O can be explained by the strikingly enlarged difference in polymerization kinetics between Boc-L-Lys α-NCA and β3-LCHG NTA in this mixed solvent than in THF (Figure 4B), which is also reflected from the 24-fold increase on the ratio of monomer reactivity (rα-NCA/rβ-NTA = 126.4 in CHCl3/H2O vs. rα-NCA/rβ-NTA = 5.36 in THF, Figures 4C and S20). The instantaneous copolymer composition Fα-NCA revealed a block-like copolymer of poly-α/β-peptides with α-amino acid residues and β-amino acid residues enriching in the first block and the second block, respectively (Figure 4C). The obtained Mn of poly-α/β-peptide from different feeding ratios of monomer/initiator [Mα + Mβ]0/[I]0 matched the theoretical values of Mn well and displayed a linear correlation with [Mα + Mβ]0/[I]0, which in combination with the narrow dispersity (Đ = 1.12–1.23) indicating a controllable polymerization in CHCl3/H2O (Figures 4D and S31). This result was echoed by the observation on the kinetics in homopolymerization that Boc-α-L-Lys NCA was consumed within 5 min, whereas about 98% percent of β3-LCHG NTA was left unreacted during the same time window (Figure 4E).
Figure 4.
Primary amine-initiated polymerization on α-NCA and β-NTA in a mixed solvent CHCl3/H2O (95/5, v/v)
(A) PEG-NH2 initiated Boc-L-Lys α-NCA and Bn-β3-LCHG NTA copolymerization in the mixed solvent of CHCl3/H2O (95/5, v/v) gives poly-α/β-peptides as block-like copolymers.
(B) Corresponding conversion of Boc-L-Lys NCA and Bn-β3-LCHG NTA ([Mα]0:[Mβ]0:[I]0 = 15:15:1, rt, [M]0 = 0.2 M) in the mixed solvent of CHCl3/H2O (95/5, v/v).
(C) The instantaneous copolymer composition of α-amino acid in the poly-α/β-peptide chains (F) versus total conversion based on the reactivity ratios of Boc-L-Lys NCA (blue) and Bn-β3-LCHG NTA (red).
(D) The relationship between Mn or Đ value of poly-α/β-peptide, prepared via PEG-NH2 initiated copolymerization on α-NCA and β-NTA in the mixed solvent of CHCl3/H2O (95/5, v/v), and the initial molar ratio of total α-NCA and β-NTA vs. initiator, ([Mα + Mβ]0/[I]0).
(E) Conversion of Boc-L-Lys NCA and Bn-β3-LCHG NTA, respectively, in tBuBnNH2-initiated homopolymerization in the mixed solvent of CHCl3/H2O (95/5, v/v) ([M]0:[I]0 = 15:1, rt, [M]0 = 0.2 M).
Sequence-controlled polymerization for polypeptide synthesis was a long-standing challenge (Jones, 2008; Lutz, 2010; Lutz et al., 2013). We found with surprise that our strategy for copolymerization on α-NCA and β-NTA in a mixed solvent CHCl3/H2O (95/5, v/v) provided a perfect solution for sequence-tunable copolymerization to prepare polypeptides based on the obvious difference in homopolymerization on α-NCA and β-NTA (Figures 4E and S21). Using PEG-NH2 as the polymerization initiator, repeated addition of a Boc-α-L-Lys/Bn-β3-LCHG mixture into the reaction afforded sequence-tunable polypeptide with alternate Boc-α-L-Lys blocks and Bn-β3-LCHG blocks for total 10 blocks of peptides (Figure 5A). The living copolymerization property and successful growing of each new blocks were confirmed by the progressive shift of GPC traces to a larger Mn and high monomer conversion ratio (>99%, HPLC analysis) in the polymerization reaction between the iterative addition (Figure 5B). Moreover, the obtained Mn of polypeptides was very close to the theoretical value, showing a linear increase after adding each two blocks and with a narrow dispersity (Đ = 1.18–1.32) (Figure 5C). In another demonstration on the sequence-tunable polymerization for polypeptide synthesis, we iteratively added Boc-α-L-Lys NCA or α-BLG NCA into the reaction of PEG-NH2-initiated β3-LCHG NTA polymerization in CHCl3/H2O (95/5, v/v), resulting in poly-α/β-peptides with insertion of α-amino acid block into predefined position along the polypeptide chain owing to the dramatically faster reaction rate of NCA in CHCl3/H2O (95/5, v/v) (Figure 5D), which was supported by the observed characteristic 1H NMR signals for both Ha, Hb in poly(α-BLG) block and H1, H2, H4 in poly(Boc-α-L-Lys) block within obtained poly-α/β-peptides (Figure 5E).
Figure 5.
Sequence-tunable copolymerization on α-NCA and β-NTA in a mixed solvent CHCl3/H2O (95/5, v/v)
(A) PEG-NH2 initiated copolymerization on α-NCA and β-NTA to prepare sequence-tunable decablock poly-α/β-peptide, with each block in the form of (poly-α-peptide)-b-(poly-β-peptide). A mixture of α-NCA:β-NTA 15:5, relative to 1 equiv. of the initiator PEG-NH2, was added to the reaction each time, using Boc-α-L-Lys NCA, Bn-β3-LCHG NTA, and Me-β3-LCHG NTA as monomers.
(B) GPC traces of reaction solutions for PEG-terminated multi-block poly-α/β-peptides, from diblock to decablock.
(C) The controlled polymerization in (a) gave the decablock poly-α/β-peptide with a narrow dispersion and an excellent match between the obtained and calculated Mn.
(D) 21-block poly-α/β-peptide with alternate β-peptide block and α-peptide block, by adding 5 mer of Boc-α-L-Lys NCA or α-BLG NCA repeatedly at intervals into the ongoing polymerization reaction of β-NTA (Bn-β3-LCHG NTA), using an initial molar ratio of Bn-β3-LCHG NTA:PEG-NH2 at 100:1.
(E) 1H NMR spectrum of PEG-terminated 21-block poly-α/β-peptide that contains total 25 Boc-L-Lysine residues and 25 BLG residues. 1H NMR spectra of PEG-terminated poly-(Boc-α-L-Lys)5, poly-(α-BLG)5 and poly-(Bn-β3-LCHG)100 were used for comparison. All NMR spectra were collected using DMSO-d6 as the solvent.
Sequence-control of block copolymers is very important for the nanostructure of the polymer materials and have diverse applications, such as drug encapsulation and delivery, which is highly relied on the reproducible synthesis of copolymers (Feng et al., 2013; Ge et al., 2020; Krumm et al., 2012; Miao et al., 2017; Nederberg et al., 2011; Wang et al., 2006; Xu and Pu, 2021; Zhang et al., 2019). Above sequence-tunable copolymerization on a mixture of Boc-α-L-Lys NCA and Bn-β3-LCHG NTA in CHCl3/H2O (95/5, v/v) was found to have excellent reproducibility, generating a PEG-terminated triblock copolymer of PEG-poly(Boc-α-L-Lys)-b-poly(Bn-β3-LCHG) with overlapped GPC traces, almost identical Mn and dispersity (Mn = 6.7–6.8KDa, Đ = 1.13–1.14) among repeats from six batches of reactions (Figures 6A and 6B and Table S1). In addition, the obtained PEG-functionalized block-like poly-α/β-peptide with different amino acid component underwent self-assembly spontaneously to form different nanostructures (Figure 6C). Specifically, DLS and TEM characterization indicated that self-assembly of PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20 in THF/H2O (15/100, v/v) gave micelles with particle size of 244 nm and PDI value of 0.25 (Figure 6D); however, the assembly of PEG-poly(α-L-Lys)15-b-poly(Bn-β3-HPhG)15 gave a fibrous structure with particle size of 328 nm and PDI value of 0.22 (Figures 6E, and S32). This proof-of-concept demonstration showed the power of this controlled-polymerization strategy to synthesize poly-α/β-peptides with predefined sequence that defines the self-assembly, resulting nanostructure and applications.
Figure 6.
Self-assembly study of PEG-terminated triblock poly-α/β-peptide prepared via one-shot copolymerization on α-NCA and β-NTA
(A) One-shot preparation of PEG-functionalized triblock poly-α/β-peptide PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20 via open-vessel copolymerization in an aqueous environment of CHCl3/H2O (95/5, v/v).
(B) GPC traces of PEG-poly(Boc-α-L-Lys)10-b-poly(Bn-β3-LCHG)20 from six batches.
(C) The different self-assembly behavior of PEG-functionalized triblock poly-α/β-peptides with different components of β-amino acid.
(D) DLS analysis on size distributions and TEM images of self-assembled PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20 (c = 0.87 mg/mL) in a mixed solvent H2O/THF 100/15 (v/v).
(E) DLS analysis on size distributions and TEM images of self-assembled PEG-poly(α-L-Lys)15-b-poly(β3-HPhG)15 (c = 1 mg/mL) in H2O solvent.
Conclusions
We demonstrate a promising strategy for sequence-tunable synthesis of polypeptides utilizing the copolymerization on α-NCA and β-NTA. The polymerization is tunable by the reaction solvent to give random-like copolymers of poly-α/β-peptides in THF and block-like copolymers of poly-α/β-peptides in a mixed solvent of CHCl3/H2O (95/5, v/v). This polymerization strategy represents a breakthrough in facile synthesis of poly-α/β-peptides with tunable sequence and resistance to enzymatic degradation, implying great potential in protein mimicking study to explore folding, self-assembly, and biological functions.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Staphylococcus epidermidis | ATCC | ATCC49134 |
| Pseudomonas aeruginosa (intermediate resistance to carbenicillin and piperacillin) | ATCC | ATCC9027 |
| Escherichia coli (E. coli R01, R07) | Shanghai Ruijin Rehabilitation Hospital | N/A |
| Acinetobacter baumannii (A. b R02, R03) | Shanghai Ruijin Rehabilitation Hospital | N/A |
| Pseudomonas aeruginosa (P. a R05, R12) | Shanghai Ruijin Rehabilitation Hospital | N/A |
| Klebsiella pneumonia (K. p R02, R11) | Shanghai Ruijin Rehabilitation Hospital | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Nε-Boc-L-lysine | Adamas | 01048879; CAS: 2418-95-3 |
| L-glutamic acid γ-benzyl ester | Adamas | 28910C; CAS: 1676-73-9 |
| β-alanine | Adamas | 13484A; CAS: 107-95-9 |
| 3-amino-3-phenylpropionic acid | Adamas | 01096013; CAS: 614-19-7 |
| 1-benzyl L-aspartate | Adamas | 01110161; CAS: 7362-93-8 |
| 1-methyl L-aspartate | Adamas | 01035418; CAS: 17812-32-7 |
| cis-2-Amino-1-cyclohexanecarboxylic acid | Bidepharm | BD121558-1g; CAS: 5691-20-3 |
| 4-tert-Butylbenzylamine | TCI | B1587-5ml; CAS: 39895-55-1 |
| mPEG-NH2 | Ruixibio | Cat#R-1001-2k |
| Software and algorithms | ||
| ChemDraw Professional 15.1 | PerkinElmer | https://www.perkinelmer.com/category/chemdraw |
| Others | ||
| SepaBean machine equipped with Sepaflash columns | Santai Technologies Inc. in China | http://www.santaitech.com/cn/ |
| Bruker AVANCE III 400 | Bruker | https://www.bruker.com/en/products-and-solutions/mr/nmr.html |
| Bruker Ascend 600 | Bruker | https://www.bruker.com/en/products-and-solutions/mr/nmr.html |
| Waters XEVO G2 TOF mass spectrometer | Waters | https://www.waters.com/nextgen/us/en.html |
| AB SCIEX TOF/TOFTM 5800 | SCIEX | https://sciex.com/ |
| Waters GPC instrument equipped with a Waters 1515 isocratic HPLC pump, a Brookhaven BI-MwA multi-angle light scattering detector and a Waters 2414 refractive index detector | Waters | https://www.waters.com/nextgen/us/en.html |
| SHIMADZU LC 20AR HPLC System | Shimadzu Scientific Instruments | https://www.ssi.shimadzu.com/ |
| Model ZEN3600 | Malvern Instruments Ltd., UK | https://www.malvernpanalytical.com/en |
| JEOL JEM-1400 TEM | JEOL Ltd. | http://www.jeol.com.cn/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Runhui Liu (rliu@ecust.edu.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Bacteria used in this study include Staphylococcus aureus USA300 (methicillin-resistant), Staphylococcus epidermidis ATCC49134, Bacillus subtilis BR-151, Escherichia coli JM109, Pseudomonas aeruginosa ATCC9027 (intermediate resistance to carbenicillin and piperacillin), and 9 clinically isolated pathogens from Shanghai Ruijin Rehabilitation Hospital including Escherichia coli (E. coli R01, R07), Acinetobacter baumannii (A. b R02, R03), Pseudomonas aeruginosa (P. a R05, R12), and Klebsiella pneumonia (K. p R02, R11). All 14 strains of Gram positive and Gram negative bacteria were cultured individually in Luria–Bertani (LB) medium for 9 h at 37°C under shaking at 200 rpm.
Method details
Synthesis of α-NCAs
Nε-tert-butyloxycarbonyl-α-L-lysine N-Carboxy Anhydride (Boc-α-L-Lys NCA)
Boc-α-L-Lys NCA was synthesized by following the previously reported method with slight modification (Zhang et al., 2018) Nε-Boc-α-L-Lys (4.9 g, 20.0 mmol), α-pinene (7.4 g, 54.0 mmol) and triphosgene (2.7 g, 9.0 mmol) were added into 200 mL anhydrous THF. The reaction suspension was stirred and heated to 50°C under a nitrogen environment for 2 h. The reaction mixture was concentrated under vacuum and the obtained residue was dissolved in ethyl acetate (EtOAc, 100 mL) followed by washing with ice water (3 × 50 mL) and cold brine (1 × 50 mL) successively. The collected organic phase was dried over anhydrous MgSO4 and concentrated under reduced pressure to give a crude product, that was purified from recrystallization using anhydrous EtOAc/n-hexane three times to give the pure Boc-α-L-Lys NCA as a needle-like colorless crystal (2.5 g, 45.9% yield). 1H NMR (400 MHz, CDCl3, Figure S1): δ 6.99 (s, 1H), 4.66 (br, 1H), 4.33 (dd, J = 6.4, 4,8 Hz, 1H), 3.13 (s, 2H), 1.96-2.07 (m, 1H), 1.79-1.90 (m, 1H), 1.47-1.61 (m, 4H), 1.45 (s, 9H), 13C NMR (100 MHz, CDCl3, Figure S2): δ 170.15, 156.85, 152.46, 80.09, 57.72, 31.01, 29.47, 28.66, 21.44. ESI-MS: m/z calculated for C12H19N2O5[M-H]-:271.1; Found 271.1.
γ-Benzyl-L-glutamate N-carboxyanhydride (α-BLG NCA)
α-BLG NCA was synthesized by following the precedent literature with slight modification. (Wu et al., 2018) L-glutamic acid γ-benzyl ester (4.46 g, 20 mmol) and triphosgene (2.7 g, 9.0 mmol) were dissolved in 200 mL anhydrous THF. The reaction was stirred and heated to 50 °C under a nitrogen environment for 2 h. The reaction mixture was concentrated and dissolved in 100 mL EtOAc following by washing with ice water (1 ×100 mL) and cold brine (1 × 100 mL) successively. The obtained organic layer was then dried over anhydrous MgSO4 and concentrated under reduced pressure to give a crude product that was purified from recrystallization in anhydrous EtOAc/n-hexane three times to give the pure α-BLG-NCA as a needle-like colorless crystal (4.0 g, 76.0% yield). 1H NMR (400 MHz, CDCl3, Figure S3): δ 7.41–7.30 (m, 5H), 6.67 (s, 1H), 5.14 (s, 2H), 4.38 (t, J = 6.0 Hz, 1H), 2.59 (t, J = 6.8 Hz, 2H), 2.32–2.23 (m, 1H), 2.16-2.04 (m, 1H). 13C NMR (100 MHz, CDCl3, Figure S4): δ 172.62, 169.60, 152.13, 135.43, 128.94, 128.82, 128.59, 67.34, 57.15, 30.03, 27.12. HRESI-MS: m/z calculated for C13H13NNaO5 [M+Na]+: 286.0691; Found 286.0692.
Synthesis of β-NTAs
β3-Benzyl-L-carboxyhomoglycine N-thiocarboxyanhydride (Bn-β3-LCHG NTA)
Bn-β3-LCHG NTA was synthesized by following a precedent procedure with modifications (Zhou et al., 2020). To a reaction flask containing 100 mL anhydrous DMF was added 1-benzyl L-aspartate (4.47 g, 20.0 mmol), S-ethoxythiocarbonyl mercapto-acetic acid (XAA, 3.6 g, 20.0 mmol), Et3N (10.1 g, 100.0 mmol) and DMAP (1.8 g, 2.0 mmol). The reaction mixture was stirred vigorously at room temperature under a nitrogen environment for 48 h. The reaction mixture was concentrated and dissolved in 100 mL EtOAc followed by washing with deionized water (3 × 50 mL) and saturated brine (1 × 50 mL) successively. The obtained organic layer was then dried over anhydrous MgSO4 and concentrated under reduced pressure to give a crude intermediate that was purified through a silica gel column chromatography to give the pure intermediate (5.4 g, 17.3 mmol). The intermediate was then dissolved in anhydrous DCM (200 mL) and treated with PBr3 (4.7 g, 17.3 mmol) at 0°C under nitrogen. The reaction mixture was allowed to stir at rt for 6 h, and then was washed sequentially with ice water (3 × 100 mL) and saturated brine (1 × 100 mL). The obtained organic phase was dried over MgSO4, filtered and concentrated to afford the crude product that was purified by recrystallization from EtOAc/n-hexane to give the pure Bn-β3-LCHG NTA as a white fluffy solid (3.8 g, 71.6% yield over two steps). 1H NMR (400 MHz, CDCl3, Figure S5): δ 7.30–7.45 (m, 5H), 6.83 (s, 1H, NH), 5.27 (s, 2H), 4.40–4.47 (dt, J = 2.8, 10.4 Hz, 1H), 3.23 (dd, J = 2.8, 16.4 Hz, 1H), 2.96 (dd, J = 10.4, 16.4 Hz, 1H). EI-MS: m/z calculated for C12H11NO4S[M]+: 265.0; Found 265.0.
β3-Methyl-L-carboxyhomoglycine N-thiocarboxyanhydride (Me-β3-LCHG NTA)
Me-β3-LCHG NTA was prepared as a white crystal in 67.5% yield over two steps from 1-methyl L-aspartate, using a similar method described above for Bn-β3-LCHG NTA synthesis. 1H NMR (400 MHz, CDCl3, Figure S6): δ 7.20 (s, 1H), 4.40–4.47 (m, 1H), 3.86 (s, 3H), 3.20 (dd, J = 3.2, 16.4 Hz, 1H), 3.01 (dd, J = 9.6, 16.4 Hz, 1H). 13C NMR (100 MHz, CDCl3, Figure S7): δ 193.84, 168.84, 164.13, 54.01, 51.23, 42.78. HREI-MS (Figure S8): m/z calculated for C6H7NO4S [M]+: 189.0096; Found 189.0094.
Cis-2-aminocyclohexanecarboxylic acid N-thiocarboxyanhydride (β2,3-CH(±) NTA)
β2,3-CH(±) NTA was prepared as a white crystal in 53.8% yield over two steps from cis-2-aminocyclohexanecarboxylic acid using the method described above for Bn-β3-LCHG NTA synthesis. 1H NMR (400 MHz, CDCl3, Figure S9): δ 7.39 (s, 1H), 3.72–3.92 (m, 1H), 2.7–2.9 (m, 1H), 1.98–2.12 (m, 1H), 1.84–1.94 (m, 1H), 1.60–1.79 (m, 4H), 1.38–1.56 (m, 2H). 13C NMR (100 MHz, CDCl3, Figure S10): δ 199.13, 166.25, 50.54, 49.68, 29.60, 24.43, 23.38, 21.20. HRESI-MS (Figure S11): m/z calculated for C8H10NO2S [M-H]-: 184.0432; Found 184.0433.
β-Alanine N-thiocarboxyanhydride (β-HG NTA)
β-HG NTA was synthesized by following a precedent procedure described above for Bn-β3-LCHG NTA synthesis with modifications. The mixture of β-alanine (1.8 g, 20.0 mmol) and S-ethoxythiocarbonyl mercapto-acetic acid (XAA, 3.6 g, 20.0 mmol), NaOH (4.0 g, 100.0 mmol) were dissolved in 100 mL deionized water. The reaction mixture was stirred vigorously at rt for 72h followed by acidification with 2 N HCl to pH = 3 and then extraction with ethyl acetate (3 × 100 mL). The combined organic phase was washed with saturated brine (1 × 100 mL) then dried over anhydrous MgSO4 and concentrated under reduced pressure to give the crude intermediate, which was purified through a silica gel column chromatography to give the pure intermediate (2.7 g, 15.2 mmol). The pure intermediate was dissolved in anhydrous DCM (200 mL) and then was treated with PBr3 (4.1 g, 15.2 mmol) at 0°C under nitrogen. The reaction mixture was allowed to stir at rt for 6 h and then washed sequentially with ice water (3 × 100 mL) and saturated brine (1 × 100 mL). The organic phase was dried over MgSO4, filtered and concentrated to afford the crude product that was further purified by recrystallization from EtOAc/n-hexane to give pure β-HG NTA as a white fluffy solid (1.2 g, 45.7% yield over two steps). 1H NMR (400 MHz, CDCl3, Figure S12): δ 7.49 (brs, 1H), 3.56–3.61 (m, 2H), 2.80–2.86 (m, 2H). 13C NMR (100 MHz, CDCl3, Figure S13): δ 196.05, 166.47, 40.22, 37.77. HREI-MS (Figure S14): m/z calculated for C4H5NO2S [M]+: 131.0041; Found 131.0027.
β3-D/L-homophenylglycine N-thiocarboxyanhydride (β3-HPhG NTA)
β3-HPhG NTA was prepared as a white crystal in 82.8% yield over two steps from 3-amino-3-phenylpropionic acid using the method described above for β-HG NTA synthesis. 1H NMR (400 MHz, CDCl3, Figure S15): δ 7.28–7.40 (m, 3H), 7.18–7.24 (m, 2H), 5.97 (brs, 1H), 4.46–4.54 (m, 1H, NHCH), 3.32 (dd, J = 4.0, 14.0 Hz, 1H), 2.91 (dd, J = 9.6, 14.0 Hz, 1H). EI-MS: m/z calculated for C10H9NO2S [M]+: 207.0; Found 207.0.
Copolymerization on α-NCA and β-NTAs in THF
To a reaction vial equipped with a magnetic stir bar was added the α-NCA and β-NTA at a predefined molar ratio in an appropriate solvent, followed by addition of a primary amine initiator. The polymerization reaction was stirred at room temperature for 2–4 days until completion as monitored by TLC and HPLC (>99% conversion). The reaction mixture was poured into 45 mL cold n-hexane to precipitate out the crude polypeptide as a light yellow solid, which was collected after centrifugation and dried under air flow. The crude product was purified via the dissolution-precipitation process thrice (2.0 mL THF/45 mL n-hexane) and then drying under vacuum. Copolymerization on α-NCA and β-NTA in THF was conducted by following the general procedure described above. To prepare poly-α/β-peptides with variable structures, α-NCA, β-NTA and the primary amine initiator were individually prepared as stock solutions at a concentration of 0.2 M in anhydrous THF under nitrogen and then mixed as needed for different copolymerization. To prepare poly-α/β-peptides with variable chain length (Table 1, entry 8–11), α-NCA, β-NTA and the primary amine initiator were individually prepared as stock solutions at a concentration of 0.78 M in anhydrous THF under nitrogen and then mixed as needed for different copolymerization. The variable ratio of α:β amino acid subunits within the polypeptide chains was controlled by the initial ratio of Boc-α-L-Lys NCA and Bn-β3-LCHG NTA in the reaction mixture.
Synthesis of α/β-peptide triblock copolymer in THF
For the synthesis of α/β-peptide triblock copolymer, the α-NCAs, β-NTAs and tBuBnNH2 were respectively dissolved in anhydrous THF to a final concentration of 0.2 M. The first block of poly-α/β-peptide was prepared from the mixture of 2 mL Boc-α-Lys NCA, 2 mL Bn-β3-LCHG NTA and 200 μL tBuBnNH2 to give the poly(Boc-α-L-Lys0.5/Bn-β3-LCHG0.5)20 (Mn = 4.7 kg/mol, Đ = 1.13, characterized by GPC) in 87.5% yield after three cycles of dissolution-precipitation process (2.0 mL THF/45.0 mL n-hexane). The purified poly(Boc-α-L-Lys0.5/Bn-β3-LCHG0.5)20 was dissolved in 100 μL anhydrous THF to a final concentration of 0.2 M as the initiator, followed by adding 1 mL of Boc-α-Lys NCA (54.4 mg, 0.2 mmol) and 1 mL of β3-HPhG NTA (41.4 mg, 0.2 mmol) into the reaction flask. The polymerization reaction was continued for 72h to give the α/β-peptide diblock copolymer poly(Boc-α-L-Lys0.5/Bn-β3-LCHG0.5)20-b-poly(Boc-α-L-Lys0.5/β3-HPhG0.5)20 (Mn = 8.1 kg/mol, Đ = 1.22, characterized by GPC) in 88.7% yield after three cycles of dissolution-precipitation process (2.0 mL THF/45.0 mL n-hexane). The purified diblock copolymer was dissolved in 100 μL anhydrous THF to a final concentration of 0.2 M as the initiator, followed by adding 1 mL of Boc-α-Lys NCA (54.4 mg, 0.2 mmol) and 1 mL of β-HG NTA (26.2 mg, 0.2 mmol) into the reaction flask. The polymerization reaction was continued for 72h to give the α/β-peptide triblock copolymer poly(Boc-α-L-Lys0.5/Bn-β3-LCHG0.5)20-b-poly(Boc-α-L-Lys0.5/β3-HPhG0.5)20-b-poly(Boc-α-L-Lys0.5/β-HG0.5)20 (Mn = 11.1 kg/mol, Đ = 1.14, characterized by GPC) in 82.3% yield after three cycles of dissolution-precipitation process (2.0 mL THF/45.0 mL n-hexane).
The synthesis of C- and N-terminal dual functionalization of poly-α/β-peptide in THF
2-(3-aminopropyl)-6-morpholino-1H-benzo[de]isoquinoline-1,3(2H)-dione (Dye-NH2)
Dye-NH2 was used as the initiator for the copolymerization of α-NCAs and β-NTAs. Dye-NH2 was synthesized by following previously reported procedure to give the pure product as a yellow solid in 25.3% yield over two steps (Zhou et al., 2020). Structure characterization of Dye-NH2 was consistent to the report in literature. 1H NMR (400 MHz, CDCl3, Figure S16): δ 8.58 (dd, J = 0.8, 7.2 Hz, 1H), 8.51 (d, J = 8.0 Hz, 1H), 8.42 (dd, J = 0.8, 8.4Hz, 1H), 7.70 (dd, J = 8.4, 7.2 Hz, 1H), 7.23 (d, J = 8.0 Hz, 1H,), 4.27 (t, J = 6.8 Hz, 2H), 4.02 (t, J = 4.4 Hz, 4H), 3.27 (t, J = 4.4 Hz, 4H), 2.76 (t, J = 6.6 Hz, 2H), 1.90 (p, J = 6.8 Hz, 2H), 1.79 (s, 2H).
The C-terminal functionalization of poly-α/β-peptide was demonstrated by the copolymerization of Boc-α-L-Lys NCA and β-HG NTA. First, Dye-NH2, Boc-α-L-Lys NCA and β-HG NTA was dissolved in anhydrous THF individually to a final concentration of 0.2 M. Then 200μL Dye-NH2 was added into the mixture of 2 mL Boc-α-L-Lys NCA and 2 mL β-HG NTA, and the reaction mixture was stirred at rt for 72 h in a dark environment. After the reaction was complete, the reaction solution was poured into cold n-hexane (45.0 mL) to precipitate out the crude product that was collected by centrifugation and dried under air flow. The purified C-terminal functionalized poly-α/β-peptide Dye-poly(Boc-α-L-Lys0.5/β-HG0.5)20 was obtained in 92.7% yield after three cycle of dissolution-precipitation in a mixed solvent (2.0 mL THF/45.0 mL n-hexane) and then was characterized by GPC (Figure S17).
The purified Dye-poly(Boc-α-L-Lys0.5/β-HG0.5)20 (31.0 mg, 10.0 mmol) and 3-Maleimidopropionic acid N-hydroxysuccinimide ester (NHS-MAL, 13mg, 50.0 mmol) were dissolved in 500 μL anhydrous DMF and the reaction mixture was stirred for 18 h at 80°C under nitrogen. When reaction was complete, the reaction mixture was cooled to rt and concentrated under reduced pressure to give the crude product. The C-and N-terminal dual functionalized poly-α/β-peptide Dye-poly(Boc-α-L-Lys0.5/β-HG0.5)2-MAL was purified vis three cycles of the dissolution-precipitation process using a mixed solvent (2.0 mL THF/45.0 mL n-hexane). The collected solid was washed with 2 mL MeCN once then was dried under vacuum to give a yellow solid (20.5mg, 62.5% yield).
Dye-poly(Boc-α-L-Lys0.5/β-HG0.5)2-MAL (20.5 mg, 6.3 mmol) was continuously treated with 2 mL of trifluoroacetic acid (TFA) at rt for 2 h under gentle shaking to remove the side-chain N-Boc protecting group. After TFA was removed under air flow, the crude product was purified via three cycles of the dissolution-precipitation process in a mixed solvent (0.5 mL MeOH/45.0 mL Et2O) to give the deprotected poly-α/β-peptide Dye-poly(α-L-Lys0.5/β-HG0.5)2-MAL as yellow solid in the form of TFA salt (20.0 mg, 88.3% yield). 1H NMR (600 MHz, D2O, Figure S18) confirmed that the morpholino-naphthalimide fluorophore group and the maleimide group were successfully conjugated to the C-terminal and N-terminal of poly-α/β-peptide, respectively.
Kinetics study on copolymerization of α-NCA and β-NTA in THF
The kinetics study on copolymerization of α-NCAs and β-NTAs in THF was performed as described here. Boc-α-Lys NCA, Bn-β3-LCHG NTA and tBuBnNH2 were dissolved in anhydrous THF individually to a final concentration of 0.2 M. To the mixture of 1.0 mL Boc-α-Lys NCA, 1.0 mL Bn-β3-LCHG NTA and 67 μL tBuBnNH2 was added triphenylmethane (TPM, 4.0 mg) as the internal standard. The reaction mixture was stirred at rt in a nitrogen purged glovebox. Real-time monitoring of remaining Boc-α-Lys NCA and Bn-β3-LCHG NTA was performed by measuring the relative ratio of the absorbance peak area at 200 nm between the Boc-α-Lys NCA/TPM and Bn-β3-LCHG NTA/TPM using HPLC to calculate the remaining percentage. A mixed solvent of MeCN and H2O was used as the mobile phase, with MeCN increasing incrementally from 50% to 90% within 9 min, holding at 90% for 8 min, and decreasing from 90% to 50% within 3 min at a flow rate of 1.0 mL/min using a Luna Omega polar C18 column. The remaining percentage of NCA and NTA versus time was calculated from below equation:
whereas [M]t is the concentration of the monomer at a given time, [M]0 is the initial concentration of the monomer.
The synthesis of HDP-mimicking amphiphilic poly-α/β-peptide libraries in THF
The synthesis and characterization of HDP-mimicking amphiphilic poly(α-L-Lysx/β2,3-CH1-x)20 libraries were performed as described here. In a nitrogen purged glovebox, tBuBnNH2, Boc-α-L-Lys NCA and β2,3-CH NTA were individually dissolved in anhydrous THF to a final concentration of 0.2 M as the stock solution. Then 100μL tBuBnNH2 was added into 2.0 mL mixture of Boc-α-L-Lys NCA stock solution and β2,3-CH NTA stock solution in a volume ratio of x/1-x, and the reaction mixture was stirred at rt for 72 h under nitrogen. When the reaction was complete, the obtained polymers were purified by repeated dissolution-precipitation process (2.0 mL THF/45.0 mL n-hexane) three times to give poly(Boc-α-L-Lysx/β2,3-CH1-x)20 in 85.7–93.5% yield. The purified poly(Boc-α-L-Lysx/β2,3-CH1-x)20 was treated with 2 mL TFA at rt for 2 h under gentle shaking to remove the side-chain N-Boc protecting group, followed by removing TFA under air flow to give the crude product. The crude product was purified by the dissolution-precipitation process (0.5 mL MeOH/45.0 mL Et2O) three times. The purified poly(α-L-Lysx/β2,3-CH1-x)20 was dissolved in milli-Q and lyophilized to give the final product in the form of TFA salts. The polymers were and characterized by 1H NMR (Figures S22–S25) and used for further antibacterial activity test.
Copolymerization on α-NCA and β-NTA in CHCl3/H2O (95/5, v/v)
Copolymerization on α-NCA and β-NTA in CHCl3/H2O (95/5, v/v) was operated at ambient condition (Song et al., 2019). To the reaction mixture of α-NCA and β-NTA (with variable α:β ratio) at total final concentration of 0.1 M in CHCl3/H2O (95/5, v/v) was quickly added a solution of the initiator in CHCl3/H2O (95/5, v/v) under vigorous stir. The reaction was stirred at room temperature in an open flask until the polymerization reaction was completed as monitored by TLC and HPLC (>99% conversation). The reaction solution was concentrated under vacuum and the obtained residue was purified by the dissolution-precipitation process (2.0 mL THF/45 mL n-hexane) thrice to give the product as a white solid.
The controlled copolymerization on Boc-α-L-lys NCA and Bn-β3-LCHG NTA in CHCl3/H2O (95/5, v/v)
The copolymerization of Boc-α-L-lys NCA and Bn-β3-LCHG NTA in CHCl3/H2O (95/5, v/v) was operated as follows: At ambient condition, Boc-α-L-Lys NCA, Bn-β3-LCHG NTA and PEG-NH2 were respectively dispersed in a mixture of 0.2 M CHCl3/H2O (95/5, v/v). 150 μL, 75 μL, 50 μL and 37.5 μL of PEG-NH2 in CHCl3/H2O (95/5, v/v) were respectively added into the reaction mixture of 750 μL of Boc-α-L-lys NCA and 750 μL of Bn-β3-LCHG NTA in CHCl3/H2O (95/5, v/v) under vigorous stir. When the polymerization reaction was complete monitored by TLC and HPLC (conversation>99%), the reaction solution was poured into cold n-hexane (45.0 mL) to precipitate out the crude product. The crude product was purified via the dissolution-precipitation process thrice (2.0 mL THF/45 mL n-hexane) and then characterized by GPC using DMF as the mobile phase at flow rate of 1 mL/min (Figures S30 and S31).
Kinetics study on copolymerization of α-NCA and β-NTA in CHCl3/H2O (95/5, v/v)
The kinetics study on the copolymerization of α-NCAs and β-NTAs in CHCl3/H2O (95/5, v/v) was described here. At ambient condition, Boc-α-L-lys NCA (54.4 mg, 0.2 mmol) and Bn-β3-LCHG NTA (53.0 mg, 0.2 mmol) were weighed out and dispersed in a mixed solvent CHCl3/H2O (2000μL/106μL, v/v) under vigorous stir. Then 67μL of 0.2M PEG-NH2 (Mn = 2000) solution in CHCl3/H2O (95/5, v/v) and 4 mg TPM was quickly added into the reaction mixture, and the reaction was stirred at rt in an open flask at an environment of 60% relative humidity. The real-time monitoring of remaining Boc-α-Lys NCA and Bn-β3-LCHG NTA was performed by measuring the relative ratio of the absorbance peak area at 200 nm between the Boc-α-Lys NCA/TPM and Bn-β3-LCHG NTA/TPM using HPLC to detect the remaining monomers percentage in copolymerization using CHCl3/H2O (95/5, v/v) as the solvent. A mixed solvent of MeCN and H2O was used as the mobile phase, with MeCN increasing incrementally from 50% to 90% within 9 min, holding at 90% for 8 min, and decreasing from 90% to 50% within 3 min at a flow rate of 1.0 mL/min using a Luna Omega polar C18 column. The reactivity ratio of Boc-α-Lys NCA and Bn-β3-LCHG NTA within the copolymerization reaction, using a mixed solvent of CHCl3/H2O (95/5, v/v), was shown in Figure S20.
The synthesis of sequenced-controlled decablock poly-α/β-peptide via α-NCAs and β-NTAs copolymerization in CHCl3/H2O (95/5, v/v)
At ambient condition with 60% relative humidity, Boc-α-L-Lys NCA, Bn-β3-LCHG NTA, and Me-β3-LCHG NTA as monomers and PEG-NH2 as initiator were respectively dispersed in a mixture of 0.2 M CHCl3/H2O (95/5, v/v). The sequence of poly-α/β-peptide was controlled by copolymerization with step-wise addition of 15:5 α-NCA:β-NTA in the form of each diblock poly-α-peptide-b-poly-β-peptide. First, 750 μL Boc-α-L-Lys NCA, 250 μL Bn-β3-LCHG NTA and 50 μL PEG-NH2 were mixtured and vigorously stirred in CHCl3/H2O (95/5, v/v) at rt in an open flask, when the polymerization reaction was complete monitored by TLC and HPLC (conversation>99%), the reaction solution was directly characterized by GPC without purification, then the mixture of 750 μL Boc-α-L-Lys NCA and 250 μL Me-β3-LCHG NTA was added into the polymer reaction to prepare the second diblock. The polymerization would not stop until to give the decablock poly-α/β-peptide and the resulting sequenced-controlled decablock poly-α/β-peptide was obtained 248.6 mg in 90.2% yield.
Synthesis of sequence-controlled poly-α/β-peptide in CHCl3/H2O (95/5, v/v) by incorporating α-peptide sequence into β-peptide backbone
In an open flask at ambient condition, 4.0 mL of 0.2 M Bn-β3-LCHG NTA in CHCl3/H2O (95/5, v/v) and 40 μL of 0.2 M PEG-NH2 in CHCl3/H2O (95/5, v/v) were mixed and vigorously stirred at room temperature. A solution of 200 μL 0.2 M Boc-α-L-Lys NCA in CHCl3/H2O (95/5, v/v) and 200 μL 0.2 M α-BLG NCA in CHCl3/H2O (95/5, v/v) was alternatively added into above reaction every 4 hours. After adding α-NCA into the reaction each time, the reaction was monitored by HPLC until the consumption of α-NCA to have a new block of α-peptide sequence. The reaction continued with the presence of Bn-β3-LCHG NTA in the reaction flask for another two hours to have a new block of the β-peptide sequence along the polypeptide chain. Then α-NCA was added to the reaction flask again as described above to obtain another new block of α-peptide sequence. Repeated addition of α-NCA into the polymerization reaction flask was conducted until total 10 blocks of α-peptide sequence were inserted into the backbone of the β-peptide to obtain the sequence-controlled poly-α/β-peptide. The obtained polymer was purified by the dissolution-precipitation process (2.0 mL THF/45 mL n-hexane) thrice to give the final product of 238.6 mg (88.5% yield) as white solid. The poly-α/β-peptide was characterized by 1H NMR using poly-(Boc-α-L-Lys)5 and poly-(α-BLG)5 for comparison.
The synthesis of self-assembling poly-α/β-peptides in CHCl3/H2O (95/5,v/v)
At ambient condition, Boc-α-L-lys NCA (27.2 mg, 0.1 mmol) and Bn-β3-LCHG NTA (53.0 mg, 0.2 mmol) was respectively weighed out and dispersed in a mixture of CHCl3/H2O (1500μL/81μL, v/v,) with vigorous stir, followed by addition of 50 μL 0.2 M PEG-NH2 (Mn = 2000) solution in CHCl3/H2O (95/5, v/v) quickly into the reaction. The reaction mixture was stirred at rt in an open flask at an environment of 60% relative humidity. When the polymerization reaction was complete, the reaction mixture was poured into 45.0 mL cold n-hexane to precipitate the crude product that was purified from the dissolution-precipitation process (2.0 mL THF/45.0 mL n-hexane) three times to give the final product PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20 in 75.2–95.5% yield within our repeats of this reaction in six batches.
PEG-poly(Boc-α-L-Lys)15-b-poly(β3-HPhG)15 was synthesized by following above procedure in preparing PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20
Minimum inhibitory concentration (MIC) assay
14 strains of Gram positive and Gram negative bacteria were cultured individually in Luria–Bertani (LB) medium for 9 h at 37°C under shaking at 200 rpm. After centrifugation at 4000 rpm for 5 min, the bacteria were collected and re-suspended in Mueller–Hinton (MH) medium to 2 × 105 CFU/mL as the working suspension. Poly(α-L-Lys)20, poly(α-L-Lys0.7/β2,3-CH0.3)20, poly(α-L-Lys0.5/β2,3-CH0.5)20 and poly(α-L-Lys0.3/β2,3-CH0.7)20 were diluted to concentrations ranging from 1.56 μg/mL to 200 μg/mL by a two-fold gradient dilution in a 96-well plate. After mixing equal volumes of bacterial cell suspension (50 μL) and polypeptide solution (50 μL) in each well, the 96-well plates were incubated at 37°C for 9 h. Then the OD value in each well on the 96-well plate was collected on a SpectraMax® M2 plate reader. MH medium only and bacteria in MH medium was used as the blank and the positive control, respectively. The percentage of bacterial cell survival was calculated from the equation , and plotted against polymer concentration to give the dose-response curves of antibacterial activities for polypeptides. Measurements were performed in duplicates, and the experiments were repeated at least twice.
Proteolytic resistance test on HDP-mimicking amphiphilic poly-α/β-peptides
Proteolytic resistance of poly(α-L-Lys)20 and poly(α-L-Lys0.5/β2,3-CH0.5)20 was evaluated with hydrolysis kinetics of polypeptide using 1H NMR analysis, by following previously reported literature with modifications (Chin et al., 2018). Specifically, 5 mg of poly(α-L-Lys)20 and 5 mg of poly(α-L-Lys0.5/β2,3-CH0.5)20 were individually dissolved in 0.5 mL PBS buffer (pH 7.4) using D2O as solvent. Trypsin was dissolved in 0.5 mL PBS buffer (pH 7.4) using D2O as solvent to a final concentration of 10 mg/mL. 0.5 mL of above polypeptide solution and 12.5 μL of above trypsin solution were mixed and the mixture solution was detected by 1H NMR at various time intervals. The integration of the CH peak on lysine backbone in proton NMR for each data collecting time is plotted against the time for data collecting after the polypeptide is incubated with the enzyme. The value of half-life in the presence of trypsin (Figures S27–S28) showed that the incorporation of β-amino acid residue, β2,3-CH, increased half-life of poly(L-Lys)20 against trypsin from 34 min to 60 min. The value of half-life of poly(α-L-Lys)20 and poly(α-L-Lys0.5/β2,3-CH0.5)20 against proteinase K23 was detected from the mixture of 5 mg of polypeptide and 0.5 mg proteinase K23 using a similar method described above for trypsin treatment. The result (Figures S29 and S30) showed that the incorporation of β-amino acid residue, β2,3-CH, increased the half-life of poly(L-Lys)20 against proteinase K23 from 340 min to 1080 min.
Characterization of self-assembled poly-α/β-peptides
PEG-poly(Boc-α-L-Lys)10-b-poly(Bn-β3-LCHG)20 and PEG-poly(Boc-α-L-Lys)15-b-poly(β3-HPhG)15 were respectively treated with 2 mL of TFA at rt for 2 h under gentle shaking to remove the side-chain N-Boc protecting group. The deprotected polypeptides were purified from the dissolution-precipitation process (2.0 mL THF/45.0 mL n-hexane) for three times. The obtained PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20 was dissolved in deionized water (1.0 mg/mL) and then was diluted with 15% (v/v) THF to a final concentration of 0.87 mg/mL. The mixture was stirred for 1h and then was allowed to stand still for 18 hours at room temperature to allow the assembly of polypeptides. The stock solution of self-assembled PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20 was filtered through a 2 μm polytetrafluoroethylene (PTFE) filter before DLS and TEM characterization. The obtained PEG-poly(α-L-Lys)15-b-poly(β3-HPhG)15 was dissolved in deionized water to the final concentration of 1.0 mg/mL, and was subjected to self-assembly by the same procedure described above for PEG-poly(α-L-Lys)10-b-poly(Bn-β3-LCHG)20.
Quantification and statistical analysis
The reactivity ratio of Boc-α-Lys NCA and Bn-β3-LCHG NTA in THF and CHCl3/H2O (95/5, v/v) was calculated respectively using the Lynd method by following a precedent procedure (Yasir et al., 2020) (Figures S19 and S20). Lynd equation for the evaluation of reactivity ratio is given below:
whereas, r1 and r2 means the reactivity ratio of Boc-α-Lys NCA and Bn-β3-LCHG NTA respectively.
[M]1 and [M]x are the concentrations of NCA and NTA at a given time, respectively.
[M]1,0 and [M]x,0 is the initial concentration of NCA and NTA, respectively.
f1,0 is the initial mole fraction of NCA.
X is the total conversion.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 22075078, 21774031, 21861162010, 31800801), Program of Shanghai Academic/Technology Research Leader (20XD1421400), Research program of State Key Laboratory of Bioreactor Engineering, the Fundamental Research Funds for the Central Universities (JKD01211520), and the Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism grant 2021 Sci & Tech 03-28 (Shanghai Municipal Education Commission). We thank the Research Center of Analysis and Test of East China University of Science and Technology for the help with the characterization. We also thank the staff members of the Mass Spectrometry System at the National Facility for Protein Science in Shanghai (NFPS), Zhangjiang Lab, China for providing technical support and assistance in data collection and analysis.
Author contributions
R.L. directed the whole project. M.Z. and R.L. conceived the idea, proposed the strategy, designed the experiments, evaluated the data, and wrote the manuscript together. M.Z. performed the majority of the experiments. J.Z. conducted polymerization kinetics study and participated in sequence-controlled copolymerization. L.L. performed DLS and TEM characterization on assembled poly-α/β-peptides. X.X. and S.D. conducted the antimicrobial assays. Y.W., J.X., Z.C. and Z.J. contributed to the synthesis of monomers and MALDI-TOF-MS characterization. All authors proofread the manuscript.
Declaration of interests
R.L. and M.Z. are co-inventors on a patent application covering reported α/β-peptide polymer synthesis and application. All remaining authors declare no competing interests.
Published: October 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103124.
Supplemental information
and Table S1
Data and code availability
This study did not generate/analyse data sets/code. All data are described in the main text and methods.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Baumann A.L., Schwagerus S., Broi K., Kemnitz-Hassanin K., Stieger C.E., Trieloff N., Schmieder P., Hackenberger C.P.R. Chemically induced vinylphosphonothiolate electrophiles for thiol–thiol bioconjugations. J. Am. Chem. Soc. 2020;142:9544–9552. doi: 10.1021/jacs.0c03426. [DOI] [PubMed] [Google Scholar]
- Chan-Seng D., Lutz J.-F. Sequence-controlled polymers: synthesis, self-assembly, and properties. ACS Symp. Ser. 2014:103–116. [Google Scholar]
- Cheng J., Deming T.J. Synthesis of polypeptides by ring-opening polymerization of α-amino acid N-carboxyanhydrides. Top. Curr. Chem. 2012;310:1–26. doi: 10.1007/128_2011_173. [DOI] [PubMed] [Google Scholar]
- Chin W., Zhong G., Pu Q., Yang C., Lou W., De Sessions P.F., Periaswamy B., Lee A., Liang Z.C., Ding X. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat. Commun. 2018;9:917. doi: 10.1038/s41467-018-03325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming T.J. Facile synthesis of block copolypeptides of defined architecture. Nature. 1997;390:386–389. doi: 10.1038/37084. [DOI] [PubMed] [Google Scholar]
- Feng F., Liu L., Wang S. Fluorescent conjugated polymer-based FRET technique for detection of DNA methylation of cancer cells. Nat. Protoc. 2010;5:1255–1264. doi: 10.1038/nprot.2010.79. [DOI] [PubMed] [Google Scholar]
- Feng L., Zhu C., Yuan H., Liu L., Lv F., Wang S. Conjugated polymer nanoparticles: preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013;42:6620–6633. doi: 10.1039/c3cs60036j. [DOI] [PubMed] [Google Scholar]
- Ge C., Yang J., Duan S., Liu Y., Meng F., Yin L. Fluorinated α-helical polypeptides synchronize mucus permeation and cell penetration toward highly efficient pulmonary siRNA delivery against acute lung injury. Nano Lett. 2020;20:1738–1746. doi: 10.1021/acs.nanolett.9b04957. [DOI] [PubMed] [Google Scholar]
- Girvin Z.C., Andrews M.K., Liu X., Gellman S.H. Foldamer-templated catalysis of macrocycle formation. Science. 2019;366:1528–1531. doi: 10.1126/science.aax7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvin Z.C., Gellman S.H. Exploration of diverse reactive diad geometries for bifunctional catalysis via foldamer backbone variation. J. Am. Chem. Soc. 2018;140:12476–12483. doi: 10.1021/jacs.8b05869. [DOI] [PubMed] [Google Scholar]
- Gleede T., Markwart J.C., Huber N., Rieger E., Wurm F.R. Competitive copolymerization: access to aziridine copolymers with adjustable gradient strengths. Macromolecules. 2019;52:9703–9714. [Google Scholar]
- Goodman C.M., Choi S., Shandler S., DeGrado W.F. Foldamers as versatile frameworks for the design and evolution of function. Nat. Chem. Biol. 2007;3:252–262. doi: 10.1038/nchembio876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjichristidis N., Iatrou H., Pitsikalis M., Sakellariou G. Synthesis of well-defined polypeptide-based materials via the ring-opening polymerization of alpha-amino acid N-carboxyanhydrides. Chem. Rev. 2009;109:5528–5578. doi: 10.1021/cr900049t. [DOI] [PubMed] [Google Scholar]
- Hamuro Y., Schneider J.P., DeGrado W.F. De novo design of antibacterial beta-peptides. J. Am. Chem. Soc. 1999;121:12200–12201. [Google Scholar]
- Hancock R.E., Sahl H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Horne W.S., Gellman S.H. Foldamers with heterogeneous backbones. Acc. Chem. Res. 2008;41:1399–1408. doi: 10.1021/ar800009n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Jiang J., Zhang X., Cui Y., Chen Z., Pan X., Wu J. Isotactic-alternating, heterotactic-alternating, and ABAA-type sequence-controlled copolyester syntheses via highly stereoselective and regioselective ring-opening polymerization of cyclic diesters. J. Am. Chem. Soc. 2021;143:4421–4432. doi: 10.1021/jacs.1c00902. [DOI] [PubMed] [Google Scholar]
- Jones R. Why nanotechnology needs better polymer chemistry. Nat. Nanotechnol. 2008;3:699–700. doi: 10.1038/nnano.2008.349. [DOI] [PubMed] [Google Scholar]
- Konai M.M., Bhattacharjee B., Ghosh S., Haldar J. Recent progress in polymer research to tackle infections and antimicrobial resistance. Biomacromolecules. 2018;19:1888–1917. doi: 10.1021/acs.biomac.8b00458. [DOI] [PubMed] [Google Scholar]
- Kricheldorf H.R. Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides. Angew. Chem. Int. Ed. 2006;45:5752–5784. doi: 10.1002/anie.200600693. [DOI] [PubMed] [Google Scholar]
- Krumm C., Fik C.P., Meuris M., Dropalla G.J., Geltenpoth H., Sickmann A., Tiller J.C. Well-defined amphiphilic poly(2-oxazoline) ABA-triblock copolymers and their aggregation behavior in aqueous solution. Macromol. Rapid Commun. 2012;33:1677–1682. doi: 10.1002/marc.201200192. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yin L. α-Amino acid N-carboxyanhydride (NCA)-derived synthetic polypeptides for nucleic acids delivery. Adv. Drug Deliv. Rev. 2020;171:139–163. doi: 10.1016/j.addr.2020.12.007. [DOI] [PubMed] [Google Scholar]
- Lutz J.-F. Sequence-controlled polymerizations: the next Holy Grail in polymer science? Polym. Chem. 2010;1:55–62. [Google Scholar]
- Lutz J.-F., Ouchi M., Liu D.R., Sawamoto M. Sequence-controlled polymers. Science. 2013;341:1238149. doi: 10.1126/science.1238149. [DOI] [PubMed] [Google Scholar]
- McGraw M.L., Clarke R.W., Chen E.Y.X. Compounded sequence control in polymerization of one-pot mixtures of highly reactive acrylates by differentiating lewis pairs. J. Am. Chem. Soc. 2020;142:5969–5973. doi: 10.1021/jacs.0c01127. [DOI] [PubMed] [Google Scholar]
- Miao Q., Xie C., Zhen X., Lyu Y., Duan H., Liu X., Jokerst J.V., Pu K. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017;35:1102–1110. doi: 10.1038/nbt.3987. [DOI] [PubMed] [Google Scholar]
- Nederberg F., Zhang Y., Tan J.P.K., Xu K., Wang H., Yang C., Gao S., Guo X.D., Fukushima K., Li L. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011;3:409–414. doi: 10.1038/nchem.1012. [DOI] [PubMed] [Google Scholar]
- Pfeifer S., Lutz J.-F. A facile procedure for controlling monomer sequence distribution in radical chain polymerizations. J. Am. Chem. Soc. 2007;129:9542–9543. doi: 10.1021/ja0717616. [DOI] [PubMed] [Google Scholar]
- Pilsl L.K.A., Reiser O. α/β-Peptide foldamers: state of the art. Amino Acids. 2011;41:709–718. doi: 10.1007/s00726-011-0894-2. [DOI] [PubMed] [Google Scholar]
- Rasines Mazo A., Allison-Logan S., Karimi F., Chan N.J., Qiu W., Duan W., O'Brien-Simpson N.M., Qiao G.G. Ring opening polymerization of alpha-amino acids: advances in synthesis, architecture and applications of polypeptides and their hybrids. Chem. Soc. Rev. 2020;49:4737–4834. doi: 10.1039/c9cs00738e. [DOI] [PubMed] [Google Scholar]
- Sang P., Zhou Z., Shi Y., Lee C., Amso Z., Huang D., Odom T., Nguyen-Tran V.T.B., Shen W., Cai J. The activity of sulfono-γ-AApeptide helical foldamers that mimic GLP-1. Sci. Adv. 2020;6:eaaz4988. doi: 10.1126/sciadv.aaz4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N.W., Jin F., Lande R., Curk T., Xian W., Lee C., Frasca L., Frenkel D., Dobnikar J., Gilliet M., Wong G.C.L. Liquid-crystalline ordering of antimicrobial peptide–DNA complexes controls TLR9 activation. Nat. Mater. 2015;14:696–700. doi: 10.1038/nmat4298. [DOI] [PubMed] [Google Scholar]
- Schmitt M.A., Weisblum B., Gellman S.H. Unexpected relationships between structure and function in α,β-peptides: antimicrobial foldamers with heterogeneous backbones. J. Am. Chem. Soc. 2004;126:6848–6849. doi: 10.1021/ja048546z. [DOI] [PubMed] [Google Scholar]
- Schmitt M.A., Weisblum B., Gellman S.H. Interplay among folding, sequence, and lipophilicity in the antibacterial and hemolytic activities of α/β-Peptides. J. Am. Chem. Soc. 2007;129:417–428. doi: 10.1021/ja0666553. [DOI] [PubMed] [Google Scholar]
- Song Z., Fu H., Wang J., Hui J., Xue T., Pacheco L.A., Yan H., Baumgartner R., Wang Z., Xia Y. Synthesis of polypeptides via bioinspired polymerization of in situ purified N-carboxyanhydrides. Proc. Natl. Acad. Sci. U S A. 2019;116:10658–10663. doi: 10.1073/pnas.1901442116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gao S., Ye W.-H., Yoon H.S., Yang Y.-Y. Co-delivery of drugs and DNA from cationic core–shell nanoparticles self-assembled from a biodegradable copolymer. Nat. Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- Wu Y., Xia G., Zhang W., Chen K., Bi Y., Liu S., Zhang W., Liu R. Structural design and antimicrobial properties of polypeptides and saccharide-polypeptide conjugates. J. Mater. Chem. B. 2020;8:9173–9196. doi: 10.1039/d0tb01916j. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang D., Ma P., Zhou R., Hua L., Liu R. Lithium hexamethyldisilazide initiated superfast ring opening polymerization of alpha-amino acid N-carboxyanhydrides. Nat. Commun. 2018;9:5297. doi: 10.1038/s41467-018-07711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Pu K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021;50:1111–1137. doi: 10.1039/d0cs00664e. [DOI] [PubMed] [Google Scholar]
- Yasir M., Liu P., Markwart J.C., Suraeva O., Wurm F.R., Smart J., Lattuada M., Kilbinger A.F.M. One-step ring opening metathesis block-like copolymers and their compositional analysis by a novel retardation technique. Angew. Chem. Int. Ed. 2020;59:13597–13601. doi: 10.1002/anie.202005366. [DOI] [PubMed] [Google Scholar]
- Zhang D., Qian Y., Zhang S., Ma P., Zhang Q., Shao N., Qi F., Xie J., Dai C., Zhou R. Alpha-beta chimeric polypeptide molecular brushes display potent activity against superbugs-methicillin resistant Staphylococcus aureus. Sci. China Mater. 2018;62:604–610. [Google Scholar]
- Zhang K., Du Y., Si Z., Liu Y., Turvey M.E., Raju C., Keogh D., Ruan L., Jothy S.L., Reghu S. Enantiomeric glycosylated cationic block co-beta-peptides eradicate Staphylococcus aureus biofilms and antibiotic-tolerant persisters. Nat. Commun. 2019;10:4792. doi: 10.1038/s41467-019-12702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Xiao X., Cong Z., Wu Y., Zhang W., Ma P., Chen S., Zhang H., Zhang D., Zhang D. Water-insensitive synthesis of poly-beta-peptides with defined architecture. Angew. Chem. Int. Ed. 2020;59:7240–7244. doi: 10.1002/anie.202001697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
and Table S1
Data Availability Statement
This study did not generate/analyse data sets/code. All data are described in the main text and methods.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.