Abstract
AIM
To investigate the role of exosomal miR-29b and Ca2+ in regulating the function of human lens epithelial cells (HLECs).
METHODS
Exosomes were isolated from human aqueous humour (AH) by ultracentrifugation, and visualized by nanoparticle tracking and transmission electron microscopy. Exosomal miRNA sequencing was performed to identify differentially expressed miRNAs between diabetes with cataracts (DMC) group and age-related cataracts (ARC) group. TargetScan was used to predict potential target of certain miRNA. The expression of CACNA1C mRNA was determined by quantitative real-time polymerase chain reaction and CACNA1C protein was determined by Western blotting. Concentration of Ca2+ in human AH and the culture supernatant of cells were detected by the calcium assay kit. Cell counting kit-8 was used to determine cell viability.
RESULTS
Exosomes were isolated from human AH, which had a typical cup-shaped phenotype and a particle size distribution in accordance with micro extracellular vesicles. Exosomal miRNA sequencing revealed that miR-29b was significantly downregulated in DMC group compared with ARC. Ca2+ concentration of human AH in DMC was higher than that in ARC. The culture supernatant of cells transfected with miR-29b inhibitors had a higher concentration of Ca2+ than that transfected with miR-29b mimics. miR-29b reduced the viability of HLECs by upregulating CACNA1C expression.
CONCLUSION
Exosomes isolated from human AH contains abundant miRNAs. A significantly expressed miRNA, miR-29b, can affect the concentration of Ca2+ and regulate HLEC processes by upregulating CACNA1C.
Keywords: exosomes, miR-29b, diabetes and cataracts, Ca2+, CACNA1C
INTRODUCTION
Cataract is the opacification of the crystalline lenses and a disease that can be caused by many factors. Diabetes is a complex metabolic disorder that also involves small blood vessels, often causing widespread damage to tissues, including the eyes. Cataracts are a common ocular complication of diabetes. Bilateral cataracts occasionally occur with rapid onset in severe juvenile diabetes, which is called true diabetic cataract. However, true diabetic cataracts are rare. Senile cataract in people with diabetes, which is called diabetes with cataracts (DMC) in our study, is more common. The potential mechanisms for the pathogenesis of diabetic cataracts are complicated and include the p38-MAPK signalling pathway[1]–[2], polyol pathway[3], and changes in inflammatory cytokines[4]–[10]. However, the precise mechanism of DMC remains unclear.
Ca2+ exists in the endocytoplasmic reticulum and plays an important role in the processing of polypeptide chains in protein translation and post-translational processing. It was reported that abnormal distribution of Ca2+ could lead to dysfunction of the endocytoplasmic reticulum and mitochondria, thus causing some metabolic diseases such as diabetes[11]. Previous studies have reported that Ca2+-CaM abnormalities exist in cataracts, and it was found that L-type calcium channels were extensively distributed in lens epithelial cells, and the inhibition of L-type calcium channels could lead to the formation of cortical cataract[12]. There are a variety of different types of channel playing a fundamental role in Ca2+ homeostasis and cell signalling which, when activated, allow Ca2+ entry into cells, including L-type calcium channels. The expression of L-type voltage-gated Ca2+ channels in both lens epithelial and fiber cells is potentially an important route of Ca2+ influx which may contribute to pathological Ca2+ overload[13]. L-type calcium channels were partially translated from the gene CACNA1C, which was also found to be mutated in DMC[14]. Given the critical role of Ca2+ in lens epithelial cells, further investigation on the role of Ca2+ in DMC is required.
Exosomes are micro extracellular vesicles (30-150 nm) that can be secreted by almost all types of cells and contain nucleus acids, proteins and lipids[15]. Exosomes have been widely studied in diabetes, neural diseases, tumours, cardiovascular diseases, etc[16]–[26]. A previous study reported that there were abundant exosomes in the human aqueous humour (AH)[27]. Exosomal microRNAs (miRNAs) play important roles in the mechanisms of diabetes[28]–[30] and diabetic retinopathy[31]–[33]. However, the function and characteristics of exosomes and miRNAs in DMC remains unknown.
In this study, we found that decreased expression of miR-29b could upregulate the expression of CACNA1C, increase the concentration of Ca2+, and affect the apoptosis of human lens epithelial cells (HLECs) in patients with DMC. The role of miR-29b and calcium signalling in diabetic cataracts has not been reported, which might suggest a potential role of exosomal miRNAs in the pathogenesis of diabetic cataracts.
The purpose of the study was to investigate the potential function of exosomal miRNAs found in AH on calcium signaling in diabetic patients with cataract.
SUBJECTS AND METHODS
Ethical Approval
The use of human AH samples from cataract eyes during surgery was approved by the Institutional Review Board of Eye and ENT hospital of Fudan University. This study was performed in accordance with the tenets of the Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from every enrolled participant.
Collection of AH and Human Lens Epithelium
We collected AH and human lens epithelium samples from 36 patients with DMC (age from 45-76 years old, fasting glucose 5.2-8.2 mmol/L, free of other ocular diseases, and lenticular opacity ranging C3-4, NO2-3, NC2-3, and P1-3 by LOCSIII) and 43 patients with age-related cataracts (ARC; age 62-85 years old, fasting glucose 5.0-6.2 mmol/L, free of other ocular diseases, C3-4, NO2-3, NC2-3, and P1-2) before cataract surgery at Eye and ENT Hospital of Fudan University. AH samples were obtained before the collection of lens epithelium samples. The lens epithelium samples were acquired by intact continuous curvilinear capsulorhexis during cataract surgery for ARC patients by the same experienced surgeon (Luo Y). All AH and human lens epithelium samples were stored in a freezer at -80°C until the next step.
Isolation of Exosomes
The AH samples from 36 patients with DMC were pooled together as the DMC group, and the AH samples from 43 patients with ARC were pooled together as the ARC group. Exosomes were isolated using ultracentrifugation. Procedures were as followed: take AH samples out and thaw in 27°C water bath; 4°C, 2000 g, 10min, and remove supernatant; 4°C, 10 000 g, 30min, and take supernatant; 4°C, 110 000 g, 75min, and discard supernatant; Resuspend pellet and filter with 0.22 µm membrane; 4°C, 110 000 g, 75min, and abandon supernatant.
TEM and NTA of AH Exosomes
Purified exosomes were diluted in PBS. Samples 5 µL were absorbed onto copper grids and dried for 5min at room temperature. After that, a drop of 2% uranyl acetate solution was added for 20min, and the sample was air-dried and examined by transmission electron microscopy (TEM; Tecnai G2 Spirit BioTwin, FEI, USA). Particle size, concentration, and distribution of exosomes were determined by nanoparticle tracking analysis (NTA; ZetaView, Particle Matrix, Germany).
Exosomal RNA Extraction and miRNA Sequencing Analysis
Exosomal RNA was extracted from the DMC group and the ARC group using the miRNeasy Micro Kit (217084, QIAGEN, Germany) according to the manufacturer's guidelines. RNA libraries were prepared and sequenced on an Illumina HiSeq 2500 platform. Read counts were obtained by FeatureCounts software. FastQC software was used for quality control. Additionally, we used Cutadapt software to remove low-quality reads and high-quality reads were used to analyse miRNAs by mapping to the human reference genome using Bowtie software. A fold change >1.2 or <0.83 was considered to indicate differentially expressed miRNAs by DESeq2.
RNA Extraction of Epithelium Samples and qRT-PCR
Lens epithelium samples from 43 patients with ARC were classified as ARC group. Epithelium samples from 36 patients with DMC were classified as DMC group. In each group, 4 to 5 epithelium samples were pooled together to obtain enough RNA. Total RNA from all epithelium samples was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed with the RT reagent Kit (Takara Bio, Inc, Japan) according to the manufacturer's protocol. Expression of mRNAs was detected using the SYBR Green detection kit (Takara, Japan) on the LightCycler 480II Real-Time PCR System (Roche, Switzerland). GAPDH was detected as the internal control. RNA expression was determined by the 2−ΔΔCT method.
HLEC Culture and Transfection
HLECs were cultured in 35-mm culture Petri dishes with growth medium containing DMEM (Gibco, USA) with 10% foetal bovine serum (FBS; Gibco, USA). MiR-29b mimics (50 nmol/L) and inhibitors (100 nmol/L) were transfected into HLECs when the cells covered 70%-80% of the entire dish.
Examination of Ca2+ in AH Samples and the Culture Supernatant of Cells
Concentration of Ca2+ in AH samples and the culture supernatant of cells was detected by using Calcium Assay Kit (Colorimetric) from Abcam (Cambridge, MA, USA) according to the manufacturer's protocol. We added 50 µL of AH samples and the culture supernatant of cells to each well of the 96-well plates. Then 90 µL of the chromogenic reagent and 60 µL of calcium assay buffer were added into each well for 10min at room temperature protected from light. Absorbance was measured at a wavelength of 575 nm using an automatic microplate reader (Tecan, Switzerland). Concentration of Ca2+ was equivalent to Abs/Vol (µg/µL). Abs referred to absorbance of AH samples and cell culture supernatant; Vol referred to the volume of AH samples and cell culture supernatant added to each well.
CCK-8 Cell Proliferation and Cytotoxicity Assay
Cell viability was determined by using cell counting kit-8 (CCK-8) kit (Dojindo, Japan) according to the manufacturer's protocol. Transfected cells were plated onto 96-well plates and cultured for 24h. CCK-8 (10 µL) was then added for 1h at 37°C. We used Tert-butyl hydroperoxide solution (TBHP) as an oxidative stimulus. Absorbance was measured at a wavelength of 450 nm using an automatic microplate reader (Tecan, Switzerland). The cell viability was equivalent to (At-Ab)/(Ac-Ab). At referred to absorbance of transfected cell groups; Ac referred to absorbance of controlled groups; Ab referred to absorbance of blank groups.
Western Blot
Protein was extracted by RIPA lysis buffer (Biotech Well, Shanghai, China). Equal amounts of proteins were resolved by SDS-PAGE using 5% acrylamide-containing gels, followed by electrophoretic transfer to PVDF membranes. The membranes were blocked with transfer buffer (Biotech Well, Shanghai, China) and incubated overnight with the monoclonal primary antibodies at a 1:200 dilution, followed by secondary antibodies at a 1:2000 dilution. The signaling of western blotting was then observed using ECL prime reagents (Biotech Well, Shanghai, China) and scanned using a Peiqing automatic gel imaging analysis system (Shanghai, China). The L-VOCC polyclonal antibody (21774-1-AP) was purchased from Proteintech Group (USA). The anti-GAPDH antibody and the goat anti-rabbit IgG (H+L) secondary antibody were purchased from Biotech Well (Shanghai, China).
MicroRNA Target Prediction
Potential targets of miR-29b were predicted by the Targetscan database (http://www.targetscan.org)[34].
Statistical Analysis
All data are shown as the mean±SD, and experiments were repeated three times. Statistical significance was determined by two-tailed Student's t-test, One-way ANOVA or Chi-square test using IBM SPSS 21.0 (USA). P<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics in DMC and ARC Groups
Totally 79 eyes were enrolled in the two groups, among which there were 36 eyes in DMC group and 43 eyes in ARC group. In DMC group, there were 22 males and 14 females, 17 right eyes and 19 left eyes. Mean age and mean fasting glucose of DMC group were 68.11±1.34y and 6.31±1.34 mmol/L. In ARC group, there were 21 males and 22 females, 21 right eyes and 22 left eyes. Mean age and mean fasting glucose of ARC group were 69.07±0.82y and 5.55±0.06 mmol/L. There were no statistical significance in the gender, right or left eye, and age between the two groups (Table 1). The fasting glucose between the two groups was statistically significant (Table 1).
Table 1. Baseline characteristics in DMC and ARC groups.
| Characteristics | DMC group | ARC group | P |
| Number | 36 | 43 | - |
| Age (y) | 68.11±1.34 | 69.07±0.82 | 0.530 |
| Gender | 0.365 | ||
| Male | 22 | 21 | |
| Female | 14 | 22 | |
| Right or left eye | 0.886 | ||
| Right eye | 17 | 21 | |
| Left eye | 19 | 22 | |
| Fasting glucose (mmol/L) | 6.31±1.34 | 5.55±0.06 | <0.001 |
DMC: Diabetes and cataracts; ARC: Age-related cataracts.
Cup-shaped Phenotype Observed Using TEM and Particle Size Distribution of Exosomes Using NTA
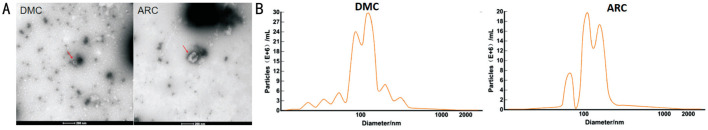
To identify the characteristics of exosomes, we used TEM to observe the morphology of exosomes isolated from AH, and observed a typical cup-shaped phenotype in both groups (Figure 1A)[35].
Figure 1. Identification of exosomes using TEM and NTA.
A: Typical cup-shaped phenotype under TEM in the DMC and the ARC group; B: Particle size distribution in DMC and ARC group by NTA.
The particle diameter was 129.8 nm in the ARC group and 116.3 nm in the DMC group. Particle sizes were mainly distributed at 80-120 nm, and the concentration reached more than E+10 particles/mL in both groups (Figure 1B)[35].
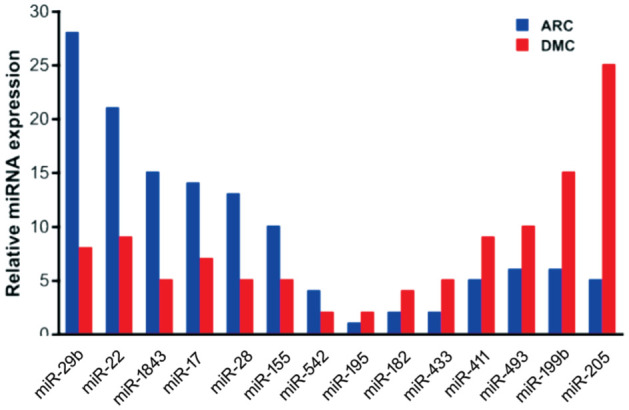
Differential Expression of miRNAs in AH Samples of the DMC and ARC Groups
miRNA sequencing was used to examine the expression of miRNAs in AH samples of the DMC and ARC groups. A total of 552 miRNAs were obtained from all samples. Of them, 119 miRNAs were unchanged; 295 miRNAs were upregulated and 138 miRNAs were downregulated in AH samples of the DMC group compared with the ARC group (fold change >1.2 in upregulated miRNAs, and fold change <0.833 in downregulated miRNAs). Among the 138 downregulated miRNAs, miR-29b was significantly downregulated in the DMC group compared with the ARC group (with fold change=0.286; Figure 2). In addition, CACNA1C was a potential target of miR-29b through the TargetScan website. We thus investigated the role of miR-29b in the regulation of HLECs function. Part of differentially expressed miRNAs were shown in Figure 2.
Figure 2. Part of downregulated and upregulated miRNA expression in the DMC group compared with the ARC group.
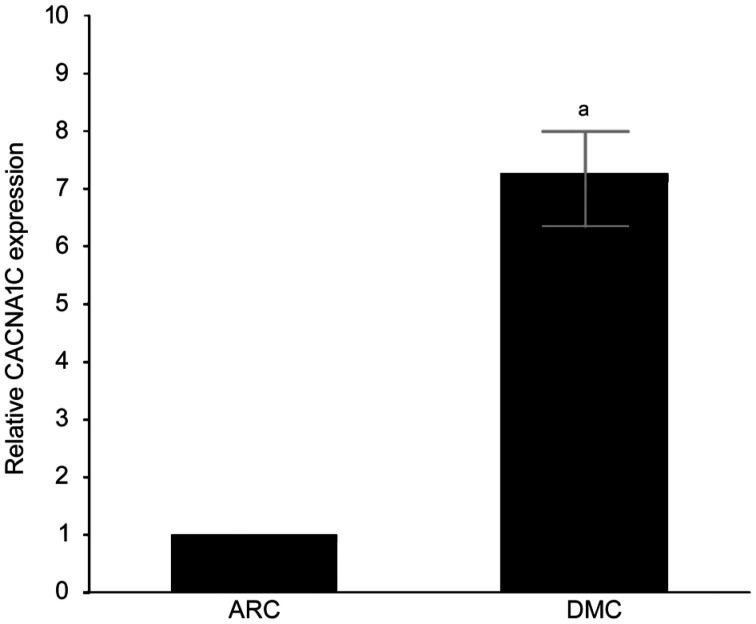
Differential CACNA1C Expression Between DMC and ARC Epithelium Samples
CACNA1C mRNA expression was upregulated in DMC epithelium samples compared with that in ARC epithelium samples by quantitative real-time PCR (qRT-PCR), and the difference was statistically significant (Figure 3).
Figure 3. Expression of CACNA1C mRNA detected by qRT-PCR.
aStatistically significant compared with ARC.
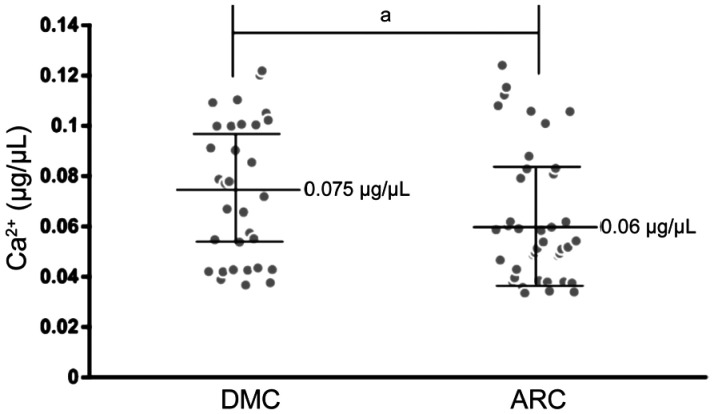
Different Concentration of Ca2+ in AH Samples in the DMC and ARC Groups
To determine the effect affected by the different expression of CACNA1C, we detected the concentration of Ca2+ in AH samples in two groups. Mean concentration of Ca2+ in AH samples was 0.075±0.005 µg/µL in the DMC group and 0.06±0.004 µg/µL in the ARC group. The concentration of Ca2+ in AH samples was higher in the DMC group than that in the ARC group (25% more), and the difference was statistically significant (P=0.024; Figure 4).
Figure 4. Concentration of Ca2+ of AH samples in the DMC and ARC groups.
aStatistically significant compared with ARC.
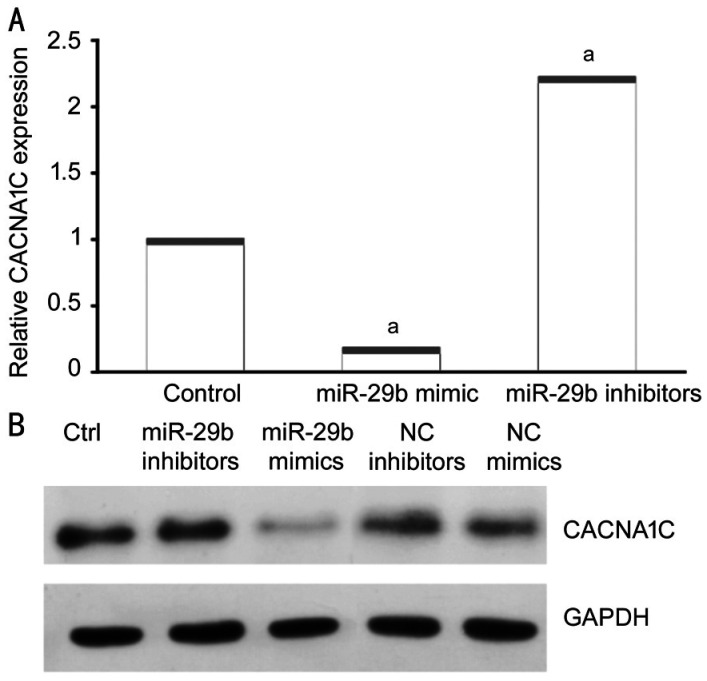
MiR-29b Mimics and Inhibitors Significantly Changed CACNA1C Expression in HELCs
To determine whether exosomal miR-29b influenced the expression of CACNA1C in HELCs, we used miR-29b mimics and inhibitors to transfect HLECs and then detect the expression of CACNA1C mRNA of HLECs. miR-29b mimics significantly downregulated CACNA1C expression in HELCs, while miR-29b inhibitors significantly upregulated CACNA1C expression in HELCs (Figure 5A). Additionally, negative control (NC) mimics and NC inhibitors were used as comparators of miR-29b mimics and inhibitors in Western blot analysis. Western blot analysis showed that transfection of miR-29b mimics led to a reduction in CACNA1C expression, while NC mimics did not. By contrast, transfection of miR-29b inhibitors led to increased CACNA1C expression, while NC inhibitors did not (Figure 5B). The results were consistent with the results observed in epithelium samples.
Figure 5. MiR-29b mimics and inhibitors changed CACNA1C expression in HELCs.
A: Expression of CACNA1C mRNA was affected by miR-29b mimics and inhibitors as demonstrated using qRT-PCR; B: Protein levels of CACNA1C were affected by miR-29b mimics and inhibitors as shown using Western blotting. aStatistically significant compared with ARC.
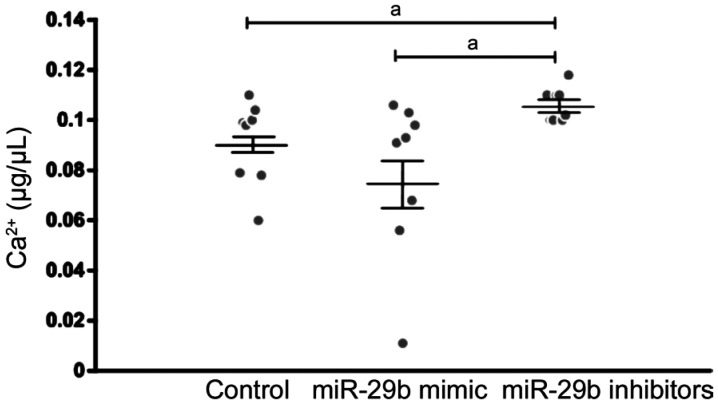
Different Concentration of Ca2+ in the Culture Supernatant of Cells Transfected with miR-29b Mimics and Inhibitors
To determine whether different expression of CACNA1C regulated by miR-29b affected the concentration of Ca2+, we then detect the concentration of Ca2+ in the culture supernatant of cells transfected with miR-29b mimics and inhibitors. Mean concentration of Ca2+ in the culture supernatant of cells was 0.09±0.005 µg/µL in the control group, and 0.078±0.01 µg/µL transfected with miR-29b mimics, and 0.106±0.002 µg/µL transfected with miR-29b inhibitors. The concentration of Ca2+ was higher in the culture supernatant of cells transfected with miR-29b inhibitors than that in normal culture supernatant of cells. The concentration of Ca2+ was 26.4% higher in the culture supernatant of cells transfected with miR-29b inhibitors than that in the culture supernatant of cells transfected with miR-29b mimics (Figure 6). The result was similar to that in AH samples.
Figure 6. Concentration of Ca2+ in normal cell culture supernatant and in cell culture supernatant transfected by miR-29b mimics and inhibitors.
aStatistically significant compared with ARC.
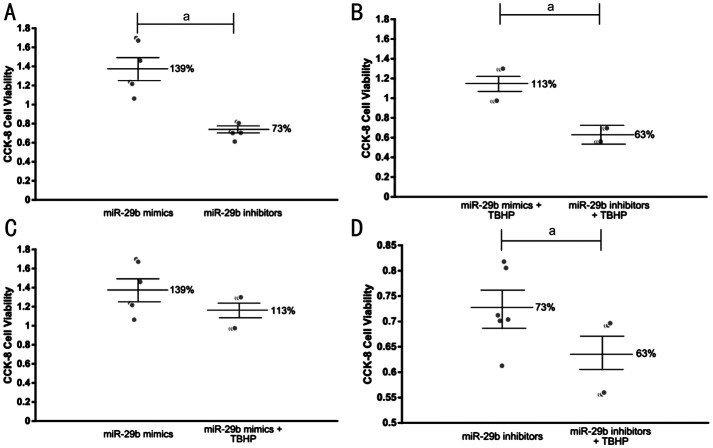
Role of miR-29b in the Regulation of Cell Viability of HLECs
We further investigated the effect of miR-29b on the viability of HLECs. Both under normal conditions and under oxidative stress, miR-29b inhibitors significantly reduced the viability of HLECs compared with miR-29b mimics, and the difference was statistically significant (Figure 7A, 7B). Under oxidative stress, the viability of HLECs transfected with miR-29b mimics was slightly reduced, but the difference was not statistically significant (Figure 7C). However, the viability of HLECs transfected with miR-29b inhibitors was significantly reduced, and the difference was statistically significant (Figure 7D).
Figure 7. Cell viability measured by CCK-8.
A: The viability of HLECs trancfected with miR-29b mimics and inhibitors in normal conditions; B: The viability of HLECs trancfected with miR-29b mimics and inhibitors under oxidative stress; C: The viability of HLECs trancfected with miR-29b mimics in normal conditions and under oxidative stress; D: The viability of HLECs trancfected with miR-29b inhibitors in normal conditions and under oxidative stress. aStatistically significant compared with ARC.
DISCUSSION
Cataracts are more common in patients with diabetes than individuals without diabetes; their symptoms are similar to those of ARC, while their progression is more rapid. Recently, exosomes have been widely studied in many fields, especially diabetes. To reveal a possible mechanism linking DMC, we investigated the role of exosomal miR-29b and Ca2+ in HLECs.
Ca2+ is an important factor in biological activities. More importantly, calcium might induce conformational damage to A-crytallin and accelerate the development of the cataracts[36]. Ca2+ influx channel, including L-type voltage-gated Ca2+ channel, is a powerful way of rapidly increasing intracellular Ca2+ during cell signalling and the possibility of different combinations of channels enables a high degree of functional diversity between cell types. The L-type Ca2+ channel blocker was found to be effective in attenuating cataract formation[13]. However, the precise mechanism by which Ca2+ plays its role still remains unclear. In our study, we detected the concentration of Ca2+ in AH samples from patients with DMC and patients with ARC, and found that the concentration of Ca2+ in the AH samples was higher in DMC than that in ARC, showing a potential relationship among Ca2+ and DMC. In addition, the mRNA expression of CACNA1C, which is partially translated into an L-type calcium channel protein that might lead to the different concentrations of Ca2+ in the two groups, was upregulated in DMC epithelial samples compared with ARC epithelial samples.
Exosomes have been widely studied in diabetes, neural diseases, tumours, cardiovascular diseases, etc[16]–[26]. Abundant exosomal miRNAs, including miR-486, miR-204, miR-184, etc., have been found in human AH[27]. Exosomal miRNAs play important roles in the mechanisms of diabetes[29]–[30]. In diabetes-associated ocular diseases (such as diabetic retinopathy), exosomal miRNAs play an important role by affecting the integrity of the vascular endothelium[32]. It has also been reported that pancreatic β cell derived exosomal miR-15a might damage the retina by targeting Akt3 and causing diabetic retinopathy[31]. In addition, plasma exosomes carrying IgG can damage retinal vessels[33], and retinal pigment epithelial (RPE) cell-derived exosomes can participate in immunoregulation by killing targeted monocytes[37]–[38], thus causing diabetic retinopathy. In addition, miRNAs play important roles in regulating the function of coding genes. Downregulation of miR-2113 inhibits high glucose-induced mesenchymal activation and fibrosis[39]. Interaction of miR-30a with circHIPK3 can regulate the expression of VEGFC, FZD4 and WNT2, thus changing the viability and apoptosis of retinal pigmented epithelial cells[40]. More importantly, our previous study showed that upregulation of miR-193a caused by downregulation of circHIPK3 can regulate cataract formation by targeting CRAYY[41]. However, there are few studies reporting the function and characterization of exosomes and miRNAs in DMC.
In our previous study, we used a bioinformatic approach to analyse the correlated functions of the differentially expressed miRNAs, and found that the coding genes of the upregulated miRNAs were mainly associated with proteoglycans in cancer and that the coding genes of the downregulated miRNAs were mainly associated with AGE-RAGE signalling pathway in diabetic complications. Among the upregulated exosomal miRNAs, miR-551b was highly expressed and could downregulated CRYAA expression, thus affecting the viability and apoptosis of HLECs[35].
In this study, we assessed the AH as a microenvironment of lens epithelial cells, and we focused on its influence on the crystalline lens and sequenced exosomal miRNAs in the DMC and ARC groups. Therefore, we isolated exosomes from the AH by ultracentrifugation and identified them through NTA and TEM. We then sequenced exosomal miRNAs; we revealed that miR-29b was downregulated in patients with DMC and compared these data with those from patients with ARC. MiR-29b was previously reported to be significantly changed across the diabetes spectrum and associated with measures of pancreatic islet β cell function and glycaemic control[42]. In cultured trancfected cells, the concentration of Ca2+ was 26.4% higher in the culture supernatant of cells transfected with miR-29b inhibitors than in the culture supernatant of cells transfected with miR-29b mimics and was almost equivalent to that in AH samples (25%). The results of our study revealed that the downregulation of miR-29b caused upregulation of CACNA1C expression and an increase in the concentration of Ca2+, resulting in decreased viability of HLECs. In addition, HLECs transfected with miR-29b inhibitors exhibited increased sensitivity to oxidative stress. However, the mechanisms of calcium homeostasis and signaling in the lens were very complicated. Evidence showed that functional expression of L-type voltage-gated Ca2+ channels in the intact lens might affect either oscillations of membrane potential or trans-lens short-circuit current in the intact rabbit lens[13]. Since endoplasmic reticulum was the store of intracellular Ca2+, the increased Ca2+ of extracellular fluid might be an interactive mechanism for increased Ca2+ within the cell. Here, we identified a novel mechanism by which the function of HLECs in DMC might be regulated through exosomal miR-29b/CACNA1C/Ca2+, in which the interaction of extracellular and intracellular Ca2+ need to be investigated in the future study (Figure 8).
Figure 8. Possible regulation of function HLECs through exosomal miR-29b/CACNA1C/Ca2+.
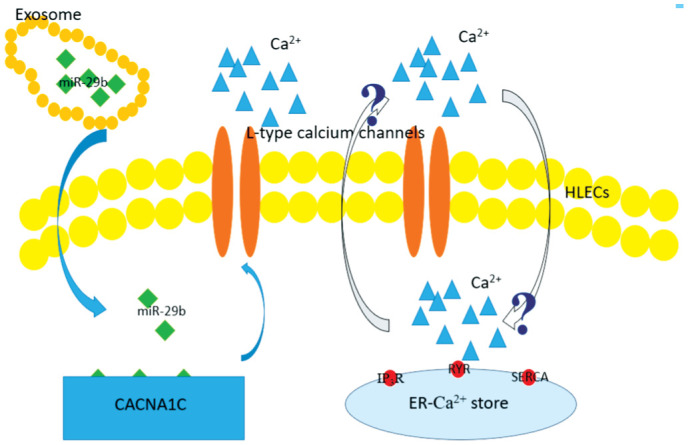
Down-regulation of exsomal miR-29b upregulated the expression of gene CACNA1C, increasing expression of L-type voltage-gated Ca2+ channels and Ca2+ of extracellular fluid. The increased Ca2+ of extracellular fluid might be an interactive mechanism for increased Ca2+ within the cell, of which the precise mechanism remained uncertain in the study.
In conclusion, exosomes isolated from human AH contained abundant miRNAs. A significantly expressed miRNA, miR-29b, could affect the concentration of Ca2+ and regulate HLEC processes by upregulating CACNA1C.
Acknowledgments
Foundation: Supported by the National Natural Science Foundation of China (No.81870645).
Conflicts of Interest: Gao C, None; Liu X, None; Fan F, None; Yang JN, None; Zhou XY, None; Mei HJ, None; Lin XL, None; Luo Y, None.
REFERENCES
- 1.Gong WF, Zhu GY, Li J, Yang X. LncRNA MALAT1 promotes the apoptosis and oxidative stress of human lens epithelial cells via p38MAPK pathway in diabetic cataract. Diabetes Res Clin Pract. 2018;144:314–321. doi: 10.1016/j.diabres.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Qi MS, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118(16):3569–3572. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- 3.Collins JG, Corder CN. Aldose reductase and sorbitol dehydrogenase distribution in substructures of normal and diabetic rat lens. Invest Ophthalmol Vis Sci. 1977;16(3):242–243. [PubMed] [Google Scholar]
- 4.Wu HL, Hwang DK, Song XD, Tao Y. Association between aqueous cytokines and diabetic retinopathy stage. J Ophthalmol. 2017;2017:1–8. doi: 10.1155/2017/9402198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi M, Sato T, Sakurai Y, Taguchi M, Harimoto K, Karasawa Y, Ito M. Association between aqueous humor and vitreous fluid levels of Th17 cell-related cytokines in patients with proliferative diabetic retinopathy. PLoS One. 2017;12(5):e0178230. doi: 10.1371/journal.pone.0178230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzmin A, Lipatov D, Chistyakov T, Smirnova O, Arbuzova M, Ilin A, Shestakova M, Dedov I. Vascular endothelial growth factor in anterior chamber liquid patients with diabetic retinopathy, cataract and neovascular glaucoma. Ophthalmol Ther. 2013;2(1):41–51. doi: 10.1007/s40123-013-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid S, Gul A, Hamid Q. Relationship of cytokines and AGE products in diabetic and non-diabetic patients with cataract. Int J Health Sci (Qassim) 2016;10(4):507–515. [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann S, Linek J, Loesenbeck G, Schüttler J, Gaedke S. TGF-β1 serum concentrations and receptor expressions in the lens capsular of dogs with diabetes mellitus. Open Vet J. 2017;7(1):12. doi: 10.4314/ovj.v7i1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Iongh RU, Gordon-Thomson C, Chamberlain CG, Hales AM, M C Avoy JW. TGFβ receptor expression in lens: implications for differentiation and cataractogenesis. Exp Eye Res. 2001;72(6):649–659. doi: 10.1006/exer.2001.1001. [DOI] [PubMed] [Google Scholar]
- 10.Lovicu FJ. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. 2002;86(2):220–226. doi: 10.1136/bjo.86.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22(3):381–397. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maddala R, Nagendran T, de Ridder GG, Schey KL, Rao PV. L-type calcium channels play a critical role in maintaining lens transparency by regulating phosphorylation of aquaporin-0 and myosin light chain and expression of connexins. PLoS One. 2013;8(5):e64676. doi: 10.1371/journal.pone.0064676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes JD, Sanderson J. The mechanisms of calcium homeostasis and signalling in the lens. Exp Eye Res. 2009;88(2):226–234. doi: 10.1016/j.exer.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Chang C, Zhang KD, Veluchamy A, Hébert HL, Looker HC, Colhoun HM, Palmer CNA, Meng WH. A genome-wide association study provides new evidence that CACNA1C gene is associated with diabetic cataract. Invest Ophthalmol Vis Sci. 2016;57(4):2246. doi: 10.1167/iovs.16-19332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuelson I, Vidal-Puig AJ. Fed-EXosome: extracellular vesicles and cell-cell communication in metabolic regulation. Essays Biochem. 2018;62(2):165–175. doi: 10.1042/EBC20170087. [DOI] [PubMed] [Google Scholar]
- 17.Liberman M, Marti LC. Vascular calcification regulation by exosomes in the vascular wall. Adv Exp Med Biol. 2017;998:151–160. doi: 10.1007/978-981-10-4397-0_10. [DOI] [PubMed] [Google Scholar]
- 18.Saeedi Borujeni MJ, Esfandiary E, Taheripak G, Codoñer-Franch P, Alonso-Iglesias E, Mirzaei H. Molecular aspects of diabetes mellitus: Resistin, microRNA, and exosome. J Cell Biochem. 2018;119(2):1257–1272. doi: 10.1002/jcb.26271. [DOI] [PubMed] [Google Scholar]
- 19.Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017;19:137–146. doi: 10.1111/dom.13027. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Fu LZ, Liu L, Xie F, Dai RC. Glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide alters bone marrow exosome-mediated miRNA signal pathways in ovariectomized rats with type 2 diabetes. Med Sci Monit. 2017;23:5410–5419. doi: 10.12659/MSM.906603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkat P, Chopp M, Chen J. Cell-based and exosome therapy in diabetic stroke. Stem Cells Transl Med. 2018;7(6):451–455. doi: 10.1002/sctm.18-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Ding X, Zhen F, Ma J, Meng F. Remedial applications of exosomes in cancer, infections and diabetes. Acta Pol Pharm. 2017;74(2):313–320. [PubMed] [Google Scholar]
- 23.Zhang C, Zhang K, Huang FF, Feng WJ, Chen J, Zhang HJ, Wang JF, Luo P, Huang H. Exosomes, the message transporters in vascular calcification. J Cell Mol Med. 2018;22(9):4024–4033. doi: 10.1111/jcmm.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal K, Saji M, Lazaroff SM, Palmer AF, Ringel MD, Paulaitis ME. Analysis of exosome release as a cellular response to MAPK pathway inhibition. Langmuir. 2015;31(19):5440–5448. doi: 10.1021/acs.langmuir.5b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson SM, Riquelme JA, Takov K, Vicencio JM, Boi-Doku C, Khoo V, Doreth C, Radenkovic D, Lavandero S, Yellon DM. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J Cell Mol Med. 2018;22(1):141–151. doi: 10.1111/jcmm.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MJ, Park DH, Kang JH. Exosomes as the source of biomarkers of metabolic diseases. Ann Pediatr Endocrinol Metab. 2016;21(3):119. doi: 10.6065/apem.2016.21.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dismuke WM, Challa P, Navarro I, Stamer WD, Liu YT. Human aqueous humor exosomes. Exp Eye Res. 2015;132:73–77. doi: 10.1016/j.exer.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Contreras M, Shah SH, Tamayo A, Robbins PD, Golberg RB, Mendez AJ, Ricordi C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration type 1 diabetes. Sci Rep. 2017;7(1):5998. doi: 10.1038/s41598-017-05787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XH, Huang W, Liu GS, Cai WF, Millard RW, Wang YG, Chang J, Peng TQ, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, Fan GC. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes. 2016;65(10):3111–3128. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamalden TA, Macgregor-Das AM, Kannan SM, et al. Exosomal microRNA-15a transfer from the pancreas augments diabetic complications by inducing oxidative stress. Antioxid Redox Signal. 2017;27(13):913–930. doi: 10.1089/ars.2016.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klingeborn M, Dismuke WM, Bowes Rickman C, Stamer WD. Roles of exosomes in the normal and diseased eye. Prog Retin Eye Res. 2017;59:158–177. doi: 10.1016/j.preteyeres.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes. 2018;67(8):1639–1649. doi: 10.2337/db17-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan B, Zhao LH, Guo JT, Zhao JL. miR-203b: a novel regulator of MyoD expression in tilapia skeletal muscle. J Exp Biol. 2013;216(Pt 3):447–451. doi: 10.1242/jeb.076315. [DOI] [PubMed] [Google Scholar]
- 35.Gao C, Fan F, Liu X, Yang JN, Zhou XY, Mei HJ, Lin XL, Luo Y. Exosomal miRNA analysis of aqueous humour of diabetes and cataract patients. Curr Eye Res. 2021;46(3):324–332. doi: 10.1080/02713683.2020.1797107. [DOI] [PubMed] [Google Scholar]
- 36.Saba S, Ghahramani M, Yousefi R. A comparative study of the impact of calcium ion on structure, aggregation and chaperone function of human αA-crystallin and its cataract- causing R12C mutant. Protein Pept Lett. 2018;24(11):1048–1058. doi: 10.2174/0929866524666170807125658. [DOI] [PubMed] [Google Scholar]
- 37.Serra AM, Waddell J, Manivannan A, Xu HP, Cotter M, Forrester JV. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. Am J Pathol. 2012;181(2):719–727. doi: 10.1016/j.ajpath.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Knickelbein JE, Liu BY, Arakelyan A, Zicari S, Hannes S, Chen P, Li ZY, Grivel JC, Chaigne-Delalande B, Sen HN, Margolis L, Nussenblatt RB. Modulation of immune responses by extracellular vesicles from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2016;57(10):4101. doi: 10.1167/iovs.15-18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue LP, Fu XL, Hu M, Zhang LW, Li YD, Peng YL, Ding P. Rg1 inhibits high glucose-induced mesenchymal activation and fibrosis via regulating miR-2113/RP11-982M15.8/Zeb1 pathway. Biochem Biophys Res Commun. 2018;501(4):827–832. doi: 10.1016/j.bbrc.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 40.Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, Zhang YY, Liu B, Zhang SJ, Wang JJ, Zhang SH, Wu JH, Zhao C, Yan B. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136(17):1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Liu BH, Zhou ML, Fan F, Yu MR, Gao C, Lu Y, Luo Y. Circular RNA HIPK3 regulates human lens epithelial cells proliferation and apoptosis by targeting the miR-193a/CRYAA axis. Biochem Biophys Res Commun. 2018;503(4):2277–2285. doi: 10.1016/j.bbrc.2018.06.149. [DOI] [PubMed] [Google Scholar]
- 42.Nunez Lopez YO, Garufi G, Seyhan AA, Seyhan AA. Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol Biosyst. 2016;13(1):106–121. doi: 10.1039/c6mb00596a. [DOI] [PubMed] [Google Scholar]