Abstract
AIM
To investigate the safety and efficacy of intravitreal dexamethasone implants (Ozurdex®/DEX) in patients with diabetic macular edema (DME) either naïve or non-naïve to anti-VEGF therapies who switched to DEX implant independent of response to anti-vascular endothelial growth factors (anti-VEGFs).
METHODS
This was an audit retrospective review of medical records of patients with DME who switched to the DEX intravitreal implant. Patients were divided into 2 groups: patients naïve to antiangiogenic therapy and patients who were previously treated with anti-VEGFs. Data regarding demographics, changes in mean best-corrected visual acuity (BCVA), central macular thickness (CMT), and intraocular pressure (IOP) was collected over 6mo. The demographic data mean changes in BCVA, CMT, and IOP were compared. Six-month follow-up data of 47 patients (57 eyes), who either switched to DEX implant irrespective of response to previous treatments or were treatment naïve before receiving DEX implant, was collected.
RESULTS
Improvement in mean BCVA was observed from 1-4mo after injection with a decreased effect at month 6 as expected, with better outcomes in naïve compared to non-naïve patients. A statistically relevant decrease in mean CMT was observed during the follow-up period. An increase in mean IOP was observed in the first 2mo after DEX therapy. The mean number of injections of the overall population during the 6mo was 1.3. A subgroup analysis showed no relevant difference between phakic versus pseudophakic patients relative to measured outcomes. There was no cataract progression during the follow-up period and no adverse events reported.
CONCLUSION
This real-life setting study shows that intravitreal DEX implant is effective and safe. The timings of greater therapeutic impact are concordant with previous studies and suggest that earlier treatment with corticosteroids may have an additional benefit in naïve patients.
Keywords: anti-VEGF therapy, diabetic macular edema, dexamethasone intravitreal implant, naïve eyes, steroids
INTRODUCTION
Diabetic retinopathy is the most common microvascular complication of diabetes leading to vision loss[1]. Diabetic macular edema (DME) is the macular thickening secondary to diabetic retinopathy present in any stage of the disease[1]. It is a critical retinal pathology affecting central visual acuity and ultimately a patient's quality of life. Different treatments are available for patients with DME[2]. Besides laser photocoagulation, intravitreal therapies with anti-vascular endothelial growth factor (anti-VEGF) agents and corticosteroids, are effective[2]–[3]. Although anti-VEGFs are efficient approved treatments for DME, not all patients respond sufficiently to these agents. Moreover, compliance to anti-VEGF treatments is normally low due to the high number of injections administered over time[4]. A review of observational real-life studies conducted in patients with DME found that visual acuity gains with anti-VEGF treatments was lower than observed in the clinical trials[5]. They attributed this difference mainly to the number of injections required for the treatment and poor compliance associated with it.
Intravitreal corticosteroids act by inhibiting inflammatory mediators like interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), and VEGF. Corticosteroids modulate the activity of Müller cells, preventing the accumulation of excess fluid in the retina and thus influencing neovascularization[6]. Dexamethasone (DEX) implant (0.7 mg; Ozurdex, Allergan, Inc., Irvine, CA, USA) is a sustained-release steroid device made specifically for intravitreal injection. The DEX implant is approved by the Food and Drug Administration (FDA) and European Medicine Agency (EMA) for the treatment of visual impairment due to DME and retinal vein occlusion, and for the treatment of non-infectious uveitis affecting the posterior segment of the eye[7].
Several studies have demonstrated that in DME patients, DEX implant improves the central macular thickness (CMT) and best-corrected visual acuity (BCVA)[8]–[12]. A clinical trial comparing DEX implant and ranibizumab demonstrated DEX implant non-inferior to ranibizumab, while the mean number of injections per patient was lower (2.85 vs 8.70) in the DEX arm)[10].
The objective of this 6-month, retrospective, single-center audit study was to assess the BCVA, CMT, and anatomical effects of DEX implant in patients who were naïve or non-naïve to anti-VEGF therapies and have been switched to DEX independent of response to anti-VEGFs. Safety was assessed through the collection of adverse events with special attention on cataract formation and increase of intraocular pressure (IOP).
SUBJECTS AND METHODS
Ethical Approval
The study was approved by the Comissão de Ética para a Saúde (CES)/Ethics Committee for Health of Setúbal Hospital Center on September 2019. All patients provided written informed consent.
This was a retrospective, real-life setting, single-center audit study reviewing medical records of patients with DME who received DEX implant between October 2018 and March 2019, irrespective of their previous response to anti-VEGF treatment. At a certain time, usage of anti-VEGF therapy was restrained in our hospital due to administrative reasons resulting that all DME patients were switched to DEX implant independently of any other condition. Because visual acuity and retinal thickness improvements were greater in the naïve patients than those observed with patients that performed previous treatments, it was decided to analyze the available sample and report the results.
The current standard of care at our hospital is anti-VEGF as first-line therapy for the treatment of DME with center involvement in a treat and extend regimen. If no response to anti-angiogenics after 3 to 6 intravitreal injections of anti-VEGF, patients switch to corticosteroid therapy, with DEX as first corticosteroid option as recommended by the Euretina guidelines. Re-treatment with DEX implant was administered in a pro-re-nata (PRN) regimen at the discretion of the attending ophthalmologist, with minimum monitoring visits every two months. After the first DEX intravitreal injection, patients were divided for statistical analysis into 2 groups: a group of naïve patients to antiangiogenic therapy and a group of non-naïve patients, previously treated with anti-VEGFs (either ranibizumab or aflibercept), without switch between anti-VEGFs. All the patients received one or more DEX implants and were followed for at least 6mo.
Participants performed full ocular examinations, including medical history, measurement of BCVA, slit-lamp biomicroscopy, tonometry (Goldmann), and optical coherence tomography (SD-OCT; Heidelberg Spectralis) at baseline (before starting on DEX implant), 2wk after the first DEX injection (safety visit) and then every month or every 2mo afterward at investigator discretion.
Ozurdex® is a single-use intravitreal biodegradable implant delivered through a disposable injection device containing 700 micrograms of DEX. The intravitreal injection procedure was always performed in an operating theatre or otherwise clean room. Before each injection, the periocular skin, eyelid, and ocular surface were disinfected with povidone-iodine solution and topical anesthesia was administered. The injection was applied through the sclera, via the pars plana, 3.5-4 mm from the limbus, followed by delivery of the implant in the vitreous cavity.
Fifty-seven eyes from 47 patients meet the inclusion criteria to integrate in this analysis: male or female patients of ≥18 years of age with a decreased visual acuity resulting from central DME involvement diagnosed and documented properly in the patient notes, with a minimum follow-up of 6mo. Moreover, informed consent must be obtained from the subject or subject's legal representative allowing the collection of data for scientific purposes.
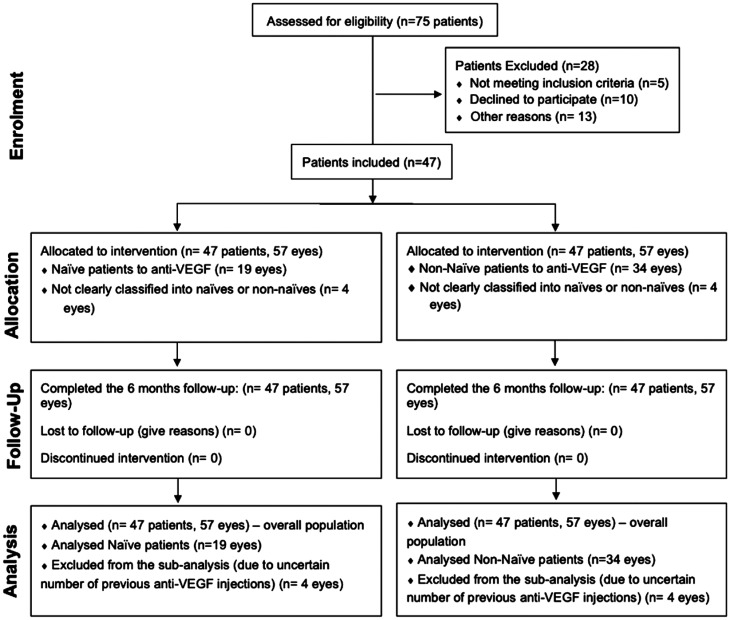
Patients were excluded from treatment if at baseline the patient had any active periocular or ocular infection or inflammation in either eye; uncontrolled glaucoma in the investigator's opinion (IOP≥30 mm Hg) and any condition or abnormality that the investigator finds might compromise the safety of the patient. Pregnant or nursing (lactating) women were also excluded. After performing a baseline visit, patients were either treated for the first time with Ozurdex or switched to DEX implant irrespective of their response to previous treatment (Figure 1).
Figure 1. Consort flow diagram.
Data was collected using a spreadsheet. Data was made anonymous by removing all the patient identifiable data. The data collected included baseline characteristics such as demographics, number of injections, phakic/pseudophakic lenses, duration of DME, and previous anti-VEGF treatment agents. Moreover, mean changes in BCVA, IOP, and CMT data were collected at baseline, 2wk, 1-4mo, and 6mo. The mean changes in BCVA, CMT, and IOP in the overall study population were analyzed. All previous treatments administered to each patient were recorded. A subgroup analysis stratified between naïve and non-naïve patients to compare mean changes in BCVA and CMT was performed to observe the effect of previous anti-VEGFs treatments. Also, a subgroup analysis of the change in CMT and BCVA in patients with and without neurosensorial detachment (NSD) was performed. Additionally, the mean changes in BCVA, CMT, and IOP from baseline to the first injection and the second injection were evaluated.
Statistical Analysis
The statistical analysis involved measures of descriptive statistics (absolute and relative frequencies, means and respective standard deviations) and inferential statistics. Per-protocol method was used to analyze the data. The level of significance for rejecting the null hypothesis was set at ≤0.05. The Chi-square, Fisher's test, independent samples t-test, paired sample t-test and ANCOVA were used. The normality of distribution was analyzed with the Shapiro-Wilk test and the homogeneity of variances with the Levene test. When the Student t assumptions were not satisfied, nonparametric tests were used as an alternative, namely Mann-Whitney independent samples and related samples Wilcoxon. The effect size was also calculated. Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) version 21.0 for Windows.
RESULTS
As per the inclusion criteria of the study, a 6-month follow-up data of 47 patients (57 eyes) with DME treated with DEX implant was collected. Baseline demographic characteristics for the overall population and naïve vs non-naïve patients are presented in Table 1. There are no statistically significant differences in all variables except in the duration of the edema, in which naïve patients have a significantly shorter duration of edema (6.2mo), t(50)=-4.446, P=0.001, n=0.283. This was found to be correlated significantly only with CMT at month 4 after the injection.
Table 1. Baseline clinical and demographic characterization in the overall population and naïve vs non-naïve patients.
| Characteristics | Overall population, n=57 eyes | Naïvea, n=34 eyes | Non-naïvea, n=19 eyes | P |
| Age (mean±SD) | 67.6±8.7 | 67.1±8.2 | 68.6±9.5 | 0.568 |
| Gender | 0.095 | |||
| Female | 22 (46.8) | 13 (38.2) | 12 (63.2) | |
| Male | 25 (53.2) | 21 (61.8) | 7 (36.8) | |
| Lens | 0.570 | |||
| Phakic | 29 (61.7) | 21 (61.8) | 10 (52.6) | |
| Pseudophakic | 18 (38.3) | 13 (38.2) | 9 (47.4) | |
| Cataract | 0.673 | |||
| Cataract with no impact on patients' vision | 2 (3.5) | 1 (2.9) | 1 (5.3) | |
| Cataract clinically relevant with impact on patient vision | 6 (10.5) | 3 (8.8) | 3 (15.8) | |
| Diabetes characterization | ||||
| Duration of diabetes mellitus (y) | 16.9 (9.0) | |||
| Diabetic patient treated with insulin | ||||
| No | 36 (76.6) | |||
| Yes | 11 (23.4) | |||
| Duration of DME (mo) | 10.6 (12.6) | 6.2 (10.2) | 20.2 (12.1) | 0.001b |
| Previous anti-VEGF injections | ||||
| Naïve patients | 34 (64.2) | 34 (100) | ||
| Non-naïve patients | 19 (35.8) | 19 (100) | ||
| BCVA (ETDRS letters) at baseline (mean±SD) | 50.56±16.50 | 51.8±15.8 | 49.8±18.6 | 0.684 |
| IOP (mm Hg) at baseline (mean±SD) | 17.28±3.60 | 17.2±2.6 | 17.2±5.2 | 0.456 |
| CMT at baseline (mean±SD) | 501.80±141.82 | 507.6±117.8 | 488.3±175.3 | 0.250 |
| Presence of neurosensorial detachment | 0.334 | |||
| No | 44 (77.2) | 24 (70.6) | 16 (84.2) | |
| Yes | 13 (22.8) | 10 (29.4) | 3 (15.8) |
DME: Diabetic macular edema; BCVA: Best corrected visual acuity; CMT: Central macular thickness; ETDRS: Early Treatment Diabetic Retinopathy Study; IOP: Intraocular pressure; SD: Standard deviation; VEGF: Vascular endothelial growth factor. aFour patients were excluded from the subanalysis due to uncertain number of previous anti-VEGF injections. bStatistically significant result.
The mean number of DEX implants for the overall population was 1.3. Additionally, also in the overall population, the mean interval between the first and second DEX implant injection was 5.52±2.9mo and 4.0±2.5mo between the second and the third DEX.
Full Analysis of the Overall Population
At month 2 (mean change=8.30 letters, Z=-3.368, P=0.001, r=0.354), month 3 (mean change=8.64 letters, Z=-2.048, P=0.041, r=0.323) and month 4 (mean change=5.53 letters, Z=-2.579, P=0.010, r=0.190), it was observed a statistically improvement of the mean BCVA. The mean BCVA decreased at month 6 (mean change=3.43 letters, Z=-1.449, P=0.147, r=0.077) as expected, due to decrease DEX levels, however, not rebounding below baseline values (Table 2).
Table 2. Mean change and difference in BCVA from baseline in the overall population.
| BCVA (ETDRS letters) | Mean±SD | Difference from baseline (ETDRS letters) | P | Effect size (r) |
| Baseline | 50.56±16.502 | -- | -- | -- |
| 2wk | 56.69±16.070 | 4.4615 | 0.413 | 0.014 |
| Month 1 | 52.75±17.748 | 5.6250 | 0.065 | 0.226 |
| Month 2 | 56.12±15.799 | 8.3030 | 0.001 | 0.354 |
| Month 3 | 57.79±14.864 | 8.6429 | 0.041 | 0.323 |
| Month 4 | 56.86±18.078 | 5.5278 | 0.010 | 0.190 |
| Month 6 | 58.86±5.546 | 3.4286 | 0.147 | 0.077 |
BCVA: Best corrected visual acuity; ETDRS: Early Treatment Diabetic Retinopathy Study; SD: Standard deviation.
Concerning CMT, a statistically significant decrease was observed at week 2, months 1, 2, 3, 4, and 6 (all with P<0.001).
Also, a statistically significant increase in IOP was observed at month 1 (mean change=4.80 mm Hg, P=0.009) and at month 2 (mean change=3.63 mm Hg, P=0.004) post-DEX implant, which decreased gradually towards month 6. All cases were successfully managed with topical treatment.
Sub-analysis Comparing Naïve and Non-Naïve Patients to Previous Anti-VEGF Therapy
The comparison of baseline demographics and clinical characteristics was similar between the two groups (naïve and non-naïve), with exception of the duration of the edema that was longer for the non-naïve patients (20.26±12.18 vs 6.20±10.25mo, respectively for non-naïve vs naïve patients; P<0.001).
The analysis of the naïve patients showed a statistically significant mean BCVA improvement in the naïve group at month 1 (Z=-2.207, P=0.027, r=0.487), at month 2 (Z=-2.906, P=0.004, r=0.541), at month 3 (Z=-2.201, P=0.028, r=0.220) and at month 4 (Z=-2.334, P=0.020, r=0.778), and a statistically significant mean BCVA improvement in the non-naïve patients' group at month 2 (Z=-2.041, P=0.0041, r=1.388; Table 3). Moreover, the naïve group showed values above the cut-off of 5 letters at all the evaluation points, with exception of month 6, suggesting a better response to treatment of this group in comparison with the non-naïve group.
Table 3. Comparison of the mean BCVA between each group and baseline values.
| BCVA (ETDRS letters) | Baseline evaluation | Following evaluation visit | P | Effect size, r |
| Naïve patients | ||||
| Baseline-2wk (n=11) | 53.18±18.519 | 59.27±15.84 | 0.313 | 0.053 |
| Baseline-month 1 (n =10) | 48.70±20.581 | 56.60±19.69 | 0.027 | 0.027 |
| Baseline-month 2 (n=23) | 50.52±17.508 | 60.91±11.53 | 0.004 | 0.541 |
| Baseline-month 3 (n=8) | 49.25±19.884 | 63.25±11.65 | 0.028 | 0.220 |
| Baseline-month 4 (n=20) | 60.60±10.065 | 54.80±12.66 | 0.020 | 0.778 |
| Baseline-month 6 (n=18) | 58.94±13.366 | 62.78±15.10 | 0.161 | 0.115 |
| Non-naïve patients | ||||
| Baseline-2wk (n=4) | 47.00±16.971 | 42.50±10.61 | 0.317 | 0.5 |
| Baseline-month 1 (n=3) | 51.00±13.856 | 49.67±18.18 | 0.655 | 0.015 |
| Baseline-month 2 (n=8) | 39.13±18.209 | 43.50±21.06 | 0.041 | 1.388 |
| Baseline-month 3 (n=4) | 47.75±19.956 | 52.00±15.56 | 0.285 | 0.142 |
| Baseline-month 4 (n=14) | 57.00±18.385 | 63.50±19.09 | 0.140 | 0.544 |
| Baseline-month 6 (n=9) | 49.67±16.462 | 52.67±15.03 | 0.498 | 0.05 |
BCVA: Best corrected visual acuity; SD: Standard deviation.
mean±SD
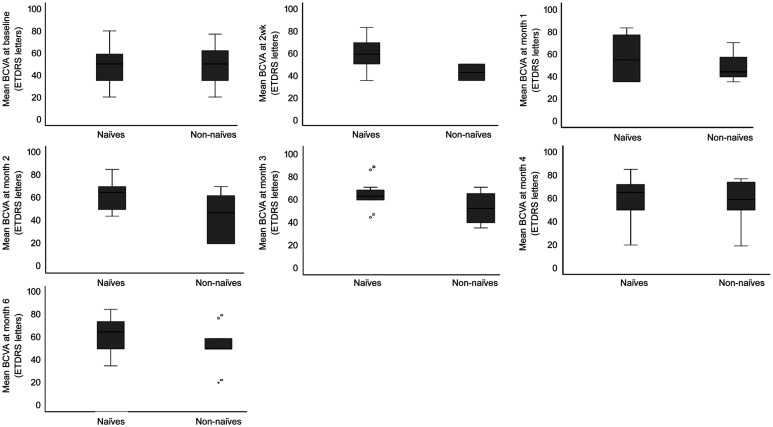
The direct comparison between the groups, naïve and non-naïve, found a significant difference at month 2, with greater BCVA in naïve patients (60.91±11.53 letters) vs non-naïve patients (43.50±21.06 letters), t (29)=2.942, P=0.006; η2=0.230 (Figure 2, Table 4).
Figure 2. Mean BCVA comparison from baseline to month 6 in naïve vs non-naïve patients (ETDRS letters).
Table 4. Direct comparison of the mean BCVA between naïve and non-naïve patients.
| BCVA (ETDRS letters) | Naïve patients | Non-naïve patients | P | Effect size, η2 |
| Baseline | 51.82±15.89 | 49.84±18.64 | 0.684 | 0.003 |
| 2wk | 59.27±15.84 | 42.50±10.61 | --a | --- |
| Month 1 | 56.60±19.69 | 49.67±18.18 | 0.598 | 0.026 |
| Month 2 | 60.91±11.53 | 43.50±21.06 | 0.006b | 0.230 |
| Month 3 | 63.25±11.65 | 52.00±15.56 | 0.186 | 0.168 |
| Month 4 | 60.15±16.24 | 55.71±18.96 | 0.470 | 0.016 |
| Month 6 | 62.78±15.10 | 52.67±15.03 | 0.113 | 0.097 |
BCVA: Best corrected visual acuity, SD: Standard deviation. aNo comparison was performed for these 2 groups due to low number of patients. bt(29)=2.942, P=0.006, η2=0.230.
mean±SD
Nevertheless, when analyzing the magnitude of the mean BCVA differences between the two groups, at each time point during the follow-up period, we did not find statistical differences between naïve and non-naïve patients (Table 5).
Table 5. Direct comparison between naïve and non-naïve patients (mean BCVA difference at all follow-up visits) mean±SD.
| Mean BCVA difference (ETDRS letters) | Naïve patients | Non-naïve patients | P | Effect size, η2 |
| 2wk | 6.09±17.68 | -4.50±6.36 | --a | -- |
| Month 1 | 7.90±9.67 | -1.33±13.05 | --a | -- |
| Month 2 | 10.39±15.42 | 4.38±3.89 | 0.567 | 0.010 |
| Month 3 | 14.00±16.54 | 4.25±9.71 | 0.391 | 0.066 |
| Month 4 | 8.20±14.92 | 2.93±7.00 | 0.138 | 0.001 |
| Month 6 | 3.83±11.79 | 3.00±10.71 | 0.860 | 0.001 |
BCVA: Best corrected visual acuity; SD: Standard deviation. aNo comparison was performed for these 2 groups due to low number of patients.
There was a statistically meaningful difference observed in the CMT change from baseline to all evaluation visits in naïve and non-naïve patients, in the following described visits (Table 6). In the naïve patients treatment group we found CMT significantly lower at 2wk (Z=-2.666, P=0.008, r=0.789), at month 1 (Z=-3.059, P=0.002, r=0.779), at month 2 (Z=-4.372, P=0.001, r=0.764), at month 3 (Z=-2.401, P=0.016, r=0.524), at month 4 (Z=-3.198, P=0.001, r=0.464), and at month 6 (Z=-2.391, P=0.017, r=0.571). The non-naïve patient group showed a significantly lower CMT at month 2 (Z=-2.666, P=0.008, r=0.454), at month 3 (Z=-2.023, P=0.043, r=0.973) and at month 4 (Z=-2.417, P=0.016, r=0.183).
Table 6. Comparison of the mean CMT between each group and baseline values.
| CMT (µm) | Baseline evaluation | Following evaluation visit | P | Effect size, r |
| Naïve patients | ||||
| Baseline-2wk (n=10) | 478.00±134.27 | 411.60±101.31 | 0.008 | 0.789 |
| Baseline-Month 1 (n=12) | 540.83±145.604 | 419.08±130.44 | 0.002 | 0.779 |
| Baseline-Month 2 (n=25) | 522.24±117.355 | 348.88±70.30 | 0.000 | 0.764 |
| Baseline-Month 3 (n=11) | 523.18±110.793 | 390.82±132.67 | 0.016 | 0.524 |
| Baseline-Month 4 (n=22) | 529.50±124.683 | 429.86±128.17 | 0.001 | 0.464 |
| Baseline-Month 6 (n=18) | 464.44±75.010 | 408.06±109.17 | 0.017 | 0.571 |
| Non-naïve patients | ||||
| Baseline-2wk (n=4) | 803.00±308.299 | 681.00±325.27 | --a | -- |
| Baseline-Month 1 (n=3) | 693.33±289.144 | 482.00±345.88 | --a | -- |
| Baseline-Month 2 (n=9) | 500.67±145.128 | 346.00±142.20 | 0.008 | 0.454 |
| Baseline-Month 3 (n=6) | 481.83±268.024 | 408.17±269.18 | 0.043 | 0.973 |
| Baseline-Month 4 (n=14) | 453.71±126.508 | 381.43±142.97 | 0.016 | 0.183 |
| Baseline-Month 6 (n=9) | 425.22±87.102 | 378.33±111.29 | 0.139 | 0.243 |
CMT: Central macular thickness; SD: Standard deviation. aNo comparison was performed for these 2 groups due to low number of patients.
mean±SD
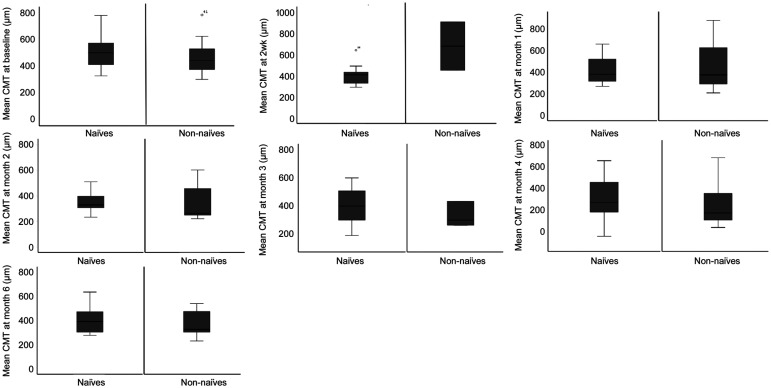
The direct comparison of the mean CMT values between naïve and non-naïve patients, showed no statistically significant differences (Figure 3, Table 7).
Figure 3. Mean CMT comparison from baseline to month 6 in naïve vs non-naïve patients.
Table 7. Direct comparison of the mean CMT between naïve and non-naïve patients.
| CMT (µm) | Naïve patients | Non-naïve patients | P | Effect size, η2 |
| Baseline | 507.6±117.8 | 488.3±175.3 | 0.250 | 0.025 |
| 2wk | 411.6±101.3 | 681.0±325.3 | --a | |
| Month 1 | 419.1±130.4 | 482.0±345.9 | --a | |
| Month 2 | 348.9±70.3 | 346.0±142.2 | 0.258 | 0.037 |
| Month 3 | 390.8±132.7 | 408.2±269.2 | 0.482 | 0.029 |
| Month 4 | 429.9±128.2 | 381.4±143.0 | 0.975b | 0.001 |
| Month 6 | 408.1±109.2 | 378.3±111.3 | 0.643 | 0.007 |
CMT: Central macular thickness; SD: Standard deviation. aNo comparison was performed for these 2 groups due to low number of patients. bAfter adjusting for significantly different baseline characteristics (duration of DME), it is suggested that the duration of edema does not influence the differences between naïve and non-naïve in respect to CMT.
mean±SD
Likewise, when analyzing the magnitude of the mean CMT differences between the two groups, at each visit during the follow-up period, we did not find statistical differences between naïve and non-naïve patients (Table 8).
Table 8. Direct comparison between naïve and non-naïve patients (mean CMT difference at all follow-up visits).
| Mean CMT difference (µm) | Naïve patients | Non-naïve patients | P | Effect size, η2 |
| 2wk | -66.40±81.23 | -122.00±16.97 | --a | |
| Month 1 | -121.75±112.83 | -211.33±58.59 | --a | |
| Month 2 | -173.36±93.84 | -154.67±65.43 | 0.585 | 0.008 |
| Month 3 | -132.36±131.92 | -73.67±55.67 | 0.191 | 0.100 |
| Month 4 | -99.64±118.27 | -72.29±86.97 | 0.721 | 0.003 |
| Month 6 | -56.39±100.95 | -46.89±93.87 | 0.797 | 0.002 |
CMT: Central macular thickness; SD: Standard deviation. aNo comparison was performed for these 2 groups due to low number of patients.
mean±SD
After adjusting for significantly different baseline characteristics, the comparison between the two groups suggested that the baseline parameter significantly different, the duration of DME, does not influence the differences between naïve and non-naïve in respect to CMT at month 4.
Sub-analysis of Patients With and Without NSD (From Baseline to Each Evaluation Visit)
The direct comparison between the groups with or without NSD showed a mean difference in the BCVA favorable to patients with NSD at month 1 (10.50±13.53 letters vs 4.00±8.81 letters, P=0.665), at month 2 (9.44±11.97 letters vs 7.88±14.05 letters, P=0.984), and month 4 (6.75±10.77 letters vs 5.18±13.21 letters, P=0.368), results shown for patients with NSD and without NSD, respectively.
The direct comparison between CMT and NSD, demonstrated a statistical significant mean CMT difference with better outcomes for the group with NSD at 2 weeks (-139.33±127.01 µm vs -54.44±45.21µm, P=0.024) and a marginally statistical significance for the group with NSD at month 3 (-103.56±115.87 µm vs -102.00±79.96 µm, P=0.062) and month 4 (-129.60±122.97 µm vs -71.43±94.58 µm, P=0.074), results shown for patients with NSD and without NSD, respectively.
Evaluation After the First and Second DEX Injection
The difference in the mean BCVA, CMT, and IOP change was analyzed after the first and second DEX implant. A significant mean BCVA change was observed from baseline to month 2 (61.31±9.71 vs 49.86±12.85, P=0.009), and to month 4 (59.25±11.11 letters vs 49.86±12.85, P=0.024) after the first DEX injection. After the second DEX injection, BCVA continued to improve, but this finding was not statistically significant. The difference in mean change in CMT was clinically significant at week 2 (-112.40 µm, P=0.068), month 1 (-200.57 µm, P=0.018), month 2 (-182.94 µm, P<0.001) and month 4 (-85.43 µm, P=0.064) after first DEX implant and at week 2 (-205.20 µm, P=0.043), month 2 (-163.56 µm, P=0.008) and month 3 (-220.25 µm, P=0.068) after second DEX implant.
The mean IOP increased from baseline visit to month 1 (26.2±8.38 mm Hg, P=0.080) and to month 2 (22.57±7.8 mm Hg, P=0.059) after the first DEX implant; decreasing afterward in the following 3, 4 and 6mo (numerical decrease, not statistically significant). After the second injection, it was observed a statistically significant increase of the IOP at month 2 (20.6 ±4.67 mm Hg, P=0.055).
Analysis of Vision Gains by ≥15, ≥10, and ≥5 Letters in the Overall Population and in Naïve vs Non-naïve Patients
At month 2, in the overall population, 15.79%, 17.54%, and 31.38% of the patients experienced BCVA gains of ≥15, ≥10, and ≥5 letters, respectively. Also, at month 2, patients with BCVA gains of ≥10 and ≥15 letters were significantly higher in the naïve patient's group compared with the non-naïve patient's group (43.5% vs 0, P=0.032 and 39.1% vs 0, P=0.068, respectively). Nevertheless, at month 2, the visual gains were not statistically significant in the group with gains ≥5 letters (P=1.000; Fisher test).
At last observation visit, naïve patients had significantly higher BCVA gains of ≥15, ≥10 and ≥5 letters compared to non-naïve patients (38.2% vs 10.5%, P=0.055; 41.2% vs 10.5%, P=0.029 and 67.6% vs 21.1%, P=0.002, respectively).
Cataract Progression and Safety
No cataract progression was reported during the period of study; however, six months follow-up may not be sufficient time to conclude the lens side effects. There were no serious adverse events reported.
DISCUSSION
The overall study result shows that DEX implants are safe and effective in the treatment of DME. Statistically, significant improvements were reported in BCVA with significant reductions of CMT. Both naïve and non-naïve patients showed improvement in BCVA. Significantly higher percentage of naïve patients had BCVA gains of ≥15, ≥10 and ≥5 letters compared to non-naïve patients (38.2% vs 10.5%, 41.2% vs 10.5%, and 67.6% vs 21.1%, respectively). These findings are consistent with the results of previously published studies that indicated comparatively greater improvement in BCVA in naïve patients than the non-naïve patients[5],[13]. This defends the hypothesis that DEX treatment may be more beneficial for the treatment-naïve eyes. Following the pattern of other studies, it was demonstrated at month 2, BCVA gains of more than 15, 10, and 5 letters in 15.79%, 17.54%, and 31.38% of patients, respectively[14]–[16].
The guidelines for the management of DME by the European Society of Retina Specialists (EURETINA), The International Algorithm, and the Consensus from Spain, Italy, and Germany recommend corticosteroids as a second-line treatment for DME patients, in non-responders to anti-VEGFs after 3-6 intravitreal injections[17]–[21]. Some guidelines also suggest the use of corticosteroids as first-line therapy in patients without the capability to attend to regular visits, with inflammatory biomarkers, pseudophakic, with cardiovascular risk, vitrectomized eyes, and patients with planned cataract surgery[17]–[21]. Nevertheless, our results suggest that naïve patients may benefit from the DEX implant, and therefore DEX first-line therapy is perhaps justifiable in certain selected patients.
This study demonstrated that the peak effectiveness of the DEX implant was at 2-3mo after the first injection. The effectiveness then gradually decreased until month 6, similar to other studies[22]. This is due to decreasing vitreous concentrations of DEX over time[22]. This confirms the fact that the DEX implant shows the best efficacy at an optimal concentration which reduces with the decrease in concentration, leading to an important consideration of shortening the reinjection interval of DEX implant from 6mo as recommended by the Summary of Product Characteristics to 4mo. Our findings, like other studies, also support earlier reinjection of the DEX implant[5]. The CHROME study supported the reinjection earlier than the recommended interval, based on their retrospective, real-world study evidence in patients with DME, retinal vein occlusion, and uveitis. They reported a mean reinjection interval of 2.3-4.9mo[23]. The reinjection interval can be adjusted as per the patients' needs based on the improvements observed, to get optimal therapeutic benefits. Additionally, in our study, it was demonstrated that the IOP increase was not cumulative from the first to the second injection (P=0.055). Comparable to the findings of Malclès et al[24] and Zarranz-Ventura et al[25], in this audit study, the mean increase of IOP was transient and manageable with topical treatment.
Concerning cataract formation, several studies reported this effect as a class effect. In the MEAD trial evaluating DEX implant, the rate of cataract surgery was reported to be 59% over 3y, and cataract-related adverse events were 67.9%[12]. Likewise, the RELDEX study reported 47% of cataract surgeries over three years period[20]. As previously referred, current guidelines recommend corticosteroid treatment for pseudophakic patients and phakic patients with scheduled cataract surgery[26]. The use of intravitreal corticosteroids in phakic patients should be discussed with the patient to determine the overall risk-benefit[26]. In the current study, 29 eyes (61.7%) were phakic at baseline and none of them underwent cataract surgery. Of course, we cannot neglect the difference in the follow-up period of our study compared to the three years follow-up of MEAD and RELDEX clinical trials. Our study showed, however, that the cataract effect may not occur within the first 1-3 treatments. The findings in BCVA and CMT in patients with and without NSD were numerically and marginally statistically significant, favoring better gains in patients with NSD.
This audit study has limitations, such as the fact of being a real-life and retrospective study. Another limitation is that the patients were selected from a single center, limiting the number and not accounting for differences in the protocol/practices in different clinical settings and practicing doctors.
In conclusion, in our retrospective study, naïve patients had better visual improvement, and almost no CME changes as compared with patients with previous anti-VEGF therapy. Considering the limitations of our research, we recommend a more extensive real-life study further to evaluate the DEX implant in the treatment-naïve population.
Acknowledgments
Medical writing assistance was provided by Manuscriptedit.
Foundation: Allergan plc provided financial support for the work conducted to prepare this manuscript but was not involved directly in preparation of the manuscript or the decision to publish.
Conflicts of Interest: Neves P, None; Ornelas M, None; Matias I, None; Rodrigues J, None; Santos M, None; Dutra-Medeiros M, None; Martins D, None.
REFERENCES
- 1.Cunha-Vaz J, Coscas G. Diagnosis of macular edema. Ophthalmologica. 2010;224(Suppl 1):2–7. doi: 10.1159/000315156. [DOI] [PubMed] [Google Scholar]
- 2.Ford JA, Lois N, Royle P, Clar C, Shyangdan D, Waugh N. Current treatments in diabetic macular oedema: systematic review and meta-analysis. BMJ Open. 2013;3(3):e002269. doi: 10.1136/bmjopen-2012-002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Wang W, Gao Y, Lan J, Xie LX. The efficacy and safety of current treatments in diabetic macular edema: a systematic review and network meta-analysis. PLoS One. 2016;11(7):e0159553. doi: 10.1371/journal.pone.0159553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. doi: 10.2147/OPTH.S151611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodjikian L, Bellocq D, Mathis T. Pharmacological management of diabetic macular edema in real-life observational studies. Biomed Res Int. 2018;2018:8289253. doi: 10.1155/2018/8289253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci. 2018;19(1):E110. doi: 10.3390/ijms19010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA Approves Revised Indication for Ozurdex for the Treatment of Diabetic Macular Edema. Drugs.com, Sep 29, 2014. https://www.drugs.com/newdrugs/fda-approves-revised-indication-ozurdex-diabetic-macular-edema-4093.html.
- 8.Matonti F, Pommier S, Meyer F, Hajjar C, Merite PY, Parrat E, Rouhette H, Rebollo O, Guigou S. Long-term efficacy and safety of intravitreal dexamethasone implant for the treatment of diabetic macular edema. Eur J Ophthalmol. 2016;26(5):454–459. doi: 10.5301/ejo.5000787. [DOI] [PubMed] [Google Scholar]
- 9.Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121(12):2473–2481. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):463–473. doi: 10.1007/s00417-016-3472-1. [DOI] [PubMed] [Google Scholar]
- 11.Iglicki M, Busch C, Zur D, et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: the international retina group real-life 24-month multicenter study. The IRGREL-DEX study. Retina. 2019;39(1):44–51. doi: 10.1097/IAE.0000000000002196. [DOI] [PubMed] [Google Scholar]
- 12.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM, Ozurdex MEAD Study Group Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Castro-Navarro V, Cervera-Taulet E, Navarro-Palop C, Monferrer-Adsuara C, Hernández-Bel L, Montero-Hernández J. Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema. BMC Ophthalmol. 2019;19(1):15. doi: 10.1186/s12886-018-1022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM, Ozurdex CHAMPLAIN Study Group Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):915–923. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 15.Pacella E, Vestri AR, Muscella R, Carbotti MR, Castellucci M, Coi L, Turchetti P, Pacella F. Preliminary results of an intravitreal dexamethasone implant (Ozurdex®) in patients with persistent diabetic macular edema. Clin Ophthalmol. 2013;7:1423–1428. doi: 10.2147/OPTH.S48364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pareja-Ríos A, Ruiz-de la Fuente-Rodríguez P, Bonaque-González S, López-Gálvez M, Lozano-López V, Romero-Aroca P. Intravitreal dexamethasone implants for diabetic macular edema. Int J Ophthalmol. 2018;11(1):77–82. doi: 10.18240/ijo.2018.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, Jonas J, Larsen M, Tadayoni R, Loewenstein A. Guidelines for the management of diabetic macular edema by the European society of retina specialists (EURETINA) Ophthalmologica. 2017;237(4):185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 18.García-Layana A, Figueroa MS, Arias L, Adán A, Cabrera F, Abraldes M, Fernández-Vega Á, Navarro R, Cervera E, Silva R, Armadá F, Donate J, Ruiz-Moreno JM. Clinical decision-making when treating diabetic macular edema patients with dexamethasone intravitreal implants. Ophthalmologica. 2018;240(2):61–72. doi: 10.1159/000486800. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini A, Parravano M, Ricci F, Bandello F. Management of diabetic macular edema with intravitreal dexamethasone implants: expert recommendations using a Delphi-based approach. Eur J Ophthalmol. 2019;29(1):82–91. doi: 10.1177/1120672118781236. [DOI] [PubMed] [Google Scholar]
- 20.Kodjikian L, Bellocq D, Bandello F, et al. First-line treatment algorithm and guidelines in center-involving diabetic macular edema. Eur J Ophthalmol. 2019;29(6):573–584. doi: 10.1177/1120672119857511. [DOI] [PubMed] [Google Scholar]
- 21.García Layana A, Adán A, Ascaso FJ, Cabrera F, Donate J, Escobar Barranco JJ, Peralta G, Reyes García R, Rodríguez Maqueda M, Ruiz-Moreno JM, Vinagre I, MOMENTUM-D Study Group Use of intravitreal dexamethasone implants in the treatment of diabetic macular edema: expert recommendations using a Delphi approach. Eur J Ophthalmol. 2020;30(5):1042–1052. doi: 10.1177/1120672119861623. [DOI] [PubMed] [Google Scholar]
- 22.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 23.Lam WC, Albiani DA, Yoganathan P, Chen JC, Kherani A, Maberley DA, Oliver A, Rabinovitch T, Sheidow TG, Tourville E, Wittenberg LA, Sigouin C, Baptiste DC. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255–1268. doi: 10.2147/OPTH.S80500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malclès A, Dot C, Voirin N, Vié AL, Agard É, Bellocq D, Denis P, Kodjikian L. Safety of intravitreal dexamethasone implant (Ozurdex): the safodex study. incidence and risk factors of ocular hypertension. Retina. 2017;37(7):1352–1359. doi: 10.1097/IAE.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 25.Zarranz-Ventura J, Sala-Puigdollers A, Velazquez-Villoria D, et al. Long-term probability of intraocular pressure elevation with the intravitreal dexamethasone implant in the real-world. PLoS One. 2019;14(1):e0209997. doi: 10.1371/journal.pone.0209997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malclès A, Dot C, Voirin N, Agard É, Vié AL, Bellocq D, Denis P, Kodjikian L. Real-life study in diabetic macular edema treated with dexamethasone implant: the reldex study. Retina. 2017;37(4):753–760. doi: 10.1097/IAE.0000000000001234. [DOI] [PubMed] [Google Scholar]