Abstract
AIM
To investigate the safety and efficacy of phacoemulsification with capsular-tension-ring implantation and posterior chamber intraocular lens implantation combined with ophthalmic endoscope-controlled goniosynechialysis (Phaco-CTR-IOL-OE-GSL) for treating secondary angle-closure glaucoma induced by traumatic lens subluxation.
METHODS
A retrospective and descriptive study was performed on patients with lens subluxation, angle closure, goniosynechia, and evaluated intraocular pressure (IOP) that cannot be controlled with medication, who underwent Phaco-CTR-IOL-OE-GSL. The postoperative best-corrected visual acuity (BCVA), IOP, range of goniosynechia and complications were retrospectively observed.
RESULTS
Nine patients with secondary angle-closure glaucoma induced by traumatic lens subluxation were included. The follow-up period was 51.1±8.6mo. The preoperative range of zonule rupture was 158.2°±33.0°, and the range of goniosynechia was 220.0°±92.5°. The baseline BCVA was 0.9±1.0 logMAR, IOP was 30.7±17.3 mm Hg, and number of anti-glaucoma medication was 3.2±1.1. Mild intraoperative hyphaemia with 8 eyes (88.8%) in the anterior chamber, and was absorbed two days postoperatively. One eye (11.1%) had postoperative ciliary body detachment and was recovered after five days of topical drug treatment. BCVA was 0.2±0.2 logMAR at 3mo postoperatively. The average IOP at the last follow-up was 16.7±2.0 mm Hg, and no anti-glaucoma medications were used. The average range of recurrent goniosynechia was 54.9°±33° at the final postoperative gonioscopic examination.
CONCLUSION
Phaco-CTR-IOL-OE-GSL is safe and effective in the treatment of secondary angle-closure glaucoma induced by traumatic lens subluxation. The use of an endoscope provides a more direct and clear examination for GSL, and 360° dissection is easily achieved.
Keywords: lens subluxation, glaucoma, tension ring, goniosynechia, ophthalmic endoscope
INTRODUCTION
In recent years, phacoemulsification (Phaco) with intraocular lens (IOL) implantation combined with goniosynechialysis (GSL) has become the primary treatment option for primary angle-closure glaucoma[1]–[4]. The reported success rate of this operation is 85.9%[5]. GSL aims to separate the peripheral iris anterior synechia from the chamber angle and recover the function of the trabecular meshwork. It is easier to handle with less tissue damage because the operation can be performed directly under an ophthalmic endoscope[6]–[8].
For angle-closure glaucoma due to lens subluxation, Phaco with capsular tension ring (CTR) and IOL implantation is currently the primary treatment for subluxated lens. Cionni and Osher[9] reported the clinical use of CTR in 1995. The implanted IOL using a CTR assistant can be placed in the capsule and securely fixed in the central pupillary zone, which fits the normal anterior segment structure[10]. However, IOL suspension is still applied in cases with a larger range of zonule rupture.
Therefore, we proposed that a one-stage combination of Phaco-CTR-IOL and ophthalmic endoscope-controlled goniosynechialysis (OE-GSL) could resolve lens subluxation and secondary angle-closure simultaneously. We investigated the effect of the combination of Phaco-CTR-IOL and OE-GSL to treat secondary angle-closure glaucoma due to traumatic lens subluxation. The change in the range of goniosynechia and related complications was evaluated.
SUBJECTS AND METHODS
Ethical Approval
This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Wenzhou Medical University. The informed consent was obtained from the patients.
Subjects
This was a retrospective and descriptive study. Nine eyes from nine patients with secondary angle-closure glaucoma due to traumatic lens subluxation from Eye Hospital, Wenzhou Medical University between November 2014 and December 2016 were included. The general information of the patients is listed in Table 1. All patients received Phaco-CTR-IOL-OE-GSL, with five of them receiving CTR implantation and the other four receiving CTR suspension. There were six male patients and three female patients. The average age was 66.1±5.9y (range 57-77y). Preoperative measurements included best-corrected visual acuity (BCVA), intraocular pressure (IOP) by Goldmann tonometer, fundoscopy, gonioscopy, ciliary body imaging by optical coherence tomography (OCT), and A and B ultrasound. If the preoperative examination could not be performed because of corneal oedema, intraoperative endoscope examination of peripheral anterior synechia (PAS) was conducted, and the fundus was examined postoperatively. The average duration of the follow-up period was 51.1±8.6mo (range 43-68mo).
Table 1. Patients' information.
| Gender | Age (y) | Eye | Kinds of drugs | Range of zonular breaking | Range of PAS | BCVA | IOP on operation day (mm Hg) | C/D | 3mo postop. BCVA | Intraoperative complications | Postop. complications | Range of recurrent PAS | 1mo postop. IOP (mm Hg) | IOP at last follow-up (mm Hg) | Time of follow-up (mo) |
| Male | 71 | OD | 2 | 7/12 | 9/12 | 0.6 | 15 | 0.4 | 0.8 | Hyphema | None | 0 | 19 | 17 | 53 |
| Male | 64 | OS | 4 | 5/12 | 12/12 | 0.9 | 50 | 0.8 | 0.9 | Hyphema | None | 5.5/12 | 15 | 20 | 56 |
| Male | 57 | OS | 3 | 6/12 | 3/12 | 0.7 | 16 | 0.3 | 0.7 | Hyphema | None | 3/12 | 10 | 13 | 45 |
| Male | 77 | OS | 3 | 4/12 | 6/12 | FC/30 cm | 21 | 1.0 | 0.3 | Hyphema | None | 0 | 19 | 16 | 44 |
| Female | 64 | OD | 5 | 4/12 | 5/12 | HM/20 cm | 57 | Unclear | 0.8 | Hyphema | 180° cyclodialysis | 1.5/12 | 18 | 17 | 44 |
| Male | 66 | OS | 2 | 5.5/12 | 6/12 | 0.2 | 24 | 0.4 | 1.0 | Hyphema | None | 1/12 | 18 | 19 | 45 |
| Male | 60 | OD | 4 | 6/12 | 7/12 | 0.3 | 50 | 0.4 | 0.7 | None | None | 0 | 17 | 17 | 43 |
| Female | 68 | OS | 2 | 4/12 | 6/12 | 0.4 | 12 | 0.5 | 1.0 | Hyphema | None | 1/12 | 14 | 17 | 68 |
| Female | 68 | OD | 4 | 6/12 | 12/12 | 0.05 | 31 | 0.7 | 0.3 | Hyphema | None | 4.5/12 | 22 | 15 | 62 |
PAS: Peripheral anterior synechia; FC: Finger count; HM: Hand motion.
Methods
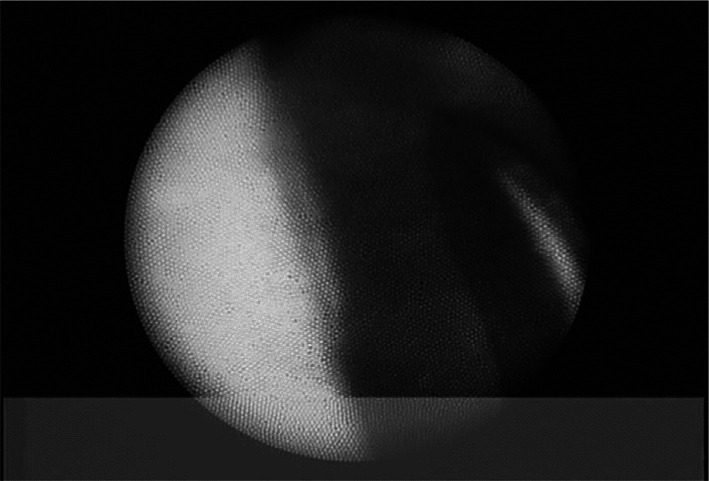
All operations were performed by the same surgeon (Pan WH). Topical anaesthesia of 0.5% alcaine was applied, and 2% lidocaine was injected for peribulbar anaesthesia. A transparent corneal incision was first made using a 2.0-mm keratome, and then the viscoelastic agent was injected into the anterior chamber. This allowed the observation of the rupture range of the zonule. Next, the viscoelastic agent was injected into the anterior chamber angle to separate the PAS first, followed by continuous circular capsulorhexis. One patient (one eye) was found to have an anterior vitreous hernia, and the vitreous body was cut from the main incision; part of it was returned to the vitreous cavity by injection of the viscoelastic agent. In situ Phaco without hydrodissection was then performed. The peripheral cortex was then drawn out, followed by the implantation of CTR in the capsule. If the rupture of the lens zonule was over 150° and was located at the superior position, a scleral flap was made as 2×2 mm2 large and 1/2 thickness at 12 o'clock. A needle was inserted at 1.5 mm posterior to the inferior limbus under the scleral flap, passing through the sclera and equatorial lens capsule under the iris, and exiting the opposite side limbal of the cornea. Next, a 10-0 Prolene suspension suture (Alcon, Sinking Spring, PA, USA) was pulled out from the main incision and ligated in the head hole of the CTR (Croma, Austria). The CTR was slowly pushed into the capsule from the main corneal incision, and the head of the ring was placed at the corresponding position, appropriately tightened by suture, and secured to the ciliary sulcus. Next, the IOL was implanted in the capsule. The viscoelastic agent was then injected into the anterior chamber, and the angle of the chamber was examined with OE (Figure 1). The remaining PAS part was separated with an iris restorer (Figure 2). For the residual PAS under the main incision, blunt separation was applied by the viscoelastic agent or iris restorer through the lateral incision until complete 360° separation of the PAS. The viscoelastic agent, blood clots, and angle pigment were removed with I/A. All the patients were administered topical tobramycin dexamethasone eye drops postoperatively four times a day (5 mL; S.A., ALCON-COUVREUR N.V., Belgium) for two weeks. Additionally, 0.5% pilocarpine was used twice daily in the first month after operation. The observed intra- and post-operative complications were recorded. BCVA, IOP and the types of medicines applied were recorded. The patients were followed monthly for half a year and every 3mo thereafter.
Figure 1. Separation of the PAS by the effect of viscoelastic agent under ophthalmic endoscope.

Figure 2. Separation of the remained PAS part using an iris restorer under ophthalmic endoscope.
Statistical Analysis
The data were analysed by descriptive analysis using SPSS20.0 software.
RESULTS
Pre- and Postoperative Best-Corrected Visual Acuity
The preoperative and average three-month postoperative BCVA were 0.9±1.0 (logMAR) and 0.2±0.2 (logMAR), respectively. BCVA was improved in seven of the eyes (77.8%) and unchanged in the remaining two eyes (22.2%) at three months postoperatively.
Pre- and Postoperative Intraocular Pressure
The preoperative average IOP was 30.7±17.3 mm Hg (range 12-50 mm Hg). On average, 3.2 types of anti-glaucoma eye drops were used preoperatively. No medication was applied at six months postoperatively, and two types of topical anti-glaucoma eye drops were needed due to intraocular hypertension secondary to anterior uveitis in one of the cases. The medication was stopped gradually after two months, and the IOP became normal. At the last follow-up, none of the cases required topical anti-glaucoma eye drops. The average IOP was significantly reduced to 16.9±3.5 mm Hg (range 10-22 mm Hg) one month after operation. The average IOP at the last follow-up was 16.7±2.0 mm Hg, which is obviously lower than the preoperative IOP.
Anterior Chamber Angle and Range of Zonule Rupture
Figure 3 indicates range of zonule rupture and PAS in each eye. Five of the nine eyes had goniosynechia less than or equal to 180° before the operations, and the other four eyes had more than 180° goniosynechia. The average range of the PAS was 220.0°±92.5°. The observed range of zonule rupture during operation was 158.2°±33.0°. PAS occurred at the same or opposite position of the zonule rupture in five of our cases (cases 3, 4, 5, 7, and 8). After visco-GSL, the residual PAS was completely separated by a blunt device under an ophthalmic endoscope. At the final postoperative gonioscopic examination, six of the nine cases (66.6%) presented recurrent PAS, with the average synechia range of 54.9°±33.0°.
Figure 3. Range of zonule rupture (blue) and PAS (green) in each eye and the recurrent range of the PAS (red).

Complications
Eight of the nine eyes (88.8%) had mild intraoperative hyphaemia in the angle of the anterior chamber, and the blood was absorbed two days postoperatively. One of the nine eyes (11.1%) had postoperative ciliary body detachment and was recovered after five days of topical drug treatment. No other significant complications were observed.
DISCUSSION
Phaco with trabeculectomy is the most common approach to treat secondary angle-closure glaucoma, which is induced by lens subluxation[11]. However, sometimes, simple lens suspension or replacement of iris-clip IOL cannot relieve the patient from intraocular hypertension[12]. Moreover, massaging the eyeball after trabeculectomy probably induces loosening of the suspended IOL. The novelty of the approach presented in this research lies in the combination of Phaco-CTR-IOL and OE-GSL to resolve lens subluxation and secondary angle-closure at the same time. To the best of our knowledge, we are the first to propose this combination surgery to treat secondary angle-closure glaucoma caused by lens subluxation. In our study group, all the patients improved BCVA postoperatively. Within the average observation period of 51.1±8.6mo (range 43-68mo), six cases had recurrent goniosynechia, but the synechia progression stopped within six months after the operations, and the IOP was well controlled until the last follow-up. None of these patients required anti-glaucoma medicine at the last follow-up. Therefore, Phaco-CTR-IOL-OE-GSL is an effective surgery. The main intraoperative complication observed was mild bleeding during GSL (88.8%). Postoperative ciliary body detachment was observed in one of the cases (11.1%). The incidence of serious complications was far less than trabeculectomy. The risk of the breakage of the suspension suture during postoperative eyeball massage after trabeculectomy was also avoided. Thus, Phaco-CTR-IOL-OE-GSL is a safer approach than trabeculectomy.
The use of both an endoscope and CTR with a suspension approach in this study improved the safety and efficacy of the surgery. The use of an ophthalmic endoscope provided direct visualization to examine the outcomes of GSL effectively and securely. In this study, we applied an endoscope to examine the status after visco-GSL directly, ensuring a complete opening of the peripheral chamber angle. Using the endoscope, the operation could be performed in more directly and clearly with higher magnification power. The entry of the endoscope reused the cataract incision; thus, no additional wound was needed[13]. The tension ring used in this study had holes at both ends, allowing the nylon suture to pass through easily. This significantly helped to place the ring at the desired position in the lens capsule under the endoscope. The use of this CTR has several advantages. First, it improves the safety of Phaco and posterior chamber IOL implantation. It reduces the loss of the vitreous body while decreasing the incidence of potential vitreous retinal complications from the IOL suspension. Second, it helps maintain the round contour of the lens capsule and the centre position of IOL. It could also restrain lens epithelial cell proliferation and migration, reducing the incidence of posterior capsule opacity (PCO)[14]. Third, this CTR has already been widely used, and it is more common and easier to obtain at most hospitals in China than modified capsular tension ring (MCTR). Werner et al[15] reported the occurrence of the dislocation of the IOL, together with the CTR and lens capsule at an average of 6.8y after implantation of CTR alone. Compared with the method of IOL suspension, our approach is simpler and offers gentle pulling of the capsule. Therefore, CTR with suspension causes less damage to the lens capsule and increases the stability of the IOL in the capsule, avoiding capsular shrinkage and enlargement of zonule rupture-caused, in-the-bag IOL dislocation during long-term postoperative observation.
We suspected that PAS might correlate with the range of zonule rupture. From our patients, PAS occurred at the same or opposite position of the zonule rupture in five of our cases (cases 3, 4, 5, 7, and 8). We speculate that after rupture of the zonule, the lens at the rupture zone was unstable. The edge of the lens wobbled and squeezed towards the anterior chamber. While the lens moved towards the opposite side, the thick body part pushed the iris to move forward so that PAS occurred at the position opposite to the ruptured zonule. Such PAS is very likely related to the trauma type, the direction of the hit to the eyeball, and the habitual head position of the patient. Additionally, the recurrent range of the PAS mostly occurred at the same position as the PAS preoperatively. The cause was probably related to the mechanical secondary damage to the iris and inflammatory reaction postoperatively. However, due to the small sample size of this study, the relevant factors and mechanism of the formation of such PAS need further investigation.
In conclusion, we suggest that Phaco-CTR-IOL-OE-GSL is a safe and effective treatment for secondary angle-closure glaucoma induced by traumatic lens subluxation. The operation is direct, simple, and convenient with mild complications. The normal out-flowing channel of aqueous humour could be recovered, and the postoperative IOP was controlled in the normal range. Both the long-term efficacy of Phaco-CTR-IOL-OE-GSL and progression of recurrent goniosynechia require further investigation. Furthermore, because this study was limited by the small sample size, further studies with a large number of cases are needed.
Acknowledgments
Authors' contributions: Pan WH were responsible for the design of the study and interpretation of the data. Dai Q and Pan WH were responsible for conducting the study. Pan WH was involved in the management and was responsible for the operations. Dai Q, Pan WH, Fu L, and Liu XY reviewed the article.
Foundation: Supported by Wenzhou Municipal Science and Technology Bureau (No.Y20180712).
Conflicts of Interest: Dai Q, None; Fu L, None; Liu XY, None; Pan WH, None.
REFERENCES
- 1.Zhang HL, Tang GX, Liu J. Effects of phacoemulsification combined with goniosynechialysis on primary angle-closure glaucoma. J Glaucoma. 2016;25(5):e499–e503. doi: 10.1097/IJG.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen Xuan H, Nguyen Dinh N, Nguyen Thu H, Nguyen van C, Aung T, Loo Y, Nguyen Do Thi Ngoc H, Do T. Comparing the safety and efficacy of phacogoniosynechialysis with phacotrabeculectomy in the management of refractory acute primary closure angle glaucoma with cataract: a multicenter randomized trial. J Glaucoma. 2021;30(7):552–558. doi: 10.1097/IJG.0000000000001868. [DOI] [PubMed] [Google Scholar]
- 3.Yu JG, Zhao F, Xiang Y. Phacoemulsification with goniosynechialysis versus phacoemulsification alone in angle-closure glaucoma: a meta-analysis of randomized controlled trials. J Ophthalmol. 2021;2021:8831479. doi: 10.1155/2021/8831479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang LJ, Ren C, Lu PR. Effects of phacoemulsification combined with goniosynechialysis on primary angle-closure glaucoma. J Glaucoma. 2020;29(12):e142. doi: 10.1097/IJG.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 5.Kameda T, Inoue T, Inatani M, Tanihara H, Japanese Phaco-Goniosynechialysis Multicenter Study Group Long-term efficacy of goniosynechialysis combined with phacoemulsification for primary angle closure. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):825–830. doi: 10.1007/s00417-012-2091-8. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues IA, Alaghband P, Beltran Agullo L, Galvis E, Jones S, Husain R, Lim KS. Aqueous outflow facility after phacoemulsification with or without goniosynechialysis in primary angle closure: a randomised controlled study. Br J Ophthalmol. 2017;101(7):879–885. doi: 10.1136/bjophthalmol-2016-309556. [DOI] [PubMed] [Google Scholar]
- 7.Maeda M, Watanabe M, Ichikawa K. Goniosynechialysis using an ophthalmic endoscope and cataract surgery for primary angle-closure glaucoma. J Glaucoma. 2014;23(3):174–178. doi: 10.1097/IJG.0b013e31826aaf3b. [DOI] [PubMed] [Google Scholar]
- 8.Qian ZB, Huang JH, Song BH, Wei LQ, Fu L, Austin MW, Spaeth GL, Pan WH, McAlinden C. Cataract surgery (phacoemulsification with intraocular lens implantation) combined with endoscopic goniosynechialysis for advanced primary angle-closure glaucoma. Ophthalmol Glaucoma. 2021;4(4):365–372. doi: 10.1016/j.ogla.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Cionni RJ, Osher RH. Endocapsular ring approach to the subluxed cataractous lens. J Cataract Refract Surg. 1995;21(3):245–249. doi: 10.1016/s0886-3350(13)80126-5. [DOI] [PubMed] [Google Scholar]
- 10.Vasavada AR, Praveen MR, Vasavada VA, Yeh RY, Srivastava S, Koul A, Trivedi RH. Cionni ring and in-the-bag intraocular lens implantation for subluxated lenses: a prospective case series. Am J Ophthalmol. 2012;153(6):1144–1153.e1. doi: 10.1016/j.ajo.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Kiuchi Y, Tsujino C, Nakamura T, Otori Y, Mochizuki H. Phacoemulsification and trabeculotomy combined with goniosynechialysis for uncontrollable chronic angle-closure glaucoma. Ophthalmic Surg Lasers Imaging. 2010;41(3):348–354. doi: 10.3928/15428877-20100430-09. [DOI] [PubMed] [Google Scholar]
- 12.Kristianslund O, Råen M, Østern AE, Drolsum L. Glaucoma and intraocular pressure in patients operated for late in-the-bag intraocular lens dislocation: a randomized clinical trial. Am J Ophthalmol. 2017;176:219–227. doi: 10.1016/j.ajo.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Fang AW, Yang XJ, Nie L, Qu J. Endoscopically controlled goniosynechialysis in managing synechial angle-closure glaucoma. J Glaucoma. 2010;19(1):19–23. doi: 10.1097/IJG.0b013e318179f79c. [DOI] [PubMed] [Google Scholar]
- 14.Halili I, Mutlu FM, Erdurman FC, Gündogan FC, Kilic S. Influence of capsular tension ring on posterior capsule opacification in myopic eyes. Indian J Ophthalmol. 2014;62(3):311–315. doi: 10.4103/0301-4738.116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner L, Zaugg B, Neuhann T, Burrow M, Tetz M. In-the-bag capsular tension ring and intraocular lens subluxation or dislocation: a series of 23 cases. Ophthalmology. 2012;119(2):266–271. doi: 10.1016/j.ophtha.2011.08.016. [DOI] [PubMed] [Google Scholar]