Abstract
AIM
To investigate the potential differences between topography-guided (TG) and wavefront-optimized (WFO) laser in situ keratomileusis (LASIK) for the treatment of myopia.
METHODS
A systematic literature search was performed to determine relevant trials comparing LASIK with TG and WFO from the time of library construction to August 2020, and The PubMed, Cochrane, Web of Science, EMBASE and Chinese databases (i.e. CNKI, CBM, WAN FANG and VIP) were accessed. The data on visual acuity, refractive status and wavefront aberration were retrieved and evaluated from three to six months after surgery. STATA (version 14.0) software was used for statistical analysis. A cumulative Meta-analysis was simultaneously performed.
RESULTS
Eleven studies with a total of 1425 eyes were incorporated. No statistically significant differences were evident between TG and WFO ablation in the proportion of eyes achieving an uncorrected distance visual acuity (UCVA) of 20/20 or better (P=0.377), gaining one line or more (P=0.05), postoperative cylinder (P=0.40), vertical coma (P=0.593) and horizontal coma (P=0.957). After TG ablation, the proportion of the patients' eyes of which postoperative refraction is within ±0.5 diopter of the target refraction was significantly higher than that undergoes WFO (P=0.003). As opposed to the WFO group, manifest refraction spherical equivalent (MRSE; P=0.000) was lower, and UCVA (P=0.005) was better in the TG group. The higher-order aberrations (HOAs; P=0.000), spherical aberration (P=0.000) and coma (P=0.000) were significantly lower in TG group. The cumulative Meta-analysis illustrated that the proportion of eyes achieving UCVA of 20/20 or better, postoperative refraction within ±0.5 diopter, and MRSE has steady between the two groups.
CONCLUSION
Both TG-LASIK and WFO-LASIK are safe, effective, and predictable for correcting myopia. TG-LASIK may produce fewer aberration and is more precise than WFO-LASIK.
Keywords: Meta-analysis, corneal topography, wavefront aberration, laser in situ keratomileusis, myopia
INTRODUCTION
Laser in situ keratomileusis (LASIK) is currently the most frequently used surgical procedure to correct myopia. However, the traditional surgical method based on the Munnerlyn formula can no longer meet the requirements of higher visual quality in modern society[1]. While correcting the refractive error of patients, reducing the introduction of higher-order aberrations and corneal irregularity, personalized cutting mode arises at the historic moment[2]–[3].
Topography-guided laser in situ keratomileusis (TG-LASIK) is used in abnormal corneal astigmatism and eccentric ablation, and its safety and effectiveness have also been confirmed in the eyes of patients undergoing primary surgery[4]–[5]. At the same time, wavefront-optimized laser in situ keratomileusis (WFO-LASIK) is also used to improve the visual quality of patients[6].
Recent clinical studies have compared the differences between the two customized ablation methods in the treatment of myopia[7]–[12]. However, the sample size of these studies is small, and there are some contradictory results from different studies. During this study, a Meta-analysis of TG-LASIK and WFO-LASIK for myopia was conducted to examine whether there were differences in refractive status and visual differences between the two ablation modes.
MATERIALS AND METHODS
By the generally accepted recommendations, we performed a Meta-analysis using the following criteria[13].
Search Strategy
The two commentators independently searched PubMed, Cochrane, Web of Science, EMBASE and Chinese databases (i.e., CNKI, CBM, WAN FANG and VIP) to compare the records of TG-LASIK and WFO-LASIK in the treatment of myopia. The key words include “myopia”, “corneal topography”, “wavefront aberration”, “LASIK”, or “laser in situ keratomileusis”. There are no language or dates restrictions on the study, and the last search was on August 18, 2020. Complete duplicates of all associated studies were accessed and evaluated to determine whether they met the standard quality criteria for inclusion. The title and abstract were selected independently by the two reviewers. Meanwhile, the differences between reviewers were solved through deliberations.
Selection Criteria
The studies were determined to be included in this Meta-analysis by the following selection criteria: 1) randomized controlled trials (RCTs) and non-randomized comparative studies Non-RCFs; 2) adult patients with the stable refractive state, no systemic disease likely to affect or cause abnormal wound healing, no history of ophthalmic surgery; 3) patients undergoing corneal surgery (TG-LASIK or WFO-LASIK); 4) a minimum follow-up period of three months; 5) original clinical publications with accessible data.
Outcome Measures
Primary outcomes
The outcome parameters were safety, efficacy and predictability. The safety indicator was postoperative refractive spherical equivalent (SE). Efficacy was measured by the proportion of eyes with uncorrected distance visual acuity (UCVA) of 20/20 or better. Predictability was gauged as the refractive SE within ±0.5 diopters (D) of the target value.
Secondary outcomes
The secondary outcomes were postoperative uncorrected distance visual acuity, the percentage of eyes gaining one or more lines after surgery, postoperative cylinder and wavefront aberration. The aberrations included high-order aberrations, spherical aberration, coma, vertical and horizontal coma. The follow-up period was 3 to 6mo. The included data were extracted and analyzed.
Data Extraction and Quality Assessment
Two researchers independently extracted data and performed a quality assessment of the articles using standardized forms. The results were compared, and any different were resolved through discussion. Each of the included studies summarized the following data: first author, publication year, country, number of eyes selected, preoperative SE, design, follow-up time, quality assessment scores. The quality of RCTs was measured by the Jadad scale[14]. The main assessment domains of the Jadad scale were these three indicators listed below: randomization, blindness, and participant withdrawal/dropout. Two points could be awarded for using the random assignment and describing the correct random method. Also, 2 points were scored for using a double-blind method and describing the appropriate blinding procedure. A detailed description of the number of participant withdrawal/ dropout and the reasons for them could earn 1 point. The total score after article quality assessment ranged between 0 and 5, and a score of 2 or less was regarded as low-quality research. Non-RCTs were evaluated using the Newcastle-Ottawa scale (NOS)[15]. The NOS evaluated article quality using three metrics: selection, comparability, and exposure or outcome measurements. The total score was 9, and studies with a score of 6 or higher were deemed to be of high quality.
Statistical Analysis
Statistical analysis of all data was accomplished using STATA (version 14.0). The continuous variables were computed by weighted mean difference (WMD) or standard mean difference (SMD), and the dichotomous outcomes were calculated by odds ratio (OR). The confidence interval (CI) was set at 95%, and the outcome was regarded as statistically significant when P<0.05. Heterogeneity was analyzed using the I2 statistic and the Chi-square test. When the heterogeneity was obvious (I2>50% or P<0.10), the random effect model (REM) was adopted. When there was no heterogeneity, the fixed effect model (FEM) was adopted. In addition, we also conducted a subgroup analysis of different follow-up periods. Publication bias was estimated by Funnel plots, Egger tests, Harbord tests and Peters tests[16]–[18]. Sensitivity analysis was used to estimate the robustness of the results by removing the individual studies and assessing their impact on the pooled estimates. At the same time, the cumulative Meta-analysis was conducted to evaluate the effect of time on the robustness of the results.
RESULTS
Search Results
According to the retrieval strategy, the electronic database was searched to screen out 360 relevant studies, and 279 studies were retained by eliminating duplicate records from the literature. The complete screening process was illustrated in Figure 1. After screening titles and abstracts, 249 studies were excluded. The remaining 30 records were systematically reviewed in their full text, and 19 studies were removed: thirteen studies did not describe the results related to the topic, three studies lacked a control group, two studies had no measurable results, and one result was a summary of the meeting. Finally, 11 full-text studies applied to this Meta-analysis, including 8 prospective comparative studies[7]–[9],[19]–[23], 2 RCTs[10],[24] and 1 retrospective study[25].
Figure 1. A flow diagram of the inclusion criteria of studies eligible.
Study Characteristics and Quality
A total of 1425 eyes were enrolled in 11 studies[7]–[10],[19]–[25], of which 701 eyes (49.19%) were TG-LASIK, and 724 eyes (50.81%) underwent WFO-LASIK. The follow-up period after surgery was 3 to 6mo. Table 1 provided a summary of the characteristics of the included studies, and Table 2 summarized the quality assessment of the non-RCTs. El Awady et al[24] was a randomized, double-blind trial, but the randomized and double-blind methods were not described, and there was no dropout and withdrawal. Zhang and Chen[10] indicated random methods but did not describe whether to use the blinding method and no loss of follow-up and withdrawal of the participants. According to the criteria of the Jadad scale, two RCTs were identified as being of high quality (score ≥3)[10],[24]. There was no significant difference in age, preoperative SE, cylindrical lens, wavefront aberration and other factors among the preoperative patients in the prospective control study and retrospective study[7]–[9],[19]–[23],[25]. The quality of the cohort was evaluated by the NOS system. The total score of all cohorts is more than 5, which is considered high-quality research.
Table 1. Characteristics and quality assessment of the included studies.
| Study | Year | Country | TG group |
WFO group |
Design | Follow-up (mo) | NOS | Jadad | ||
| Eyes (n) | Preop. SE (D) | Eyes (n) | Preop. SE (D) | |||||||
| El Awady et al | 2011 | Egypt | 84 | -5.23±1.60 | 84 | -5.42±3.70 | RCT | 6 | - | 3 |
| Chen et al | 2016 | China | 35 | -5.44±2.02 | 35 | -6.08±1.62 | Non-RCT | 3 | 7 | - |
| Zhang et al | 2016 | China | 44 | -5.19±1.77 | 43 | -5.39±1.34 | Non-RCT | 6 | 7 | - |
| Jain et al | 2016 | India | 35 | -4.19±1.92 | 35 | -3.89±1.85 | Non-RCT | 6 | 8 | - |
| Shetty et al | 2017 | India | 30 | -4.93±2.47 | 30 | -5.08±2.50 | Non-RCT | 6 | 8 | - |
| Tiwari et al | 2018 | India | 100 | -3.51±1.94 | 100 | -3.46±2.14 | Non-RCT | 3 | 7 | - |
| Zhang et al | 2019 | China | 42 | -5.94±1.99 | 36 | -5.49±1.91 | Non-RCT | 6 | 7 | - |
| Kim et al | 2019 | South Korea | 43 | -4.17±1.77 | 43 | -4.12±1.84 | Non-RCT | 3 | 7 | - |
| Ozulken et al | 2019 | Turkey | 32 | - | 32 | - | Non-RCT | 3 | 7 | - |
| Zhang et al | 2019 | China | 216 | -6.17±1.62 | 216 | -6.09±1.68 | RCT | 6 | - | 3 |
| Kim et al | 2020 | South Korea | 40 | -4.18±1.95 | 70 | -4.45±1.75 | Non-RCT | 3 | 6 | |
RCT: Randomized controlled trial; Non-RCT: Nonrandomized controlled trial; Preop: Preoperative; SE: Spherical equivalent; SD: Standard deviation; D: Diopters; TG: Topography-guided; WFO: Wavefront-optimized.
mean±SD
Table 2. NOS for non-randomized controlled trials.
| Study | Selection | Comparability | Outcome/exposure | Sum of score |
| Chen et al | 4 | 1 | 2 | 7 |
| Zhang et al | 3 | 1 | 3 | 7 |
| Jain et al | 4 | 1 | 3 | 8 |
| Shetty et al | 4 | 1 | 3 | 8 |
| Tiwari et al | 4 | 1 | 2 | 7 |
| Zhang et al | 4 | 1 | 2 | 7 |
| Kim et al | 4 | 1 | 2 | 7 |
| Ozulken et al | 4 | 1 | 2 | 7 |
| Kim et al | 2 | 1 | 3 | 6 |
The NOS includes the following three aspects: selection, comparability, exposure, or outcome. The study was considered high quality when scoring >5 points in the NOS.
Primary Outcomes
Postoperative mean refractive spherical equivalent
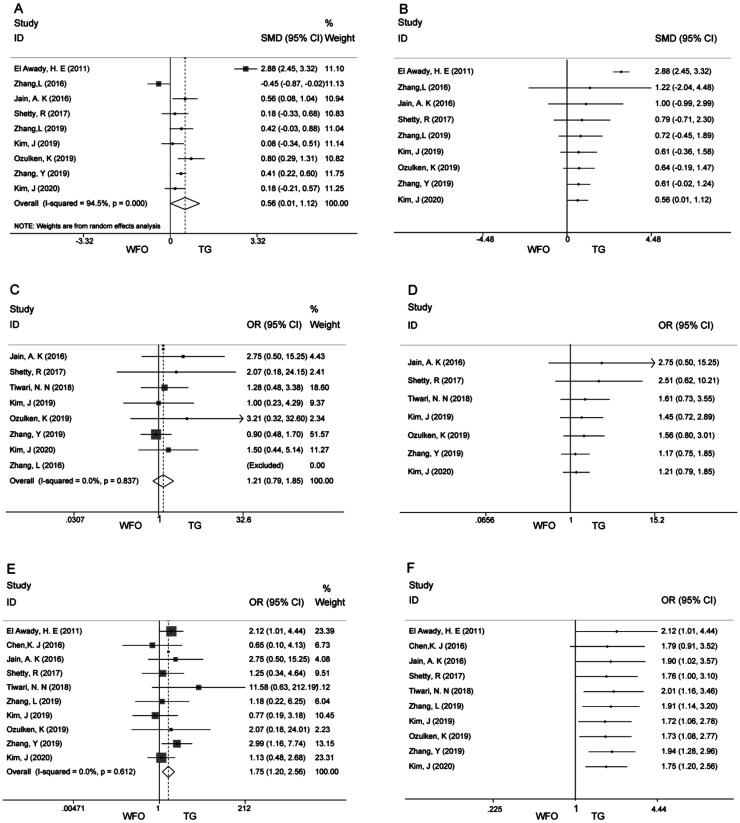
Nine articles reported postoperative diopter SE[7]–[10],[20]–[22],[24]–[25]. Postoperative mean refractive SE was statistically different between the two ablations (SMD 0.56; 95%CI: 0.01, 1.12; P=0.000; Figure 2A). Excluding Zhang et al[20] and El Awady et al[24], the heterogeneity was decreased (I2 from 94.5% to 11.2%). But it did not have a significant impact on the results (SMD 0.37; 95%CI: 0.22, 0.52; P=0.000). In addition, we conducted a cumulative Meta-analysis. Starting with study 9 in 2020 (SMD 0.56; 95%CI: 0.01, 1.12; P=0.000; Figure 2B), a significant difference between the two groups emerged, and the results demonstrated a greater advantage of TG ablation. The 95%CI gradually narrowed, and the effect tended to be stable.
Figure 2. Forest plots for Meta-analysis of primary outcomes and cumulative Meta-analysis.
A: Forest plot of SMD of postoperative mean refractive spherical equivalent; B: Cumulative Meta-analysis of postoperative mean refractive spherical equivalent; C: Forest plot of OR of uncorrected distance visual acuity of 20/20 or better; D: Cumulative Meta-analysis of uncorrected distance visual acuity of 20/20 or better; E: Forest plot of OR of postoperative refraction within ±0.5 D of the target refraction; F: Cumulative Meta-analysis of postoperative refraction within ±0.5 D of the target refraction.
Uncorrected distance visual acuity of 20/20 or better
Eight articles reported that the percentage of patients with uncorrected distance visual acuity of 20/20 or better after treatment had no significant difference between the TG and the WFO ablation (OR 1.21; 95%CI: 0.79, 1.85; P=0.377; Figure 2C)[8]–[10],[20]–[23],[25]. There was no heterogeneity (I2=0, P=0.837). Cumulative Meta-analysis indicated no statistically significant difference between the two ablation modalities (Figure 2D).
Postoperative refraction within ±0.5 D of the target refraction
Cumulatively, ten studies reported the percentage of patients with Postoperative refraction within ±0.5 D of the target refraction, and the period of follow-up time varied from 3 to 6mo[7]–[10],[19],[21]–[25]. Meta-analysis showed that the number of patients with target refraction of ±0.5 D was significantly greater in the TG group than in the WFO ablation modality (OR 1.75; 95%CI: 1.20, 2.56; P=0.003; Figure 2E). The results suggested that no heterogeneity was generated (I2=0, P=0.612). We also performed a cumulative Meta-analysis, and the first significant difference between the two groups was observed in the fifth study in 2018 (OR 2.01; 95%CI: 1.16, 3.46). The 95%CI gradually shrinks, and the effect tends to be stable (Figure 2F).
Secondary Outcomes
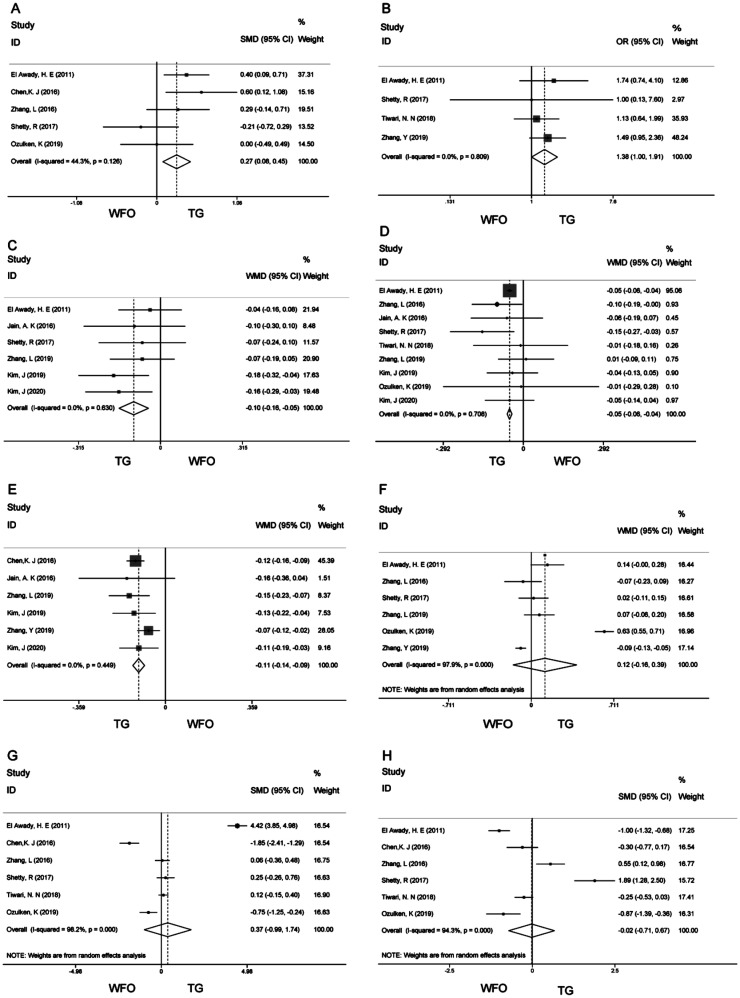
The forest map of postoperative UDVA showed that there was a significant difference between the two ablation modes (SMD 0.27; 95%CI: 0.08, 0.45; P=0.005; Figure 3A)[9],[19]–[20],[22],[24]. The proportion of eyes gaining one or more lines after surgery (OR 1.38; 95%CI: 1.00, 1.91; P=0.05; Figure 3B)[10],[22]–[24]; the higher-order aberrations (WMD -0.10; 95%CI: -0.16, -0.05; P=0.000; Figure 3C)[7]–[8],[21]–[22],[24]–[25]; spherical aberration (WMD -0.05; 95%CI: -0.06, -0.04; P=0.000; Figure 3D)[7]–[9],[20]–[23],[24]–[25] and coma (WMD -0.11; 95%CI: -0.14, -0.09; P=0.000; Figure 3E)[7]–[8],[10],[19],[21],[25]. Outcomes in the remaining group were analyzed using a REM because there was high heterogeneity (postoperative cylinder, vertical coma, horizontal coma). postoperative cylinder (WMD 0.12; 95%CI: -0.16, 0.39; P=0.40; Figure 3F)[7],[9]–[10],[20],[22],[24]; vertical coma (SMD 0.37; 95%CI: -0.99, 1.74; P=0.593; Figure 3G)[9],[19]–[20],[22]–[24]; horizontal coma (SMD -0.02; 95%CI: -0.71, 0.67; P=0.957; Figure 3H)[9],[19]–[20],[22]–[24].
Figure 3. Forest plots for Meta-analysis of secondary outcomes.
A: Forest plot of SMD of postoperative uncorrected distance visual acuity; B: Forest plot of OR of proportion of eyes gaining one or more lines after surgery; C: Forest plot of WMD of higher-order aberrations; D: Forest plot of WMD of spherical aberration; E: Forest plot of WMD of coma; F: Forest plot of WMD of postoperative cylinder; G: Forest plot of SMD of vertical coma; H: Forest plot of SMD of horizontal coma.
Subgroup Analysis
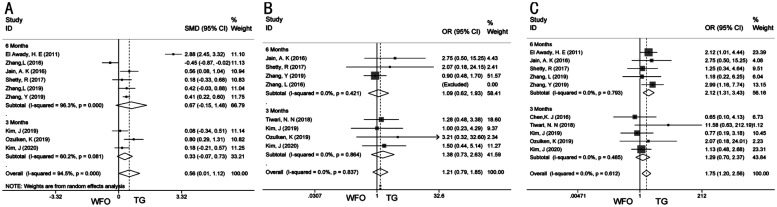
Subgroup analysis was performed for the primary outcomes indicators. Two subgroups were divided according to the duration of follow-up (3 vs 6 mo). In the sixth month group, the Meta-analysis results of postoperative mean refractive SE (SMD 0.67; 95%CI: -0.15, 1.48; P=0.109; Figure 4A)[7],[10],[20]–[22],[24] and uncorrected visual acuity of 20/20 or better (OR 1.09; 95%CI: 0.62, 1.93; P=0.771; Figure 4B)[10],[20]–[22] remained stable. There was statistical significance on postoperative refraction within ±0.5 D of the target refraction (OR 2.12; 95%CI: 1.31, 3.43; P=0.002; Figure 4C) in 6mo group[7],[10],[21]–[22],[24]. The size of the effect will gradually become stable with the inclusion of more relevant studies, and the 95%CI will narrow.
Figure 4. Subgroup analysis of primary outcomes.
A: Subgroup analysis of postoperative mean refractive spherical equivalent; B: Subgroup analysis of uncorrected distance visual acuity of 20/20 or better; C: Subgroup analysis of postoperative refraction within ±0.5 D of the target refraction.
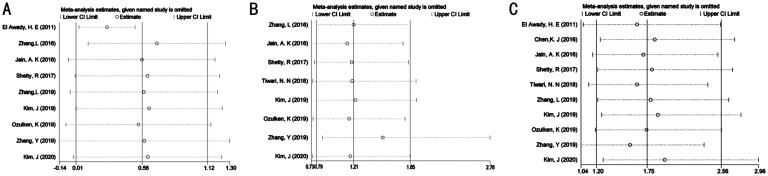
Publication Bias and Sensitivity Analysis
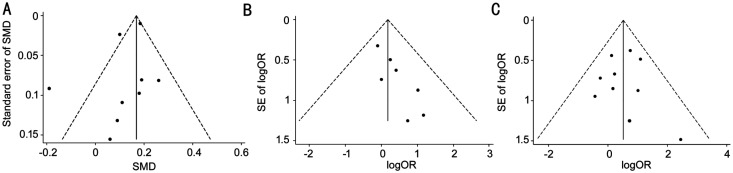
The funnel chart proved that the primary outcomes were symmetrical (Figure 5). The publication bias of UCVA of 20/20 or better may be caused by the small sample study. According to the Cochrane Handbook, only 6 articles were included in this study, and the publication bias was assessed to be insufficient. The remaining outcome indicators were evaluated using Harbord tests, Peters tests, and Egger tests (Table 3), and the pooled results indicated that no publication bias was discovered among the studies. The robustness of the results was also confirmed by sensitivity analysis (Figure 6).
Figure 5. Funnel chart of primary outcomes.
A: Funnel chart of postoperative mean refractive spherical equivalent; B: Funnel chart of uncorrected distance visual acuity of 20/20 or better; C: Funnel chart of postoperative refraction within ±0.5 D of the target refraction. SE: Spherical equivalent.
Table 3. The P value of Egger tests, Harbord tests and Peters tests.
| Outcomes | Egger tests | Harbord tests | Peters tests |
| UCVA of 20/20 or better | - | 0.021 | 0.016 |
| MRSE | 0.233 | - | - |
| Postoperative refraction within ±0.5 D | - | 0.761 | 0.039 |
| Gained one line or more of CDVA | - | 0.828 | 0.592 |
| UCVA | 0.372 | - | - |
| Cylinder | 0.588 | - | - |
| HOAs | 0.924 | - | - |
| Spherical aberration | 0.805 | - | - |
| Coma | 0.522 | - | - |
| Vertical coma | 0.784 | - | - |
| Horizontal coma | 0.287 | - | - |
UCVA: Uncorrected visual acuity; MRSE: Mean refractive spherical equivalent; CDVA: Corrected distance visual acuity; HOAs: Higher-order aberrations.
Figure 6. Sensitivity analysis of primary outcomes.
A: Sensitivity analysis of postoperative mean refractive spherical equivalent; B: Sensitivity analysis of uncorrected distance visual acuity of 20/20 or better; C: Sensitivity analysis of postoperative refraction within ±0.5 D of the target refraction.
DISCUSSION
Due to the side effects such as reduced night vision and glare after surgery, traditional LASIK surgery can no longer meet the demand for better visual quality, and customized refractive surgery will definitely become one of the mainstream[26]–[27]. We were the first to use Meta-analysis to compare TG-LASIK and WFO-LASIK, and it was to guide the choice of myopia surgery. In this Meta-analysis, the effect of WFO-LASIK was similar to that of TG-LASIK. There was no significant difference in the results of the postoperative cylinder, vertical coma, and horizontal coma between the two operations. Less refraction remained after the TG group, and less higher-order aberrations (HOAs), spherical aberration and coma were introduced. At the same time, a cumulative Meta-analysis was also applied in this paper to investigate the trend effect of time on the above results. The outcomes supported that the size of the effect tended to be stable.
According to the accepted method of Meta-analysis, all articles that meet the inclusion criteria were included, and the collected data were analyzed. When the primary outcomes were found to have high heterogeneity (I2>50%), it was solved by sensitivity analysis. Heterogeneity of secondary outcomes was addressed by changing the effect model calculation. Also, due to the difference of follow-up time in different studies, we also conducted a subgroup analysis. Cumulative Meta-analysis was used to evaluate the effect of time on the stability of results. Publication bias was estimated by forest map, Harbord, Peters, and Egger tests.
In this study, the postoperative mean refractive SE was used as an index to measure the safety of operation, and the results showed that it had significant heterogeneity. Through sensitivity analysis, it was found that the source of heterogeneity was the study of El Awady et al[24] and Zhang et al[20]. Instead of using a femtosecond laser, El Awady et al[24] used the Moria2 microkeratome to make the corneal flap. Zhang et al[20] assigned the surgical population of the TG and WFO group according to the patients' subjective requirements, so the clinical heterogeneity and methodological heterogeneity led to high heterogeneity. After excluding these two studies by sensitivity analysis, the heterogeneity decreased significantly, and the results did not change, which proved that the outcome was robust and reliable. The summary results suggested that the TG group was superior to the WFO group in safety.
The curative effect of the operation was evaluated by the proportion of the UCVA of 20/20 or better. The forest map revealed that the two laser ablation modes were not significantly different from each other. Moreover, there was no statistical difference in subgroup analysis between the two groups for 3mo and 6mo. However, through the Harbord test and peters test, it was found that there may be publication bias in the study. Because only 8 articles were included, according to the Cochran Handbook, when less than 10 articles were included, the evaluation of publication bias was not accurate. More RCTs were expected to be included to evaluate the authenticity of the outcome.
In terms of predictability, we evaluated the proportion of postoperative refraction within ±0.5 D of the target refraction. TG group was significantly better than the WFO group. Subgroup analysis showed that 3mo after the operation, the predictability between the two groups was similar, and 6mo after the operation, the TG-LASIK showed an advantage, which was consistent with the summary results. The cumulative Meta-analysis also proved that the advantage tended to be stable with time.
Simple vision improvement can no longer meet the needs of the public. It is very essential to minimize the introduction of high-order aberrations. The results of the forest map indicated that the TG-LASIK was significantly lower than the WFO-LASIK in the introduction of HOAs, spherical aberration, and coma. There was no heterogeneity, and the result was robust. Also, the forest map showed that there was high heterogeneity between vertical and horizontal coma between the two groups. The REM was used to evaluate the results, and the summary results showed no statistical difference between the two laser ablation modes. The forest map of uncorrected distance visual acuity after operation demonstrated that TG-LASIK was superior to WFO-LASIK. In the correction of astigmatism and postoperative gain one line or more, the two groups had the same effect.
This Meta-analysis still has some limitations which cannot be avoided in the meantime. First of all, the aberration measurement derived from different instruments, which increased the generation of deviation. Second, the study was followed up for 3-6mo, lacking long-term follow-up results, so the longsighted term effect could not be evaluated. Finally, some parameters were highly heterogeneous, which might be due to different surgical methods, follow-up time and measurement methods. Although some of the outcome indicators were highly heterogeneous, we still believed that the results were robust after subgroup analysis and sensitivity analysis.
To sum up, based on the current Meta-analysis, the two surgical methods were effective. TG-LASIK was safer and provided better postoperative predictability than WFO-LASIK. Compared with WFO-LASIK, the introduction of HOAs was lower. These results pointed out that TG-LASIK was superior to WFO-LASIK in the treatment of myopia. Additional randomized, prospective contralateral eye studies are required as a way to measure the long-term differences between TG and WFO treatments.
Acknowledgments
Conflicts of Interest: Hu PC, None; Li L, None; Wu XH, None; Li YQ, None; Li KW, None.
REFERENCES
- 1.Jiménez JR, Alarcón A, Anera RG, Del Barco LJ. Hyperopic Q-optimized algorithms: a theoretical study on factors influencing optical quality. Biomed Opt Express. 2017;8(3):1405–1414. doi: 10.1364/BOE.8.001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schallhorn JM, Seifert S, Schallhorn SC. SMILE, topography-guided LASIK, and wavefront-guided LASIK: review of clinical outcomes in premarket approval FDA studies. J Refract Surg. 2019;35(11):690–698. doi: 10.3928/1081597X-20190930-02. [DOI] [PubMed] [Google Scholar]
- 3.Du CX, Shen Y, Wang Y. Comparison of high order aberration after conventional and customized ablation in myopic LASIK in different eyes of the same patient. J Zhejiang Univ Sci B. 2007;8(3):177–180. doi: 10.1631/jzus.2007.B0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallerstein A, Caron-Cantin M, Gauvin M, Adiguzel E, Cohen M. Primary topography-guided LASIK: refractive, visual, and subjective quality of vision outcomes for astigmatism ≥2.00 diopters. J Refract Surg. 2019;35(2):78–86. doi: 10.3928/1081597X-20181210-01. [DOI] [PubMed] [Google Scholar]
- 5.Wiley LA, Randleman JB. Topography-guided custom ablation photorefractive keratectomy treatment of irregular astigmatism resulting from decentered SMILE. J Refract Surg. 2020;36(11):766–771. doi: 10.3928/1081597X-20200820-02. [DOI] [PubMed] [Google Scholar]
- 6.Kung JS, Manche EE. Quality of vision after wavefront-guided or wavefront-optimized LASIK: a prospective randomized contralateral eye study. J Refract Surg. 2016;32(4):230–236. doi: 10.3928/1081597X-20151230-01. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Zhai CB, Zhou YH, Qi Y, Zhang J, Zheng Y, Liu Q. Comparision of visual quality after topography-guided and wavefront-optimized FS-LASIK in myopic eyes. Chin J Exp Ophthalmol. 2019;37(11):914–920. [Google Scholar]
- 8.Kim J, Choi SH, Lim DH, et al. Topography-guided versus wavefront-optimized laser in situ keratomileusis for myopia: surgical outcomes. J Cataract Refract Surg. 2019;45(7):959–965. doi: 10.1016/j.jcrs.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Ozulken K, Yuksel E, Tekin K, et al. Comparison of wavefront-optimized ablation and topography-guided contoura ablation with LYRA protocol in LASIK. J Refract Surg. 2019;35(4):222–229. doi: 10.3928/1081597X-20190304-02. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Chen YG. A randomized comparative study of topography-guided versus wavefront-optimized FS-LASIK for correcting myopia and myopic astigmatism. J Refract Surg. 2019;35(9):575–582. doi: 10.3928/1081597X-20190819-01. [DOI] [PubMed] [Google Scholar]
- 11.Falavarjani KG, Hashemi M, Modarres M, Sanjari MS, Darvish N, Gordiz A. Topography-guided vs wavefront-optimized surface ablation for myopia using the WaveLight platform: a contralateral eye study. J Refract Surg. 2011;27(1):13–17. doi: 10.3928/1081597X-20100310-02. [DOI] [PubMed] [Google Scholar]
- 12.Faria-Correia F, Ribeiro S, Lopes BT, Salomão MQ, Ambrósio R., Jr Outcomes comparison between wavefront-optimized and topography-guided PRK in contralateral eyes with myopia and myopic astigmatism. J Refract Surg. 2020;36(6):358–365. doi: 10.3928/1081597X-20200416-01. [DOI] [PubMed] [Google Scholar]
- 13.Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998;351(9095):47–52. doi: 10.1016/S0140-6736(97)08461-4. [DOI] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Wells G, Shea B, O'Connell D, Peterson J, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in Meta-analysis. 2000.
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 18.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 19.Chen KJ, Bai J, Liu T, Ye JX, Kan QX, Liu LN. The effectiveness of topography-guided FS-LASIK in treating asymmetric corneal astigmatism. Chin J Optom Ophthalmol Vis Sci. 2016;18(07):394–398. [Google Scholar]
- 20.Zhang L, Zhou YH, Xu W, Li Y, Wang Y, Zhang QL, Liu J, Hu YB. Comparison of corneal topography after topography-guided FS-LASIK and wavefront-optimized FS-LASIK. Chin J Optom Ophthalmol Vis Sci. 2016;18(07):399–403. [Google Scholar]
- 21.Jain AK, Malhotra C, Pasari A, Kumar P, Moshirfar M. Outcomes of topography-guided versus wavefront-optimized laser in situ keratomileusis for myopia in virgin eyes. J Cataract Refract Surg. 2016;42(9):1302–1311. doi: 10.1016/j.jcrs.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Shetty R, Shroff R, Deshpande K, Gowda R, Lahane S, Jayadev C. A prospective study to compare visual outcomes between wavefront-optimized and topography-guided ablation profiles in contralateral eyes with myopia. J Refract Surg. 2017;33(1):6–10. doi: 10.3928/1081597X-20161006-01. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari NN, Sachdev GS, Ramamurthy S, Dandapani R. Comparative analysis of visual outcomes and ocular aberrations following wavefront optimized and topography-guided customized femtosecond laser in situ keratomileusis for myopia and myopic astigmatism: a contralateral eye study. Indian J Ophthalmol. 2018;66(11):1558–1561. doi: 10.4103/ijo.IJO_507_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Awady HE, Ghanem AA, Saleh SM. Wavefront-optimized ablation versus topography-guided customized ablation in myopic LASIK: comparative study of higher order aberrations. Ophthalmic Surg Lasers Imaging. 2011;42(4):314–320. doi: 10.3928/15428877-20110421-01. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Choi SH, Lim DH, Yoon GJ, Chung TY. Correction to: comparison of outcomes after topography-modified refraction versus wavefront-optimized versus manifest topography-guided LASIK. BMC Ophthalmol. 2020;20(1):492. doi: 10.1186/s12886-020-01459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awwad ST. Wavefront-guided ablation: evidence for efficacy compared to traditional ablation. Am J Ophthalmol. 2006;141(6):1169; author reply 1169. doi: 10.1016/j.ajo.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Lackner B, Pieh S, Schmidinger G, Hanselmayer G, Simader C, Reitner A, Skorpik C. Glare and halo phenomena after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29(3):444–450. doi: 10.1016/s0886-3350(02)01816-3. [DOI] [PubMed] [Google Scholar]