Abstract
Polyamines are required for maintenance of intestinal epithelial integrity, and a decrease in cellular polyamines increases the cytoplasmic levels of RNA-binding protein HuR stabilizing p53 and nucleophosmin mRNAs, thus inhibiting IEC (intestinal epithelial cell) proliferation. The AMPK (AMP-activated protein kinase), an enzyme involved in responding to metabolic stress, was recently found to be implicated in regulating the nuclear import of HuR. Here, we provide evidence showing that polyamines modulate subcellular localization of HuR through AMPK-regulated phosphorylation and acetylation of Impα1 (importin α1) in IECs. Decreased levels of cellular polyamines as a result of inhibiting ODC (ornithine decarboxylase) with DFMO (D,L-α-difluoromethylornithine) repressed AMPK activity and reduced Impα1 levels, whereas increased levels of polyamines as a result of ODC overexpression induced both AMPK and Impα1 levels. AMPK activation by overexpression of the AMPK gene increased Impα1 but reduced the cytoplasmic levels of HuR in control and polyamine-deficient cells. IECs overexpressing wild-type Impα1 exhibited a decrease in cytoplasmic HuR abundance, while cells overexpressing Impα1 proteins bearing K22R (lacking acetylation site), S105A (lacking phosphorylation site) or K22R/S105A (lacking both sites) mutations displayed increased levels of cytoplasmic HuR. Ectopic expression of these Impα1 mutants also prevented the increased levels of cytoplasmic HuR following polyamine depletion. These results indicate that polyamine-mediated AMPK activation triggers HuR nuclear import through phosphorylation and acetylation of Impα1 in IECs and that polyamine depletion increases cytoplasmic levels of HuR as a result of inactivation of the AMPK-driven Impα1 pathway.
Keywords: AMP-activated protein kinase (AMPK), growth arrest, HuR, importin α1, intestinal epithelium, ornithine decarboxylase
INTRODUCTION
The mammalian intestinal mucosa has the most rapid turnover rate of any tissue in the body and undergoes a continual renewal process, characterized by active proliferation of stem cells localized near the base of the crypts, progression of these cells up the crypt–villus axis with cessation of proliferation and subsequent differentiation and apoptosis [1,2]. This dynamic and constant state of intestinal epithelial renewal is highly regulated and critically controlled by numerous factors, including cellular polyamines [3,4]. The natural polyamines spermidine and spermine and their precursor putrescine are organic cations found in all eukaryotic cells [5], and the regulation of cellular polyamines has been recognized for many years as a central convergence point for the multiple signalling pathways driving different IEC (intestinal epithelial cell) functions [3,6]. It has been shown that IEC proliferation in the intestinal mucosa depends on the supply of polyamines to the dividing cells in the crypts [3,4] and that decreasing cellular polyamines inhibits cell renewal in vivo [3,4,9] as well as in vitro [7–11]. Although few specific functions of polyamines at the molecular level have been identified to date, an increasing body of evidence indicates that polyamines regulate IEC proliferation by virtue of their ability to modulate expression of various growth-related genes [3,5,12–15]. Polyamines positively regulate the transcription of growth-promoting genes such as c-fos, c-jun and c-myc [7,12,13], but negatively modulate expression of growth-inhibiting genes, including p53 [9], p21 [14,15], the gene coding for NPM (nucleophosmin) [11], junD [8,43] and the genes coding for TGFβ (transforming growth factor β) receptors [6,16] through modifications at the post-transcriptional level.
Although stress stimuli trigger alterations in gene expression patterns via transcriptional and post-transcriptional mechanisms, the important contribution of post-transcriptional events, such as mRNA processing, transport, turnover and translation, has been becoming increasingly recognized recently [17,18]. Among these post-transcriptional processes, mRNA stability and translation are tightly regulated by the association of the mRNAs with specific RBPs (RNA-binding proteins) and are emerging as highly effective means of controlling the profiles of expressed gene products [17,19,20]. The best-characterized cis-acting elements regulating mRNA stability and translation are U-rich elements and AREs (AU-rich elements) that are usually located in the 3′-UTRs (3′-untranslated regions) of many labile mRNAs [17]. Likewise, different RBPs selectively recognize and bind to AREs of target mRNAs, leading to either an increase or decrease in the transcript half-life and translation rate [21,22]. HuR is a ubiquitously expressed member of the Hu/ELAV (embryonic lethal abnormal vision in Drosophila melanogaster) family of RBPs, which also comprises the neuron-specific proteins HuC, HuD and Hel-N1 [20]. HuR is predominantly nuclear in unstimulated cells, but it rapidly translocates to the cytoplasm in response to various stress stimuli and binds with great affinity and specificity to AREs in a variety of mRNAs that typically present one or several hits of a recently identified RNA motif [23–25]. Although the precise processes regulating HuR function remain to be fully understood, it is clear that HuR subcellular localization is intimately linked to its effects on target transcripts. We [3,26] have recently demonstrated that polyamines modulate the HuR shuttle between the nucleus and the cytoplasm in IECs and that depletion of cellular polyamines increases cytoplasmic levels of HuR-stabilizing p53 and NPM mRNAs, thus contributing to the inhibition of IEC proliferation. However, the exact mechanism by which polyamines regulate HuR subcellular distribution remains elusive.
The AMPK (AMP-activated protein kinase) is a cellular sensor of metabolic stress, by which cells in both mammals and lower organisms sense and respond to changes in energy status [27]. Mammalian AMPK is a heterotrimer of three subunits: one catalytic (α) and two regulatory (β and γ) subunits [28,29], and its activity is ubiquitous, although different isoforms of AMPK exhibit tissue-specific distribution and preferential subcellular localization [30,31]. Recently, several studies have revealed that AMPK is implicated in regulating the nuclear import of HuR and that decreased AMPK activity results in an elevation of cytoplasmic HuR levels; conversely, AMPK activation causes a reduction in cytoplasmic HuR [32–35]. Previous studies also have shown that Impα1 (importin α1), an adaptor protein, is a critical downstream target of AMPK and functions as a key mediator of AMPK-mediated HuR nuclear import [33–35]. Given that polyamines are absolutely required for IEC proliferation and their cellular levels are tightly regulated by the status of cell growth and stress stimulation [3] and given that AMPK-triggered reduction in cytoplasmic HuR levels profoundly affects the stability of target mRNAs [32], we sought to determine whether polyamines modulate subcellular localization of HuR by altering AMPK-regulated Impα1 activity in IECs. The results presented in the current study demonstrate that decreased levels of cellular polyamines repressed AMPK expression, whereas increased polyamines induced AMPK levels. The present study further shows that activated AMPK following increased polyamines triggered HuR nuclear import through phosphorylation and acetylation of Impα1 and that polyamine depletion increased cytoplasmic HuR levels as a result of inactivation of the AMPK-driven Impα1 pathway.
MATERIALS AND METHODS
Chemicals and supplies
Disposable culture ware was purchased from Corning Glass Works (Corning, NY, U.S.A.). Tissue culture medium and dFBS [dialysed FBS (fetal bovine serum)] were purchased from Invitrogen (Carlsbad, CA, U.S.A.), and biochemicals were obtained from Sigma Chemical (St. Louis, MO, U.S.A.). Antibodies against HuR, β-tubulin and lamin B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.), and antibodies against pAMPKα (phosphorylated AMPKα), AMPKα and phospho-acetyl-CoA-carboxylase (Ser79) were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). The monoclonal antibody against Impα1 or ODC (ornithine decarboxylase) was from Sigma. DFMO (D,L-α-difluoromethylornithine) was purchased from Genzyme (Cambridge, MA, U.S.A.).
Cell culture
The IEC-6 cell line was purchased from the A.T.C.C. at passage 13. The cell line was derived from normal rat intestine and was developed and characterized by Quaroni et al. [54]. IEC-6 cells originated from intestinal crypt cells as judged by morphological and immunological criteria. They are non-tumorigenic and retain the undifferentiated characteristics of intestinal crypt cells. Passages 15–20 were used in experiments, which exhibit a stable phenotype [8–10]. ODC-IEC cells (ODC-overexpressing IEC-6 cells) cells were developed as described in our previous publications [26,55] and expressed a more stable ODC variant with full enzyme activity [36]. Stock cells were maintained in T-150 flasks in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 5% (v/v) heat-inactivated FBS, 10 μg of insulin/ml and 50 μg/ml gentamicin. Flasks were incubated at 37°C in a humidified atmosphere of 90% air/10% CO2.
Recombinant viral construction and infection
Adenoviral vectors were constructed by using the Adeno-X Expression System (Clontech, Mountain View, CA, U.S.A.) according to the protocol provided by the manufacturer. Briefly, the full-length cDNA of human AMPKα was cloned into the pShuttle by digesting with BamHI/HindIII and ligating the resultant fragments into the XbaI site of the pShuttle vector. AdAMPKα (pAdeno-AMPKα) was constructed by digesting the pShuttle construct with PI-SceI/I-CeuI and ligating the resultant fragment into the PI-SceI/I-CeuI sites of the pAdeno-X adenoviral vector. Recombinant adenoviral plasmids were packaged into infectious adenoviral particles by transfecting HEK-293 cells (human embryonic kidney cells) using Lipofectamine™ Plus reagent (Gibco-Bethesda Research Laboratory, Gaithersburg, MD, U.S.A.). The adenoviral particles were propagated in HEK-293 cells and purified by caesium chloride ultracentrifugation [14000 rev./min for 1 h in a type SA-600 rotor (Sorvall)]. Titres of the adenoviral stock were determined by standard plaque assay. Recombinant adenoviruses were screened for the expression of the introduced gene by Western-blot analysis using anti-AMPKα antibody. pAdeno-X, which was the recombinant replication-incompetent adenovirus carrying no AMPKα cDNA insert (Adnull), was grown and purified as described above and served as a control adenovirus. IEC-6 cells were infected with AdAMPKα or Adnull, and expression of AMPKα was assayed at 24 or 48 h after the infection.
Generation of stable Impα1-transfected IECs
The WT (wild-type)-Impα1 and the Impα1 proteins bearing either a lysine-to-arginine mutation on residue 22 [Impα1(K22R)] or a serine-to-alanine mutation on residue 105 [Impα1(S105A)] or both mutations [Impα1(S105A/K22R)] were generated using the QuikChange® site-directed mutagenesis kit (Stratagene) and were expressed in the plasmid pcDNA3.1/V5-His-TOPO (Invitrogen). All these constructs were verified by sequencing before being used for transfection. IEC-6 cells were transfected with either the WT-Impα1, various point-mutated Impα1 constructs or the control vector lacking Impα1 cDNA by using Lipofectamine™ 2000, and the transfected cells were selected by incubation with the selection medium containing G418 (600 μg/ml). Clones resistant to the selection medium were isolated, cultured and screened for Impα1 expression by Western-blot analysis using a specific anti-His-tag antibody.
Preparation of cytoplasmic and nuclear proteins and Western-blot analysis
Cytoplasmic and nuclear proteins were prepared via the procedure described previously [11,26], and the protein contents in different preparations were measured using the Bradford [37] method. Cell samples, placed in SDS sample buffer [250 mM Tris/HCl, pH 6.8, 2% (w/v) SDS, 20% (v/v) glycerol and 5% mercaptoethanol], were sonicated and then centrifuged (10000 g) at 4°C for 15 min. The supernatant from cell samples was boiled for 5 min and then subjected to SDS/PAGE (7.5% polyacrylamide) by the method of Laemmli [38]. After the transfer of protein on to nitrocellulose filters, the filters were incubated for 1 h in 5% (w/v) non-fat dry milk in PBS-T [1×PBS/Tween 20: 15 mM NaH2PO4, 80 mM Na2HPO4, 1.5 M NaCl, pH 7.5, and 0.5% (v/v) Tween 20]. Immunological evaluation was then performed for 1 h in 1% BSA/PBS-T buffer containing 1 μg/ml of the specific antibody against HuR, AMPKα or Impα1 proteins. The filters were subsequently washed with 1×PBS-T and incubated for 1 h with the secondary antibody conjugated with peroxidase by protein cross-linking with 0.2% glutaraldehyde. After extensive washing with 1×PBS-T, the immunocomplexes on the filters were developed by the ECL® method according to the manufacturer’s instructions (Amersham Biosciences, Arlington Heights, IL, U.S.A.).
Immunofluorescence staining
The immunofluorescence staining procedure was carried out according to the methods described previously [39,40]. After the monolayers of control and polyamine-deficient cells were fixed in 3.7% (v/v) formaldehyde in PBS and rehydrated, they were incubated with the primary antibody against AMPKα or Impα1 in the block buffer [5% (w/v) non-fat dried milk in 1% TBS-T (25 mM Tris base, 137 mM NaCl, 3 mM KCl and 0.1% (v/v) Tween 20), pH 7.5)] at a 1:300 dilution at 4°C overnight and then incubated with secondary antibody conjugated with Alexa Fluor® 594 (Molecular Probes, Eugene, OR, U.S.A.) for 2 h at room temperature (22°C). After rinsing three times, the slides were incubated with TO-PRO3 (Molecular Probes) at a concentration of 1 μM for 10 min to stain the cell nuclei. Finally, the slides were washed, mounted and viewed through a Zeiss confocal microscope (model LSM410). Images were processed using Photoshop software (Adobe, San Jose, CA, U.S.A.).
Polyamine analysis
The cellular polyamines content was analysed by HPLC analysis as previously described [8,35]. Briefly, after 0.5 M HClO4 was added, the cells were frozen at −80°C until ready for extraction, dansylation and HPLC analysis. The standard curve encompassed 0.31–10 μM. Values that fell >25% below the curve were considered undetectable. The results are expressed as nanomoles of polyamines per milligram of protein.
Statistics
All data are expressed as means ± S.E.M. for three or six dishes. Immunoblotting results and immunofluorescence staining were repeated three times. The significance of the difference between means was determined by ANOVA. The level of significance was determined using Duncan’s multiple-range test [41].
RESULTS
Reduced levels of AMPK and Impα1 after polyamine depletion
Polyamine depletion increases the levels of cytoplasmic HuR, which in turn stabilizes p53 and NPM mRNAs in IECs [3,26], and increasing AMPK activity lowers cytoplasmic HuR levels by promoting Impα1-mediated nuclear import of HuR. Therefore we hypothesized that decreasing cellular polyamines might induce HuR cytoplasmic translocation by altering AMPKα/Impα1 signalling. As reported in our previous studies [8,15,42], exposure of IEC-6 cells to 5 mM DFMO for 4 and 6 days completely inhibited ODC enzyme activity and almost totally depleted cellular polyamines. The levels of putrescine and spermidine were undetectable on days 4 and 6 after treatment with DFMO, and spermine was decreased by ~60% (results not shown). The results presented in Figure 1(A) show that polyamine depletion by DFMO decreased the levels of total (by%), cytoplasmic (by ~60%) and nuclear (by ~50%) AMPKα and lowered its activity as indicated by decreased levels (by ~65% on day 4 and by ~95% on day 6 respectively) of pAMPKα at Thr172 (pT172AMPKα). The reduced AMPKα activity in polyamine-deficient cells was associated with a significant decrease in the levels of total (by ~60%), cytoplasmic (by ~70%), and nuclear (by ~25%) Impα1. Putrescine (10 μM) given together with DFMO prevented the decreases in levels of AMPKα and Impα1. Addition of spermidine (5 μM) had an effect equal to putrescine on AMPKα and Impα1 when it was added to cultures containing DFMO (results not shown). To monitor the quality and abundance of the cytoplasmic and nuclear fractions, the levels of β-tubulin (a cytoplasmic protein) and lamin B (a nuclear protein) were also examined and served as loading controls. Assessment of these markers revealed that there was no contamination between cytoplasmic and nuclear fractions.
Figure 1. Changes in levels and cellular distribution of AMPK and Impα1 in control IEC-6 cells and cells treated with either DFMO (5 mM) alone or DFMO plus putrescine (Put; 10 μM) for 4 and 6 days.

Whole-cell lysates and cytoplasmic and nuclear fractions were prepared for Western blotting. (A) Representative Western blots in total (top), cytoplasmic (middle) and nuclear (bottom) proteins. Total, cytoplasmic and nuclear proteins (30 μg of each) were subjected to SDS/PAGE (10% polyacrylamide), and levels of AMPKα and Impα1 were measured by using specific antibody against AMPKα or Impα1. The pAMPKα was identified by using the antibody that specifically recognizes pAMPKα at Thr172 (pT172AMPKα). After detecting AMPKα and Impα1, blots were reprobed to detect β-actin in whole-cell lysates, β-tubulin in cytoplasmic proteins or lamin B in nuclear proteins to control for the quality of the fractionation procedure and the even loading of samples. Three experiments were performed and they showed similar results. (B) Cellular distribution of AMPKα (left) and Impα1 (right) in cells described in (A). (a) Control; (b) DFMO treatment for 6 days; (c) DFMO plus putrescine treatment for 6 days. AMPKα and Impα1 were first detected using specific antibodies recognizing either AMPKα or Impα1 and then with anti-IgG conjugated with Alexa Fluor® 594 (red fluorescence), while nuclei were stained with TO-PRO3 (green fluorescence). The images shown result from the merging of these two individual signals. Yellow signals reveal the areas where red has merged with green in the nuclear area; and brighter yellow colour indicates a decrease in AMPKα or Impα1 in the area. Original magnification, × 1000. (C) Time course of levels of pT172AMPKα in controls (a) and cells exposed to DFMO (b). Whole-cell lysates were harvested at different time points after treatment with 5 mM DFMO, and levels of pT172AMPKα were measured by Western-blot analysis.
These changes in levels of AMPKα and Impα1 after polyamine depletion were further studied by immunofluorescence staining. In control IEC-6 cells (Figure 1Ba), AMPKα and Impα1 immunoreactive signals were localized in both the cytoplasm and nucleus. Consistent with the Western blotting results, the levels of cytoplasmic and nuclear AMPKα and Impα1 immunostainings decreased significantly in polyamine-deficient cells (Figure 1Bb). Combined treatment with DFMO and putrescine prevented the decreased AMPKα and Impα1 signals, rendering the subcellular staining patterns similar to those of untreated cells (Figure 1Bc). The results presented in Figure 1(C) further show the time course of pT172AMPKα and Impα1 levels after polyamine depletion. Significant decreases in levels of pT172AMPKα and Impα1 were observed on day 3, while the maximal reduction of both AMPKα and Impα1 occurred on day 4 after treatment with DFMO. In contrast, there were no significant changes in the levels of pT172AMPKα or Impα1 between days 1 and 4 after plating in control cells (without DFMO treatment). These results indicate that decreasing cellular polyamines repressed AMPKα expression and decreased Impα1 levels.
Increasing cellular polyamines by ODC overexpression increases the cytoplasmic levels of AMPK and Impα1
Given our previous findings that increasing cellular polyamines decreased the cytoplasmic levels of HuR in IEC-6 cells [26], we sought to examine if there were changes in the levels of AMPKα and Impα1 in stable ODC-IEC cells that were recently developed in our laboratory [15,55]. These ODC-IEC cells expressed high levels of ODC protein (Figure 2, top), exhibited greater than 50-fold higher ODC enzyme activity and had elevated cellular polyamines putrescine, spermidine and spermine (by 12-fold, 2-fold and 25% compared with control populations respectively). Increased levels of cellular polyamines by ODC overexpression induced the levels of total and cytoplasmic AMPKα, although there were no significant changes in the levels of nuclear AMPKα in ODC-IEC cells (Figure 2). On the other hand, ODC overexpression increased the cytoplasmic levels of Impα1 (Figure 2, middle) and correspondingly decreased its nuclear abundance (Figure 2, bottom); no net changes in whole-cell Impα1 levels (Figure 2, top) were seen in ODC-IEC cells compared with control cells (vector alone). The effect of ODC overexpression on the levels and subcellular distribution of AMPKα and Impα1 were not simply due to clonal variation, since two stable clones, ODC-IEC-C1 and ODC-IEC-C2, showed identical responses. These results indicate that increasing cellular polyamines not only increases AMPKα expression but also promotes the cytoplasmic accumulation of AMPKα and Impα1 in IECs.
Figure 2. Changes in levels of AMPKα and Impα1 proteins in stable ODC-IEC cells.

Whole-cell lysates were harvested from clonal (C) populations of ODC-IEC cells and in control IEC-6 cells (vector alone), and cytoplasmic and nuclear fractions were prepared for Western-blot analysis. β-Actin signals served to assess the equality of protein loading in whole-cell lysates; β-tubulin served as loading of cytoplasmic proteins, while lamin B immunoblotting served as loading of nuclear preparations. Three experiments were performed and they showed similar results.
AMPK activation increases Impα1 and decreases cytoplasmic HuR levels
To define the exact role of polyamine-modulated AMPKα in regulating HuR subcellular trafficking, the following two studies were carried out. First, we examined the effects of increasing AMPKα by treatment with its chemical activator antimycin A [27] on the cytoplasmic levels of HuR in IEC-6 cells. The results presented in Figure 3(A) show that exposure of control and polyamine-deficient cells (pretreatment with DFMO for 6 days) to 5 μM antimycin A not only increased the levels of total and pAMPKα protein but it also stimulated its kinase activity as indicated by increased levels of pACC (phospho-acetyl-CoA carboxylase), which is a specific AMPK substrate, and its phosphorylation at Ser79 is exclusively performed by active AMPK. Induced AMPK phosphorylation and its activity by antimycin A were associated with an increase in Impα1. Significant inductions of AMPK activation and Impα1 occurred by 4–6 h after administration of antimycin A. Treatment with antimycin A also reduced the cytoplasmic levels of HuR in control (Figure 3A, left) and polyamine-deficient cells (Figure 3A, right). The levels of cytoplasmic HuR in control cells decreased by ~60% at 4 h and by ~80% at 6 h after treatment with antimycin A, while the cytoplasmic levels of HuR in polyamine-deficient cells decreased by ~83% at 4 h and 95% at 6 h respectively.
Figure 3. Effect of stimulation of AMPKα by treatment with its chemical activator antimycin A (Ant-A) and ectopic expression of WT AMPKα gene on cytoplasmic levels of HuR in control and polyamine-deficient cells.
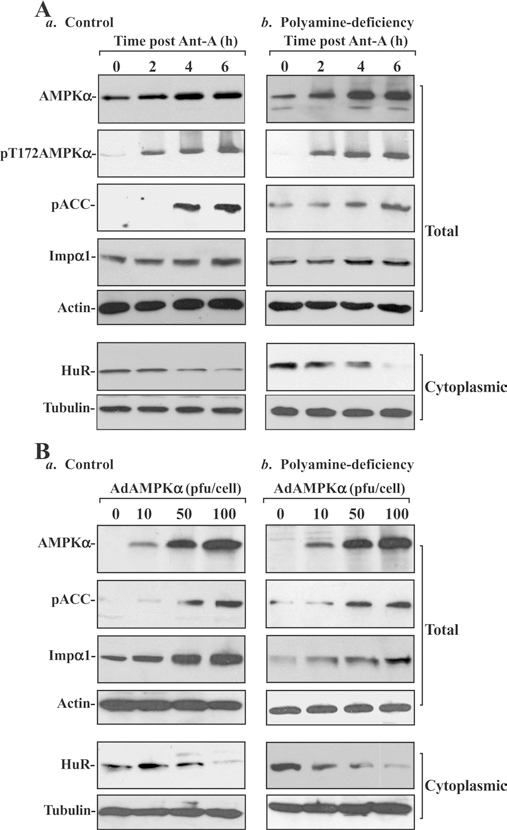
(A) Changes in levels of total and pAMPKα, pACC, Impα1 and HuR after treatment with Ant-A. (a) Control; (b) polyamine-deficient cells. IEC-6 cells were cultured in the control medium and a medium containing DFMO for 6 days and then exposed to Ant-A (5 μM). Whole-cell lysates were harvested at different times after administration of Ant-A, and total and cytoplasmic fractions were prepared for Western-blot analysis. Levels of AMPKα, pAMPKα, pACC, Impα1 and HuR were identified by using the specific antibodies, and equal loading was monitored by immunoblotting of β-actin in total proteins and β-tubulin in cytoplasmic proteins. Three experiments were performed and they showed similar results. (B) Changes in levels of AMPKα, pACC, Impα1 and HuR after overexpression of the AMPKα gene. (a) Control; (b) polyamine-deficient cells. After cells were grown in control cultures and cultures containing 5 mM DFMO for 4 days, they were infected with the adenoviral expression vector encoding the complete open reading frame of the human AMPKα cDNA (AdAMPKα) or adenoviral vector lacking AMPKα cDNA (Adnull) at an MOI (multiplicity of infection) of 10–100 pfu per cell. Whole-cell lysates were harvested 48 h after the infection in the presence or absence of DFMO, and levels of AMPKα, pACC, Impα1 and HuR were measured by Western-blot analysis. Three experiments were performed and they showed similar results.
Secondly, we examined changes in the levels of cytoplasmic HuR after overexpression of the AMPKα gene. An adenoviral vector containing the corresponding full-length human AMPKα cDNA under the control of the cytomegalovirus immediate early gene promoter (AdAMPKα) was constructed as described previously [34]. This delivery vector was chosen over other delivery methods because adenoviruses have been shown to infect a variety of cultured rat and human IECs with nearly 100% efficiency [10,15]. More than 95% of IEC-6 cells were positive by 24 h following infection with a control adenoviral vector encoding GFP (green fluorescent protein; results not shown). As shown in Figure 3(B), AMPKα protein levels increased in cells infected with progressively higher viral loads. When AdAMPKα was used to infect at 10, 50 and 100 pfu (plaque-forming units)/cell, the levels of AMPKα protein increased by ~2-fold (controls) or 3-fold (polyamine-deficient cells) at 10 pfu, ~10-fold at 50 pfu and 15-fold at 100 pfu respectively. The increased levels of AMPKα protein after AdAMPKα infection were paralleled by significant increases in levels of pACC, indicating that this overexpressed AMPKα has sufficient kinase activity. An adenovirus that lacked an insert (Adnull) was used as a negative control and did not induce AMPKα levels (results not shown). Overexpression of the AMPKα gene increased Impα1 and decreased the cytoplasmic levels of HuR. When control cells were infected with different concentrations of AdAMPKα (Figure 3B, left), the whole-cell levels of Impα1 increased by ~2-fold at 10 pfu, 8-fold at 50 pfu and 11-fold at 100 pfu respectively. Conversely, the levels of cytoplasmic HuR decreased by ~60% at 50 pfu and 93% at 100 pfu after the infection. In polyamine-deficient cells (Figure 3B, right), infection with AdAMPKα decreased cytoplasmic HuR levels by ~47% at 10 pfu, ~75% at 50 pfu and ~91% at 100 pfu respectively. These findings indicate that increased AMPKα activity elevates Impα1 abundance and decreases the levels of cytoplasmic HuR in the presence or absence of cellular polyamines.
Polyamine depletion increases cytoplasmic HuR levels by inhibiting AMPK-regulated phosphorylation and acetylation of Impα1
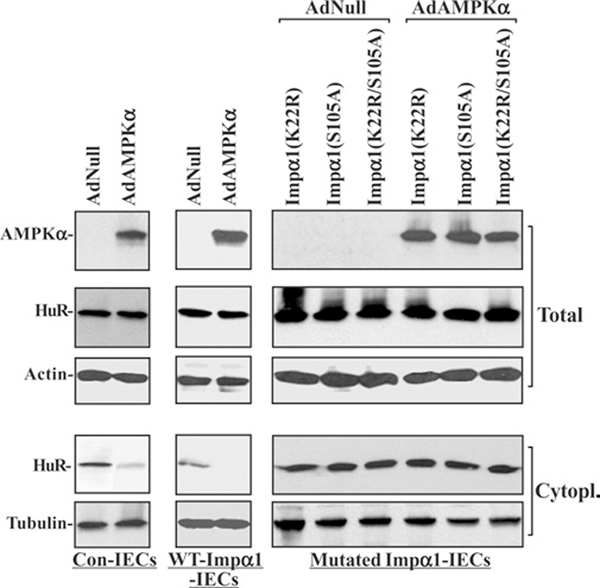
To determine whether increased levels of cytoplasmic HuR following polyamine depletion result from inactivation of AMPK-regulated phosphorylation and acetylation of Impα1, stable WT and point-mutant Impα1-transfected IECs (mutated Impα1-IECs) were developed and characterized. His-tagged Impα1 constructs were prepared from pcDNA3.1/V5-His-TOPO to express either WT-Impα1 or Impα1 carrying specific amino acid mutations: Impα1 mutants lacking Lys22 [Impα1(K22R)], Ser105 [Impα1(S105A)] alone or in combination [Impα1(K22R/S105A)] as described in our previous publication [34]. Previous studies showed that Ser105 and Lys22 in Impα1 are necessary for its phosphorylation and acetylation respectively and that point mutations in these modification sites impair the functional activity of Impα1 [32,34]. As shown in Figure 4, four clones resistant to the selection medium containing 0.6 mg/ml of G418 expressed high levels of either WT- or mutated-Impα1 as indicated by an increase in levels of their His tag. Overexpression of WT-Impα1 did not change total HuR levels, but it significantly reduced cytoplasmic HuR levels and increased HuR nuclear abundance. In contrast, IECs overexpressing Impα1 bearing mutations at K22R (which abrogated acetylation at Lys22), S105A (which abrogated phosphorylation at Ser105) or K22R/S105A (which prevented both modifications) displayed a significant increase in the levels of cytoplasmic HuR, although there were no changes in whole-cell HuR levels.
Figure 4. Effect of overexpression of WT and point-mutated Impα1 on subcellular distribution of HuR in IEC-6 cells.

His-tagged Impα1 constructs were generated from the vector pcDNA3.1/V5-His-TOPO to express either WT-Impα1 or Impα1 bearing point mutations. IEC-6 cells were transfected with the constructs indicated by using Lipofectamine™, and clones resistant to the selection medium were isolated and screened for Impα1 expression by Western-blot analysis using a specific anti-His-tag antibody. Total, cytoplasmic and nuclear proteins were prepared, and levels of HuR were measured by using a specific antibody. Equal loading in total, cytoplasmic and nuclear proteins was monitored by β-actin, β-tubulin and lamin B respectively. Three experiments were performed and they showed similar results.
Overexpression of Impα1 mutants, including Impα1(K22R), Impα1(S105A) and Impα1(K22R/S105A), prevented the AMPKα-induced nuclear import of HuR, as indicated by a decrease in cytoplasmic HuR levels in IECs. Ectopic expression of the AMPKα by infection with AdAMPKα decreased cytoplasmic HuR levels in both control cells (Figure 5, left) and cells expressing WT-Impα1 (Figure 5, middle), and had no effect on total HuR levels. However, neither cytoplasmic HuR levels nor its whole-cell levels were changed after overexpression of AMPKα in various stable IEC clones ectopically expressing Impα1 point mutants (Figure 5, right). There were no significant differences in the levels of cytoplasmic and total HuR between mutated Impα1-IECs infected with Adnull (lacking AMPKα cDNA) and those infected with AdAMPKα.
Figure 5. Effect of ectopic expression of AMPKα gene on the levels of cytoplasmic HuR in stable WT and point-mutated Impα1-transfected IEC-6 cells.
Control (Con) IEC-6 cells transfected with the empty vector (left), stable WT-Impα1-transfected cells (middle) and stable point-mutated Impα1-transfected cells (right) were infected with the AdAMPKα or Adnull at an MOI of 100 pfu per cell. Whole cell lysates were harvested 48 h after the infection, and levels of total and cytoplasmic HuR were measured by Western-blot analysis. Equal loading of samples was monitored by β-actin in total cell lysates and β-tubulin in cytoplasmic lysates. Three experiments were performed and they showed similar results.
The results presented in Figure 6 further show that inhibition of phosphorylation and acetylation of Impα1 by overexpression of its various mutants totally blocked the polyamine depletion-induced cytoplasmic translocation of HuR. In control IECs, depletion of cellular polyamines by treatment with DFMO for 6 days not only decreased AMPKα but also increased the cytoplasmic levels of HuR, which was completely prevented by putrescine given together with DFMO (Figure 6, left). Consistently, polyamine depletion also decreased the levels of AMPKα and increased cytoplasmic HuR levels in cells expressing WT-Impα1 (Figure 6, middle). On the other hand, in IEC clones expressing mutant Impα1 proteins, polyamine depletion by DFMO did not alter cytoplasmic HuR levels, although it significantly decreased the levels of total and cytoplasmic AMPKα (Figure 6, right). The levels of cytoplasmic HuR in the Impα1 mutant IEC clones exposed to DFMO alone or DFMO plus putrescine were indistinguishable from those observed in control cells. The finding that overexpression of all Impα1 mutants (K22R, S105A or both mutations) have similar inhibitory effects on the cytoplasmic translocation of HuR following polyamine depletion suggests that both phosphorylation and acetylation of Impα1 through AMPKα contribute to the polyamine-modulated subcellular trafficking of HuR in IECs.
Figure 6. Effect of polyamine depletion on the levels of cytoplasmic HuR in stable WT and point-mutated Impα1-transfected IEC-6 cells.

Whole cell lysates were harvested after control (Con) IEC-6 cells (left), stable WT-Impα1-transfected cells (middle) and stable point-mutated Impα1-transfected cells (right) were grown in the control cultures and cultures containing DFMO (5 mM) alone or DFMO plus putrescine (10 μM) for 6 days. Total and cytoplasmic proteins were prepared and levels of AMPKα and HuR were measured by Western-blot analysis. Immunoblottings of β-actin in total cell lysates and β-tubulin in cytoplasmic lysates were examined to serve as equal loading controls. Three experiments were performed and they showed similar results.
Based on the results presented here and our previous studies [26,34], we propose a model delineating the role of polyamines in the regulation of the subcellular localization of HuR in IECs (Figure 7). In this model, increased levels of cellular polyamines stimulate AMPK activity, in turn triggering HuR nuclear import through the activation of both phosphorylation (Ser105) and acelylation (Lys22) of Impα1, whereas polyamine depletion increases the cytoplasmic levels of HuR by inactivating the AMPK-mediated dual modifications of Impα1.
Figure 7. Proposed model depicting the role of cellular polyamines in modulating the subcellular localization of HuR through AMPK-regulated phosphorylation (P) and acetylation (Ac) of Imp-α1.
Increased polyamines result in AMPK activation that enhances the nuclear accumulation of HuR through a mechanism involving Impα1. The activation of AMPK by polyamines promotes Impα1-mediated nuclear import of HuR via modification of Impα1 on two residues. In addition, Impβ is also proposed to participate in the formation of HuR–Impα1 complexes for HuR nuclear import.
DISCUSSION
Increases in the levels of cellular polyamines enhance IEC proliferation, at least partially, by suppressing the expression of growth-inhibiting genes such as p53 [9], NPM [11] and junD [8,43,44], whereas decreases in the levels of cellular polyamines activate the expression of these growth-inhibiting genes. Our previous studies [3,8,9] and others [5,14,45] further indicate that polyamines negatively regulate expression of these growth-inhibiting genes at the post-transcriptional level and that depletion of cellular polyamines stabilizes these mRNAs without affecting their transcription rates [6,8,9]. Induced levels of nuclear p53, NPM and JunD in polyamine-deficient cells are shown to interact directly with their downstream target genes [e.g. they increase p21 but repress CDK4 (cyclin-dependent kinase 4)] [8,11,44], leading to increased arrest in the G1-phase of the cell cycle. Our recent studies [26] have further demonstrated that polyamine depletion increases HuR abundance in the cytoplasm, where it binds to the 3′-UTR of p53 and NPM, in turn controlling the stability of these mRNAs and influencing their protein levels. The silencing of HuR prevented the stabilization of p53 and NPM mRNAs and hence lowered p53 and NPM protein expression in polyamine-deficient cells. In the present study, we provide new evidence showing that polyamines modulate the subcellular trafficking of HuR in IECs through AMPK-regulated phosphorylation and acetylation of Impα1, thereby advancing our understanding of the molecular functions of cellular polyamines.
The results reported here show that cellular polyamines critically influence AMPK expression and activity. Depletion of cellular polyamines by inhibiting ODC with DFMO decreased AMPK protein and repressed its activity, as indicated by a reduction in pT172AMPKα levels (Figure 1). Because decreases in AMPK expression and its activity in DFMO-treated cells were completely prevented by the addition of exogenous putrescine, these observed changes in AMPK are most likely related to polyamine depletion rather than to the non-specific effect of DFMO. Furthermore, increases in the levels of cellular polyamines by ectopic ODC overexpression induced AMPK levels, which was predominantly located in the cytoplasm (Figure 2). Although AMPK was discovered almost three decades ago, details of its regulation have only recently begun to be elucidated. In mammalian cells, AMPK lies at the foot of an activating signal transduction cascade, in which the AMPKα subunit is phosphorylated on Thr172 by an upstream kinase [AMPKK (AMPK kinase)] [46]. Although not yet defined in molecular terms, an AMPKK activity has been purified from liver extracts [47], and is co-immunoprecipitated by antibodies against the β-subunit of AMPK, suggesting that two kinases, AMPKK and AMPK, are normally associated in a complex. In addition to covalent modification, AMPK activity is also activated directly by elevation of AMP and repressed by high concentrations of ATP [27,46]. Although how polyamines modulate AMPK expression and activity remains an open question, it is plausible that these compounds influence multiple distinct signalling pathways leading to alterations of gene transcription [12,13], post-transcriptional events [3,8], protein phosphorylation [48], and protein trafficking [49,50], at least in part through AMPK-elicited processes. Given the pleiotropic effects of AMPK on the cell’s metabolism, further efforts to elucidate the role of AMPK in the processes mediated by polyamines within the intestinal epithelium are warranted.
The results from the present study also show that polyamine-mediated AMPK modulates subcellular trafficking of HuR through a process involving Impα1 in IECs. It has been reported that Impα1 functions as an adaptor that associates with Impβ, which transports bound cargoes through the nuclear pore complex [51]. Recently, Impα1 was identified as a key cytoplasmic HuR ligand mediating nuclear import of HuR [33–35]. In the present study, inhibition of AMPK expression levels and function by polyamine depletion decreased Impα1 abundance (Figure 1), which was associated with an increase in the cytoplasmic accumulation of HuR but did not alter whole-cell HuR levels [3,26]. In contrast, increased AMPK by ODC overexpression induced Impα1 expression and decreased the levels of cytoplasmic HuR, with a corresponding increase in nuclear HuR abundance [26]. Activation of AMPK by either treatment with antimycin A or ectopic overexpression of a constitutively active isoform of AMPK not only increased Impα1 but also decreased cytoplasmic HuR levels regardless of the presence (controls) or absence (DFMO treatment for 6 days) of cellular polyamines (Figure 3), suggesting that decreased levels of Impα1 and subsequently increased levels of cytoplasmic HuR result primarily from AMPK inactivation following polyamine depletion. These findings are consistent with previous observations [33–35] that show that decreased AMPK activity led to an elevation in cytoplasmic HuR levels and that AMPK activation through interventions such as treatment with AICAR (5-amino-4-imidazolecarboxamide riboside) and ectopic expression of the AMPK gene results in a reduction of cytoplasmic HuR. The results presented in Figure 3(A) also show that treatment of IEC-6 cells with antimycin A significantly increased levels of total AMPKα protein regardless of the presence or absence of cellular polyamines. To our knowledge, this is the first report showing changes in total AMPKα protein after exposure to antimycin A. Consistent with the findings reported by others [27], treatment of IEC-6 cells with antimycin A also increased AMPKα phosphorylation. Although the mechanisms underlying the induction of AMPKα by antimycin A remain unknown, our results suggest that increased AMPK activation by antimycin A in IECs results from inductions of both total AMPKα protein levels and its phosphorylation. Because the decrease in cytoplasmic HuR in polyamine-deficient cells by transfection with its specific siRNA (small interfering RNA) is accompanied by a reduction in complexes of HuR bound to p53 and NPM mRNAs, a decrease in the stability of such mRNAs, a lessening of the expression of the corresponding protein products and an ensuing inhibition of cell growth [3,11,26], the subcellular distribution of HuR via an AMPK-regulated Impα1 pathway following changes in the levels of cellular polyamines is of great biological significance in maintaining the intestinal epithelial integrity.
The findings presented here further indicate that phosphorylation and acetylation of Impα1 are crucial for regulating the relative subcellular localization of HuR by polyamine-mediated AMPK activation. Impα1 has an N-terminal Impβ-binding domain that also includes an autoinhibitory region, followed by a nuclear localization sequence-binding domain and a C-terminal domain that is necessary for binding its export factor CAS (cellular apoptosis susceptibility protein) [52,53]. AMPK was found to phosphorylate Impα1 at Ser105 and also indirectly acetylate Impα1, through phosphorylation of p300, which in turn acetylates Impα1 at Lys22 [34]. Phosphorylation and/or acetylation of Impα1 increase its affinity for Impβ and also increase its affinity for nuclear import cargoes such as HuR [32,34]. Consistent with these observations, the results presented in Figure 4 show that overexpression of WT-Impα1, but not point mutants of Impα1 lacking the phosphorylation and acetylation sites, enhanced the nuclear import of HuR in IECs. In contrast, overexpression of Impα1 bearing mutations K22R (to disrupt its acetylation), S105A (to disrupt its phosphorylation) or K22R/S105A (to disrupt both modifications) displayed a significant increase in the levels of cytoplasmic HuR. Importantly, ectopic overexpression of different point mutants of Impα1, including K22R, S105A and K22R/S105A, also prevented the increased levels of cytoplasmic HuR following polyamine depletion in IEC-6 cells (Figure 6), suggesting that both AMPK-regulated phosphorylation and acetylation of Impα1 are required for the stimulation of HuR nuclear import following polyamine-mediated AMPK activation.
In summary, these results indicate that polyamines modulate the subcellular localization of HuR through AMPK-driven modification of Impα1 function in IECs. Depletion of cellular polyamines repressed AMPK expression and activity, whereas increased polyamines by ODC overexpression increased AMPK levels. In the absence of cellular polyamines, decreased levels of Impα1 were associated with an increase in the levels of cytoplasmic HuR, which was completely overcome by AMPK activation through ectopic expression of the WT AMPK gene. The results obtained in the present study further show that overexpression of Impα1 mutants, lacking either phosphorylation site, acetylation site, or both, suppressed the nuclear accumulation of HuR and also prevented the increased levels of cytoplasmic HuR in polyamine-deficient cells. Since cellular polyamines are highly regulated by the status of epithelial cell growth and stress stimuli, these findings suggest that polyamine-modulated HuR subcellular distribution through AMPK-regulated phosphorylation and acetylation of Impα1 plays an important role in regulating IEC proliferation and epithelial integrity under physiological and pathological conditions.
Acknowledgments
This work was supported by a Merit Review Grant (to J.-Y.W.) from the Department of Veterans Affairs and by NIH grants DK-57819, DK-61972, DK-68491 (to J.-Y.W.) and AI-68432 and AT-4148 (to H.Z.). J-Y.W. is a Research Career Scientist at the Medical Research Service, the U.S. Department of Veterans Affairs. M.G. is supported by the NIA-IRP, NIH.
Abbreviations used:
- AdAMPKα
pAdeno-AMPKα
- AMPK
AMP-activated protein kinase
- AMPKK
AMPK kinase
- ARE
AU-rich element
- DFMO
D,L-α-difluoromethylornithine
- FBS
fetal bovine serum
- HEK-293 cell
human embryonic kidney cell
- IEC
intestinal epithelial cell
- Impα1
importin α1
- MOI
multiplicity of infection
- NPM
nucleophosmin
- ODC
ornithine decarboxylase
- ODC-IEC cells
ODC-overexpressing IEC-6 cells
- pACC
phospho-acetyl-CoA carboxylase
- pAMPKα
phosphorylated AMPKα
- PBS-T
1 × PBS/Tween 20
- pfu
plaque-forming units
- RBP
RNA-binding protein
- WT
wild-type
- UTR
untranslated region
REFERENCES
- 1.Radtke F and Clevers H (2005) Self-renewal and cancer of the gut: two sides of a coin. Science 307, 1904–1909 [DOI] [PubMed] [Google Scholar]
- 2.Gregorieff A and Clevers H (2005) Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 19, 877–890 [DOI] [PubMed] [Google Scholar]
- 3.Wang JY (2007) Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 33, 241–252 [DOI] [PubMed] [Google Scholar]
- 4.Wang JY, McCormark SA, Viar MJ and Johnson LR (1991) Stimulation of proximal small intestinal mucosal growth by luminal polyamines. Am. J. Physiol. Gastrointest. Liver Physiol. 261, G504–G511 [DOI] [PubMed] [Google Scholar]
- 5.Casero RA Jr and Marton LJ (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 6, 373–390 [DOI] [PubMed] [Google Scholar]
- 6.Patel AR, Li J, Bass BL and Wang JY (1998) Expression of the transforming growth factor-β gene during growth inhibition following polyamine depletion. Am. J. Physiol. Cell Physiol. 275, C590–C598 [DOI] [PubMed] [Google Scholar]
- 7.Patel AR and Wang JY (1997) Polyamines modulate transcription but not posttranscription of c-myc and c-jun in IEC-6 cells. Am. J. Physiol. Cell Physiol. 273, C1020–C1029 [DOI] [PubMed] [Google Scholar]
- 8.Li L, Liu L, Rao JN, Esmaili A, Strauch ED, Bass BL and Wang JY (2002) JunD stabilization results in inhibition of normal intestinal epithelial cell growth through p21 after polyamine depletion. Gastroenterology 123, 764–779 [DOI] [PubMed] [Google Scholar]
- 9.Li L, Rao JN, Li J, Patel AR, Bass BL and Wang JY (2001) Polyamine depletion stabilizes p53 resulting in inhibition of normal intestinal epithelial cell proliferation. Am. J. Physiol. Cell Physiol. 281, C941–C953 [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Li L, Rao NJ, Zou T, Zhang HM, Boneva D, Bernard MS and Wang JY (2005) Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am. J. Physiol. Cell Physiol. 288, C89–C99 [DOI] [PubMed] [Google Scholar]
- 11.Zou T, Rao JN, Liu L, Marasa BS, Keledjian KM, Zhang AH, Lan X, Bass BL and Wang JY (2005) Polyamine depletion induces nucleophosmin modulating stability and transcriptional activity of p53 in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 289, C686–C696 [DOI] [PubMed] [Google Scholar]
- 12.Celano P, Baylin SB and Casero RA Jr (1989) Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J. Biol. Chem. 264, 8922–8927 [PubMed] [Google Scholar]
- 13.Wang JY, McCormack SA, Viar MJ, Wang HL, Tzen CY, Scott RE and Johnson LR (1993) Decreased expression of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 265, G331–G338 [DOI] [PubMed] [Google Scholar]
- 14.Kramer DL, Vujcic S, Diegelman P, Alderfer J, Miller JT, Black JD, Bergeron RJ and Porter CW (1999) Polyamine analogue induction of the p53–p21WAF1/CIP1–Rb pathway and G1 arrest in human melanoma cells. Cancer Res. 59, 1278–1286 [PubMed] [Google Scholar]
- 15.Zhang AH, Rao JN, Zou T, Liu L, Marasa BS, Xiao L, Chen J, Turner DJ and Wang JY (2007) p53-dependent NDRG1 expression induces inhibition of intestinal epithelial cell proliferation but not apoptosis after polyamine depletion. Am. J. Physiol. Cell Physiol. 293, C379–C389 [DOI] [PubMed] [Google Scholar]
- 16.Rao JN, Li L, Bass BL and Wang JY (2000) Expression of the TGF-β receptor gene and sensitivity to growth inhibition following polyamine depletion. Am. J. Physiol. Cell Physiol. 279, C1034–C1044 [DOI] [PubMed] [Google Scholar]
- 17.Garneau NL, Wilusz J and Wilusz CJ (2007) The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 8, 113–126 [DOI] [PubMed] [Google Scholar]
- 18.Abdelmohsen K, Pullmann R Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA et al. (2007) Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell 23, 543–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan J, Yang X, Wang W, Wood WH, Becker KG and Gorospe M (2002) Global analysis of stress-regulated mRNA turnover by using cDNA arrays. Proc. Natl. Acad. Sci. U.S.A. 99, 10611–10616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorospe M (2003) HuR in the mammalian genotoxic response: post-transcriptional multitasking. Cell Cycle 2, 412–414 [PubMed] [Google Scholar]
- 21.Zhu H, Zhou HL, Hasman RA and Lou H (2007) Hu proteins regulate polyadenylation by blocking sites containing U-rich sequences. J. Biol. Chem. 282, 2203–2210 [DOI] [PubMed] [Google Scholar]
- 22.Li XL, Andersen JB, Ezelle HJ, Wilson GM and Hassel BA (2007) Post-transcriptional regulation of RNase-L expression is mediated by the 3′-untranslated region of its mRNA. J. Biol. Chem. 282, 7950–7960 [DOI] [PubMed] [Google Scholar]
- 23.Lopez de Silanes IL, Zhan M, Lal A, Yang X and Gorospe M (2004) Identification of a target RNA motif for RNA-binding protein HuR. Proc. Natl. Acad. Sci. U.S.A. 101, 2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazan-Mamczarz K, Galban S, de Slianes IL, Martindale JL, Atasoy U, Keene JD and Gorospe M (2003) RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc. Natl. Acad. Sci. U.S.A. 100, 8354–8359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran H, Maurer F and Nagamine Y (2003) Stabilization of urokinase and urokinase receptor mRNAs by HuR is linked to its cytoplasmic accumulation induced by activated mitogen-activated protein kinase-activated protein kinase 2. Mol. Cell. Biol. 23, 7177–7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang AH, Xiao L, Pullmann R, Gorospe M and Wang JY (2006) Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J. Biol. Chem. 281, 19387–19394 [DOI] [PubMed] [Google Scholar]
- 27.Rutter GA, Silva Xavier GS and Leclerc I (2003) Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem. J. 375, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung PC, Salt IP, Davies SP, Hardie DG and Carling D (2000) Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem. J. 346, 659–669 [PMC free article] [PubMed] [Google Scholar]
- 29.Stapleton D, Woollatt E, Mitchelhill KI, Nichol JK, Fernandez CS, Michell BJ, Witters LA, Power DA, Sutherland GR and Kem BE (1997) AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Lett. 409, 452–456 [DOI] [PubMed] [Google Scholar]
- 30.Hardie DG, Carling D and Carlson M (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell?. Annu. Rev. Biochem. 67, 821–855 [DOI] [PubMed] [Google Scholar]
- 31.Hardie DG and Hawley SA (2001) AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays 23, 1112–1119 [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Fan J, Yang X, Furer-Galban S, de Silanes IL, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG et al. (2002) AMP-activated kinase regulates cytoplasmic HuR. Mol. Cell. Biol. 22, 3425–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Chantar ML, Vazqez-Chantada M, Garnacho M, Latasa MU, Varela-Rey M, Dotor J, Santamaria M, Martinez-Cruz LS, Parada LA, Lu SC and Mato JM (2006) S-adenosylmethionine regulates cytoplasmic HuR via AMP-activated kinase. Gastroenterology 131, 223–232 [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Yang X, Kawai T, de Sianes IL, Mazan-Mamczarz K, Chen P, Chook YM, Quensel C, Kohler M and Gorospe M (2004) AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1: involvement in the nuclear import of RNA-binding protein HuR. J. Biol. Chem. 279, 48376–48388 [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Yang X, de Silanes IL, Carling D and Gorospe M (2003) Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J. Biol. Chem. 278, 27016–27023 [DOI] [PubMed] [Google Scholar]
- 36.Ghoda L, van Daalen Wetters T, Macrae M, Ascherman D and Coffino P (1989) Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science 243, 1493–1495 [DOI] [PubMed] [Google Scholar]
- 37.Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 39.Vielkind U and Swierenga SH (1989) A simple fixation procedure for immunofluorescent detection of different cytoskeletal components within the same cell. Histochemistry 91, 81–88 [DOI] [PubMed] [Google Scholar]
- 40.Guo X, Rao JN, Liu L, Zou T, Keledjian KM, Boneva D, Marasa BS and Wang JY (2005) Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1159–G1169 [DOI] [PubMed] [Google Scholar]
- 41.Harter JL (1960) Critical values for Duncan’s new multiple range test. Biometric 16, 671–685 [Google Scholar]
- 42.Liu L, Santora R, Rao JN, Guo X, Zou T, Zhang HM, Turner DJ and Wang JY (2003) Activation of TGF-β-Smad signaling pathway following polyamine depletion in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G1056–G1067 [DOI] [PubMed] [Google Scholar]
- 43.Patel AR and Wang JY (1999) Polyamine depletion is associated with an increase in JunD/AP-1 activity in small intestinal crypt cells. Am. J. Physiol. Gastrointest. Liver Physiol. 276, G441–G450 [DOI] [PubMed] [Google Scholar]
- 44.Xiao L, Rao JN, Zou T, Liu L, Marasa BS, Chen J, Turner DJ, Passaniti A and Wang JY (2007) Induced JunD in intestinal epithelial cells represses CDK4 transcription through its proximal promoter region following polyamine depletion. Biochem. J. 403, 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grudzien E, Kalek M, Jemielity J, Darzynkiewicz E and Rhoads RE (2005) Differential inhibition of mRNA degradation pathways by novel cap analogs. J. Biol. Chem. 281, 1857–1867 [DOI] [PubMed] [Google Scholar]
- 46.Hardie DG, Salt IP, Hawley SA and Davies SP (1999) AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem. J. 338, 717–722 [PMC free article] [PubMed] [Google Scholar]
- 47.Hawley SA, Davison M, Wood A, Davies SP, Beri RK, Carling D and Hardie DG (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 271, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 48.Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ and Wang JY (2004) Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J. Biol. Chem. 279, 22539–22547 [DOI] [PubMed] [Google Scholar]
- 49.Li L, Rao JN, Bass BL and Wang JY (2001) NF-κB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G992–G1004 [DOI] [PubMed] [Google Scholar]
- 50.Pfeffer L, Yang CH, Murti A, McCormack SA, Viar MJ, Ray RM and Johnson LR (2001) Polyamine depletion induces rapid NF-κB activation in IEC-6 cells. J. Biol. Chem. 276, 45909–45913 [DOI] [PubMed] [Google Scholar]
- 51.Kohler M, Speck C, Christiansen MM, Bischoff FR, Prehn S, Haller H, Gorlich D and Hartmann E (1999) Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol. Cell. Biol. 19, 7782–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chook YM and Blobel G (2001) Karyopherins and nuclear import. Curr. Opin. Struct. Biol. 11, 703–715 [DOI] [PubMed] [Google Scholar]
- 53.Fanara P, Hodel MR, Corbett AH and Hodel AE (2000) Quantitative analysis of nuclear localization signal (NLS)–importin-α interaction through fluorescence depolarization. Evidence for auto-inhibitory regulation of NLS binding. J. Biol. Chem. 275, 21218–21223 [DOI] [PubMed] [Google Scholar]
- 54.Quaroni A, Wands J, Trelstad RL and Isselbacher KJ (1979) Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J. Cell Biol. 80, 248–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Guo X, Rao JN, Zou T, Marasa BS, Chen J, Greenspon J, Casero RA Jr and Wang J-Y (2006) Polyamine-modulated c-Myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region. Biochem. J. 398, 257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]