Abstract
We describe a previously healthy, 22-year-old man who, after a closed fracture of the femur and subsequent operation, developed chronic osteomyelitis. Within a few days, infected bone fragments, bone, and wound drainage repeatedly yielded three different filamentous fungi: Aspergillus fumigatus, Aspergillus flavus, and Chalara ellisii. Histologic examination of the bone revealed septate hyphae. After sequential necrotomies of the femur and irrigation-suction drainage with added antimycotic therapy, the infection ceased and the fracture healed. This case is unique in that it is the only known instance in which a long bone was affected in an immunocompetent individual, with no evidence of any systemic infection, by a mixed population of two different Aspergillus spp. and the rare filamentous fungus C. ellisii. Environmental factors that could potentiate the infection include blood and edema fluid resulting from the surgical procedure and the presence of the osteosynthetic plate.
Fungi have emerged as significant pathogens only during the past few decades. Before the mid 1960s, accounts of fungal infections were mainly limited to sporadic case reports and autopsy series spanning many years (2, 11).
Currently the number of fungal species reported as being opportunistic agents of human disease is increasing by about 10 to 15 per year (8). Possible explanations are the general availability of broad-spectrum parenteral antibiotics, which results in fungal superinfections (5), and an increasing number of immunocompromised patients.
Fungi of the genus Aspergillus are ubiquitous in nature and are normally harmless inhabitants of the upper respiratory tract (19). Occasionally they can cause human disease, and they thus have been classified as opportunistic pathogens (8). These infections are most commonly seen in immunocompromised hosts, and they involve the skin, lungs, gastrointestinal tract, heart, thyroid, and brain, or they disseminate (3, 7, 23).
Aspergillus species are seldom mentioned as etiologic agents of osteomyelitis. When they are, it is mainly a consequence of immunosuppression, malignancy with neutropenia, transplantation, use of antibiotics and corticosteroids, or occasionally trauma followed by surgical manipulation (9, 11). Aspergillus osteomyelitis usually results from direct invasion from a contiguous infection such as a pulmonary or nasal sinus abscess, and it almost always involves the ribs, spine, or orbit (3, 9). In adults, the vertebrae are the most frequent and common site of osteomyelitis, which sometimes causes spinal cord compression (10, 23). Although secondary destruction of long bones by Aspergillus has been occasionally described, in very few cases did the bones show primary involvement (9). Posttraumatic long-bone aspergillosis in immunocompetent hosts is extremely rare, as are infections with multiple fungal species (2, 3, 9, 10).
This paper reports the only known occurrence of a primary mixed Aspergillus and Chalara osteomyelitis in one of the long bones in a healthy individual with no evidence of any other primary fungal infection.
CASE REPORT
A 22-year-old man sustained a closed transverse fracture of the left femur in a traffic accident. Immediately after the admission to the trauma center, ostheosynthesis of the fractured femur was performed. The operative wound healed per primam, and the patient’s treatment continued with standard physiotherapy. After 10 days, he developed fever and his left thigh became reddish and painful. No clinical signs of abscess were evident. The patient received broad-spectrum antibiotic therapy: a combination of gentamicin (120 mg every 8 h) and metronidazole (500 mg every 6 h). At discharge from the hospital, he did not show any signs of infection. Two months later he was readmitted because of a spontaneous refracture of the left femur and the implant. A second ostheosynthesis was performed, and a 3- by 5-mm bone particle was taken for histologic analysis. Although it showed chronic inflammation, the operative wound healed without further complications. Three months later a fistulation on the postoperative wound appeared. On classical tomography, several avascular necrotic fragments of the bone near the fracture line were seen. The ostheosynthetic plate was operatively removed, bone necrectomy was performed, the fracture gap was bridged by using an external fixator (Orthofix), and suction-irrigation drainage with Ringer solution and gentamicin was started. Samples of fluid and necrotic bone fragments were taken from the fracture site for microbiological and histologic analyses. The irrigation drainage was continued for 7 days. The outgoing fluid was sampled for microbiological analyses after 5 days and again after 7 days, when the inflow had been removed and only the outflow remained. The fluid showed presence of methicillin-susceptible Staphylococcus aureus, Aspergillus fumigatus, Aspergillus flavus, and Chalara ellisii. Within 3 days the incubated bone fragments yielded two different fungi, which were later identified as A. fumigatus and A. flavus. Three months later the patient was readmitted because of loosened external fixator screws. The external fixator was changed, autologous spongy bone was inserted, and suction-irrigation drainage with added amphotericin B was performed for 10 days. The patient was than discharged without any signs of chronic inflammation. The fracture healed 4 months later, and in 3 months the external fixator was removed. One year later the patient did not show any signs of chronic osteitis, and his left lower limb was functioning well.
MATERIALS AND METHODS
Evaluation of immune competence.
The basic screening laboratory tests of immune competence, including tests of T cells, B cells, natural killer (NK) cells, complement, and phagocytes, were performed (FACSort; Becton Dickinson).
Microbiological studies.
The cortical bone fragment removed during necrectomy was both plated and spread on potato dextrose agar (PDA) nutrient medium, with 0.05 g of added chloramphenicol per liter for bacterial growth prevention. Samples of the irrigation-suction drainage outgoing liquid were inoculated on PDA, Sabouraud glucose agar, and malt yeast extract agar media (7), as well as filtered with a 0.45-μm-pore-size membrane filter (Millipore), and subsequently placed on PDA medium and incubated at 37°C for 30 days.
All isolated fungal cultures were identified and deposited at the Microbiological Culture Collection of the National Institute of Chemistry (MZKI), Ljubljana, Slovenia. Identifications were also confirmed by the International Mycological Institute, Egham, United Kingdom, and the Chalara strain was also confirmed at Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
Histology.
Microscopic observations of the bone particles were performed after staining with standard hematoxylin-eosin, methenamine silver, and periodic acid-Schiff stain.
RESULTS
The patient was a healthy young man who had never had symptoms and signs related to immunodeficiency. He had had no recurrent infections or infections caused by uncommon bacterial, fungal, protozoal, or viral organisms. The total peripheral leukocyte count and differential were normal. There was no granulocytopenia, which predisposes patients to fungal infections. The percentages of circulating B cells, NK cells, T cells, and the two major T cell subsets, CD4+ and CD8+ cells, remained within the normal range, as did the quantitative immunoglobulin G (IgG), IgM, and IgA levels. Antibodies against tetanus toxoid and measles virus were present in high titers. Hemolytic complement activity (CH50) was normal. Results of the nitroblue tetrazolium test and chemiluminescense were within the normal ranges, indicating normal phagocytic function. With the thorough history, a complete physical examination, and laboratory assessment of host defenses, recognizable risk factors for fungal infections were ruled out.
However, microscopic observations of bone fragments, after staining with standard hematoxylin-eosin, showed chronic osteitis with irregular destruction of cortial and trabecular bone. The cavity was filled with necrotic debris, leukocytes, and mononuclear cells. Staining with methenamine silver and the periodic-acid Schiff stain revealed uniform, hyaline, septate, acutely branching hyphae, consistent with Aspergillus, between fragments of necrotic bone, scattered through the edematous fibrovascular tissue around the central cavity. Hyphae invaded sections of the cortical bone with communication between the marrow cavity and adjacent muscle.
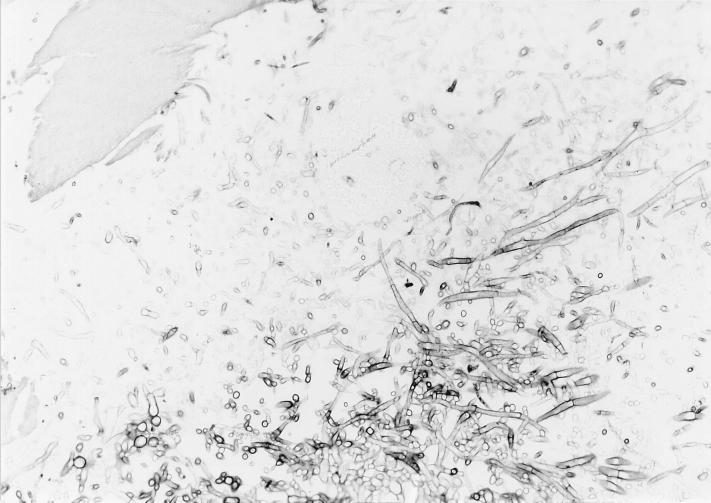
After 3 days of incubation of the cortical bone fragment on PDA at 37°C, the microbiological analyses showed a rapidly growing fungus (Fig. 1), which was later identified as A. flavus MZKI A-403. The fungal growth started on the bone fragment, completely overgrew it in 2 days, and from there spread to the entire plate. Similar colonies appeared on the PDA medium after the bone fragments were spread over the surface.
FIG. 1.
Histologic preparation (periodic acid-Schiff reaction; magnification, ca. ×125) of the bone fragment after incubation for 3 days at 37°C on PDA medium. Note the fungal hyphae.
The irrigation-suction drainage outgoing liquid was analyzed as well. After 12 days of incubation of the outgoing fluid at 37°C on the PDA medium, only two different colonies appeared. They started to sporulate after 2 days and were identified as A. fumigatus (MZKI A-402) and A. flavus (MZKI A-403). No fungal colony appeared from the incubated filter membrane after filtration of the outgoing fluid even after 30 days on PDA. After 5 days of irrigation-suction drainage, the inflow was removed and only the outgoing fluid remained, which was inoculated again onto PDA medium and filtered as well. After 10 days of incubation of the outgoing fluid at 37°C, 12 colonies were observed and were individually isolated on the PDA medium. Six of them were identified as A. fumigatus, five were identified as A. flavus, and one was identified as C. ellisii (MZKI B-726). The last fungus also appeared on the incubated filter on the PDA medium after 10 days of incubation, together with an A. fumigatus colony.
Mycology.
Colonies of A. fumigatus MZKI A-402 grew quickly as dark blue-green colonies with a felt-like surface. Conidiophores were smooth walled and green in the upper part. Conidial heads were columnar and uniseriate. Vesicles were broadly clavate and 20 to 25 μm wide. Conidia were verrucose, spherical, and subspherical, from 2.5 to 3.0 μm in diameter.
Colonies of A. flavus MZKI A-403 were rapidly growing and yellow-greenish and had a felt-like surface. Microscopic examination revealed the presence of uniseriate or biseriate phialides covering the entire surface of a spherical vesicle 25 to 40 μm in diameter; conidiophors were typically coarsely roughened, and conidia were globose to subglobose, pale green, conspicously echinulate, and 3.5 to 4.5 μm.
Colonies of C. ellisii MZKI B-726 grew slowly and superficially, with effuse, grayish brown, and woolly mycelium. Phialophores were simple, cylindrical, septate, 30 to 150 μm long, and 2.5 to 3 μm wide. They were dark brown and verrucose at the base, becoming paler above and terminating in a subcylindrical phialide. A subcylindrical collarette was present. After isolation from the incubated suction drainage liquid, the strain was sporulating, producing cylindrical, unicellular, and hyaline phialoconidia with smooth walls, 3 to 12 by 1.5 to 2 μm, which were extruded singly. During successive serial transfers, the strain lost its sporulation ability.
DISCUSSION
A review of the literature on fungal osteomyelitic infections revealed fewer than 40 cases (3, 9, 10, 23). A. fumigatus was the most commonly isolated species (1, 9). Other Aspergillus spp. isolated were A. flavus, A. nidulans, A. terreus, A. niger, and A. flavipes (23).
The definitive documentation of bone infection due to an ordinary, ubiquitous saprophytic fungus requires three procedures: (i) cultural isolation and accurate identification of the organisms, preferably repeated, from the actual site of infection; (ii) direct microscopic demonstration of the pathogenic invasive form of the fungus in material from the site where the cultures were obtained (a pus smear or histologic section of the tissue); and (iii) correlation of the results of the culture to the osteomyelitic process (20). In this case, prior to the installation of the irrigation-suction drainage, S. aureus was isolated once as well, but it did not appear when the successive microbiological analyses were performed. Both Aspergillus species were repeatedly isolated from the necrotic bone fragments as well as from the irrigation-suction drainage liquid. C. ellisii was isolated only from the irrigation-suction drainage outgoing fluid and was not observed in the bone fragments, even though it is a melanized fungus and its hyphae should appear darker in the bone tissue. Melanized fungi in early developmental stages may be uncolored (22); thus, Ramos et al. (18) suggest Fontana-Masson staining, which was not performed in this case. Therefore, C. ellisii does not meet all requirements of being a bone infection etiologic agent in this case (20). Although fungi can occur in multiple infections (2), especially in opportunistic infections, the etiologic role of the individual fungus is unknown. C. ellisii is otherwise a rare anamorphic fungus, which has so far been isolated only once from soil in Canada (14), and therefore its clinical significance is unknown.
Opportunistic pathogens more frequently involved in osteomyelitis have a preference for environments with low water activity (aw) (6). The isolated Aspergillus species are considered to be xerotolerant fungi, adapted to grow in media with low aw potential: A. fumigatus at aws of 0.85 to 0.94 and A. flavus at aws of 0.78 to 0.80 (14).
In most cases of fungal osteomyelitis, gross defects in immunocompetence are present. Among children, chronic granulomatous disease is the most common defect, and among adults, immunosuppressive drug therapy is the most important predisposing condition (9, 12, 23). In healthy individuals the skin and mucous membranes are first-line barriers against infections, but these barriers are regularly violated by surgeons during operative procedures. Thus, fungal osteoarthritis and osteomyelitis following surgical procedures or trauma have been occasionally described (2, 16). Surgery (in particular multiple procedures of the abdomen) after trauma, burns, and treatment with broad-spectrum antibiotics are conditions associated with a significant compromise of one or more host defenses (11, 21). Descriptions in the literature of osteomyelitis cases caused by Aspergillus show that surgical debridement of the infection site together with the administration of systemic antimycotics has a higher success rate than therapy without surgery, which may be due to low penetration of most drugs into the bone tissue (9, 10). In the case of multiple infections, a continued therapy with different antimycotics should be applied (2).
Operative procedures on otherwise healthy patients can transiently inhibit systemic host defenses. Local tissue environmental factors and their duration may also play a role in the pathogenesis of infection, because these factors may further inhibit host defense. Fungal infections tend to occur late after an operation (1 to 3 months) (11), as in this case. Although not immunosuppressing in the commonly used sense, the presence of a prosthetic device or surgical wound may well predispose patients, even otherwise healthy individuals, to such infections (5, 11, 23). It is known that the presence of implants increases the incidence of gram-negative bacterial infections (4, 17). The relationship between internal fixation locally decreasing immunocompetence and the exact role of the simultaneous presence of various infecting organisms is at the moment unknown and deserves further exploration (4).
ACKNOWLEDGMENTS
We thank S. Kozakiewicz (International Mycological Institute) and W. Gams (Centraalbureau voor Schimmelcultures) for helping with fungal taxonomic identifications, G. S. de Hoog for carefully reading the manuscript, J. Tomazic for performing immunocompetence tests, and B. Kastelic for technical assistance.
REFERENCES
- 1.Alvarez L, Calvo E, Abril C. Articular aspergillosis: case report. Clin Infect Dis. 1995;20:457–460. doi: 10.1093/clinids/20.2.457. [DOI] [PubMed] [Google Scholar]
- 2.Bodey G P, Anaissie E J. Opportunistic infections: a major problem in immunocompromised patients. In: Richardson R G, editor. Opportunistic fungal infections: focus on fluconazole. International Congress and Symposium series 153. London, United Kingdom: Royal Society of Medicine; 1989. pp. 1–16. [Google Scholar]
- 3.Casscells S W. Aspergillus osteomyelitis of the tibia. J Bone Joint Surg. 1978;60:994–995. [PubMed] [Google Scholar]
- 4.Chapman M W. Open fracture: future directions. In: Lane J M, editor. Bristol-Myers/Zimmer Orthopaedic Symposium—Fracture healing. New York, N.Y: Churchill Livingstone; 1987. pp. 261–266. [Google Scholar]
- 5.Cohen M, Bonfiglio, Campbell C J. Orthopedic pathophysiology in diagnosis and treatment. New York, N.Y: Churchill Livingstone; 1996. Inflammation and infection; pp. 19–52. [Google Scholar]
- 6.de Hoog G S, Tan C S, Stalpers J A, Stegehuis G. The spectrum of opportunistic filamentous fungi present in the CBS culture collection. Mycoses. 1992;35:209–214. doi: 10.1111/j.1439-0507.1992.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 7.de Hoog G S, Guarro J, editors. Atlas of clinical fungi. Baarn, The Netherlands: Centraalbureau voor Shimmelcultures/Universitat Rovira I Virgili; 1995. [Google Scholar]
- 8.de Hoog G S. Risk assessment of fungi reported from humans and animals. Mycoses. 1996;39:407–417. doi: 10.1111/j.1439-0507.1996.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 9.de Vuyst D, Surmont I, Verhaegen J, Vanhaecke J. Tibial osteomyelitis due to Aspergillus flavus in heart transplant patient. Infection. 1992;20:48–49. doi: 10.1007/BF01704898. [DOI] [PubMed] [Google Scholar]
- 10.Hoore K D, Hoogmartens M. Vertebral aspergillosis. A case report and review of the literature. Acta Orthopaed Belg. 1993;59:306–314. [PubMed] [Google Scholar]
- 11.Howard R J. Infections in the immunocompromised patients. Surg Clin N Am. 1994;74:620. [PubMed] [Google Scholar]
- 12.Meis J F G M, Kullberg B, Pruszczynski M, Veth R P H. Severe osteomyelitis due to the zygomycete Apophysomyces elegans. J Clin Microbiol. 1994;32:3078–3081. doi: 10.1128/jcm.32.12.3078-3081.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller J. Pathogenesis of opportunistic fungal infections. In: Richardson R G, editor. Opportunistic fungal infections: focus on fluconazole. International Congress and Symposium series 153. London, United Kingdom: Royal Society of Medicine; 1989. pp. 25–28. [Google Scholar]
- 14.Nag Raj T R, Kendrick B. A monograph of Chalara and allied genera. Waterloo, Ontario, Canada: Wilfrid Laurier University Press; 1975. pp. 113–115. [Google Scholar]
- 15.Northolt M D, Frisvad J C, Samson R A. Occurrence of food-borne fungi and factors for growth. In: Samson R A, Hoekstra E S, Frisvad J C, Filtenborg O, editors. Introduction to food-borne fungi. 4th ed. Wageningen, The Netherlands: Ponsen & Looyen; 1995. pp. 243–250. [Google Scholar]
- 16.Padhye A A, Godfrey J H, Chandler F W, Peterson S W. Osteomyelitis caused by Neosartorya pseudofischeri. J Clin Microbiol. 1994;32:2832–2836. doi: 10.1128/jcm.32.11.2832-2836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patzakis M J, Wilkins J, Kumar J, Holtom P, Geenbaum B, Ressler R. Comparison of the results of bacterial cultures from multiple sites in chronic osteomyelitis of long bones. J Bone Joint Surg. 1994;76:664–666. doi: 10.2106/00004623-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Ramos A M, Sales D O, de Andrade M C, Bittencourt J F, Ramos C C. A simple method for detecting subcutaneous phaeohyphomycosis with light-colored fungi. A study of eight cases. Am J Surg Pathol. 1995;19:109–114. doi: 10.1097/00000478-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Raper K B, Fennel D I. The genus Aspergillus. Baltimore, Md: The Williams and Wilkins Company; 1965. [Google Scholar]
- 20.Simpson M B, Merz W G, Kurlinski J P, Solomon M. Opportunistic mycotic osteomyelitis: bone infection due to Aspergillus and Candida species. Medicine. 1977;56:475–482. [PubMed] [Google Scholar]
- 21.Sugar A M. Problems in the diagnosis of invasive candidiasis in the immunocompromised patient. In: Richardson R G, editor. Opportunistic fungal infections: focus on fluconazole. International Congress and Symposium series 153. London, United Kingdom: Royal Society of Medicine; 1989. pp. 17–24. [Google Scholar]
- 22.Sutton D A, Slifkin M, Yakulis R, Rinaldi M G. U.S. case report of cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (R. mackenziei): criteria for identification, therapy, and review of other known dematiaceous neurotropic taxa. J Clin Microbiol. 1998;36:708–715. doi: 10.1128/jcm.36.3.708-715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tack K J, Rhame F S, Brown B, Thompson R C. Aspergillus osteomyelitis. Report of four cases and review of the literature. Am J Med. 1982;73:295–300. doi: 10.1016/0002-9343(82)90192-9. [DOI] [PubMed] [Google Scholar]