Abstract
Despite the changing paradigms of melanoma treatment in recent years, there remains a relative paucity of data regarding subungual melanoma in the literature. From 2002–2018, 25 patients with subungual melanoma were surgically treated at our facility. A retrospective chart review was conducted to collect relevant demographic, clinical, pathologic, and outcomes data. The median age at diagnosis was 69 years. Most patients (60%) were male, and the melanoma lesion was most often located on the foot (68%). Acral-lentiginous was the most common histologic subtype (59%), and the median Breslow thickness was 3.4 mm. Fifteen patients (63%) underwent a sentinel lymph node biopsy as part of their surgical resection, and four of these patients (27%) had metastatic disease in the lymph nodes. In total, 10 patients underwent lymph node dissection of the involved basin. The median follow up was 21 months in this patient population. Age, gender, tumor location, ulceration, and lesion histology were not significantly associated with recurrence free survival (RFS). Increasing Breslow thickness was found to be significantly associated with shorter RFS (HR: 1.07, CI: 1.03–1.55). In total, 13 patients developed a disease recurrence, and RFS rates were 66% at 1 year and 40% at 3 years. Additionally, 91 and 37% of patients were alive at one year and three years, respectively. Subungual melanomas are rare lesions that often have a more advanced stage at diagnosis, which contributes to the poor prognosis of these cutaneous malignancies.
Keywords: subungual, melanoma, acral lentiginous, nail, skin cancer
1. Introduction
The melanoma treatment landscape has undergone widespread changes in recent years with the emergence of immune checkpoint blockade and therapies targeting the BRAF pathway [1,2], the Food and Drug Administration (FDA) approval of the first oncolytic virus (Talimogene laherperepvec [T-VEC]) [3], and the move away from completion lymph node dissections secondary to the results of the second Multicenter Selective Lymphadenectomy Trial (MSLT-2) [4]. However, despite these shifts in treatment paradigms, there remains a relative paucity of data regarding subungual melanomas in the literature.
Subungual melanomas arise from the nail apparatus [5] and are rare tumors with an annual incidence of approximately 0.1 per 100,000 individuals [6], which accounts for up to 3% of all cutaneous melanomas [7]. Interestingly, there are differences in the rates of subungual melanoma between different racial and ethnic groups where they account for approximately 1–2% of all cutaneous melanomas in non-Hispanic Caucasians, but they can account for upwards of 20% in Asian and African American populations [6,8,9,10]. Both whole genome and next generation sequencing have revealed that acral melanomas harbor mutational signatures that are unique from other cutaneous melanomas (often a lower tumor mutational burden), demonstrating less reliance on the BRAF pathway and ultraviolet radiation as sources of carcinogenesis [11,12]. Additionally, hair salon UV lamps and recurrent trauma have been suggested as having a potential role in the pathogenesis of subungual melanoma [13,14].
Subungual melanomas present unique clinical challenges, in part due to diagnostic difficulties resulting in delays in diagnosis or misdiagnosis [15,16]. Due to these challenges, many subungual melanomas are identified at later stages and consequently have worse prognoses and survival outcomes compared to cutaneous melanomas subtypes [10,17]. Five year overall survival rates have been reported to range from 15–59%, depending on the series [17,18].
In this report, we describe our institutional series of 25 subungual melanoma patients, including the demographic makeup, clinicopathologic characteristics, and treatment factors associated with survival.
2. Materials and Methods
2.1. Patients
From 2002–2018, 25 patients with subungual melanoma were surgically treated at City of Hope National Medical Center, a National Cancer Institute (NCI) designated Comprehensive Cancer Center. After obtaining approval from our Institutional Review Board (IRB), we conducted a retrospective chart review to collect relevant demographic, clinicopathologic, treatment-related, and outcome data. Recurrence free survival (RFS) was calculated as the time from the date of diagnosis to the first evidence of disease recurrence. If the patient did not experience a recurrence, they were censored at their last tumor follow-up date. Overall survival was calculated from their date of diagnosis to their date of death. Patients alive at last contact were censored at their last contact date.
2.2. Statistical Analysis
Descriptive statistics were used to characterize the patient population. Univariate Cox proportional hazards models were used to determine the association between demographic and clinical risk factors with recurrence-free and overall survival. Survival was further characterized using Kaplan–Meier plots. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Demographic and Histopathologic
There were 25 patients included in this retrospective study with a median age at diagnosis of 69 years (Table 1). The majority of patients were male (60%), and the melanoma lesion was located on the foot (68%) more often than on the hand. Of those patients with histology reports available for review (17 of 25), acral-lentiginous was the most common histologic subtype (59%), followed by nodular (18%) and superficial spreading (6%). Additionally, 18% of patients had in situ disease. For those patients with Breslow thickness available for review (18 of 25), the median thickness was 3.4 mm, with 50% of the patients having intermediate thickness melanomas (2–4 mm) and 30% having a thick melanoma (>4 mm). When recorded in the pathology reports, 76% of lesions were found to be ulcerated and 69% had an elevated mitotic index (>1 per mm2). No patient had AJCC Stage IV disease, and the majority of patients (52%) were found to have Stage III disease after their initial surgical resection.
Table 1.
Baseline Patient Characteristics.
| All | ||
|---|---|---|
| N = 25 | ||
| N (%) | ||
| Age at Diagnosis | Median (IQR+) | 69 (58–76) |
| Sex | Male | 15 (60) |
| Female | 10 (40) | |
| Histologic Subtype | Acral-lentiginous | 10 (59) |
| Melanoma in situ | 3 (18) | |
| Nodular | 3 (18) | |
| Superficial Spreading | 1 (6) | |
| Unknown/NR | 8 | |
| Breslow Thickness (mm) | Median (IQR+) | 3.4 (2.3–4.3) |
| Breslow Thickness (mm) | <2 | 4 (20) |
| 2–4 | 10 (50) | |
| >4 | 6 (30) | |
| In situ | 3 | |
| Unknown/NR | 2 | |
| AJCC Stage | 0 | 3 (13) |
| I | 2 (9) | |
| II | 6 (26) | |
| III | 12 (52) | |
| IV | 0 | |
| Unknown/NR | 2 | |
| Laterality | Right | 10 (40) |
| Left | 15 (60) | |
| Hand/Foot | Hand | 8 (32) |
| Foot | 17 (68) | |
| Ulceration | Present | 13 (76) |
| Absent | 4 (24) | |
| Unknown/NR | 8 | |
| PNI | Yes | 1 (4.0) |
| No | 8 (32.0) | |
| Unknown/NR | 16 (64.0) | |
| LVI | Yes | 2 (8.0) |
| No | 8 (32.0) | |
| Unknown/NR | 15 (60.0) | |
| Mitotic Index (>1 per mm2) | Present | 9 |
| Absent | 4 | |
| Unknown/NR | 12 | |
| SLNB Performed | Yes | 15 (63) |
| No | 9 (38) | |
| Unknown/NR | 1 | |
| Lymph Node Dissection | Yes | 10 (42) |
| No | 14 (58) | |
| Unknown/NR | 1 | |
| Received Adjuvant Therapy | Yes | 8 (33) |
| No | 16 (67) | |
| Disease Recurrence | Yes | 13 (52) |
| No | 12 (48) | |
IQR: Interquartile Range, NR: Not Recorded, PNI: Perineural Invasion, LVI: Lymphovascular Invasion, SLNB: Sentinel Lymph Node Biopsy.
3.2. Regional and Systemic Management
Fifteen patients (63%) underwent a sentinel lymph node biopsy as part of their surgical resection. Of the remaining 10 patients, three had in situ disease, six went directly to a completion lymph node dissection (due to the presence of clinically positive/palpable lymph nodes upon initial presentation), and one patient’s sentinel node status was unknown. Four patients (27%) who underwent a sentinel lymph node biopsy had evidence of metastatic disease upon pathologic review and subsequently underwent a completion lymph node dissection of the involved basin. Eight patients (33%) went on to receive adjuvant therapy in the form chemotherapy (dacarbazine-based), immunotherapy (interferon alpha, ipilimumab, or pembrolizumab), radiation therapy to the regional nodal basin, or a melanoma vaccine trial (Supplementary Table S1).
3.3. Recurrence Free Survival
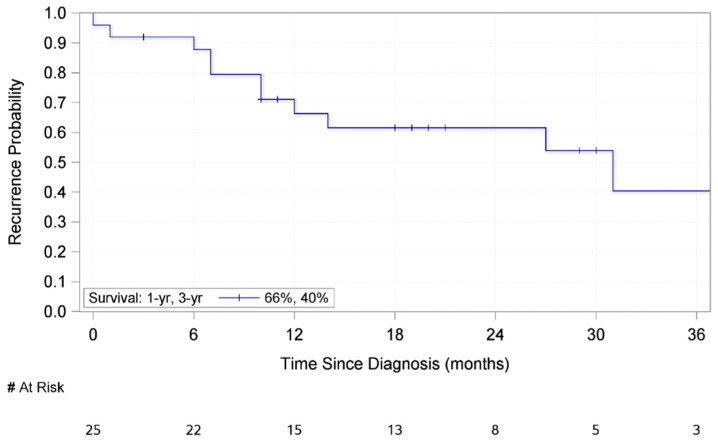
The median follow up was 21 months (interquartile range (IQR): 18–29 months) in this patient population. Table 2 outlines the association between demographic and clinicopathologic factors and recurrence-free survival (RFS). Age, gender, tumor location (hand or foot), tumor laterality, ulceration, and lesion histology were not significantly associated with RFS. Increasing Breslow thickness was found to be significantly associated with shorter RFS (HR: 1.27, CI: 1.03–1.55). Additionally, patients who underwent a completion lymph node dissection either at time of presentation with clinically matted/palpable nodes or with a positive sentinel lymph node biopsy demonstrated a marginally significant association with shorter RFS (HR: 2.87, CI: 0.83–9.89). The mean RFS was 33.6 months. Additionally, the median RFS of all patients in this study was 31 months (IQR: 10–41 months) and RFS rates were 66% at 1 year and 40% at 3 years (Figure 1). In the study population, four patients recurred with in-transit disease, four developed a regional nodal basin recurrence, and six developed distant metastases as their initial recurrence (Supplementary Table S1).
Table 2.
Univariate Cox Proportional Hazards on Recurrence Free Survival.
| All N = 25 N (%) |
Hazard Ratio (95% CI) |
p-Value | ||
|---|---|---|---|---|
| Age at Diagnosis | Median (IQR+) | 69 (58–76) | 1.01 (0.97–1.05) | 0.5492 |
| Sex | Male | 15 (60) | -reference- | - |
| Female | 10 (40) | 1.29 (0.34–4.90) | 0.7119 | |
| Breslow Thickness (mm) | Median (IQR+) | 3.4 (2.5–4.5) | 1.27 (1.03–1.55) | 0.0228 |
| Laterality | Right | 10 (40) | -reference- | - |
| Left | 15 (60) | 1.40 (0.40–4.93) | 0.6027 | |
| Hand/Foot | Hand | 8 (32) | -reference- | - |
| Foot | 17 (68) | 0.64 (0.18–2.28) | 0.4935 | |
| AJCC Stage | 0-II | 11 (44) | -reference- | - |
| III | 12 (48) | 3.89 (0.83–18.32) | 0.0859 | |
| Unknown/NR | 2 (8) | 9.80 (0.82–117.06) | 0.0713 | |
| Ulceration | Yes | 13 (52) | -reference- | - |
| No | 4 (16) | 2.47 (0.45–13.53) | 0.2965 | |
| Unknown/NR | 8 (32) | 1.49 (0.35–6.28) | 0.5844 | |
| Histology | Acral-lentiginous | 10 (40) | -reference- | - |
| Melanoma in situ | 3 (12) | UND | UND | |
| Nodular | 3 (12) | 2.68 (0.44–16.39) | 0.2872 | |
| Superficial Spreading | 1 (4) | 4.42 (0.44–44.48) | 0.2074 | |
| Unknown/NR | 8 (32) | 2.34 (0.52–10.61) | 0.2707 | |
| PNI | No | 8 (32) | -reference- | - |
| Yes | 1 (4) | 1.24 (0.11–13.41) | 0.8601 | |
| Unknown/NR | 16 (64) | 0.94 (0.23–3.92) | 0.9351 | |
| LVI | No | 8 (32) | -reference- | - |
| Yes | 2 (8) | 1.47 (0.23–9.50) | 0.6842 | |
| Unknown/NR | 15 (60) | 0.86 (0.20–3.75) | 0.8446 | |
| SLNB | No | 9 (36) | -reference- | - |
| Yes | 15 (60) | 0.57 (0.17–1.91) | 0.3649 | |
| Unknown/NR | 1 (4) | UND | UND | |
| CLND | No | 14 (56) | -reference- | - |
| Yes | 10 (40) | 2.87 (0.83–9.89) | 0.0950 | |
| Unknown/NR | 1 (4) | UND | UND | |
| Adjuvant Therapy | No | 16 (64) | -reference- | - |
| Yes | 8 (32) | 0.97 (0.26–3.68) | 0.9687 | |
| Unknown/NR | 1 (4) | UND | UND | |
IQR: Interquartile Range, NR: Not Recorded, PNI: Perineural Invasion, LVI: Lymphovascular Invasion, SLNB: Sentinel Lymph Node Biopsy, CLND: Completion Lymph Node Dissection.
Figure 1.
Kaplan–Meier Curve for Recurrence Free Survival of 25 patients who Underwent Surgical Resection of a Subungual Melanoma.
3.4. Overall Survival
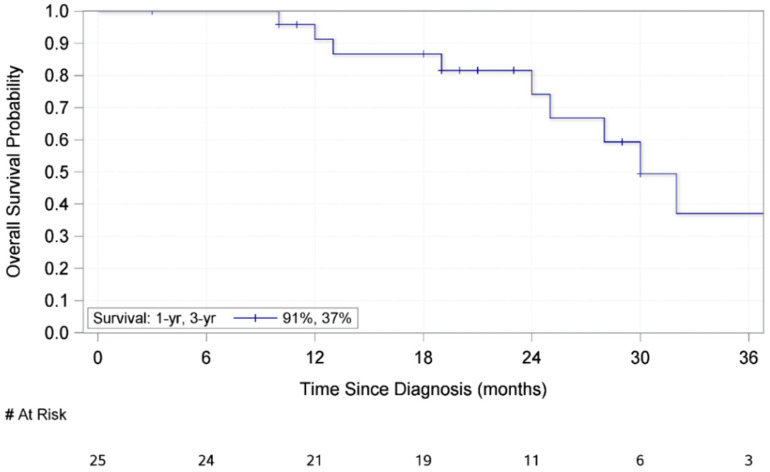
Table 3 outlines the association between demographic and clinicopathologic factors and overall survival. No variable had a statistically significant association with overall survival; however, a marginally significant association was found between increasing Breslow thickness and shorter overall survival (HR 1.17, CI: 1.00–1.37). For these patients, the mean overall survival was 48.4 months. Furthermore, the median overall survival was 30 months (IQR: 24–81 months) (Figure 2) with 91% and 37% of patients alive at one year and three years, respectively, after initial resection. In total, there were 11 deaths in this patient population, and nine of these were attributed to melanoma disease progression.
Table 3.
Univariate Cox Proportional Hazards on Overall Survival.
| All N = 25 N (%) |
Hazard Ratio (95% CI) |
p-Value | ||
|---|---|---|---|---|
| Age at Diagnosis | Median (IQR+) | 69 (58–76) | 1.03 (0.98–1.09) | 0.2694 |
| Sex | Male | 15 (60) | -reference- | - |
| Female | 10 (40) | 1.27 (0.30–5.43) | 0.7443 | |
| Breslow Thickness (mm) | Median (IQR+) | 3.4 (2.3–4.3) | 1.17 (1.00–1.37) | 0.0576 |
| Laterality | Right | 10 (40) | -reference- | - |
| Left | 15 (60) | 1.70 (0.42–6.94) | 0.4610 | |
| Hand/Foot | Hand | 8 (32) | -reference- | - |
| Foot | 17 (68) | 0.70 (0.17–2.83) | 0.6161 | |
| AJCC Stage | 0-II | 11 (44) | -reference- | - |
| III | 12 (48) | 5.52 (0.68–45.12) | 0.1108 | |
| Unknown/NR | 2 (8) | 29.45 (2.24–386.65) | 0.0100 | |
| Ulceration | Yes | 13 (52) | -reference- | - |
| No | 4 (16) | 2.95 (0.27–31.93) | 0.3726 | |
| Unknown/NR | 8 (32) | 2.05 (0.51–8.24) | 0.3116 | |
| Histology | Acral-lentiginous | 10 (40) | -reference- | - |
| Melanoma in situ | 3 (12) | UND | UND | |
| Nodular | 3 (12) | 5.63 (0.49–65.08) | 0.1662 | |
| Superficial Spreading | 1 (4) | 10.00 (0.59–169.87) | 0.1112 | |
| Unknown/NR | 8 (32) | 5.71 (0.67–48.94) | 0.1120 | |
| PNI | No | 8 (32) | -reference- | - |
| Yes | 1 (4) | 2.10 (0.12–37.76) | 0.6152 | |
| Unknown/NR | 16 (64) | 2.06 (0.24–17.63) | 0.5107 | |
| LVI | No | 8 (32) | -reference- | - |
| Yes | 2 (8) | 2.43 (0.20–29.69) | 0.4861 | |
| Unknown/NR | 15 (60) | 1.98 (0.23–17.30) | 0.5357 | |
| SLNB | No | 9 (36) | -reference- | - |
| Yes | 15 (60) | 0.40 (0.11–1.49) | 0.1713 | |
| Unknown/NR | 1 (4) | 2.43 (0.25–24.10) | 0.4468 | |
| CLND | No | 14 (56) | -reference- | - |
| Yes | 10 (40) | 2.53 (0.62–10.30) | 0.1955 | |
| Unknown/NR | 1 (4) | 7.05 (0.64–78.24) | 0.1116 | |
| Adjuvant Therapy | No | 16 (64) | -reference- | - |
| Yes | 8 (32) | 0.90 (0.21–3.96) | 0.8932 | |
| Unknown/NR | 1 (4) | 3.97 (0.41–38.75) | 0.2349 | |
IQR: Interquartile Range, NR: Not Recorded, PNI: Perineural Invasion, LVI: Lymphovascular Invasion, SLNB: Sentinel Lymph Node Biopsy, CLND: Completion Lymph Node Dissection.
Figure 2.
Kaplan–Meier Curve for Overall Survival of 25 Patients who Underwent Surgical Resection of a Subungual Melanoma.
4. Discussion
When compared to non-nail apparatus cutaneous melanoma, subungual melanoma represents a rare disease subtype with a relative paucity of clinical data. We present our institutional experience on subungual melanoma to add to the collective literature and confirm key trends in patient presentation and survival.
The incidence of melanoma has been increasing in recent years, and the vast majority of melanoma patients have thin melanomas that are less than 1 mm in depth [19,20]. This is in contrast to patients with subungual melanomas who typically present at later stages and have even been found to have worse survival outcomes when staged-matched and compared to non-acral cutaneous melanomas [21]. For instance, the median Breslow thickness in our patient population was 3.4 mm, which is consistent with previously published series of subungual melanoma [6,7]. In addition, we found the most common histologic subtype of subungual melanoma to be acral lentiginous (59%), and this is comparable to other institutional series [6,22,23]. Furthermore, the rate of positive sentinel lymph node biopsies increases with the thickness of the primary lesions [24]. Our data demonstrates that 26.7% of patients in our study had a positive sentinel lymph node biopsy, which is within the range reported by other series [9,22,25]. In total, 40% of patients in our study had metastases in regional nodal basins on final pathologic analysis.
The overall survival rate in our study was 37% at three years. This is somewhat less than other reports in the literature which have demonstrated near 60% survival rate at five years [7,18]. However, similar to other studies, our data demonstrates a marginally significant association between shorter survival and regional nodal metastases [6]. Additionally, increasing Breslow thickness was associated with shorter RFS (p < 0.05).
All the patients in our series (except for one individual with in situ disease who had a wide local excision) underwent an amputation of the affected digit. Historically, amputation has been the treatment of choice with much debate centering on the level of amputation necessary to maximize survival. Some reports have demonstrated that the level of amputation does not matter so long as that the surgical margins are negative [7,18]. Additionally, there has been a push to further minimize the extent of resection when possible and to avoid amputation in favor of wide local excision [26]. In one of the largest series of conservative surgical approach, Moehrle et al. did not find any significant decrease in overall survival for patients who underwent local excision (compared to amputation); however, the local excision group did have a lower mean lesion thickness [27].
One of the limitations of this study is its retrospective nature with certain information not available in patients’ chart, despite an exhaustive review of print and electronic medical records. The patients were referred and treated at a tertiary cancer center and may not reflect the general population. In addition, the limited number of patients included in this study (partially a result of the rarity of the disease) and non-uniform distribution of certain variables limited the statistical power of the study.
5. Conclusions
In conclusion, our study results add to the body of literature and support findings previously published on subungual melanoma. Patients are often diagnosed at later disease stages, and this finding likely contributes to the unfavorable prognosis of subungual melanoma, which is particularly poor in those patients with thick primary lesions and lymph node metastases. Given the unique molecular characteristics and tumor microenvironment of subungual melanoma (when compared to other cutaneous melanomas), future studies will need to identify the adjuvant therapy regimens that will be most efficacious for patients with advanced disease.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/medsci9030057/s1, Table S1: Supplementary Table-Patient Treatment Factors.
Author Contributions
Conceptualization—C.J.L. and B.C.; Chart Review/Data collection—C.J.L. and B.C.; Statistical analysis—R.A.N.; Data interpretation—C.J.L., L.L., R.A.N., B.M., B.C.; Prepa-ration of original draft—C.J.L.; Critical revision of manuscript—all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board at City of Hope National Medical Center (Protocol # 17104).
Informed Consent Statement
Patient consent was waived due to retrospective study and aggregate reporting of data.
Data Availability Statement
Data is contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luke J.J., Flaherty K.T., Ribas A., Long G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 3.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S., et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 4.Faries M.B., Thompson J.F., Cochran A.J., Andtbacka R.H., Mozzillo N., Zager J.S., Jahkola T., Bowles T.L., Testori A., Beitsch P.D., et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017;376:2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochran A.M., Buchanan P.J., Bueno R.A., Jr., Neumeister M.W. Subungual melanoma: A review of current treatment. Plast. Reconstr. Surg. 2014;134:259–273. doi: 10.1097/PRS.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 6.Chakera A.H., Quinn M.J., Lo S., Drummond M., Haydu L.E., Bond J.S., Stretch J.R., Saw R.P.M., Lee K.J., McCarthy W.H., et al. Subungual Melanoma of the Hand. Ann. Surg. Oncol. 2019;26:1035–1043. doi: 10.1245/s10434-018-07094-w. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen J.T., Bakri K., Nguyen E.C., Johnson C.H., Moran S.L. Surgical management of subungual melanoma: Mayo clinic experience of 124 cases. Ann. Plast. Surg. 2013;71:346–354. doi: 10.1097/SAP.0b013e3182a0df64. [DOI] [PubMed] [Google Scholar]
- 8.Kato T., Suetake T., Sugiyama Y., Tabata N., Tagami H. Epidemiology and prognosis of subungual melanoma in 34 Japanese patients. Br. J. Dermatol. 1996;134:383–387. doi: 10.1111/j.1365-2133.1996.tb16218.x. [DOI] [PubMed] [Google Scholar]
- 9.Dika E., Patrizi A., Fanti P.A., Chessa M.A., Reggiani C., Barisani A., Piraccini B.M. The Prognosis of Nail Apparatus Melanoma: 20 Years of Experience from a Single Institute. Dermatology. 2016;232:177–184. doi: 10.1159/000441293. [DOI] [PubMed] [Google Scholar]
- 10.Banfield C.C., Redburn J.C., Dawber R.P. The incidence and prognosis of nail apparatus melanoma. A retrospective study of 105 patients in four English regions. Br. J. Dermatol. 1998;139:276–279. doi: 10.1046/j.1365-2133.1998.02365.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayward N.K., Wilmott J.S., Waddell N., Johansson P.A., Field M.A., Nones K., Patch A.M., Kakavand H., Alexandrov L.B., Burke H., et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 12.Elefanti L., Zamuner C., Del Fiore P., Stagni C., Pellegrini S., Dall’Olmo L., Fabozzi A., Senetta R., Ribero S., Salmaso R., et al. The Molecular Landscape of Primary Acral Melanoma: A Multicenter Study of the Italian Melanoma Intergroup (IMI) Int. J. Mol. Sci. 2021;22:3826. doi: 10.3390/ijms22083826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohrle M., Hafner H.M. Is subungual melanoma related to trauma? Dermatology. 2002;204:259–261. doi: 10.1159/000063354. [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan N.A., Tait C.P. Tanning bed and nail lamp use and the risk of cutaneous malignancy: A review of the literature. Australas. J. Dermatol. 2014;55:99–106. doi: 10.1111/ajd.12145. [DOI] [PubMed] [Google Scholar]
- 15.Levit E.K., Kagen M.H., Scher R.K., Grossman M., Altman E. The ABC rule for clinical detection of subungual melanoma. J. Am. Acad. Dermatol. 2000;42:269–274. doi: 10.1016/S0190-9622(00)90137-3. [DOI] [PubMed] [Google Scholar]
- 16.Leppard B., Sanderson K.V., Behan F. Subungual malignant melanoma: Difficulty in diagnosis. Br. Med. J. 1974;1:310–312. doi: 10.1136/bmj.1.5903.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson R.H., Helwig E.B. Subungual malignant melanoma: A clinical-pathologic study. Cancer. 1980;46:2074–2087. doi: 10.1002/1097-0142(19801101)46:9<2074::AID-CNCR2820460928>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Heaton K.M., el-Naggar A., Ensign L.G., Ross M.I., Balch C.M. Surgical management and prognostic factors in patients with subungual melanoma. Ann. Surg. 1994;219:197–204. doi: 10.1097/00000658-199402000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaikh W.R., Dusza S.W., Weinstock M.A., Oliveria S.A., Geller A.C., Halpern A.C. Melanoma Thickness and Survival Trends in the United States, 1989 to 2009. J. Natl. Cancer. Inst. 2016;108 doi: 10.1093/jnci/djv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimotty P.A., Botbyl J., Soong S.J., Guerry D. A population-based validation of the American Joint Committee on Cancer melanoma staging system. J. Clin. Oncol. 2005;23:8065–8075. doi: 10.1200/JCO.2005.02.4976. [DOI] [PubMed] [Google Scholar]
- 21.Bello D.M., Chou J.F., Panageas K.S., Brady M.S., Coit D.G., Carvajal R.D., Ariyan C.E. Prognosis of acral melanoma: A series of 281 patients. Ann. Surg. Oncol. 2013;20:3618–3625. doi: 10.1245/s10434-013-3089-0. [DOI] [PubMed] [Google Scholar]
- 22.Reilly D.J., Aksakal G., Gilmour R.F., Gyorki D.E., Chauhan A., Webb A., Henderson M.A. Subungual melanoma: Management in the modern era. J. Plast. Reconstr. Aesthet. Surg. 2017;70:1746–1752. doi: 10.1016/j.bjps.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Tan K.B., Moncrieff M., Thompson J.F., McCarthy S.W., Shaw H.M., Quinn M.J., Li L.X., Crotty K.A., Stretch J.R., Scolyer R.A. Subungual melanoma: A study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am. J. Surg. Pathol. 2007;31:1902–1912. doi: 10.1097/PAS.0b013e318073c600. [DOI] [PubMed] [Google Scholar]
- 24.Morton D.L., Thompson J.F., Cochran A.J., Mozzillo N., Elashoff R., Essner R., Nieweg O.E., Roses D.F., Hoekstra H.J., Karakousis C.P., et al. Sentinel-node biopsy or nodal observation in melanoma. N. Engl. J. Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 25.Nunes L.F., Mendes G.L.Q., Koifman R.J. Subungual melanoma: A retrospective cohort of 157 cases from Brazilian National Cancer Institute. J. Surg. Oncol. 2018;118:1142–1149. doi: 10.1002/jso.25242. [DOI] [PubMed] [Google Scholar]
- 26.Sureda N., Phan A., Poulalhon N., Balme B., Dalle S., Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: Report of seven cases and literature review. Br. J. Dermatol. 2011;165:852–858. doi: 10.1111/j.1365-2133.2011.10477.x. [DOI] [PubMed] [Google Scholar]
- 27.Moehrle M., Metzger S., Schippert W., Garbe C., Rassner G., Breuninger H. “Functional” surgery in subungual melanoma. Dermatol. Surg. 2003;29:366–374. doi: 10.1046/j.1524-4725.2003.29087.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and supplementary material.