Abstract
Background: Off-pump coronary artery bypass grafting (OPCAB) is believed to limit inflammatory reaction. Neutrophil to lymphocyte ratio (NLR) is one of the more common and easily accessible markers of inflammatory response. The aim of the study was to compare postoperative results of NLR with mid-term OPCAB results. Methods: In total, 224 patients (198 (88%) men and 26 (12%) women) with mean age 65 +/− 9 years who underwent OPCAB though median full sternotomy in our department in 2018 enrolled into the study. We scrupulously collected the postoperative mid-term results, including survival rate, clinical status and risk for major adverse events, and compared them with perioperative laboratory results. Results: A three-year follow-up was completed by 198 individuals (90% survival rate) with 12 (5%) showing major adverse cardiovascular (MACE) events risk. In the multivariable analysis, the laboratory parameters noticed on the 1st postoperative day were statistically significantly predictive of survival, including neutrophils (HR 1.59, 1.33–1.89 95%CI, p < 0.0001), platelets (HR 1.01, 1.01–1.01 95%CI, p = 0.0065), NLR (HR 1.47, 1.3–1.65 95%CI, p < 0.0001) and postoperative ejection fraction (HR 0.9, 0.87–0.95 95%CI, p < 0.0001). Conclusions: Postoperative NLR above 4.6, as an inflammatory reaction marker, is related to mid-term mortality in OPCAB patients.
Keywords: NLR 1, OPCAB 2, platelets 3, LVEF 4
1. Introduction
The surgical revascularization of coronary artery disease can be performed with cardiopulomnary bypass, or with the “beating heart” technique called off-pump coronary artery bypass grafting (OPCAB). It allows one to omit the cardiopulmonary circuit, along with all possible side effects, including inflammatory reaction activation [1].
Off-pump surgical revascularization of complex coronary artery disease has gained worldwide attention [2,3,4]. The major advantage of off-pump coronary artery bypass grafting (OPCAB) consists in the avoidance of non-physiological blood flow and minimization of the risk of complications related to inflammatory response. Inflammation plays a significant role in atherosclerosis progression and its complications [5,6].
One of the more easily accessible markers of inflammatory response is neutrophil-to-lymphocyte ratio (NLR). Its capacity to predict adverse outcomes in patients with coronary artery disease has already been postulated [7].
The aim of the study was to compare postoperative results of NLR with mid-term OPCAB results, since there are no medical references evaluating the correlation between postoperative NLR and mid-term mortality risk in off-pump surgery.
2. Materials and Methods
2.1. Study Population
In total, 224 patients (198 (88%) men and 26 (12%) women) with a mean age of 65 ± 9 years who had undergone OPCAB (off-pump coronary artery bypass grafting) through median full sternotomy at our Department in 2018 were enrolled into the study. Ethical approval was obtained from the local university’s bioethics committee (273/21). All patients were provided with a form of written informed consent for a surgical procedure, and the research was conducted according to the principles laid down in the Declaration of Helsinki.
The presented group was subdivided in terms of 3-year survival into 2 subgroups. Detailed demographical and clinical information are presented in Table 1.
Table 1.
Demographical and clinical data.
| Parameter | Group 1 Survivors (n = 198) |
Group 2 Deceased (n = 26) |
p-Value |
|---|---|---|---|
| Gender M/F | 174 (88%)/25 (12%) | 24 (92%)/2 (8%) | p = 0.5483 |
| Age | 65 +/− 9 | 67 +/− 9 | p = 0.2869 |
| Concomitant diseases: | |||
| 1. arterial hypertension | 166 (84%) | 21 (81%) | p = 0.6973 |
| 2. DM | 78 (39%) | 9 (65%) | p = 0.0116 |
| 3. Stroke | 5 (3%) | 8 (31%) | p < 0.0001 |
| 4. Hypercholesterolemia | 134 (68%) | 18 (70%) | p = 0.8368 |
| 5. PAD | 29 (15%) | 11 (42%) | p = 0.0008 |
| Surgical indication: | p = 0.5650 | ||
| 1. LM disease | 102 (52%) | 12 (46%) | p = 0.5629 |
| 2. 3 vessels disease | 88 (44%) | 13 (50%) | p = 1.0000 |
| 3. 2 vessels disease | 8 (4%) | 1 (4%) | |
| Echocardiographic results | |||
| 1. LV diameter (mm) | 47 +/− 6 | 48 +/− 6 | p = 0.4257 |
| 2. LVEF (%) | 54 +/− 8 | 50 +/− 7 | p = 0.0159 |
| Surgery: | |||
| 1. overall time (min) | 141 +/− 42 | 139 +/− 39 | p = 0.8182 |
| 2. Mean anastomosis | 2.3 +/− 0.7 | 2.3 +/− 0.7 | p = 1.0000 |
| Hospitalization time (days) | 8.8 +/− 3 | 13 +/− 10 | p = 0.0433 |
| (excluding in hospital mortality) | (10 +/− 4) |
Abbreviations: COPD—chronic obstructive pulmonary disease, DM—diabetes mellitus, LV—left ventricle, LVEF—left ventricle ejection fraction, PAD—peripheral artery disease.
The mean follow-up time was 2.5 years. The data were collected from initial hospitalization, followed by regular checks at an outpatient clinic and telephone survey. The exclusion criteria included temporary mechanical support prior to surgery, including intraortic balloon counterpulsation, the need for respiratory support, kidney failure requiring hemodialysis, a previous cardiac surgery, an active infection, or an acute phase of myocardial infarction. Chronic inflammatory diseases and a history of oncological diseases were also added to the exclusion criteria.
2.2. Patient and Public Partnership
The patients were first enlisted into the study by verification of their survival, and their participation was voluntary, subject to being informed of the study. Survival was additionally assessed using national electronic verification data available for our country.
2.3. Laboratory Analysis
Laboratory results were obtained prior to surgery, as well as on day 1 and day 7 following the surgery. The perioperative laboratory results are presented in Table 2. The whole blood count was the only standard laboratory test presenting inflammatory reaction on the 1st and 7th postoperative days. The other inflammatory parameters, such as C-reactive protein (CRP) or procalcitonine, were assessed only if infection was suspected.
Table 2.
Perioperative laboratory results.
| Parameters | Group 1 Survivors (n = 198) |
Group 2 Deceased (n = 26) |
p-Value |
|---|---|---|---|
| Preoperative: | |||
| 1. WBC, ×109/L (mean ± SD) | 8.4 +/− 3.3 | 7.7 +/− 1.9 | p = 0.6780 |
| 2. Neutrophils, ×109/L (mean ± SD) | 5.3 +/− 1.7 | 5.1 +/− 1.5 | p = 0.7849 |
| 3. Lymphocyte, ×109/L (mean ± SD) | 2.2 +/− 2.5 | 1.8 +/− 0.7 | p = 0.4513 |
| 4. Hb, mmol/L (mean ± SD) | 8.7 +/− 0.9 | 8.6 +/− 1.1 | p = 0.9893 |
| 5. Plt, ×109/L (mean ± SD) | 229 +/− 63 | 233 +/− 63 | p = 0.9397 |
| 6. NLR (mean ± SD) | 3.3 +/− 1.8 | 3.2 +/− 1.5 | p = 0.7119 |
| 7. Troponin, ng/mL (mean ± SD) | 0.23 +/− 2.8 | 0.02 +/− 0.4 | p = 0.1442 |
| Postoperative 1st day): | |||
| 1. WBC, ×109/L (mean ± SD) | 9.1 +/− 5 | 12.1 +/− 13 | p = 0.0331 |
| 2. Neutrophils, ×109/L (mean ± SD) | 5.2 +/− 2 | 8.7 +/− 11 | p = 0.0012 |
| 3. Lymphocyte, ×109/L (mean ± SD) | 2.5 +/− 3.4 | 1.8 +/− 0.7 | p = 0.0779 |
| 4. Hb, mmol/L (mean ± SD) | 6.9 +/− 0.6 | 7 +/− 0.5 | p = 0.3570 |
| 5. Plt, ×109/L (mean ± SD) | 304 +/− 92 | 354 +/− 107 | p = 0.0157 |
| 6. NLR (mean ± SD) | 2.8 +/− 1.6 | 5.1 +/− 3.6 | p = 0.0003 |
| 7. Troponin, ng/mL (mean ± SD) | 4 +/− 6.3 | 9.9 +/− 11 | p = 0.1206 |
| Postoperative 7th day): | |||
| 1. WBC, ×109/L (mean ± SD) | 9.1 +/− 4.9 | 9.2 +/− 2.6 | p = 0.4047 |
| 2. Neutrophils, ×109/L (mean ± SD) | 5.1 +/− 1.9 | 5.5 +/− 2.1 | p = 0.3956 |
| 3. Lymphocyte, ×109/L (mean ± SD) | 2.5 +/− 3.4 | 2.4 +/− 1.7 | p = 0.4216 |
| 4. Hb, mmol/L (mean ± SD) | 6.9 +/− 0.6 | 7.1 +/− 0.7 | p = 0.1483 |
| 5. Plt, ×109/L (mean ± SD) | 305.7 +/− 93.4 | 283.5 +/− 70.6 | p = 0.2592 |
| 6. NLR (mean ± SD) | 2.7 +/− 1.4 | 3 +/− 1.9 | p = 0.6694 |
| 7. Troponin, ng/mL (mean ± SD) | 0.2 +/− 1.0 | 0.1 +/− 0.5 | p = 0.7638 |
Hb—hemoglobin, Me—mediana, NLR—neutrophil to lymphocyte ratio, Plt—platelets, Q—quartile, WBC—white blood count.
Postoperative mid-term results, including survival rate, clinical status, and the risk of major adverse events, were meticulously collected.
Postoperative myocardial infarction (type 5) diagnosis was based on the following criteria: elevated serum Troponin-I levels combined with a decrease in left ventricle ejection fraction in accordance with fourth universal definition [8].
2.4. Surgical Technique
Procedures were performed through median sternotomy without cardiopulmonary bypass application (OPCAB technique) by a team of experienced surgeons. Prior to performing anastomosis, heparin was administered according to the results of ACT (activating clotting time) with a mean value of 458 ± 42 s. The Octopus III (Medtronic, Minneapolis, MN, USA) local stabilizer was used in combination with a deep pericardial stitch to elevate the beating heart. Following the application of intraluminal shunts into coronary arteries, the anastomoses were performed with a monofilament 7-0 suture. A double dose of protamine was routinely administered after performing the anastomoses in order to reverse heparin action (mean ACT 132 ± 36 s).
2.5. Clinical Endpoint
To identify the effect of the post-procedural NLR on mid-term mortality in patients undergoing off-pump surgical revascularization.
2.6. Statistical Analysis
Continuous variables were reported as mean ± standard deviation (SD). Categorical data were presented as numbers and percentages. The comparison of interval parameters between the survivors and the deceased group was performed by Student’s t-test, and, if the data did not follow the normal distribution, the Mann–Whitney test was used as an alternative. Categorical data were analyzed using the test of proportions. The Cox’s proportional hazard regression model was used to check whether the analyzed demographical and clinical data could be a risk factor for all-cause mortality. Both univariate and multivariate analyses were performed. Additionally, for significant factors, the ROC analysis was performed to find the optimal cut-off point for continuous parameters. In the next step, a multivariable Cox’s model was used, wherein all data were binary. The analysis was performed using MedCalc® Statistical Software version 19.6 (MedCalc Software Ltd., Ostend, Belgium). All tests were considered significant at p < 0.05.
3. Results
There were two (1%) perioperative deaths and seven (3%) myocardial infarctions in the presented groups. A three-year follow-up was not completed by 24 individuals, with a 10% mortality rate, and major adverse cardiovascular (MACE) events combined with percutaneous interventions were found in 12 (5%) more patients.
There were 26 deaths in the presented analysis related to 15 (58%) coronary syndromes and 11 (42%) secondary to stroke.
The two groups (the deceased (non-survivors) and the survivors) were statistically different regarding concomitant diseases, including: history of stroke (p < 0.0001), peripheral artery disease (p = 0.0008), and left ventricle ejection fraction (p = 0.002).
The median values of the maximum Troponin-I serum level following the surgery were 4 ± 6 vs. 9.9 ± 11 mcg/L in groups 1 and 2, respectively. Detailed results are presented in Table 2.
The preoperative NLR results were comparable in both groups (3.3 ± 1.8 vs. 3.2 ± 1.5 (p = 0.7119) in the survivors vs. the deceased group, respectively). A postoperative NLR increase on post-op day 1 was observed in the survivors and the deceased group (2.8 ± 1.6 vs. 5.1 ± 3.6 (p = 0.0003) and 2.7 ± 1.4 vs. 3 ± 1.9 (p = 0.6694), respectively). The NLR increase on post-op day 1 decreased back to preoperative values on day 7. The preoperative NLR and its values on post-op day 7 in the survivors group was lower (3.3 ± +/−1.8 vs. 2.7 ± 1.4 (p = 0.0002) and comparable in the deceased group, 3.2 ± 1.5 vs. 3 ± 1.9 (p = 0.6754).
Interestingly, the maximal serum levels of myocardial injury markers (Troponin-I) were comparable between both groups (4 ± 6.3 mcg/L and 9.9 ± 11 mcg/L (p = 0.1206)). Preoperative and postoperative whole blood count data, including the commonly accepted marker of inflammatory reaction, such as neutrophil-to-lymphocyte ratio (NLR), are presented in Table 2.
Univariable and multivariable Cox’s proportional hazard regression analysis was performed and is presented in Table 3.
Table 3.
Univariable and multivariable Cox’s proportional hazard regression analysis.
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Parameter | HR | 95%CI | p-Value | HR | 95%CI | p-Value |
| Gender M/F Ref. =F | 2.55 | 0.60–10.79 | 0.2034 | |||
| Age | 1.03 | 0.97–1.07 | 0.2823 | |||
| Concomitant diseases: | ||||||
| Arterial hypertension | 1.32 | 0.49–3.51 | 0.5736 | |||
| DM | 0.98 | 0.43–2.23 | 0.9668 | |||
| Stroke | 14.07 | 6.34–31.22 | <0.0001 | |||
| Hypercholesterolemia | 1.35 | 0.59–3.11 | 0.4752 | |||
| PAD | 3.9 | 1.77–8.60 | 0.0007 | |||
| Surgical indication: | ||||||
| LM disease | 0.85 | 0.39–1.85 | 0.6906 | |||
| 3 vessels disease | 1.14 | 0.52–2.50 | 0.7314 | |||
| 2 vessels disease | 1.74 | 0.80–3.81 | 0.1597 | |||
| Echocardiographic results: | ||||||
| LV diameter (mm) | 1.08 | 1.02–1.16 | 0.0191 | |||
| LVEF (%) | 0.88 | 0.85–0.91 | <0.0001 | 0.92 | 0.87–0.95 | <0.0001 |
| Preoperative: | ||||||
| (mean ± SD) | ||||||
| WBC, ×109/L | 0.92 | 0.77–1.09 | 0.3248 | |||
| Neutrophils, ×109/L | 0.94 | 0.75–1.19 | 0.6364 | |||
| Lymphocyte, ×109/L | 0.78 | 0.45–1.35 | 0.3734 | |||
| Hb, mmol/L | 0.98 | 0.62–1.52 | 0.9218 | |||
| Plt, ×109/L | 1 | 0.99 -1.01 | 0.7882 | |||
| NLR | 1.04 | 0.84–1.29 | 0.6986 | |||
| Troponin, ng/mL | 0.91 | 0.34–2.41 | 0.8498 | |||
| Postoperative 1st day: | ||||||
| (mean ± SD) | ||||||
| WBC, ×109/L | 1.05 | 1.01–1.08 | 0.0059 | 1.18 | 1.07–1.30 | 0.0006 |
| Neutrophils, ×109/L | 1.59 | 1.33–1.89 | <0.0001 | 0.36 | 0.22–0.58 | <0.0001 |
| Lymphocyte, ×109/L | 0.64 | 0.37–1.10 | 0.1047 | |||
| Hb, mmol/L | 1.14 | 0.61–2.15 | 0.6848 | |||
| Plt, ×109/L | 1.01 | 1.01–1.01 | 0.0065 | 1.01 | 1.01–1.01 | 0.0038 |
| NLR | 1.47 | 1.30–1.65 | <0.0001 | 1.61 | 1.18–2.18 | 0.0022 |
| Troponin, ng/mL | 0.99 | 0.99–1.01 | 0.8088 | |||
| Postoperative 7th day: | ||||||
| (mean ± SD) | 1.01 | 0.93–1.08 | 0.9178 | |||
| WBC, ×109/L | 1.11 | 0.93–1.32 | 0.2687 | |||
| Neutrophils, ×109/L | 0.99 | 0.87–1.12 | 0.8528 | |||
| Lymphocyte, ×109/L | 1.53 | 0.85–2.74 | 0.1524 | |||
| Hb, mmol/L | 0.98 | 0.99–1.01 | 0.3514 | |||
| Plt, ×109/L | 1.1 | 0.88–1.38 | 0.412 | |||
| NLR | 0.94 | 0.56–1.57 | 0.8105 | |||
| Troponin, ng/mL | ||||||
Abbreviations: F—female, DM—diabetes mellitus, Hb—hemoglobin, LV—left ventricle, LVEF—left ventricle ejection fraction, M—male NLR—neutrophil to lymphocyte ratio, PAD—peripheral artery disease, Plt—platelets.
In the univariable analysis, stroke in medical history (HR 14.07, 6.34–31.22, 95%CI, p < 0.0001) and peripheral artery disease (HR 3.9, 1.77–8.6 95% CI, p = 0.0007) were found to be significant demographical factors affecting survival. Postoperative echocardiographic results, including left ventricle diastolic dimension (HR 1.08, 1.02–1.16, 95%CI, p = 0.0191) and ejection fraction (HR 0.88, 0.85–0.91, 95%CI, p < 0.0001). Interestingly, among the laboratory parameters, those measured on post-op day 1 were statistically significantly predictive of survival, including neutrophils (HR 1.59, 1.33–1.89 95%CI, p < 0.0001), platelets (HR 1.01, 1.01–1.01 95%CI, p = 0.0065), and NLR (HR 1.47, 1.3–1.65 95%CI, p < 0.0001).
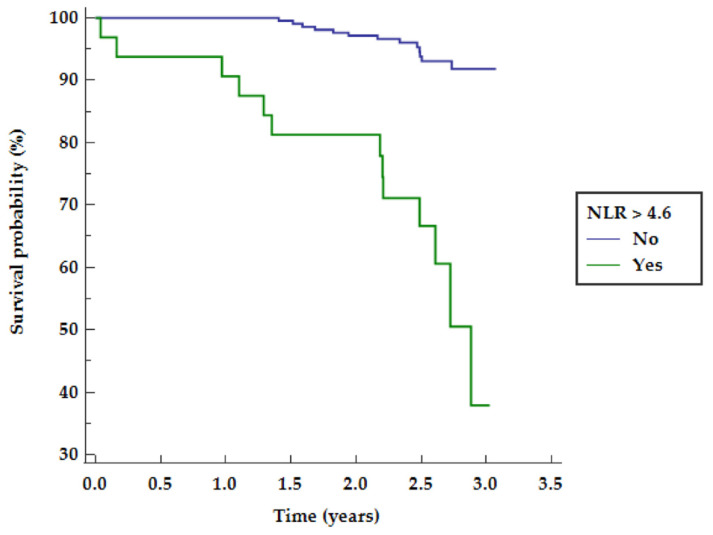
NLR above 4.6 was found to be related to mid-term survival as presented in Figure 1.
Figure 1.
NLR probability of mid-term survival. NLR—neutrophil to lymphocyte ratio.
Postoperative ejection fraction (HR 0.9, 0.87–0.95 95%CI, p < 0.0001) and laboratory parameters noted on post-op day 1 were found to represent significant factors in the multivariable analysis (Table 3).
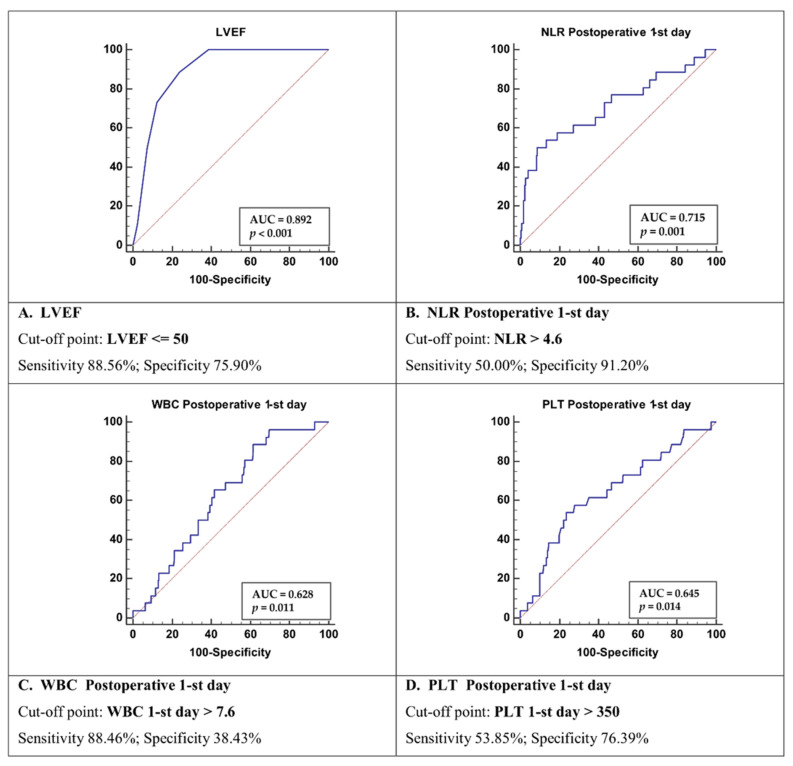
Receiver operating characteristic curves for Cox’s model revealed the following significant parameters: LVEF ≤ 50% with AUC = 0.892 (sensitivity 88.6% and specificity 76%), WBC above 7.6 10E9/L with AUC = 0.628 (sensitivity 88.5% and specificity 38%), Plt above 350 k/L with AUC = 0.645 (sensitivity 54% and specificity 76%), and NLR above 4.6 with AUC = 0.715 (sensitivity 50% and specificity 91%) (Figure 2).
Figure 2.
Receiver operating characteristic curves for Cox’s model LVEF (A). NLR on first postoperative day (B). WBC on first postoperative day (C). PLT on first postoperative day (D). Abbreviations: LVEF—left ventricle ejection fraction, NLR—neutrophil to lymphocyte ratio, PLT—platelets count, WBC—white blood count.
The multivariable Cox’s proportional hazard regression analysis was performed once again, taking all significant parameters as a binary. The results were presented in Table 4, including left ventricle ejection fraction ≤50% (HR 12.6, 3.69–42.72 95%CI, p = 0.0001), WBC > 7.6 (HR 1.04, 0.32–3.45 95%CI, p = 0.9409), platelets > 350 k/L (HR 2.7, 1.19–6.15 95%CI, p = 0.018) and NLR > 4.6 (HR 9.3, 3.6–24.02 95%CI, p < 0.0001).
Table 4.
Multivariable Cox’s proportional hazard regression analysis—binary covariates.
| Parameter | HR | 95%CI | p-Value |
|---|---|---|---|
| LVEF ≤ 50 | 12.56 | 3.69–42.72 | 0.0001 |
| WBC > 7.6 | 1.04 | 0.32–3.45 | 0.9409 |
| Plt > 350 | 2.70 | 1.19–6.15 | 0.0180 |
| NLR > 4.6 | 9.30 | 3.60–24.02 | <0.0001 |
Abbreviations: LVEF—left ventricle ejection fraction, NLR—neutrophil to lymphocyte ratio, PLT—platelets, WBC—white blood count.
4. Discussion
The unique result of the study is the relationship between the NLR increase on the first postoperative days after off-pump surgical revascularization and mid-term survival in multivariable analysis. In our analysis, four parameters were found to be related to mid-term mortality, including postoperative ejection fraction below 50%, NLR above 4.6, WBC above 7.6, and platelet count above 350 k per liter. The strongest predictor for mid-term survival was left ventricle ejection fraction (AUC = 0.895). The results of our study present the NLR as an independent mortality predictor following the OPCAB procedure with moderate significance (AUC = 0.715). This study points out the significance of inflammatory reaction activation as one of the possible prognostic factors.
Surgical stress by cellular immunity suppression may result in postoperative systemic leukocytic alterations, including leukocytosis, neutrophilia, lymphopenias, or inflammatory mediator overproduction [9].
The high NLR (above 4.6) on post-op day 1 was positively correlated with 3-year mortality. The results indicate that inflammatory processes occurring one day after the surgery have a significant negative impact on the patient’s survival. The postoperative NLR results were related to ICU stay and length of hospitalization in previous studies [10]. The correlation between the risk of MACCE following surgical revascularization in CPB was presented with a cut-off value of 4.32 in the multivariable analysis [11]. We present the results of the study with a similar cut-off value for mid-term mortality as the end-point analysis. We would like to point out that NLR reverted to preoperative values on post-op day 7. This suggests that after a surgical procedure, inflammatory processes are temporarily activated, and may trigger an unknown cascade leading to increased mortality within 2.5 years’ time. The overactivation of inflammatory system was transient, but further investigation seems to be required to distinguish between the prevalence of the patients’ hyperreactivity and a single inducible reaction. The association between increased preoperative NLR and cardiovascular risk, as well as cardiovascular and cerebrovascular events (MACE), in the 30-day postoperative period in non-cardiac surgery was presented by Larman [12].
Tan in his review postulated the association of increased preoperative values of NLR (>3.3 in cardiac surgery) with increased mortality at a mean follow-up of 34.8 months [13].
Increased NLR represents neutrophil activation combined with lymphocyte depletion. Neutrophils, as short-lived phagocytic cells, are characterized by a broad spectrum of biologically active molecules (myeloperoxidase, proteinases) [14]. According to Zernecke, the increased neutrophil content in plaques is associated with apoptosis and a proinflammatory phenotype [15]. In his study, the activation of circulating neutrophils was investigated, as labeled neutrophils were noted in atherosclerotic plaques after 8 weeks.
Myocardial ischemia/reperfusion injury occurring in the early postoperative phase involves the activation of neutrophils (and other cellular blood elements) combined with complementary system activation and molecular oxygen [16]. Lymphopenia, as observed in high NLR values, is another independent factor for atherosclerosis progression, as presented in previous studies [17,18].
The results of our study found a statistically significant relationship between WBC and survival in multivariable analysis. There are other studies in the literature presenting total white blood cell count (WBC) as a mortality predictor after surgical revascularization [19]. Other variables in outcome prediction include subtypes of WBC or specific ratios, such as the neutrophil-to-lymphocyte ratio [20]. If the whole blood count parameters are easily affected by the patient’s hydration level, NLR is believed to be relatively stable.
Contrarily, the Troponin-I serum level, a commonly used marker for myocardial injury, was not found in our results to be a mid-term mortality risk factor. This corroborates with the results obtained by Yan Li [21].
We present the relationship between perioperative NLR ratio and 3-year survival as the perioperative indicator of inflammatory response to the procedure. Chronic inflammatory processes are linked to atherosclerosis progression and plaque rupture, as presented in the review by Yuhua Zhu [22]. Inflammation initiates and promotes the development of atherosclerotic changes. We believe that the increased inflammatory activation during the perioperative period may strongly affect results, since surgical injury induces endogenous mediators that alter the immuno-inflammatory response [23].
Avoiding cardiopulmonary bypass during surgical revascularization has been proven to limit inflammatory response [24,25]. Contrarily, the complementary cascade of activation was on a similar level, according to Ascione’s study [26]. Additionally, neurohumoral activation triggering the systemic stress response was presented in a prospective study by Velissaris [27]. Shulze revealed the significantly lower expression of the TNF-system and Il-2r in OPCAB patients compared to CBP, except for similar Il-6 levels [28].
Postoperative high serum levels of C-reactive protein—another inflammatory marker commonly used in clinical practice—were found by Min [29] to be associated with a risk of MACE in the long-term.
Our study results present a negative correlation between left ventricle ejection fraction and mid-term survival. Decreased LVEF levels below 50% were found to be a mortality predictor. Postoperative deterioration of the left ventricle ejection fraction represents a myocardial perioperative injury significantly related to long-term survival, as postulated in previous studies [30,31].
The results from our retrospective analysis present the link between inflammatory reaction activation and risk for mid-term mortality. The NLR was found be a marker of moderate significance in the presented study. The significance of NLR in the perioperative period of cardiovascular procedures was already presented [32]. Nevertheless, the deaths were related to cardiovascular complications with known linkages to inflammatory processes [22].
We present a statistically significant relationship between the platelet count and mortality risk. Increased platelet counts are closely related to inflammatory reactions and increased NLR [33]. Contact between platelets and neutrophils modulates the thrombotic and inflammatory reaction [34]. High NLR may also increase platelet activity in the patient after coronary artery interventions, and both combined may help identify the patients with high risk of recurrent acute syndrome [35,36]. Therefore, we believe that the close relationship between platelets and NLR indicate that one of them should be considered a predictor.
This was a retrospective study, but it highlights possible new approaches to patients undergoing OPCAB procedures. We intend to follow up more closely this group of patients (NLR > 4.6) with more frequent tests, including treadmill tests and angiographies, if indicated. This group will be more closely monitored regarding cardiovascular risk factors, including hypertension.
5. Conclusions
Postoperative increases in NLR, as an inflammatory reaction marker, are related to mid-term mortality in OPCAB patients.
Author Contributions
Conceptualization, T.U., M.M., B.P. and M.J.; methodology, T.U., M.M., A.G. and B.P.; software, T.U., M.R. and M.B.; validation, M.M.; formal analysis, M.M.; investigation, T.U., A.G., M.R. and M.B.; resources, T.U., A.G., M.R. and M.B.; data curation, T.U., A.G., M.R. and M.B.; writing—original draft preparation, T.U., M.M., A.G. and B.P.; writing—review and editing, E.S.-M. and M.J.; supervision, B.P., E.S.-M. and M.J.; project administration, T.U; funding acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee) of Poznan University of Medical Sciences (protocol code 55/20, 16th January 2020—date of approval).
Informed Consent Statement
Informed consent for surgery was obtained from all subjects involved in the study as standard procedure. The study is a retrospective analysis.
Data Availability Statement
All data will be available under the correspondence e-mail address for 3 years following the publication after justifiable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bronicki R.A., Hall M. Cardiopulmonary Bypass-Induced Inflammatory Response: Pathophysiology and Treatment. Pediatr. Crit. Care Med. 2016;17:S272–S278. doi: 10.1097/PCC.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 2.Kuwahara G., Tashiro T. Current Status of Off-Pump Coronary Artery Bypass. Ann. Thorac. Cardiovasc. Surg. 2020;26:125–132. doi: 10.5761/atcs.ra.18-00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chivasso P., Guida G.A., Fudulu D., Bruno V.D., Marsico R., Sedmakov H., Zakkar M., Rapetto F., Bryan A.J., Angelini G.D. Impact of off-pump coronary artery bypass grafting on survival: Current best available evidence. J. Thorac. Dis. 2016;8:808–817. doi: 10.21037/jtd.2016.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puskas J.D., Williams W.H., Mahoney E.M., Huber P.R., Block P.C., Duke P.G., Staples J.R., Glas K.E., Marshall J.J., Leimbach M.E., et al. Off-pump vs conventional coronary artery bypass grafting: Early and 1-year graft patency, cost, and quality-of-life outcomes: A randomized trial. JAMA. 2004;291:1841–1849. doi: 10.1001/jama.291.15.1841. [DOI] [PubMed] [Google Scholar]
- 5.Wirtz P.H., von Känel R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr. Cardiol. Rep. 2017;19:111–117. doi: 10.1007/s11886-017-0919-x. [DOI] [PubMed] [Google Scholar]
- 6.Sawant A.C., Adhikari P., Narra S.R., Srivatsa S.S., Mills P.K., Srivatsa S.S. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol. J. 2014;21:500–508. doi: 10.5603/CJ.a2013.0148. [DOI] [PubMed] [Google Scholar]
- 7.Azab B., Zaher M., Weiserbs K.F., Torbey E., Lacossiere K., Gaddam S., Gobunsuy R., Jadonath S., Baldari D., McCord D., et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am. J. Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D. The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 9.Kim W.H., Jin H.S., Ko J.S., Hahm T.S., Lee S.M., Cho H.S., Kim M.H. The effect of anesthetic techniques on neutrophil-to-lymphocyte ratio after laparoscopy-assisted vaginal hysterectomy. Acta Anaesthesiol. Taiwanica. 2011;49:83–87. doi: 10.1016/j.aat.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Özer A., Mardin B., Kılıç Y., Oktar L., İriz E., Arslan M., Ünal Y., Alkan M. The effect of neutrophil-lymphocyte ratio on the postoperative course of coronary artery bypass graft surgery. Turk. J. Med. Sci. 2018;48:1036–1040. doi: 10.3906/sag-1804-94. [DOI] [PubMed] [Google Scholar]
- 11.Gurbuz O., Kumtepe G., Ozkan H., Karal I.H., Velioglu Y., Ercan A., Yüksel A., Ener S. Predictive Value of Neutrophil-Lymphocyte Ratio for Long-Term Cardiovascular Event Following Coronary Artery Bypass Grafting. Braz. J. Cardiovasc. Surg. 2020;35:274–284. doi: 10.21470/1678-9741-2018-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larmann J., Handke J., Scholz A.S., Dehne S., Arens C., Gillmann H.J., Uhle F., Motsch J., Weigand M.A., Janssen H. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non-cardiac surgery. BMC Cardiovasc. Disord. 2020;20:230.:230. doi: 10.1186/s12872-020-01500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan T.P., Arekapudi A., Metha J., Prasad A., Venkatraghavan L. Neutrophil-lymphocyte ratio as predictor of mortality and morbidity in cardiovascular surgery: A systematic review. ANZ J. Surg. 2015;85:414–419. doi: 10.1111/ans.13036. [DOI] [PubMed] [Google Scholar]
- 14.Galkina E., Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zernecke A., Bot I., Djalali-Talab Y., Shagdarsuren E., Bidzhekov K., Meiler S., Krohn R., Schober A., Sperandio M., Soehnlein O., et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborti T., Mandal A., Mandal M., Das S., Chakraborti S. Complement activation in heart diseases: Role of oxidants. Cell Signal. 2000;12:607–617. doi: 10.1016/S0898-6568(00)00111-X. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y.L., Chung H.T., Chang C.J., Yeh K.W., Chen L.C., Huang J.L. Lymphopenia is a risk factor in the progression of carotid intima-media thickness in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2009;60:3766–3775. doi: 10.1002/art.25019. [DOI] [PubMed] [Google Scholar]
- 18.Ducloux D., Challier B., Saas P. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J. Am. Soc. Nephrol. 2003;14:767–772. doi: 10.1097/01.ASN.0000048718.43419.44. [DOI] [PubMed] [Google Scholar]
- 19.Bagger J.P., Zindrou D., Taylor K.M. Leukocyte count: A risk factor for coronary artery bypass graft mortality. Am. J. Med. 2003;115:660–663. doi: 10.1016/S0002-9343(03)00438-8. [DOI] [PubMed] [Google Scholar]
- 20.Horne B.D., Anderson J.L., John J.M., Weaver A., Bair T.L., Jensen K.R., Renlund D.G., Muhlestein J.B. Intermountain Heart Collaborative Study Group. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Li Y., Hu Q., Zheng S., Tian B., Meng F., Chen Z., Han J., Wang S., Zhang H., et al. Association of early elevated cardiac troponin I concentration and longitudinal change after off-pump coronary artery bypass grafting and adverse events: A prospective cohort study. J. Thorac. Dis. 2020;12:6542–6551. doi: 10.21037/jtd-20-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y., Xian X., Wang Z., Bi Y., Chen Q., Han X., Tang D., Chen R. Research Progress on the Relationship between Atheroscler. Inflamm. Biomol. 2018;8:80–91. doi: 10.3390/biom8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsi C.H.-H., Wang J.J. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol. Taiwanica. 2015;15:23–28. doi: 10.1016/j.aat.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Raja S.G., Berg G.A. Impact of off-pump coronary artery bypass surgery on systemic inflammation: Current best available evidence. J. Card. Surg. 2007;5:445–455. doi: 10.1111/j.1540-8191.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 25.Al-Ruzzeh S., Hoare G., Marczin N., Asimakopoulos G., George S., Taylor K., Amrani M. Off-pump coronary artery bypass surgery is associated with reduced neutrophil activation as measured by the expression of CD11b: A prospective randomized study. Heart Surg. Forum. 2003;6:89–93. doi: 10.1532/hsf.1205. [DOI] [PubMed] [Google Scholar]
- 26.Ascione R., Lloyd C.T., Underwood M.J., Lotto A.A., Pitsis A.A., Angelini G.D. Inflammatory response after coronary revascularization with or without cardiopulmonary bypass. Ann. Thorac. Surg. 2000;69:1198–1204. doi: 10.1016/S0003-4975(00)01152-8. [DOI] [PubMed] [Google Scholar]
- 27.Velissaris T., Tang A.T., Murray M., Mehta R.L., Wood P.J., Hett D.A., Ohri S.K. A prospective randomized study to evaluate stress response during beating-heart and conventional coronary revascularization. Ann. Thorac. Surg. 2004;78:506–512. doi: 10.1016/S0003-4975(03)01360-2. [DOI] [PubMed] [Google Scholar]
- 28.Stoppe C., Werker T., Rossaint R., Dollo F., Lue H., Wonisch W., Menon A., Goetzenich A., Bruells C.S., Coburn M., et al. What is the significance of perioperative release of macrophage migration inhibitory factor in cardiac surgery? Antioxid. Redox Signal. 2013;19:231–239. doi: 10.1089/ars.2012.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min J.J., Nam K., Kim T.K., Kim H.J., Seo J.H., Hwang H.Y., Kim K.B., Murkin J.M., Hong D.M., Jeon Y. Relationship between early postoperative C-reactive protein elevation and long-term postoperative major adverse cardiovascular and cerebral events in patients undergoing off-pump coronary artery bypass graft surgery: A retrospective study. Br. J. Anaesth. 2014;113:391–401. doi: 10.1093/bja/aeu099. [DOI] [PubMed] [Google Scholar]
- 30.Awan N.I., Jan A., Rehman M.U., Ayaz N. The effect of ejection fraction on mortality in Coronary Artery Bypass Grafting (CABG) patients. Pak. J. Med. Sci. 2020;36:1454–1459. doi: 10.12669/pjms.36.7.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popovic B., Agrinier N., Voilliot D., Voilliot D., Elfarra M., Villemot J.P., Maureira P. Ventricular Dysfunction in Patients with Acute Coronary Syndrome Undergoing Coronary Surgical Revascularization: Prognostic Impact on Long-Term Outcomes. PLoS ONE. 2016;11:e0168634. doi: 10.1371/journal.pone.0168634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serra R., Ielapi N., Licastro N., Provenzano M., Andreucci M., Bracale U.M., Jiritano F., de Franciscis S., Mastroroberto P., Serraino G.F. Neutrophil-to-lymphocyte Ratio and Platelet-to-lymphocyte Ratio as Biomarkers for Cardiovascular Surgery Procedures: A Literature Review. Rev. Recent Clin. Trials. 2021;16:173–179. doi: 10.2174/1574887115999201027145406. [DOI] [PubMed] [Google Scholar]
- 33.Gasparyan A.Y., Ayvazyan L., Mukanova U., Yessirkepov M., Kitas G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019;39:345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lako S., Dedej T., Nurka T., Ostreni V., Demiraj A., Xhaxho R., Prifti E. Hematological Changes in Patients Undergoing Coronary Artery Bypass Surgery: A Prospective Study. Med. Arch. 2015;69:181–186. doi: 10.5455/medarh.2015.69.181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verdoia M., Nardin M., Gioscia R., Negro F., Marcolongo M., Suryapranata H., Kedhi E., De Luca G. Novara Atherosclerosis Study Group (NAS). Higher neutrophil-to-lymphocyte ratio (NLR) increases the risk of suboptimal platelet inhibition and major cardiovascular ischemic events among ACS patients receiving dual antiplatelet therapy with ticagrelor. Vascul. Pharmacol. 2020;132:106765. doi: 10.1016/j.vph.2020.106765. [DOI] [PubMed] [Google Scholar]
- 36.Adatia K., Farag M.F., Gue Y.X., Srinivasan M., Gorog D.A. Relationship of Platelet Reactivity and Inflammatory Markers to Recurrent Adverse Events in Patients with ST-Elevation Myocardial Infarction. Thromb. Haemost. 2019;119:1785–1794. doi: 10.1055/s-0039-1695007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available under the correspondence e-mail address for 3 years following the publication after justifiable request.