Abstract
With sustainable development of biotechnology, increasing attention has been placed on utilization of solid-state fermented feed (SFF). Solid-state fermented feed has been a candidate strategy to alleviate the contradiction between supply and demand of feed resources, ensure food hygiene safety, promoting energy conservation, and emission reduction. In production of SFF, a variety of organic acids, enzymes, vitamins, peptides, and other unknown growth factors are produced, which could affect performance of animals. Solid-state fermented feed produced by different fermentation techniques has great instability on different physiological stages of different animals, which hinders the application and standardized production of SFF. Herein, we summarize the current advances in the role of the characteristics of SFF prepared by different manufacturing technique and its research progress in animal experiments on growth performance, gastrointestinal ecology, and immune system, so as to provide references for further acquiring a relatively perfect set of SFF production and evaluation systems.
Keywords: Solid-state fermented feed, Manufacturing technique, Growth performance, Gastrointestinal ecology, Immune system
1. Introduction
Fermentation has been used for food processing (Nout, 1994; Soccol et al., 2017; Marco et al., 2017; Şanlier N et al., 2017; De et al., 2018) and preservation (Winsen et al., 2001; Wang et al., 2014a, Wang et al., 2014b; Soccol et al., 2017; Yang et al., 2018) for thousands of years around the world. Moreover, this technique recently gained increasing interest as a tool for adjusting nutritive value of feed and output of livestock products (Chen et al., 2013; Pedersen et al., 2010). Additionally, solid-state fermented feed (SFF) has been defined as a raw feed ingredient or commercial feed in which macromolecular substances and anti-nutritional factors are converted into more efficient and non-toxic nutrients by metabolic activities of microorganisms. Solid-state fermented feed refers to the fermentation of feed substrate by using natural or artificially added microorganisms under artificial control conditions (water content is generally controlled below 70%), so as to change the nutritional characteristics, digestibility, palatability and safety of feed. Meanwhile, SFF is potential to be a candidate strategy for replacing antibiotics in livestock feed (Wang et al., 2011a, Wang et al., 2011b; Ying et al., 2010). The nutritional properties of fermented feed depend on the fermentation starter (bacteria culture used to start fermentation), substrates, and fermentation conditions (temperature and incubation time) used (Awati et al., 2006; Niba et al., 2009; Missotten et al., 2016). Although studies have confirmed that fermentation could be an approach to improve nutritional value of ingredients before being offered to animals (Shimelis and Rakshit (2010); Shi et al., 2017), the quality of feed produced by different manufacturing techniques and their effects on animal performance have not been consistent (Feng et al., 2007a, Feng et al., 2007b; Wang et al., 2014a, Wang et al., 2014b). This inconsistency has encouraged industry professionals to explore SFF.
In recent years, researchers have carried out a large number of experiments in vivo and in vitro to explore applications of SFF (Hu et al., 2008; Yu et al., 2010; Wang et al., 2010; Shi et al., 2017). However, a perfect set of SFF production and evaluation systems is unavailable currently because of the instability of SFF. Our review herein summarizes nutritional characteristics of SFF produced by different manufacturing techniques and their effects on growth performance, gastrointestinal ecology, and immune system, hoping to guide researchers to make objective choices in the application of SFF.
2. Manufacturing technique
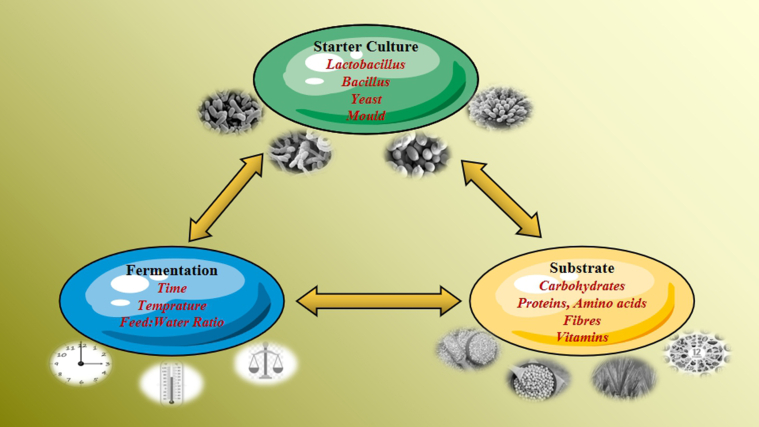
Interactions among starter cultures, incubation parameters, and substrate characteristics affect end-products of SFF (Fig. 1, Niba et al., 2009). Moreover, the effect of SFF on animals produced by different technologies has been inconsistent (Feng et al., 2007a, Feng et al., 2007b; Kim et al., 2010; Zhang et al., 2013; Liu et al., 2014; Jakobsen et al., 2015a, Jakobsen et al., 2015b). Therefore, to ensure correct management of SFF production to capture its potential, a thorough knowledge of the processes taking place during fermentation is required.
Fig. 1.
Interactions in fermented feed among micro-organisms present, fermentation parameters, and substrate quantity and quality that influence final end products.
2.1. Starter cultures and substrate characteristics
The most important factors for successful manufacturing of SFF are the choices of substrates and starter culture. Over the past few years, researchers have developed many substrates for SFF such as swill (Zhang et al., 2012; Zeng et al., 2020), seaweed (Zhao et al., 2018; Dong et al.,2020), beet pulp (Połec et al., 2010; Lu et al., 2020), vinasse (Shen et al., 2018), soybean meal (Zhang et al., 2018a, Zhang et al., 2018b) and complete feed (Liang et al., 2012; Li et al., 2019), so as to meet manufacturers' requirements for diverse end-products. Certainly, when the same substrate was fermented by different starter cultures, the end-products different (Table 1).
Table 1.
The effect of different starter cultures on the end fermentation products.
| Substrate | Microorganism | Product | Productivity | References |
|---|---|---|---|---|
| Wheat straw | Bacillus sp. BBXS-2 | Amylase | 6,900 U/g, 5 d | Qureshi et al. (2016) |
| Aspergillus lentulus | Xylanase | 158.4 U/g, 4 d | Kaushik et al. (2014) | |
| Wheat bran | Aspergillus oryzae | Amylase | 1,491 U/g, 3 d | Chen et al. (2014) |
| Rhizopus oryzae SN5 | Cellulase | 437 U/g, 5 d | Pandey et al. (2016) | |
| Aspergillus niger NS-2 | Cellulase | 395 U/g (CMCase), 28 U/g (FPase), 46 U/g | Bansal et al. (2012) | |
| Pleurotus ostreatus | Laccase | 32,450 U/g, 7 d | El-Batal et al. (2015) | |
| Coriolus sp. | Laccase | 2,661 U/g, 10 d | Mathur et al. (2013) | |
| Aspergillus niger LBA 02 | Protease | 262.78 U/g, 2 d | Castro et al. (2015) | |
| Trichoderma viride-IR05 | Xylanase | 72.4 U/g, 168 h | Irfan et al. (2014) | |
| Bacillus sp. PKD-9 | Xylanase | 98,000 U/g, 120 h | Panwar et al. (2014) | |
| Aspergillus oryzae (P6B2) | Xylanase | 2,830.7 U/g, 1 d | Pirota et al. (2013) | |
| Mucor petrinsularis, Mucor dimorphosporus, Mucor circinelloides, Mucor hiemalis | β-Carothene and γ-linolenic acid | 8.5 μg/g of β-carothene and 12.1 mg of γ-linolenic acid, 180 h | Certík et al. (2013) | |
| Apple pomace | Macrophomina | Amylase | 3,309 U/g, 120 h | Kaur et al. (2012) |
| Aspergillus niger NRRL-567 | Cellulase | 134 U/g (FPase), 60 U/g (β-glucosidase), 172 U/g (CMCase), 2 d | Dhillon et al., 2012a, Dhillon et al., 2012b | |
| Aspergillus niger NRRL 567 | Citric acid | 294.2 g/kg of dried apple pomace, 5 d | Dhillon et al. (2013) | |
| Rhizopus oryzae 1526 | Fumaric acid | 52.0 g/kg of dry weight substrate, 21 d | Das et al. (2015) | |
| Saccharomyces | Volatile | Esters, lactones, acids, terpenoids, aldehydes, ketones and alcohols | Madrera et al. (2015) | |
| Consortium of Aspergillus ornatus | Citric acid | 13.32 mg/g of substrate, 2 d | Ali et al. (2016) | |
| Sugarcane bagasse | Burkholderia | Lipase | 72.3 U/g, 4 d | Liu et al. (2016) |
| Thermomucor indicae | Lipase | 15 U/g, 3 d | Ferrarezi et al. (2014) | |
| Aspergillus oryzae CPQBA 394-12 DRM 01 | Pectinase | 40 U/g, 18 to 24 h | Biz et al. (2016) | |
| Thermoascus aurantiacus var. levisporus KKU-PN-I2-1 | Xylanase | 176 U/g, 196 h | Chanwicha et al. (2015) | |
| Pleurotus ostreatus | Laccase | 167 U/g, 5 d | Karp et al. (2012) | |
| Rice straw | Pyrenophora phaeocomes | Laccase | 10,859 U/g, 4 d | Rastogi et al. (2016) |
| Aspergillus niger NRRL 2001 | Cellulase | 401 U/g (FPase), 545 U/g (CMCase), 285 U/g | Dhillon et al., 2012a, Dhillon et al., 2012b | |
| Pleurotus sajor-caju | Protease | 85 U/mL, 8 d | Ravikumar et al. (2012) | |
| Promicromonospora sp. MARS | Xylanase | 85.0 U/g, 4 d | Kumar et al. (2011) | |
| Citrus peel | Aspergillus niger F3 | Pectinase | 265 U/g, 4 d | Rodríguez et al. (2011) |
| Aspergillus oryzae CPQBA 394-12 DRM 01 | Pectinase | 40 U/g, 18 to 24 h | Biz et al. (2016) | |
| Corn cob powder | Monascus purpureus KACC 42430 | Pigments (red) | 25.42 OD units/g, 7 d | Velmurugan et al. (2011) |
| Aspergillus niger van Tieghem KACC 44333 | Oxalic acid | 120 g/kg of dry weight substrate, 7 d | Mai et al. (2016) | |
| Sorghum straw | Aspergillus tubingensis FDHN1 | Xylanase | 5,177.23 U/g, 5 d | Adhyaru et al. (2016) |
| Saccharomyces cerevisiae | Ethanol | 6.56 g/100 g | Zai et al. (2009) | |
| Bread waste | Thermomyces sp. | Amylase | 39,900 U/g, 4 d | Cerda et al. (2016) |
| Aspergillus awamori | Protease and glucoamylase | 102.8 U/g (glucoamylase), 63.7 U/g (protease), 7 d | Melikoglu et al. (2013) | |
| Monascus purpureus | Pigment, glucoamylase and protease | 24 AU (absorbance units)/g, 8 and 117 U for pigments, glucoamylase and protease, respectively, 7 d | Haque et al. (2016) |
2.1.1. Starter cultures
Starter cultures that are widely used for SFF are Lactobacillus, Yeast, bacillus, and Moulds. Amylase, protease, lipase, cellulase, pectinase and glucanase, which degrade macromolecular substances into small compounds that are more conducive to animal absorption, are produced during the production of SFF. Effective utilization of fiber by microorganisms is a primary advantage of SFF. Lactic acid, short-chain fatty acid (SCFA) and other metabolites improve palatability of feed, and play an important role in promoting intestinal health. A previous study showed that mixed culture of Cellulose monomonas and Bacillus foecalis alkaligenes reduced cellulose concentration in the substrate (Dawson, 1987). Liao et al. (2009) found that when Aspergillus niger, Trichoderma and yeast were inoculated into corn straw feed at a ratio of 1:2:1 for 6 d (inoculation amount was 12%, incubation temperature was 31 °C), crude protein (CP) content in the medium increased 10 times, and content of crude fiber (CF) decreased from 36.2% to 18.47%.
2.1.2. Degradation of non-starch polysaccharides
Recent gradual development and deepening of our understanding of intestinal flora, dietary fiber as “food” of flora has attracted great attention. Microorganisms can decompose dietary fiber during production of SFF, and produce a variety of monosaccharides which are more easily used by intestinal flora. In addition, the monosaccharides can nourish growth of microorganisms, acting as prebiotics and probiotics. Degradation of different types of fiber is closely related to the difference of fiber components. According to characteristics of fiber, we could select microbial strains with high efficiency to degrade a certain component to establish a synergistic degradation system (Table 2).
Table 2.
The effect of microbial strains on fermentation of raw materials with different non-starch polysaccharides.
| Items | Typical raw materials | Fermentation strain | Enzymes | Changes in indicators | References |
|---|---|---|---|---|---|
| Araboxylan | Wheat bran, Rice bran, Maize, Sorghum | Agaricus blazei murill, Aspergillus niger, Lentinus edodes, Trichoderma, Fomes lignosus. | Endo-β-1,4-D-xylanase, β-D-xylosidase; α-L-arabinofuranase, Xylan acetylesterase, Glucuronidase | Soluble sugar↑, Crude Protein↑, Ferulic acid↑ | Shen et al. (2012); Zhang et al. (2003); Ghoneum (1998); Cui et al. (2005) |
| β-glucan | Barley, Oats, Rye, Brewer's yeast | Thermoascus aurantiacus, NFEg16A, Caldicellulosiruptor sp. F32, Paenibacillus sp. S09 | Endo-β-1,3-glucanase, Endo-β-1,4-glucanase, Exo-β-1,3-glucanase, Exo-β-1,4-glucanase |
Oligosaccharide ↑, Glucose ↑, Viscosity ↓ | Qiao et al. (2018); Ali et al. (2018); Feng et al. (2019); Chen (2014) |
| Mannan and Glucomannan | Palm meal, Yeast cell wall, Konjac | Enterococcus faecalis, Lactobacillus plantarum, Cladosporium velox, Aspergillus nidulans, Neosartorya fischeri, Aspergillus oryzae, Trichoderma virens, Penicillium oxalicum | β-1,4-D-Mannanase, Mannosidase, Glucomannanase | Short chain fatty acids↑, Lactic acid↑, Mannan oligosaccharide↑, Mannose↑ | Wang et al. (2016); David et al. (2016); Zhang et al., 2018a, Zhang et al., 2018b; Wang et al., 2014a, Wang et al., 2014b; Sin et al. (2016); Liao et al. (2014); Zhu et al. (2018) |
| Pectin | Sugar beet pulp, Citrus peel, Peanut meal, Ramie | Bacillus cereu, Bacillus megaterium, Pectobacterium, Aspegr illus tubingensis, Rahnella aquatilis, Aspergillus niger | Pectin methylesterase, Polygalacturonase, Pectin lyase | Utilization of pectin and Pectinic acid↑, Soluble sugar↑, Organic acids↑ | Mukhopadhyay et al. (2013); Duan et al. (2016); Na et al. (2018); Long et al. (2017); Chang (2020); Debing et al. (2006) |
| Fructan | Chicory, Onion, Jerusalem artichoke | Aspergillus niger, Penicillium, Coriolusversiolor | β-fructofuranase | Fructooligosaccharides↑, Lactic acid↑, Short chain fatty acids↑ | Cao et al. (2009); Liu et al. (2018); Liu and Cao (1996) |
| Galactomannan | Soybean hulls, Soybean meal, Nut | Bispora, Lachancea thermotolerans, Penicillium simplicissimum, Neosartorya fischeri, Talaromyces leycettanus, Alicyclobacillus, Lactobacillus fermentum, Talaromyces flavus | α-galactosidase, and β-galactosidase | Mannan oligosaccharides↑, Mannose↑ | Wang (2014); Wang, 2010a, Wang, 2010b; Carrera-Silva et al. (2006); Simersk et al. (2007) |
2.1.3. Substrate characteristics
Many scholars have reported that soybean meal can be fermented by Aspergillus oryzae, Yeast, and Lactobacillus in solid state, which could increase concentration of crude protein (Chen et al., 2011; Rombenso et al., 2013; Hassaan et al., 2015). This concentration increase occurs because microorganisms consume the organic materials, resulting in the “concentration effect” of protein. Furthermore, the effective utilization of ammonium salts and the increase of bacterial protein also contribute to the increase of crude protein. Another study reported that phytic acid, an anti-nutrient factor in soybean meal, can be degraded nearly completely by fermentation with Aspergillus usamii (Hirabayashi et al., 1998). Similarly, fermentation of soybean meal using Bacillus subtilis as the starter culture extensively hydrolyzes protein to amino acids and degrades inhibitors of trypsin and chymotrypsin (Feng et al., 2007a, Feng et al., 2007b). Organisms used in SFF can degrade potentially hazardous raw materials and transform them into products that can improve storage qualities of the ingredient and reduced risk of causing illness (Adams et al., 2002; Guanghui et al., 2017; Yang et al., 2018; Godoy et al., 2018; Dong et al., 2018). However, the extent to which SFF are safe and how fermentation processes should be conducted to achieve the required level of safety are crucial. Occasionally, the fermentation process can cause loss of nutrients such as vitamins and amino acids, especially synthetic amino acids (Brooks et al., 2003; Niven et al., 2006; Joris et al., 2010; Canibe and Jensen, 2003). Therefore, some investigators proposed that fermentation of complete feed was defective (Joris et al., 2010; Brookst eal., 2003; Scholten et al., 2002; Moran et al., 2006; Canibe et al., 2007).
Numerous investigations strongly suggested that use of multiple substrates and starter cultures will enlarge scope of feed resources that can be developed with SFF, promote directional conversion of feed, and alleviate competition between human beings and livestock for grain. However, what is worth emphasizing is that further studies are needed to assess the underlying mechanisms of the detailed dynamic change regulation process during the during incubation.
2.2. Incubation parameters
Characteristics of SFF are related closely to temperature, moisture content, and time of incubation which leads to diverse quality of feed.
2.2.1. Temperature
Appropriate temperature guarantees proper growth and metabolism of microorganisms. Liu et al., 2010a, Liu et al., 2010b adjusted temperature from 30 °C (optimal temperature for enzyme production) to 45 °C (optimal hydrolysis temperature for proteases) in fermentation of soybean meal by A. oryzae A-9005. Conversion rate of soybean peptide increased from 50% to 54.51% after 72 h of incubation (Liu et al., 2010a, Liu et al., 2010b). Appropriate temperature can shorten the stable time of fermentation and improve fermentation products (Gu, 2010; Zhang et al., 2013; Li, 2010; Dujardin et al., 2014). From the point of view of enzymatic kinetics, increasing system temperature accelerates reaction speed and growth and metabolism of microorganisms (Pandey, 2003). However, the enzyme is easily inactivated by excessive temperature. Also, rapid growth of microorganisms generates additional heat. Poor heat transfer efficiency of solid-state fermentation feed leads to a sharp rise in temperature of substrates. If excess heat cannot be dissipated in time, growth and metabolism of microorganisms is limited. Another interesting study reported that while soybean meal was fermented by compound bacteria, incubation temperature exceeded the optimal temperature for hydrolysis of soybean by protease, and the koji-heating would appear (Wang et al., 2014a, Wang et al., 2014b). In addition, this situation could also occur in the production of liquid fermented feed (LFF).
2.2.2. Moisture content
Moisture content of substrates is also a crucial factor affecting qualities of final end products (Nagel et al., 2015; Liu et al., 1999; Plahar et al., 2010). The biggest shortcoming of SFF is lack of free water. Low moisture content reduces diffusion of nutrients and metabolites and affects activity of enzymes, resulting in limited growth of microorganisms. Conversely, excessive moisture content reduces porosity of substrates, reduces oxygen and heat transfer, and increases risk of mycotoxin contamination. Inappropriate moisture content is not conducive to growth of microorganisms and stability of pH. With increased of moisture content, dry matter recovery of SFF gradually decreases (Lin et al., 2015; Zhou et al., 2013; Zhao et al., 2015). An interesting study showed that the content of total volatile basic nitrogen (TVB-N) which react the of loss of amino acids and degree of corruption increased with the increasing of moisture content in fermented soybean meal feed (Saccharomyces cerevisae:A. oryzae:B. subtilis = 5:1:2) (Hu et al., 2013). Studies over the past years have strongly demonstrated that moisture content of fermentation substrates should be adjusted according to properties of substrates (granularity, hydraulics; Nagel et al., 2015; Wang et al., 2016; Qin et al., 2017), microbial characteristics (anaerobic, aerobic or facultative anaerobic; Liu et al., 2017; Wardynski et al., 1993), temperature (Hamidi-Esfahani et al., 2004; Park et al., 2018; Mcquestin et al., 2009), and time (Pojanagaroon et al., 2007; Nagel et al., 2015). Vinegar lees, wheat bran, corn flour and soybean meal were mixed in the ratio of 9:2:1:1, and the moisture content was controlled to 33.8%. After 5 d of anaerobic fermentation at room temperature, Lactobacillus plantarum, Bacillus licheniformis and Saccharomyces boulardii could reach 2.7 × 107, 1.4 × 108 and 3.4 × 106 cfu/g, respectively (Yang et al., 2020). Under this incubation condition, the quality of SFF can be guaranteed and the shelf life can be extended to the maximum extent.
2.2.3. Incubation time
The effect of incubation time on quality of SFF is also crucial (Hong et al., 2004; Chen et al., 2010). In early stages of incubation, the substrate contains enough nutrients to make microorganisms grow vigorously. If fermentation is terminated prematurely, fermentation would be incomplete and concentration of the end product would be too low (Gao et al., 2009; Sun, 2008). However, if fermentation time is too long, nutrients would be consumed in large quantities, and bacteria numbers would decline and autophagy would occur (Zhang et al., 2015; Wang, 2014). Wang et al., 2014a, Wang et al., 2014b (Wang et al., 2014a, Wang et al., 2014b) studied the effects of incubation conditions on nutritional quality of fermented soybean meal by single factor design. The results showed that the optimal fermentation conditions were as follows: initial water content was 40%; sugar content was 0.5%; the ratio of neutral protease to acid protease was 3:1; exogenous protease was 0.3%; anaerobic fermentation at 40 °C for 5 d.
In conclusion, defining more precisely the optima of the environmental variables is required to build a complex kinetic model for the bacterial strains to ensure robust, repetitive, and safe fermentation cycles over long periods of time.
3. Feedback from applied research
In recent years, finding new unconventional feed sources has become a major emphasis in animal husbandry to reduce dependency on conventional feed. Coincidently, interest in SFF for adjusting health of animals increased dramatically after the European Union banned use of antibiotics as antimicrobial growth promoters for swine.
3.1. Growth performance
Under the action of microorganisms, complex macromolecular organic compounds in feed are degraded into small molecular substances which can be easily utilized by animals. Meanwhile, nutritious bacterial proteins and various metabolites are produced (Mao et al., 2020; Yang et al., 2021). Solid-state fermented feed has a sour fragrance, has the potential to stimulate appetite, and logically might improve animal production performance. However, studies on growth performance of animals fed SFF have not yielded consistent responses which confuses nutritionists and livestock farmers (Chi et al., 2019; Tang et al., 2020).
Nevertheless, the vast majority of reports have shown positive effects of SFF. Lu et al. (2014) found that feeding a diet containing 6% fermented soybean meal (FSBM, Streptococcus thermophiles, Saccharomy cescerevisiae and Bacillus subtilis MA139 were used for start culture) resulted in greater average daily gain and average daily feed intake in weanling pigs (Liu et al., 2014). Jiang et al. (2014) fed 10% FSBM instead of soybean meal (SBM) to piglets and observed responses similar to Lu et al. (2014). More attractively is that the palatability of animals ameliorates with the increase of lactic acid in SFF (Kil et al., 2006). Indeed, positive effects of SFF on growth performance of animals have been reported in several studies (Feng et al., 2007a, Feng et al., 2007b; Kim et al., 2010; Zhang et al., 2013).
Because of lack of endogenous hydrolyzing enzymes, non-starch polysaccharides (NSP) cannot be digested by monogastric animals (Jakobsen et al., 2015a, Jakobsen et al., 2015b). Studies reported that NSP could increase viscosity of digesta and reduce nutrient digestibility in intestines (Refstie et al., 1999; Choct et al., 2015; Suhermiyati et al., 2011). Feeding trials have shown that fermentation of rapeseed meal (Chiang et al., 2010), wheat (Steenfeldt et al., 1998; Wei et al., 2019), oats (Svihus et al., 1997; Cui et al., 2019), and barley (Skrede et al., 2003; Huang et al., 2019) improve animal performance compared with unfermented grains, presumably due to a reduction of soluble NSP. Therefore, it seems logical to conclude that degradation of soluble NSP during fermentation improves nutritive value and digestibility of feed components and is an important factor in improving performance of monogastric animals.
Positive effects of SFF for pigs (Lei et al., 2018), broilers (Akinola et al., 2015), rabbits (Li, 2016), Landes goose (Xian et al., 2013), lamb (Zhong et al., 2013) and beef cattle (Shi et al., 2015) also have been reported (Table 3). However, different voices also appeared (Cho et al., 2007; Sungsam et al., 2009; Kim et al., 2010). Some investigators believe that the low pH and high concentration of some metabolites (e.g., acetic acid, biogenic amines) in SFF impair palatability and consequently, decrease feed intake (Brooks et al., 2001; Moran, 2001). In addition, it was reported that the disappearance of free amino acids, mainly lysine, by microbial fermentation in SFF was probably the main reason for the negative effect of feeding it on growth performance (Canibe et al., 2012; Pedersen, 2001). Last but not the least, it is worth emphasizing that the safety evaluation of SFF, such as the change regulation of mycotoxin in SFF, should also be paid great attention by investigators, yet this aspect has only scarcely been explored in the field (Yang et al., 2018).
Table 3.
The effect of solid-state fermented feed (SFF) on animal production performance.
| Animal | Period | Substrate | Starter culture | Supplementation, % | Effects | References |
|---|---|---|---|---|---|---|
| Pig | 21 to 42 d | Soybean meal | Lactobacillus Plantarum, Bacillus subtilis, Saccharomyces cerevisiae | 15 | Diarrhea↓, abundance of Lactobacillus and Prevotella↑ | Xie et al. (2017) |
| 19 to 40 d | Wheat grain | Lactobacillus reuteri TMW1.656 | 50 | Short chain fatty acids in intestine↑ | Le et al. (2016) | |
| 21 to 30 d | Wheat | Lactobacillus Plantarum; Lactobacillus buchneri | 43.75 | The digestibility of organic matter, starch and phosphorus↑ | Koo et al. (2018) | |
| 35.1 ± 1.8 kg | DDGS | Without | 60 | The digestibility of DM, CP and non-starch polysaccharides↑ | Jakobsen et al., 2015a, Jakobsen et al., 2015b | |
| 64 to 144 d | Corn straw | Saccharomyces cerevisiae | 10 | There was no difference in growth performance and microbial diversity. | Jiang et al. (2016) | |
| 35 to 65 d | Soybean meal | Lactobacillus casei, Bacillus subtilis, Hymenochaete anomala | 3.75 | Diarrhea↓, average daily gain and feed conversion rate↑ | Yuan et al. (2017) | |
| Poultry | 1 to 28 d | Red ginseng | Monascus | 1 | Body weight and feed conversion rate↑ | Chung and Choi (2016) |
| 1 to 35 d | Rice bran | Bacillus amyloliquefaciens | 5 | Body weight and feed conversion rate↑ | Mussatto et al. (2012) | |
| 120 to 155 d | Complete feed | Bacillus subtilis, Enterococcus faecium, Bacillus subtilis | 25 | Average daily feed intake↑, the digestibility of crude protein and ether extract↑ | Lian (2016) | |
| 1 to 21 d | Soybean meal | Bacillus subtilis | 5 | Apparent metabolic rate↑ | Wang et al., 2011a, Wang et al., 2011b | |
| 1 to 42 d | Basal diet | Bacillus subtilis | – | Average daily gain↑, average daily feed intake↑, feed conversion rate↑ | Bai et al. (2017) | |
| 1 to 42 d | Sour cherry kernel | Aspergillus niger | 1 | Structure of intestinal flora↑ | Gungor et al. (2020) |
DDGS = distillers dried grains with solubles.
3.2. Gastrointestinal ecology
Increasing attention has been placed to use of SFF which could influence gastrointestinal bacterial ecology (René et al., 2001). Effects of SFF on gastrointestinal ecology are reflected mainly in gastrointestinal flora and metabolites.
Intestinal microflora of an animal is the first barrier in protecting the host from diseases caused by colonization of pathogens in the gastrointestinal tract (Patterson et al., 2003). Solid-state fermented feed because of their unique characteristics, lead to acidification of the upper gastrointestinal tract and provide appropriate conditions for establishment of bacteria beneficial to livestock (Niba et al., 2009; Chen et al., 2013). Moreover, SCFA were generated in the production of SFF which could reduce pH of the gastrointestinal track and create a competitive exclusion against infection by pathogenic bacteria (Engberg et al., 2009; Niba et al., 2009). Several studies have shown that SFF can reduce levels of Enterobacteriaceae (Liang et al., 2012; Roubosvan et al., 2010) and Salmonella (Heres et al., 2003; Mulder et al., 1997) in different segments of the gastrointestinal tract (René et al., 2001), yet Lactobacilli increased (Savvidou et al., 2009; Sun et al., 2013). Many scholars believe that these phenomena resulted because that SFF contains increased concentrations of lactic acid and SCFA, leading a lower gut pH (Scholten et al., 2010; Winsen et al., 2001; Missotten et al., 2009; Lyberg et al., 2006; Canibe et al., 2007).
The proposed reduction of Enterobacteriaceae and Salmonella is related to un-dissociated lactic acid and SCFA, because they cross the membrane of bacteria freely but dissociated acids do not (Russell et al., 1998). Inside the bacterial cell, the acid dissociates and pH drops, leading to collapse enzymatic and the proton motive forces. Additionally, the anion itself may damage the bacteria. Several studies have shown that reduction in Enterobacteriaceae and Salmonella are related to concentration of SCFA, yet the correlations were not clear (Shaw et al., 1937; Burnett et al., 1963; Kershaw et al., 1966; Mikkelsen et al., 1997; Mathew et al., 1998). Recent studies have shown that xylitol metabolism key enzymes exist in some bacteria, which form a mutually trophic relationship with other bacteria to increase the production of short chain fatty acids. In addition, xylitol can promote the transcription of phosphoacetyltransferase to increase the production of propionate, thereby reducing the pH value to inhibit the growth of Escherichia and Staphylococcus (Liu, 2015; Xiang et al., 2021).
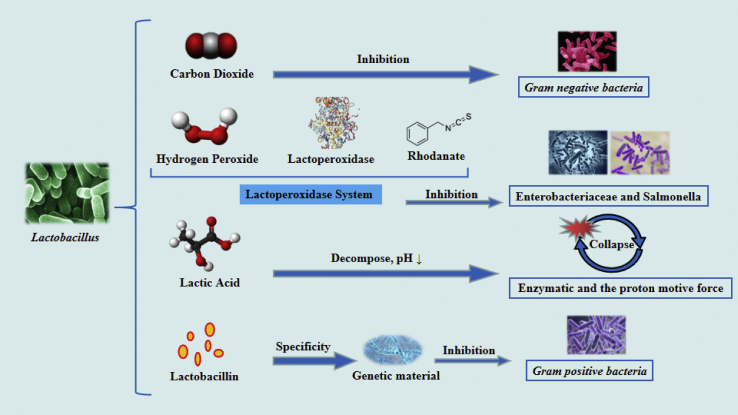
Inhibitory effects of Lactobacillus on Enterobacteriaceae and Staphylococcus aureus were not caused by a drop in pH alone (Li et al., 2009). Lactobacillus can secrete Lactobacillin and produce organic acids, CO2, and H2O2 which can inhibit growth of pathogenic bacteria (Li, 2002; Zhang, 2006). Lactobacillin is a bactericidal peptide, that inhibits Gram-positive bacteria by selectively entering the body of pathogenic bacteria and destroying its genetic material or important metabolic pathways (Cleveland et al., 2001; Quan et al., 2006; Turner et al., 2013). In addition, H2O2 can activate the peroxidase-thiocyanate system, combine lactate peroxidase with hydrogen peroxide and react with thiocyanate to produce oxidative intermediates, that inhibit growth of pathogenic bacteria inhibited (Li et al., 2002; Yu et al., 2011). Furthermore, CO2 can inhibit growth of some gram-negative bacteria (Li et al., 2009, Fig. 2). Alternatively, reductions in pathogenic bacteria may be due to a reduction of available substrates for microbial fermentation in the gastrointestinal tract and the increased digestibility of nutrients in the small intestine by feed fermentation, which could partially explain the reduction (Morishita et al., 1970; Urlings et al., 1993; Fransen et al., 1995). All these studies mentioned have explained the finding that SFF may act in a similar manner as antibiotic substitutes, by improving the gastrointestinal ecology and general health of animals.
Fig. 2.
The regulation mechanism of Lactobacillus in solid-state fermented feed (SFF) on gastrointestinal ecology.
Because of the differences about nutrient and moisture in SFF, the animal manure would be diversity. If the high-throughput sequencing technology is used alone and the proportion of strains would be overemphasized, the effect of SFF on total bacterial flora was neglected. Solid-state fermented feed have a greater impact on the number of intestinal microorganisms, and the environment it provides is universally applicable to a larger number of intestinal microorganisms. Perhaps, researchers could try to obtain more accurate quantitative relationships among intestinal microorganisms based on the total amount of animal defecation combined with the results of high-throughput sequencing.
In addition, SFF could affect not only the absorption of nutrients, but also appetite. The axis of brain-gut-microorganism (BGM) could link microorganisms to body metabolism. High acetic acid produced in fermentation, which could be absorbed by the brain through the blood–brain barrier, thus promoting the expression of appetite-suppressing neuropeptides, and appetite would be decreased (Kimura et al., 2013). Irregular dietary behavior will lead to obesity or food addiction, accompanied by BGM, and the BGM interaction mechanism is stable (Gong et al., 2020; Jais et al., 2020). It disrupts the brain homeostasis mediated by satiety and intestinal inflammation, leading to a significant impact on the hedonic feedback mechanism and inhibition mechanism of food intake (Chen et al., 2020). At the same time, continue to stimulate the body to choose high calorie food, worsen intestinal disorders. Therefore, the combination of multi node therapy targeting BGM may be a desirable way to alleviate obesity or food addiction in the future (Gupta et al., 2020).
In summary, SFF has a great impact on gastrointestinal ecology, including changes in microflora and metabolic behavior, leading a vital role in metabolism to host. Accordingly, it is reasonable that feeding SFF would be also an effective strategy to improve gastrointestinal ecology and reduce the infection vulnerabilities of enteric diseases for animals.
3.3. Immune system
The internal environment of the organism is holistic, and changes in composition of enteric microorganisms affect immune responses of animals (Missotten et al., 2013; Nathan, 2008). As an independent antigen, microorganism could play a vital role in stimulating the immune defense function and improving the ability to mitigate oxidative stress. Although only limited data are available, it has been accepted by more and more researchers that feeding SFF decreases mortality rates (Ranjitkar et al., 2016) and positively affects immune responses of animals (Sugiharto et al., 2018; Miao et al., 2013; Ahmed et al., 2016). In Lactobacillus mediated immune responses, SFF also stimulates cellular-mediated immune responses (Xijie et al., 2007; Gao et al., 2009). Feeding SFF leads to an increase in content of Lactobacillus in the intestine, which has been described above. Although, the exact mechanism of Lactobacillus mediated immunomodulatory activities is as yet unclear, they may stimulate mucosal immunity in the intestines, humoral immunity, and cellular immunity all of which play a crucial role in the induction and regulation of immune responses (Kabir, 2009; XiuLin et al., 2017).
As resident flora in the intestinal tract, Lactobacillus in SFF could bind to specific receptors on intestinal epithelial surfaces, and colonize intestinal epithelial surfaces stably and orderly, which acts as an effective mucosal barrier (Shiyan et al., 2014). In addition, Lactobacillus could also promote proliferation of B-cells in small intestinal lymphoid tissue, enhance mucosal immune responses, and induce plasmocytes to produce a large concentration of IgA, thereby enhancing immune function of animals (Kabir, 2009). Furthermore, reports also indicate that probiotics and their products of metabolism in SFF can stimulate lymphocytes in intestinal mucosa and promote production of interleukin, tumor necrosis factor and interferon (Ko et al., 1962; Roselli et al., 2007; Puwen, 2011; Liu et al., 2014). Bacillus subtilis used in SFF can activate development of the immune system and stimulate B lymphocyte which improve antibody levels (Yu et al., 2011). Similar conclusions have been confirmed by other researchers (Wang et al., 2011a, Wang et al., 2011b; Xinxu et al., 2013). Different study characteristics, experimental approaches, and levels of SFF seem to partially explain these discrepancies in inferences.
4. Challenges
SFF plays an increasingly vital role in today's ecological animal husbandry. However, the innovative research and industrialization level of SFF still need to be improved, and many problems need to be solved. In terms of starter culture, due to the complexity and diversity of strains for SFF, the contamination of miscellaneous bacteria, the transfer of drug-resistant genes, the generation of toxic metabolites and excessive immunity continue to appear (Ezekiel et al., 2019). In order to ensure the sustainable and healthy development of SFF, strict screening and identification of starter culture are essential.
In addition, improper handled of SFF production process is an important factor limiting the application of SFF. First, the control of moisture. If the water content of SFF is too low, the diffusion of nutrients and metabolites will slow down, and the growth of microorganisms will be limited. On the contrary, excessive moisture content will reduce the porosity of matrix, reduce the transfer of oxygen and heat, and increase the risk of mycotoxin contamination (Lin et al., 2015; Zhao et al., 2015). Secondly, time control. If the fermentation process is terminated too early, the product concentration will be too low. If the time is too long, nutrients will be consumed in large quantities, resulting in the reduction of the number of microorganisms. At the same time, the rapid growth of microorganisms will also lead to the limitation of their own growth and metabolism (Gao et al., 2009; Zhang et al., 2015).
It should be emphasized that if the concentration of acetic acid and biogenic amine in SFF is too high, the palatability of feed will be greatly reduced (Brooks et al., 2001; Moran, 2001). Also, the disappearance of free amino acids during SFF fermentation may also be the main reason for its negative effect on growth performance (Canibe et al., 2012). Therefore, the dialogue mechanism between microorganisms in the fermentation process and the optimization of process parameters need to be further studied to maximize the effectiveness of SFF. In addition, there are still some problems about SFF, such as imperfect product quality standards and feeding mode to be improved. In terms of standards of SFF, there is no unified standard for the quality evaluation of fermented products in the world (Yu et al., 2019). And it is difficult to identify the quality of SFF, such as the type and content of active substances. In the storage of SFF, the moisture content and the number of microorganisms increase after fermentation. With the increase of storage time, microbial metabolism will consume most of the nutrients in SFF, resulting in the decline of its nutritional value. In the aspect of application technology, the awareness of the synergy and safety of microbial and animal nutrition needs to be improved, the nutrition database and appropriate addition amount need to be further improved. In the future, the research and development of SFF will focus on the screening of characteristic strains with strong antinutritional factor degradation ability, colonization ability and rich metabolites, the evaluation of biological potency for digestion and absorption of raw materials, improvement of body health and improvement of animal product quality, and the dynamic monitoring of fermentation process and product quality. We hope that readers, after understanding the advantages and disadvantages of SFF, can make the right decision on whether and how to apply SFF.
5. Conclusions
Our review was mainly motivated by the needs to meet the strong pragmatism toward a better control and insights on SFF for modern agriculture. As a candidate strategy, SFF has positive effects on growth performance, gastrointestinal ecology and immune system. Predictably it is reasonable that a technology so elegantly simple could raise attention of scientists around the globe. More data on the production engineering and application are needed to have a more solid set of results to add to the existing ones.
Author contributions
Lijie Yang: Formal analysis, Investigation, Writing – original draft. Xiangfang Zeng: Data curation, Software, Writing-Reviewing and Editing, Visualization. Shiyan Qiao: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U180220167) and the Beijing Swine Innovation Team of Modern Agriculture Industry Technological System.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adhyaru D.N., Bhatt N.S., Modi H.A., Divecha J. Insight on xylanase from Aspergillus tubingensis FDHN1: production, high yielding recovery optimization through statistical approach and application. Biocatalysis and Agricultural Biotechnology. 2016;6:51–57. [Google Scholar]
- Ahmed S.T., Mun H.S., Islam M.M., Ko S.Y., Yang C.J. Effects of dietary natural and fermented herb combination on growth performance, carcass traits and meat quality in grower-finisher pigs. Meat Sci. 2016;122:7–15. doi: 10.1016/j.meatsci.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Akinola O.S., Onakomaiya A.O., Agunbiade J.A., Oso A.O. Growth performance, apparent nutrient digestibility, intestinal morphology and carcass traits of broiler chickens fed dry, wet and fermented-wet feed. Livest Sci. 2015;177:103–109. [Google Scholar]
- Ali S.R., Anwar Z., Irshad M., Mukhtar S., Warraich N.T. Bio-synthesis of citric acid from single and co-culturebased fermentation technology using agro-wastes. Journal of Radiation Research and Applied Sciences. 2016;9:57–62. [Google Scholar]
- Ali B., Yi Z., Fang Y., Chen L.C., Zhao H. Characterization of a fungal thermostable endoglucanase from Chinese Nong-flavor daqu by metatranscriptomic method. Int J Biol Macromol. 2018;121:183–190. doi: 10.1016/j.ijbiomac.2018.09.173. [DOI] [PubMed] [Google Scholar]
- Awati A., Williams B.A., Bosch M.W., Li Y.C., Verstegen M.W.A. Use of the in vitro cumulative gas production technique for pigs:an examination of alterations in fermentation products and substrate losses at various time points. J Anim Sci. 2006;84:1110–1118. doi: 10.2527/2006.8451110x. [DOI] [PubMed] [Google Scholar]
- Bai K., Qiang H., Zhang J., He J., Tian W. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poultry Sci. 2017;96:74–82. doi: 10.3382/ps/pew246. [DOI] [PubMed] [Google Scholar]
- Bansal N., Tewari R., Soni R., Soni S.K. Production of cellulases from Aspergillus Niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manag. 2012;32:1341–1346. doi: 10.1016/j.wasman.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Biz A., Finkler A.T.J., Pitol L.O., Medina B.S., Krieger N., Mitchell D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem Eng J. 2016;111:54–62. [Google Scholar]
- Brooks P.H., Beal J., Niven S. Liquid feeding of pigs. Potential for improving pig health and food safety. Anim Sci Pap Rep. 2003;21:23–29. [Google Scholar]
- Burnett G.S., Hanna J. Effect of dietary calcium lactate and lactic acid on faecal Escherichia coli counts in pigs. Nature. 1963;197:815. [Google Scholar]
- Canibe N., Jensen B.B. Fermented and nonfermented liquid feed to growing pigs:effect on aspects of gastrointestinal ecology and growth performance. J Anim Sci. 2003;81:2019. doi: 10.2527/2003.8182019x. [DOI] [PubMed] [Google Scholar]
- Canibe N., Højberg O., Badsberg J.H., Jensen B.B. Effect of feeding fermented liquid feed and fermented grain on gastrointestinal ecology and growth performance in piglets. J Anim Sci. 2007;85:29–59. doi: 10.2527/jas.2006-744. [DOI] [PubMed] [Google Scholar]
- Canibe N., Jensen B.B., Ravindran V. Fermented liquid feed-microbial and nutritional aspects and impact on enteric diseases in pigs. Anim Feed Sci Technol. 2012;173:17–40. [Google Scholar]
- Cao Z.H., Dong Y.W., Miao J.Z. Study on the technological conditions of inulinase production by Aspergillus Niger. Chinese Journal of Bioengineering. 2009;29:97–101. [Google Scholar]
- Carrera E.A., Silvestroni A., Leblanc J.G., Piard J.C., Giori G.S.D., Sesma F. A thermostable α-galactosidase from Lactobacillus fermentum crl722:genetic characterization and main properties. Curr Microbiol. 2006;53:374–378. doi: 10.1007/s00284-005-0442-y. [DOI] [PubMed] [Google Scholar]
- Castro R.J.S., Ohara A., Nishide T.G., Bagagli M.P., Dias F.F.G., Sato H.H. A versatile system based on substrate formulation using agroindustrial wastes for protease production by Aspergillus Niger under solid state fermentation. Biocatalysis and Agricultural Biotechnology. 2015;4:678–684. [Google Scholar]
- Cerda A., El-Bakry M., Gea T., Sánchez A. Long term enhanced solid-state fermentation: inoculation strategies for amylase production from soy and bread wastes by Thermomyces sp. in a sequential batch operation. Journal of Environmental Chemical Engineering. 2016;4:2394–2401. [Google Scholar]
- Certík M., Adamechová Z., Guothová L. Simultaneous enrichment of cereals with polyunsaturated fatty acids and pigments by fungal solid state fermentations. J Biotechnol. 2013;168:130–134. doi: 10.1016/j.jbiotec.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Chanwicha N., Katekaew S., Aimi T., Boonlue S. Purification and characterization of alkaline xylanase from Thermoascus aurantiacus var. levisporus KKU-PN-I2-1 cultivated by solid-state fermentation. Mycoscience. 2015;56:309–318. [Google Scholar]
- Chen R. Nanjing University of Science and technology; 2014. Production of Glucanases by salecan-degrading bacteria. [Google Scholar]
- Chen C.C., Shih Y.C., Chiou P.W.S., Yu B. Evaluating nutritional quality of single stage- and two stage-fermented soybean meal:(online) Asian-Australas J Anim Sci. 2010;23:598–606. [Google Scholar]
- Chen Z.P., Zhou A.G., Wang Z.S., Liu D.C., Peng D.Y. Evaluation of nutritional quality of soybean meals fermented by Aspergillus oryzae. Chinese Journal of Animal Science. 2011;9:40–44. [Google Scholar]
- Chen W., Zhu X.Z., Wang J.P., Wang Z.X., Huang Y.Q. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae fermented liquid feed on growth performance, relative organ weight, intestinal microflora, and organ antioxidant status in Landes geese. J Anim Sci. 2013;91:978–985. doi: 10.2527/jas.2012-5148. [DOI] [PubMed] [Google Scholar]
- Chen B., Wu Q., Xu Y. Filamentous fungal diversity and community structure associated with the solid state fermentation of Chinese Maotai-flavor liquor. Int J Food Microbiol. 2014;179:80–84. doi: 10.1016/j.ijfoodmicro.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Chen J., Cheng M., Wang L., Zhang L., Zhan C. A vagal-NTS neural pathway that stimulates feeding. Curr Biol. 2020;7:1–13. doi: 10.1016/j.cub.2020.07.084. [DOI] [PubMed] [Google Scholar]
- Chi Z.S., Zhang H.J., Guo A.H., Li X.W., Geng X., Wu Y.P. Application of fermented feed in chicken production. Guangdong Feed. 2019;234:17–20. [Google Scholar]
- Chiang G., Lu W.Q., Piao X.S., Hu J.K., Thacker P.A. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas J Anim Sci. 2010;23:263–271. [Google Scholar]
- Cho J.H., Min B.J., Chen Y.J., Chen J.S., Wang Y.Q., Kim J.D. Evaluation of FSP (fermented soy protein) to replace soybean meal in weaned pigs:growth performance, blood urea nitrogen and total protein concentrations in serum and nutrient digestibility. Asian-Australas J Anim Sci. 2007;20:1874–1879. [Google Scholar]
- Choct M. Feed non-starch polysaccharides for monogastric animals:classification and function. Anim Prod Sci. 2015;55:1360–1366. [Google Scholar]
- Chung T.H., Choi I.H. Growth performance and fatty acid profiles of broilers given diets supplemented with fermented red ginseng marc powder combined with red koji. Rev.bras.cienc.avic. 2016;18:733–738. [Google Scholar]
- Cleveland J., Montville T.J., Nes I.F. Bacteriocins:safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- Cui Y.H., Wang S.C., Huang Y. Evaluation of NSP enzymatic hydrolysis. Feed Industry. 2005;2:46–48. [Google Scholar]
- Cui Y.Y., Tian Z.M., Lu H.J., Deng D., Ma X.Y., Chen W.D. Nutritional value of bran and application of fermented feed in animal production. Chinese animal husbandry and veterinary. 2019;10:2902–2915. [Google Scholar]
- Das R.K., Brar S.K., Verma M. A fermentative approach towards optimizing directed biosynthesis of fumaric acid by Rhizopus oryzae 1526 utilizing apple industry waste biomass. Fungal Biology. 2015;119:1279–1290. doi: 10.1016/j.funbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- David R.C., Patricia R.M., Abelardo M., Miguel G., Clara R.G., Nuria S. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K.A. Current and future role of yeast culture in animal production. Proceedings of Alltech’s Ninth Annual Symposium. 1987:269–292. [Google Scholar]
- De J.R., De L.V. Acetic acid bacteria in fermented foods and beverages. Curr Opin Biotechnol. 2018;49:115. doi: 10.1016/j.copbio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Debing J., Peijun L., Stagnitti F., Xiong X.Z., Li L. Pectinase production by solid fermentation from Aspergillus Niger by a new prescription experiment. Ecotoxicol Environ Saf. 2006;64:244–250. doi: 10.1016/j.ecoenv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Dhillon G.S., Brar S.K., Kaur S., Metahni S., M’hamdi N. Lactoserum as a moistening medium and crude inducer for fungal cellulase and hemicellulase induction through solidstate fermentation of apple pomace. Biomass Bioenergy. 2012;41:165–174. [Google Scholar]
- Dhillon G.S., Kaur S., Brar S.K., Verma M. Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind Crop Prod. 2012;38:6–13. [Google Scholar]
- Dhillon G.S., Kaur S., Sarma S.J., Brar S.K. Integrated process or fungal citric acid fermentation using apple processing wastes and sequential extraction of chitosan from waste stream. Ind Crop Prod. 2013;50:346–351. [Google Scholar]
- Dong J.J., Han R.Z., Xu G.C., Gong L., Xing W.R. Detoxification of furfural residues hydrolysate for butanol fermentation by Clostridium saccharobutylicum DSM 13864. Bioresour Technol. 2018;259:40–45. doi: 10.1016/j.biortech.2018.02.098. [DOI] [PubMed] [Google Scholar]
- Dong Z.Q., Zhang X.S., Dong Q., Guo H.F., Gong J.S., Shi J.S. Breeding, identification and characterization of trehalase producing strain. J Microbiol. 2020;40:51–57. [Google Scholar]
- Duan S., Feng X., Cheng L. Bio-degumming technology of jute bast by Pectobacterium sp. DCE-01. Amb Express. 2016;6 doi: 10.1186/s13568-016-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin M., Elain A., Lendormi T., Le F.M., Le T.Y., Sire O. Keeping under control a liquid feed fermentation process for pigs:A reality scale pilot based study. Anim Feed Sci Technol. 2014;194:81–88. [Google Scholar]
- El-Batal A.I., ElKenawy N.M., Yassin A.S., Amin M.A. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnology Reports. 2015;5:31–39. doi: 10.1016/j.btre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg R.M., Hammershøj M., Johansen N.F., Abousekken M.S., Steenfeldt S., Jensen B.B. Fermented feed for laying hens:effects on egg production, egg quality, plumage condition and composition and activity of the intestinal microflora. Br Poultry Sci. 2009;50:228–239. doi: 10.1080/00071660902736722. [DOI] [PubMed] [Google Scholar]
- Ezekiel C.N., Ayeni K.I., Ezeokoli O.T., Sulyok M., Deidre A.B.W., Oluwawapelumi A.O., Oluwatosin M.A., Ihuoma E.C.O., Rasheed A.A., Cyril C.N., Jana H., Christopher T.E., Rudolf K. High-throughput sequence analyses of bacterial communities and multi-mycotoxin profiling during processing of different formulations of kunu, a traditional fermented beverage. Front Microbiol. 2019;9:32–82. doi: 10.3389/fmicb.2018.03282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Liu X., Xu Z.R., Lu Y.P., Liu Y.Y. Effect of fermented soybean meal on intestinal morphology and digestive enzyme activities in weaned piglets. Dig Dis Sci. 2007;52:1845–1850. doi: 10.1007/s10620-006-9705-0. [DOI] [PubMed] [Google Scholar]
- Feng J., Liu X., Xu Z.R. The effect of Aspergillus oryzae, fermented soybean meal on growth performance, digestibility of dietary components and activities of intestinal enzymes in weaned piglets. Anim Feed Sci Technol. 2007;134:295–303. [Google Scholar]
- Feng J., Hamed I., Hamouda N.A. β-glucan degrading hydrolases from Caldicellulosiruptor sp. F32 and influence of glycosylation on F32EG5 thermostability. Acta Microbiol Sin. 2019;59:2144–2154. [Google Scholar]
- Ferrarezi A.L., Ohe T.H.K., Borges J.P., Brito R.R., Siqueira M.R., Vendramini P.H. Production and characterization of lipases and immobilization of whole cell of the thermophilic Thermomucor indicae seudaticae N31 for transesterification reaction. J Mol Catal B Enzym. 2014;107:106–113. [Google Scholar]
- Fransen N.G., Urlings B.A., Bijker P.G., Van B.G.M. Utilization of fermented flocculated poultry sludge as a feed constituent for pigs. Poultry Sci. 1995;74:1948–1960. doi: 10.3382/ps.0741948. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H.J., Wu S.G., Yu S.H., Yoon I., Moore D. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeriatenella. Poultry Sci. 2009;88:2141–2151. doi: 10.3382/ps.2009-00151. [DOI] [PubMed] [Google Scholar]
- Ghoneum M. Enhancement of human natural killer cell activity by modified arabinoxylan from rice bran (MGN-3) Int J Immunother. 1998;14:89–99. [Google Scholar]
- Godoy M.G., Amorim G.M., Barreto M.S., Freire D.M.G. Chapter 12-agricultural residues as animal feed :protein enrichment and detoxification using solid-state fermentation. Current Developments in Biotechnology & Bioengineering. 2018;10:235–256. [Google Scholar]
- Gong R., Xu S., Hermundstad A., Yu Y., Sternson S.M. Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell. 2020;182:1589–1605. doi: 10.1016/j.cell.2020.07.031. [DOI] [PubMed] [Google Scholar]
- Gu B. Jiangsu University; 2010. Research on production of bio-feedstuff with rich peptide from rapeseed meal by mixed solid-state fermentation. [Google Scholar]
- Guanghui Z., Yujie C., Qing K., Ma Y.X., Yang L. Detoxification of aflatoxin B1 by zygosaccharomyces rouxii with solid state fermentation in peanut meal. Toxins. 2017;9:42. doi: 10.3390/toxins9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor E., Erener G. Effect of dietary raw and fermented sour cherry kernel (Prunus cerasus L.) on growth performance,carcass traits, and meat quality in broiler chickens. Poultry Sci. 2020;99:301–309. doi: 10.3382/ps/pez490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Osadchiy V., Mayer E.A. Brain-gut-microbiome interactions in obesity and food addiction. Nat Rev Gastroenterol Hepatol. 2020;5:8–27. doi: 10.1038/s41575-020-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi E.Z., Shojaosadati S.A., Rinzema A. Modelling of simultaneous effect of moisture and temperature on A. Niger, growth in solid-state fermentation. Biochem Eng J. 2004;21:265–272. [Google Scholar]
- Haque M.A., Kachrimanidou V., Koutinas A., Lin C.S.K. Valorization of bakery waste for biocolorant and enzyme production by Monascus purpureus. J Biotechnol. 2016;231:55–64. doi: 10.1016/j.jbiotec.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Hassaan M.S., Soltan M.A., Abdelmoez A.M. Nutritive value of soybean meal after solid state fermentation with Saccharomyces cerevisiae for Nile tilapia. Oreochromis niloticus. Animal Feed Science & Technology. 2015;201:89–98. [Google Scholar]
- Heres L., Engel B.V., Knapen F., Jong D., Wagenaar J.A., Urlings H.A. Fermented liquid feed reduces susceptibility of broilers for Salmonella enteritidis. Poultry Sci. 2003;82:603. doi: 10.1093/ps/82.4.603. [DOI] [PubMed] [Google Scholar]
- Hirabayashi M., Matsui T., Yano H., Nakajima T. Fermentation of soybean meal with Aspergillus usamii reduces phosphorus excretion in chicks. Poultry Sci. 1998;77:552–556. doi: 10.1093/ps/77.4.552. [DOI] [PubMed] [Google Scholar]
- Hong K. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 2004;7:430–435. doi: 10.1089/jmf.2004.7.430. [DOI] [PubMed] [Google Scholar]
- Hu J.K., Lu W.Q., Wang C.L., Zhu R.H., Qiao J.Y. Characteristics of solid-state fermented feed and its effects on performance and nutrient digestibility in growing-finishing pigs. Asian-australasian journal of animal sciences. 2008;21:1635–1641. [Google Scholar]
- Hu R., Chen Y., Wang Z.S. Optimization of process parameters of probiotics-fermented soybean meal and effects of coordination action between compound probiotics and enzyme on fermentation quality. Animal Nutrition. 2013;25:1896–1903. [Google Scholar]
- Huang J., Liu Y.L., Chen J., Wang S., Zang S.B. 2019. An environmentally friendly and emission reducing bio fermented feed for pigs:CN110050895a. [Google Scholar]
- Irfan M., Nadeem M., Syed Q. One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride-IR05 in solid-state fermentation. Journal of Radiation Research and Applied Sciences. 2014;7:317–326. [Google Scholar]
- Jais A., Paeger L., Sotelo-Hitschfeld T., Wunderlich F.T., Peter K., Jens C.B. PNOCARC neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron. 2020;106:1009–1025. doi: 10.1016/j.neuron.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen G.V., Jensen B.B., Knudsen K.B., Canibe N. Impact of fermentation and addition of non-starch polysaccharide-degrading enzymes on microbial population and on digestibility of dried distillers grains with solubles in pigs. Livest Sci. 2015;178:216–227. [Google Scholar]
- Jakobsen G.V., Jensen B.B., Knudsen K.E.B., Canibe N. Improving the nutritional value of rapeseed cake and wheat dried distillers grains with solubles by addition of enzymes during liquid fermentation. Anim Feed Sci Technol. 2015;208:198–213. [Google Scholar]
- Jiang H.J., Sun H., Jiang F.C. Application of fermented soybean meal in the production of weaned piglets in Suhuai pigs. China Swine Industry. 2014;12:58–60. [Google Scholar]
- Jiang H.L., Cai W.B., Mokgawa D.L. Impact of fermented corn straw on growth performance, digestibility and cecal micro flora of grower pigs. Asian J Anim Vet Adv. 2016;11:461–468. [Google Scholar]
- Joris A.M., Missotten J.M., Anneke O. Fermented liquid feed for pigs. Arch Anim Nutr. 2010;64:437–466. doi: 10.1080/1745039X.2010.512725. [DOI] [PubMed] [Google Scholar]
- Kabir S.M. The role of probiotics in the poultry industry. Int J Mol Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp S.G., Faraco V., Amore A., Birolo L., Giangrande C., Soccol V.T. Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour Technol. 2012;114:735–739. doi: 10.1016/j.biortech.2012.03.058. [DOI] [PubMed] [Google Scholar]
- Kaur S., Dhillon G.S., Brar S.K., Chauhan V.B. Carbohydrate degrading enzyme production by plant pathogenic mycelia and microsclerotia isolates of Macrophomina phaseolina through koji fermentation. Ind Crop Prod. 2012;36:140–148. [Google Scholar]
- Kaushik P., Mishra A., Malik A. Dual application of agricultural residues for xylanase production and dye removal through solid state fermentation. Int Biodeterior Biodegrad. 2014;96:1–8. [Google Scholar]
- Kershaw G.F., Luscombe J.R., Cole D.J.A. Lactic acid and sodium acrylate:effect on growth rate and bacterial flora in the intestines of weaned pigs. Vet Rec. 1966;79 [Google Scholar]
- Kil D.Y., Piao L.G., Long H.F., LiM J.S., Kim Y.Y. Effects of organic or inorganic acid supplementation on growth performance, nutrient digestibility and white blood cell counts in weanling pigs. Asian-Australas J Anim Sci. 2006;19:252–261. [Google Scholar]
- Kim S.W., Van H.E., Ji F., Lee C.H., Mateo R.D. Fermented soybean meal as a vegetable protein source for nursery pigs:I. Effects on growth performance of nursery pigs. J Anim Sci. 2010;88:214–224. doi: 10.2527/jas.2009-1993. [DOI] [PubMed] [Google Scholar]
- Kimura I., Ozawa K., Inoue D., Imamura T., Kumi K., Takeshi M. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829–1841. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B., Kim J.W., Nyachoti C.M. Nutrient and energy digestibility, and microbial metabolites in weaned pigs fed diets containing Lactobacillus-fermented wheat. Animal Feed ence & Technology. 2018;11:27–37. doi: 10.1093/jas/sky344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Joshi A., Kashyap R., Khanna S. Production of xylanase by Promicromonospora sp MARS with rice straw under non sterile conditions. Process Biochem. 2011;46:1614–1618. [Google Scholar]
- Le M.H.A., Galle S., Yang Y., Landero J.L., Beltranena E., Ganzle M.G., Zijlstra R.T. Effects of feeding fermented wheat with on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs. J Anim Sci. 2016;94:4677–4687. doi: 10.2527/jas.2016-0693. [DOI] [PubMed] [Google Scholar]
- Lei X.J., Yun H.M., Kim I.H. Effects of dietary supplementation of natural and fermented herbs on growth performance, nutrient digestibility, blood parameters, meat quality and fatty acid composition in growing-finishing pigs. Ital J Anim Sci. 2018;17:1–10. [Google Scholar]
- Li T.J. Advances in antibacterial mechanisms of lactic acid bacteria. Microbiology China. 2002;29:81–85. [Google Scholar]
- Li Z.T. Hebei University of Engineering; 2016. Optimization study on production of fermented feed of fungus chaff of pleurotus nebrodensis with growth performance in rabbit. [Google Scholar]
- Li N., Li N. Inhibitory effects of Lactobacillus metabolites on Escherichia coli and Staphylococcus aureus. China Brew. 2009;5:49–52. [Google Scholar]
- Li L., Chen X.L., Xu J.X. Research on process parameters and quality of the complex probiotics fermented feed. J Shanghai Jiaot Univ. 2010 [Google Scholar]
- Li L., Chou X.X., Yang H.J. Optimization of fermentation conditions for broiler feed and its application effect. Feed Res. 2019;491:19–22. [Google Scholar]
- Lian J.H. South China Agricultural University; 2016. Effect of Polysaccharide on the growth of probiotics in vitro and the effect of fermented feed on the quality of Xuefeng black bone chicken. [Google Scholar]
- Liang R., Zhen L.I., Gang X.U., Yang Y. Effects of fermentation feed on layer chicks growth performance and physiological indexes. Feed Review. 2012;4:5–8. [Google Scholar]
- Liao X.Y., Dai Q., Yu H.Z. Study on the production of protein feed by mixed fermentation of multi strains. China Feed. 2009;16:8–10. [Google Scholar]
- Liao H.P., Li S.X., Zheng H.P., Zhong W., Xu Y.C. A new acidophilic thermostable endo-1,4-β-mannanase from Penicillium oxalicum GZ-2:cloning, characterization and functional expression in Pichia pastoris. BMC Biotechnol. 2014;14:90. doi: 10.1186/s12896-014-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B.S., Lu J., Li Y.M., Yang X.Y. Optimization of key factors influencing microbial fermented feed production and analysis of composition variation during fermentation process. Chinese Agricultural Science Bulletin. 2015;31:1–6. [Google Scholar]
- Liu L. Zhejiang University; 2015. Antibacterial effect of xylitol on common pathogenic bacteria of animal origin. [Google Scholar]
- Liu B., Cao Y.Q. Lsolation, ldentification and lnulinase production of a basidiomycete strain. J Microbiol. 1996;16:14–19. [Google Scholar]
- Liu B.L., Tzeng Y.M. Water content and water activity for the production of cyclodepsipeptides in solid-state fermentation by Metarhizium anisopliae. Biotechnol Lett. 1999;21:657–661. [Google Scholar]
- Liu C., Sheng J.P., Zou J.H., Wang H.L., Ding Q., Lin S. Effects of microbial changes on physical and chemical characteristics of agaricus bisporus compost during fermentation. Food Sci (N Y) 2010;7:270–271. [Google Scholar]
- Liu T.M., Jun M.S., Si S.Q. Effects of temperature changes on the production processing of soybean peptides by solid state fermentation of soybean meal. China Brew. 2010;29:111–112. [Google Scholar]
- Liu H., Zhang J., Zhang S. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J Agric Food Chem. 2014;62:860–866. doi: 10.1021/jf403288r. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li C., Meng X., Yan Y. Biodiesel synthesis directly catalyzed by the fermented solid of Burkholderia cenocepacia via solid state fermentation. Fuel Process Technol. 2016;106:303–309. [Google Scholar]
- Liu Y., Ren H.H., Ma X.M., Xu Y.F., Zhang J., Yao J. Normal NW. Isolation, identification & ferment condition optimization of an inulinase-producing strain. J Microbiol. 2018;38:70–75. [Google Scholar]
- Long Z.D., Chen H.R., Liu H., Zou K.X., Sun J.S., Li J.G. Application of pectin-degrading fungus Aspergillus tubingensis GYC 501 in improvement of tobacco cut-stem quality and optimization of its fermentation conditions. Acta Agriculturae Jiangxi. 2017;29:95–98. [Google Scholar]
- Lyberg K., Lundh T., Pedersen C., Lindberg J.E. Influence of soaking, fermentation and phytase supplementation on nutrient digestibility in pigs offered a grower diet based on wheat and barley. Anim Sci. 2006;82:853–858. [Google Scholar]
- Madrera R.R., Bedrinana R.P., Valles B.S. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT-Food Science and Technology. 2015;64:1342–1353. [Google Scholar]
- Mai H.T.N., Lee K.M., Choi S.S. Enhanced oxalic acid production from corncob by a methanol-resistant strain of Aspergillus Niger using semi solid-sate fermentation. Process Biochem. 2016;51:9–15. [Google Scholar]
- Mao Y., Chen Z., Lu L., Jin B., Chen T. Efficient solid-state fermentation for the production of 5-aminolevulinic acid enriched feed using recombinant Saccharomyces cerevisiae. J Biotechnol. 2020;322:29–32. doi: 10.1016/j.jbiotec.2020.06.001. [DOI] [PubMed] [Google Scholar]
- Marco M.L., Heeney D., Binda S., Cifelli C.J., Cotter P.D., Foligne B. Health benefits of fermented foods:microbiota and beyond. Curr Opin Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Mathew A.G., Chattin S.E., Robbins C.M., Golden D.A. Effects of a direct-fed yeast culture on enteric microbial populations, fermentation acids, and performance of weanling pigs. J Anim Sci. 1998;76:2138–2145. doi: 10.2527/1998.7682138x. [DOI] [PubMed] [Google Scholar]
- Mathur G., Mathur A., Sharma B.M., Chauhan R.S. Enhanced production of laccase from Coriolus sp. using Plackett Burman design. J Pharm Res. 2013;6:151–154. [Google Scholar]
- Mcquestin O.J., Shadbolt C.T., Ross T. Quantification of the relative effects of temperature, pH, and water activity on inactivation of Escherichia coli in fermented meat by meta-analysis. Applenvironmicrobiol. 2009;75:6963–6972. doi: 10.1128/AEM.00291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikoglu M., Lin C.S.K., Webb C. Kinetic studies on the multi-enzyme solution produced via solid-state fermentation of waste bread by Aspergillus awamori. Biochem Eng J. 2013;80:76–82. [Google Scholar]
- Miao Y.U., Yan J.X., Peng Z.L. Effects of microbial fermented feed on immune function parameter of beef cattle. China Animal Husbandry & Veterinary Medicine. 2013;40:114–117. [Google Scholar]
- Mikkelsen L.L., Jensen B.B. vol. 88. 1997. (Effect of fermented liquid feed (FLF) on growth performance and microbial activity in the gastrointestinal tract of weaned piglets). [Google Scholar]
- Missotten J.A.M., Goris J., Michiels J., Coillie E.V., Herman L., Smet S.D. Screening of isolated lactic acid bacteria as potential beneficial strains for fermented liquid pig feed production. Anim Feed Sci Technol. 2009;150:122–138. [Google Scholar]
- Missotten J.A., Michiels J., Degroote J., Stefaan D.S. Fermented liquid feed for pigs:an ancient technique for the future. J Anim Sci Biotechnol. 2016;6:4–12. doi: 10.1186/2049-1891-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C.A., Scholten R.H., Tricarico J.M., Brook P.H., Verstegen M.W.A. Fermentation of wheat:effects of backslopping different proportions of pre-fermented wheat on the microbial and chemical composition. Arch Anim Nutr. 2006;60:158–169. doi: 10.1080/17450390600562700. [DOI] [PubMed] [Google Scholar]
- Morishita Y., Ogata M. Studies on the alimentary flora of pig. 5. Influence of starvation on the microbial flora. Nihon Juigaku Zasshi the Japanese Journal of Veterinary Science. 1970;32:19–24. doi: 10.1292/jvms1939.32.19. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A., Dutta N., Chattopadhyay D., Chakrabarti K. Degumming of ramie fiber and the production of reducing sugars from waste peels using nanoparticle supplemented pectate lyase. Bioresour Technol. 2013;137:202–208. doi: 10.1016/j.biortech.2013.03.139. [DOI] [PubMed] [Google Scholar]
- Mulder R.W.A.W., Havenaar R.J.H.J., Veld Huis I. vol. 94. Springer; Dordrecht: 1997. Intervention strategies:the use of probiotics and competitive exclusion microfloras against contamination with pathogens in pigs and poultry; pp. 187–207. (Probiotics 2). [Google Scholar]
- Mussatto S.I., Ballesteros L.F., Martins S., Teixeira J.A. InTech; 2012. Use of agro-industrial wastes in solid-state fermentation processes. [Google Scholar]
- Na J., Zhang L., Ge Q.P. Induction of pectinolytic enzyme from Bacillus cereus HDYM-02 and application of flax degumming. J Nat Sci Heilongjiang Univ. 2018;35:467–472. [Google Scholar]
- Nagel F.J., Tramper J., Bakker M.S. Model for on-line moisture-content control during solid-state fermentation. Biotechnol Bioeng. 2015;72:231–243. doi: 10.1002/1097-0290(20000120)72:2<231::aid-bit11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Nathan C. Microbiology. An antibiotic mimics immunity. Science. 2008;322:1337–1338. doi: 10.1126/science.1167452. [DOI] [PubMed] [Google Scholar]
- Niba A.T., Beal J.D., Kudi A.C., Brooks P.H. Potential of bacterial fermentation as a biosafe method of improving feeds for pigs and poultry. Afr J Biotechnol. 2009;8:1758–1767. [Google Scholar]
- Niven S.J., Beala J.D., Brooksa P.H. The effect of controlled fermentation on the fate of synthetic lysine in liquid diets for pigs. Anim Feed Sci Technol. 2006;129:304–315. [Google Scholar]
- Nout M.J.R. Fermented foods and food safety. Food Res Int. 1994;27:291–298. [Google Scholar]
- Pandey A. Solid-state fermentation. Biochem Eng J. 2003;13:81–84. doi: 10.1016/s1369-703x(00)00065-6. [DOI] [PubMed] [Google Scholar]
- Pandey A.K., Edgard G., Negi S. Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew Energy. 2016;98:51–56. [Google Scholar]
- Panwar D., Srivastava P.K., Kapoor M. Production, extraction and characterization of alkaline xylanase from Bacillus sp. PKD-9 with potential for poultry feed. Biocatalysis and Agricultural Biotechnology. 2014;3:118–125. [Google Scholar]
- Park S.E., Seo S.H., Kim E.J., Na C.S., Son H.S. Effects of different fermentation temperatures on metabolites of Kimchi. Food Bioscience. 2018;23:100–106. [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poultry Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Pedersen A. vol. 510. Danish Bacon and Meat Council; Copenhagen, Denmark: 2001. (Fermented liquid feed to piglets). [Google Scholar]
- Pedersen C., Stein H.H. Effects of liquid and fermented liquid feeding on energy, dry matter, protein and phosphorus digestibility by growing pigs. Livest Sci. 2010;134:59–61. [Google Scholar]
- Pirota R.D.P.B., Tonelotto M., Delabona P.S., Fonseca R.F., Paixão D.A.A., Baleeiro F.C.F. Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under controlled operation conditions. Ind Crop Prod. 2013;45:465–471. [Google Scholar]
- Plahar W.A., Leung H.K. Effect of moisture content on the development of carboxylic acids in traditional maize dough fermentation. J Sci Food Agric. 2010;33:555–558. [Google Scholar]
- Pojanagaroon S. Proceedings of the 45th kasetsart university annual conference, bangkok, Thailand, 30 january-2 february 2007. Subject:Plants. 2007. Effects of pH of fermented water, fermentation and aging time on Krachai-Dam (Kaempferia parviflora) honey wines qualities; pp. 311–318. [Google Scholar]
- Połec B., Baryga A., Szyman´Ski T., Wolynska W., Tobola A. Biogas generation capabilities from beet pulp methane fermentation process. Part II. Semi-continuous beet pulp fermentation. Gazeta Cukrownicza. 2010;118:120–125. [Google Scholar]
- Puwen X.U. Fermented liquid feed:effects on weaner piglet intestinal health. Chinese Journal of Animal Nutrition. 2011;23:2105–2108. [Google Scholar]
- Qiao J.Y., Hongye Y., Jiang Z.Q., Liu S.Q. A novel thermostable β-1,3-1,4-glucanase from Thermoascus aurantiacus and its application in oligosaccharide production from oat bran. Carbohydr Res. 2018;11:31–37. doi: 10.1016/j.carres.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Qin Q., Kun H., Hong X., Jiang M., Sheng D.M. Low-field nuclear magnetic resonance for online determination of water content during sausage fermentation. J Food Eng. 2017;11:291–297. [Google Scholar]
- Quan C.S., HongTao X.U., Jun-Hua W., Liu C.J., Fan S.D. Lactobacteriocin-safety and natural food preservative. J Microbiol. 2006;26:86–89. [Google Scholar]
- Qureshi A.S., Khushk I., Ali C.H., Chisti Y., Ahmad A., Majeed H. Coproduction of protease and amylase by thermophilic Bacillus sp. BBXS-2 using open solid-state fermentation of lignocellulosic biomass. Biocatalysis and Agricultural Biotechnology. 2016;8:146–151. [Google Scholar]
- Ranjitkar S., Karlsson A.H., Petersen M.A., Bredie W.L.P., Engberg R.M. The influence of feeding crimped kernel maize silage on broiler production, nutrient digestibility and meat quality. Br Poultry Sci. 2016;57:12–23. doi: 10.1080/00071668.2015.1115468. [DOI] [PubMed] [Google Scholar]
- Rastogi S., Soni R., Kaur J., Soni S.K. Unravelling the capability of Pyrenophora phaeocomes S-1 for the produc tion of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresour Technol. 2016;222:458–469. doi: 10.1016/j.biortech.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Refstie S., Svihus B., Shearer K.D., Trond S. Nutrient digestibility in Atlantic salmon and broiler chickens related to viscosity and non-starch polysaccharide content in different soyabean products. Anim Feed Sci Technol. 1999;79:331–345. [Google Scholar]
- René L.V.W., Urlings B.A.P., Lipman L.J.A. Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl Environ Microbiol. 2001;67:3071–3076. doi: 10.1128/AEM.67.7.3071-3076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Fernández D.E., Rodríguez-León J.A., Carvalho J.C., Sturm W., Soccol C.R. The behavior of kinetic parameters in production of pectinase and xylanase by solid-state fermentation. Bioresour Technol. 2011;102:10657–10662. doi: 10.1016/j.biortech.2011.08.106. [DOI] [PubMed] [Google Scholar]
- Rombenso A., Crouse C., Trushenski J. Comparison of traditional and fermented soybean meals as alternatives to fish meal in hybrid striped bass feeds. N Am J Aquacult. 2013;75:197–204. [Google Scholar]
- Roselli M., Finamore A., Britti M.S., Konstantinov S.R., Smidt H., Willem M. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J Nutr. 2007;137:2709–2716. doi: 10.1093/jn/137.12.2709. [DOI] [PubMed] [Google Scholar]
- Roubosvan D.H.P.J., Nout M.J.R., Beumer R.R., Meulen J.V.D., Zwietering M.H. Fermented soya bean (tempe) extracts reduce adhesion of enterotoxigenic Escherichia coli to intestinal epithelial cells. J Appl Microbiol. 2010;106:1013–1021. doi: 10.1111/j.1365-2672.2008.04068.x. [DOI] [PubMed] [Google Scholar]
- Russell J.B., Diezgonzalez F. The effects of fermentation acids on bacterial growth. Adv Microb Physiol. 1998;39:205–234. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]
- Şanlier N., Gökcen B.B., Sezgin A.C. Health benefits of fermented foods. Crit Rev Food Sci Nutr. 2017;9:1–22. doi: 10.1080/10408398.2017.1383355. [DOI] [PubMed] [Google Scholar]
- Savvidou S.E., Beal J.D., Brooks P.H. Liquid feed fermented with Lactobacillus salivarius reduces susceptibility of broiler chickens to Salmonella enterica typhimurium Sal 1344 nalr. British Poultry Abstracts. Taylor & Francis. 2009;5:43–44. [Google Scholar]
- Scholten R.H., Peet-Schwering C.M., Hartog L.A., Balk M., Schrama J.W., Verstegen M.W. Fermented wheat in liquid diets:effects on gastrointestinal characteristics in weanling piglets. J Anim Sci. 2002;80:1179–1186. doi: 10.2527/2002.8051179x. [DOI] [PubMed] [Google Scholar]
- Scholten R.H., Rijnen M.M., Schrama J.W., Boer H., Vesseur P.C., Hartog L.A.D. Fermentation of liquid coproducts and liquid compound diets:Part 1. Effects on chemical composition during a 6-d storage period. J Anim Physiol Anim Nutr. 2010;85:111–123. doi: 10.1046/j.1439-0396.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Shaw J.N., Muth O.H. The use of acidophilus milk in the treatment of dysentery of young animals. J Am Vet Med Assoc. 1937;90:171–175. [Google Scholar]
- Shen H.S., Chen J.C., Tang B.S. 2012. A method for biodegradation of arabinoxylan from wheat bran. [Google Scholar]
- Shen D.C., Yinglin L.U., Zhou W.L., Chen D.W., Ao J.H., Wang Q. Analysis of vinasse with biological fermentation and hydroponics experiment. Sugarcane & Canesugar. 2018;1:12–16. [Google Scholar]
- Shi C., Zhang Y., Yin Y., Wang C., Wang Y. Amino acid and phosphorus digestibility of fermented corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium fed to pigs. J Anim Sci. 2017;95:3996–4004. doi: 10.2527/jas2017.1516. [DOI] [PubMed] [Google Scholar]
- Shiliang Y., Donghe L., Yuhang B., Xuena L., Zheng T., Chen Z. Experimental report of feeding fattening cattle with fermented feed. Modern Journal of Animal Husbandry & Veterinary Medicine. 2015;7:31–34. [Google Scholar]
- Shimelis E.A., Rakshit S.K. Influence of natural and controlled fermentations on α-galactosides, antinutrients and protein digestibility of beans (Phaseolus vulgaris L. Int J Food Sci Technol. 2010;43:658–665. [Google Scholar]
- Shiyan Q., Chengli H., Xiangfang Z. Regulation and mechanism of lactic acid bacteria on porcine intestinal barrier function. Chinese Journal of Animal Nutrition. 2014;26:3052–3063. [Google Scholar]
- Sin Y.C., Farah D.A.B., Nor M.M. A thermotolerant Endo-1,4-β-mannanase from Trichoderma virens UKM1:Cloning, recombinant expression and characterization. J Mol Catal B Enzym. 2016 [Google Scholar]
- Skrede G., Herstad O., Sahlstr M.S., Holck A., Slinde E., Skrede A. Effects of lactic acid fermentation on wheat and barley carbohydrate composition and production performance in the chicken. Anim Feed Sci Technol. 2003;105 0-148. [Google Scholar]
- Soccol C.R., Costa E.S.F.D., Letti L.A.J. Recent developments and innovations in solid state fermentation. Biotechnology Research & Innovation. 2017;1:52–71. [Google Scholar]
- Steenfeldt S., Marianne H., Anette M. Enzyme supplementation of wheat-based diets for broilers:2. Effect on apparent metabolisable energy content and nutrient digestibility. Anim Feed Sci Technol. 1998;75:45–64. [Google Scholar]
- Sugiharto S., Ranjitkar S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses:A review. Animal Nutrition. 2018;11:1. doi: 10.1016/j.aninu.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhermiyati S., Iriyanti N. The effect of natuzyme in the diets containing non-starch polysaccharides on meat quality of native chicken. Journal of Animal Production. 2011;13:76–82. [Google Scholar]
- Sun L. Anhui Agricultural University; 2008. Studies on solid-state fermentation of rapeseed meal with microorganism. [Google Scholar]
- Sun H., Tang J.W., Fang C.L., Yao X.H., Wu Y.F., Wang X. Molecular analysis of intestinal bacterial microbiota of broiler chickens fed diets containing fermented cottonseed meal. Poultry Sci. 2013;92:392–401. doi: 10.3382/ps.2012-02533. [DOI] [PubMed] [Google Scholar]
- Sungsam K., Germanbueno G., Minhanh P., Jang J.W., Lee K.J. Effects of dietary supplementation of a Meju, fermented soybean meal, and Aspergillus oryzae for juvenile parrot fish (Oplegnathus fasciatus. Asian-Australas J Anim Sci. 2009;22:849–856. [Google Scholar]
- Svihus B., Herstad O., Newman C.W. Effect of high-moisture storage of barley, oats, and wheat on chemical content and nutritional value for broiler chickens. Acta Agric Scand Sect A Anim Sci. 1997;47:39–47. [Google Scholar]
- Tang J.P., Xiao J.F., Yang W.C., Zhou T., Wu Y.L. 2020. A kind of bio fermentation feed for pig and its preparation method which can replace antibiotics:CN111011632A[P] [Google Scholar]
- Turner D.L., Lamosa P., Rodríguez A., Martinez B. Structure and properties of the metastable bacteriocin Lcn972 from Lactococcus lactis. J Mol Struct. 2013;1031:207–210. [Google Scholar]
- A new strain of larnia aquatica with pectin degradation function Gj-4:CN202010030253. 2020;9:5–8. [Google Scholar]
- Urlings H.A., Mul A.J., Klooster A.T.V.'. Microbial and nutritional aspects of feeding fermented feed (poultry by-products) to pigs. Vet Q. 1993;15:146–151. doi: 10.1080/01652176.1993.9694394. [DOI] [PubMed] [Google Scholar]
- Velmurugan P., Hur H., Balachandar V., Kamala-Kannan S., Lee K.J., Lee S.M. Monascus pigment production by solid-state fermentation with corn cob substrate. J Biosci Bioeng. 2011;112:590–594. doi: 10.1016/j.jbiosc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- ang H., W Study on a-galactosidases and b-galactosidase from bispora sp. MEY-1. Chinese Academy of Agricultural Sciences. 2010 [Google Scholar]
- ng H.M., Wa Gene cloning and characterization of α-galactosidases from thermophilic microorganisms. Chinese Academy of Agricultural Sciences. 2010 [Google Scholar]
- Wang Y. China Agricultural University; 2014. Study on solid-state fermentation conditions of soybean meal and its application on weaned pigs. [Google Scholar]
- Wang T.Y., Wu Y.H., Jiang C.Y. Solid state fermented potato pulp can be used as poultry feed. Br Poultry Sci. 2010;51:229–234. doi: 10.1080/00071661003781864. [DOI] [PubMed] [Google Scholar]
- Wang J.J., Wang S.X., Lu W.Q. Effects of antibiotic-free microbial fermented feed on immune and antioxidant function of piglets. China Feed. 2011;16:25–27. [Google Scholar]
- Wang J.P., Liu N., Song M.Y., Qin C.L., Ma C.S. Effect of enzymolytic soybean meal on growth performance, nutrient digestibility and immune function of growing broilers. Anim Feed Sci Technol. 2011;169:224–229. [Google Scholar]
- Wang H., Shi P., Luo H., Huang H., Yang P.L., Yao B. A thermophilic α-galactosidase from Neosartorya fischeri P1 with high specific activity, broad substrate specificity and significant hydrolysis ability of soymilk. Bioresour Technol. 2014;153:361–364. doi: 10.1016/j.biortech.2013.11.078. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu X.T., Wang H.L., Li D.F., Piao X.S., Lu W.Q. Optimization of processing conditions for solid-state fermented soybean meal and its effects on growth performance and nutrient digestibility of weanling pigs. Livest Sci. 2014;170:91–99. [Google Scholar]
- Wang M., Jiang M., Li H., Lu Z.M., Luo C.Q., Xu Z.H. Investigation on the regular patterns of mannan oligosaccharides degradation and utilization by lactic acid bacteria. Food Ferment Ind. 2016;42:20–24. [Google Scholar]
- Wardynski F.A., Rust S.R., Yokoyama M.T. Effect of microbial inoculation of high-moisture corn on fermentation characteristics, aerobic stability, and cattle performance. J Anim Sci. 1993;71:2246–2252. doi: 10.2527/1993.7182246x. [DOI] [PubMed] [Google Scholar]
- Wei L.K., Zheng B., Wang H.H. Application of liquid fermented feed in pig breeding. Feed Res. 2019;42:122–125. [Google Scholar]
- Winsen R.L.V., Urlings B.A.P., Lipman L.J.A. Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl Environ Microbiol. 2001;67:3071–3076. doi: 10.1128/AEM.67.7.3071-3076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S., Ye K., Li M., Ying J., Zhu X. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome. 2021;9:3–18. doi: 10.1186/s40168-021-01029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xianzhang Z., Lulu L., Zhixiang W. Effects of fermented feed on growth Performance,Fatty liver Performance,Intestinal mucosa structure and organ tissue ATPase activity of landaise geese. Chinese Journal of Animal Nutrition. 2013;25:2668–2674. [Google Scholar]
- Xie Z., Hu L., Li Y., Geng S.J., Cheng S.S., Fu X.F. Changes of gut microbiota structure and morphology in weaned piglets treated with fresh fermented soybean meal. World J Microbiol Biotechnol. 2017;33:213–224. doi: 10.1007/s11274-017-2374-7. [DOI] [PubMed] [Google Scholar]
- Xijie Z., Hua L., Jianliang L., Xie D.X., Xu J., Huo C.M. A study on the benefit of feeding Hy-line white egg with fermented feed. Southwest China J Agric Sci. 2007;20:529–533. [Google Scholar]
- Xinxu H., Yinghua Z., Huizhi L., Wang S.P., Gao S.F., Zhou X.L. Effects of fermented feed without antibiotic on growth Performance, Intestinal Flora, Blood biochemical parameters and immune function of weaner piglets. Chinese Journal of Animal Nutrition. 2013;25:2989–2997. [Google Scholar]
- XiuLin Z., XiaoBing W., ChangBo O., Wang Q.X., Liu M.C., Chen Q. Research progress of probiotics fermented feed effects on growth performance and immune function in piglets. China Animal Husbandry & Veterinary Medicine. 2017;44:476–481. [Google Scholar]
- Yang L.J., Yang Z., Yang W., Li H.R., Zhang C.Y., Jiang S.Z. Conventional solid fermentation alters mycotoxin contents and microbial diversity analyzed by high-throughput sequencing of a Fusarium mycotoxin-contaminated diet. Can J Anim Sci. 2018;98:354–361. [Google Scholar]
- Yang L.J., Xue X.S., Song Q.L., Wei L.J., Zeng X.F., Qiao S.Y. Effects of vinegar Lees by solid state fermentation on growth performance, nutrient apparent digestibility, serum indexes and volatile fatty acid content in feces of finishing pigs. Animal Nutrition. 2020;32:119–128. [Google Scholar]
- Yang H., Wang B., Zhang Q., Cheng H., Yu H. Improvement of fermentation quality in the fermented total mixed. Ration with Oat Silage. 2021;9:420. doi: 10.3390/microorganisms9020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W., Zhu R., Lu W., Gong L. A new strategy to apply Bacillus subtilis, MA139 for the production of solid-state fermentation feed. Lett Appl Microbiol. 2010;49:229–234. doi: 10.1111/j.1472-765X.2009.02647.x. [DOI] [PubMed] [Google Scholar]
- Yu Z., Dong B., Lu W. Dynamics of bacterial community in solid-state fermented feed revealed by 16S rRNA. Lett Appl Microbiol. 2010;49:166–172. doi: 10.1111/j.1472-765X.2009.02636.x. [DOI] [PubMed] [Google Scholar]
- Yu J., Wang W.H., Menghe B.L.G. Diversity of lactic acid bacteria associated with traditional fermented dairy products in Mongolia. J Dairy Sci. 2011;94:3229–3241. doi: 10.3168/jds.2010-3727. [DOI] [PubMed] [Google Scholar]
- Yu B., Lan X.Y., He Z.J., Ji Y.X., Han L.S., Chen G.H. Research progress of microbial fermented feed in China. Modern Animal Husbandry Science & Technology. 2019;10:6–8. [Google Scholar]
- Yuan L., Chang J., Yin Q., Lu M., Di Y.R., Wang P. Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Animal Nutrition. 2017;3:19–24. doi: 10.1016/j.aninu.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y.Y., Fang Q., Huang Z.Y., Huang D.W. Study on acid production of different kinds of hogwash waste oil by anaerobic fermentation. Appl Chem Ind. 2020;336:72–76. [Google Scholar]
- Zhang L.F. Discusstion on bacteriostatic mechanism of lactic acid bacteria metabolites in vitro. Feed Review. 2006;12:4–6. [Google Scholar]
- Zhang J., Ou S.Y., Zhang N. Processing study on ferulic acid and oligosaccharides prepared from enzymatin hydrolysis of destorched wheat bran. Food Sci (N Y) 2003;24:63–68. [Google Scholar]
- hang H.B., Z, Feng-Lei L.I., Ying L.U., Ming Y.E. Condition optimization and result analysis of swill fermentation by mixed fermentation. J Hefei Univ Technol (Nat Sci) 2012 [Google Scholar]
- Zhang H.Y., Yi J.Q., Piao X.S., Li P.F., Zeng Z.K., Wang D. The metabolizable energy value, standardized ileal digestibility of amino acids in soybean meal, soy protein concentrate and fermented soybean meal, and the application of these products in early-weaned piglets. Asian-australasian Journal of Animal Sciences. 2013;26:691–699. doi: 10.5713/ajas.2012.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Yang G.H., Wei L.J. Study on solid-state fermentation of rice husk powder to produce protein feed. China Feed. 2015;8:25–27. [Google Scholar]
- Zhang J., Zhang X., Lijun L.I., Xiao Q., Weng H.F., Ni H. Solid-fermentation process of soybean meal with different probiotics. J Jimei Univ. 2018 [Google Scholar]
- Zhang P.F., Li X.L., Wang Z.Q., Yuan X.H., Li J.J., Du Y.G. Overexpression and characterization of endo-β-1, 4-mannanase from Aspergillus nidulans in Pichia pastoris. Acta Microbiol Sin. 2018;58:391–400. [Google Scholar]
- Zhao H., Wang X.T., Tang J.Y., Tang X.P., Gang J., Liu G.M., Wang K.N. Nutritional improvement of sweet potato residue by solid-state fermentation with mixed microbe strains. Animal Nutrition. 2015;27:1191–1198. [Google Scholar]
- Zhongli P., Chunhua G., Xue B., Zhu W.L., Fu X.S., Wang Y.L. Effects of fermented feed on production performance, digestibility of dietary nutrients, blood biochemical indices in goat. J Agric Sci Technol. 2013;15:106–113. [Google Scholar]
- Zhou M.J., Li T., Zeng Y. Effect of moisture content on fermented feed quality. China Feed. 2013;7:12–14. [Google Scholar]
- Zhu H., Wu W.R., He L.M., Lei X., Zhou J.P., Huang Z.X. Biochemical characterization of mannanase produced by Cladosporium velox in culture medium of plam kernal meal. Guangdong Journal of Animal and Veterinary Science. 2018;43:43–47. [Google Scholar]