Abstract
Background
Patient decision aids should help people make evidence-informed decisions aligned with their values. There is limited guidance about how to achieve such alignment.
Purpose
To describe the range of values clarification methods available to patient decision aid developers, synthesize evidence regarding their relative merits, and foster collection of evidence by offering researchers a proposed set of outcomes to report when evaluating the effects of values clarification methods.
Data Sources
MEDLINE, EMBASE, PubMed, Web of Science, the Cochrane Library, and CINAHL.
Study Selection
We included articles that described randomized trials of 1 or more explicit values clarification methods. From 30,648 records screened, we identified 33 articles describing trials of 43 values clarification methods.
Data Extraction
Two independent reviewers extracted details about each values clarification method and its evaluation.
Data Synthesis
Compared to control conditions or to implicit values clarification methods, explicit values clarification methods decreased the frequency of values-incongruent choices (risk difference, –0.04; 95% confidence interval [CI], –0.06 to –0.02; P < 0.001) and decisional conflict (standardized mean difference, –0.20; 95% CI, –0.29 to –0.11; P < 0.001). Multicriteria decision analysis led to more values-congruent decisions than other values clarification methods (χ2 = 9.25, P = 0.01). There were no differences between different values clarification methods regarding decisional conflict (χ2 = 6.08, P = 0.05).
Limitations
Some meta-analyses had high heterogeneity. We grouped values clarification methods into broad categories.
Conclusions
Current evidence suggests patient decision aids should include an explicit values clarification method. Developers may wish to specifically consider multicriteria decision analysis. Future evaluations of values clarification methods should report their effects on decisional conflict, decisions made, values congruence, and decisional regret.
Keywords: decision making, values clarification, shared decision making, preference elicitation
Highlights.
Current evidence suggests patient decision aids should include an explicit values clarification method.
To support health decisions that align with values, patient decision aid developers may wish to specifically consider multicriteria decision analysis.
Introduction
Shared decision making is appropriate in many situations and is particularly indicated in clinical situations where the “best” option may differ between people, depending on what matters to them.1–3 What is important to one person might be different from what is important to others, and determining what is important can be difficult even with the appropriate information and evidence at hand. The process of shared decision making therefore aims to help people make health-related decisions that are informed by high-quality, well-presented, and well-understood evidence,4–8 aligned with what matters to the person or people affected by the decision and acted upon.9–12 It follows that the process of clarifying and expressing values is an important aspect of shared decision making and thus of patient decision aids. Within patient decision aids, this process is supported by explicit values clarification methods.
Explicit values clarification methods require users to interact with something such as a worksheet or an interactive website to clarify what matters to them relevant to a health decision. Such methods have been shown to encourage desirable outcomes such as better alignment with patients’ values13,14 and reduced decisional regret, the latter particularly among people with lower health literacy. 15 However, explicit values clarification methods are extremely diverse, 16 and there has been little guidance regarding their comparative effects on users’ decision making processes or outcomes, 17 making it difficult for patient decision aid developers to know which explicit method to use. Patient decision aid developers might look toward the preference elicitation literature for guidance, but the guidance available 18 is often tailored toward aggregate-level decision making, such as regulatory decisions 19 or health technology assessment, 20 not for supporting individual-level decision making.
This updated review sought to build upon previous versions of the International Patient Decision Aids Standards’ chapter on values clarification21,22 as well as previous evidence syntheses that have established the advantages of explicit values clarification methods over implicit methods or no values clarification.13,14 We sought to advance the science and practice of values clarification methods in 3 ways. First, we aimed to offer clear definitions and an annotated summary of existing approaches that have been or could be used as values clarification methods. Second, we aimed to synthesize evidence of different techniques’ effects on health decision outcomes. Third, we aimed to foster future evidence by offering researchers a proposed set of outcomes to consider when evaluating the effects of values clarification methods.
Definitions
Part of the challenge in studying or using values clarification methods is that definitions vary and terms like values are used imprecisely in the patient decision support literature.23,24 Another challenge is that there is substantial overlap between values clarification methods used in patient decision support and preference elicitation methods used in health economics. To bring clarity to this imprecision and overlap, we adopt working definitions in Table 1 for use in this article.
Table 1.
Definitions of Terms
| Term | Definition Adopted in This Article |
|---|---|
| Values | An umbrella term referring to what matters to an individual relevant to a health decision. Values may be directly relevant to decisions (e.g., “beliefs, feelings, or perceptions regarding attributes of a treatment option”) or indirectly relevant (e.g., goals; worldviews; family, religious, or cultural values). 25 Values may be represented qualitatively or, in some cases, quantitatively. This definition is deliberately broad. |
| Values clarification | “The process of sorting out what matters to an individual relevant to a given health decision.” 16 This definition emphasizes that what matters to an individual may be broader than attribute-specific values. What matters may also include preferences, concerns (e.g., concerns about changes in health status), and issues to do with the context of a person’s life within which they would need to implement a decision (e.g., fitting a treatment plan into one’s work schedule). 16 |
| Values clarification methods | “Strategies that are intended to help patients evaluate the desirability of options or attributes of options within a specific decision context, in order to identify which option [they] prefer.” 22 |
| Implicit values clarification methods | Strategies for facilitating values clarification that do not require people to interact with anything or anyone—for example, describing “options in enough detail that clients can imagine what it is like to experience the physical, emotional, and social effects,” 14 or simply encouraging people to think about what matters to them. |
| Explicit values clarification methods | Strategies for facilitating values clarification that require people to interact with something or someone (e.g., filling out a worksheet, using an interactive website, having a semistructured conversation with another person with the explicit purpose of clarifying values, or engaging in another structured exercise). |
| Preferences | The extent to which a decision option or health state is desirable or acceptable, either in the abstract or in comparison to other options or health states. Preferences may be represented qualitatively or, more commonly, quantitatively. 26 |
| Preference elicitation methods | Processes by which preferences are drawn out. 16 Preference elicitation methods may vary according to the theory informing them. They are highly related to values clarification methods. Although older terms revealed and stated preference elicitation methods are no longer recommended, readers who encounter these terms in previous preference elicitation literature should note that these may overlap with implicit and explicit values clarification methods, respectively. |
| Tradeoffs | When multiple desirable outcomes cannot all be achieved, one must forgo (or trade off) some potential benefits or options to avail oneself of others. When users are explicitly required to engage with tradeoffs, this means they must consider and indicate what they are willing to give up to get something else or, in other words, which potential harms are acceptable in exchange for their associated potential benefits. 16 For example, a user might indicate in a ranking exercise that they would prioritize greater comfort at the end of life over additional months of life. |
As noted above, we continue to use the term values clarification even though this is sometimes misinterpreted as implying a narrow definition of values. Changing terms makes it difficult for people who are new to a field to connect the dots across decades of previous research. It is clear that previous research in values clarification addressed issues that were broader than valuation of treatment-specific attributes. 21 In this update, we therefore move forward with the older terms, now with more clarity about what they mean in our presentation of the evidence.
Theoretical Rationale
Our interdisciplinary team determined that the theoretical rationale for values clarification required only a small edit, shown in square brackets, to reflect the focus on explicit methods. Like Fagerlin et al., 22 we assert the theoretical rationale for explicit values clarification methods as being that they “should aim to [explicitly] facilitate at least one or more of the following six decision making processes: 1) Identifying options, which can include either the narrowing down of options, or the generation of options that were not offered at the outset, 2) Identifying attributes of the situation and/or the options which ultimately affect the patient’s preference in a specific decision context, 3) Reasoning about options or attributes of options, 4) Integrating attributes of options using either compensatory or both compensatory and noncompensatory decision rules, 5) Making holistic comparisons, and 6) Helping decision makers retrieve relevant values from long-term memory.” Pieterse et al. 27 provided theory-based recommendations on processes that values clarification methods could aim to facilitate.
Although reasoning is one of the potential processes supported by values clarification, neither the definition nor the theoretical rationale of values clarification methods requires that people who are being supported in making a personal health decision must rationally deliberate about each option, or that the goal must always be a fully rational choice. In some decision making situations, rational deliberation and rational choice may be desired, while in others, they may not.28,29
Explicit Values Clarification Methods
Table 2 organizes strategies that can be used as explicit values clarification methods in patient decision aids, building upon previously developed lists of types of values clarification methods3,16 and reviews of preference elicitation methods.30,31 Methods range from highly structured strategies that can also be used for preference elicitation in the context of health policy decision making to substantially less structured strategies. While not every use of a given method will be exactly the same, we deemed them functionally similar in terms of how they might be used and what the user experience might be in a patient decision aid. Patient decision aids may use multiple strategies. For example, a user may be asked to use a rating scale or visual analog scale whose values are then used in a decision-analytic model.
Table 2.
Explicit Values Clarification Methods
| Method | Description |
|---|---|
| Adaptive conjoint analysis (example 32 ) | The user rates a series of sets of attributes and their levels, where choices presented are tailored to earlier answers. |
| Allocation of points (example 33 ) | The user has a “budget” to “spend” on decision attributes, according to their importance. |
| Analytical hierarchy process (example 34 ) | The user is asked to compare sets of options relative to predefined decision criteria. |
| Best–worst scaling (example 35 ) | The user is asked to indicate the best and the worst object in repeated subsets of a finite number of objects (case 1, also known as object scaling or MaxDiff), the best and worst attributes within each of a number of profiles that systematically vary across a multiple attributes and levels (case 2), or the best and worst profiles from among 3 or more profiles (case 3). |
| Decision analysis or multicriteria decision analysis (umbrella term a ) (resource36,37) | The user is asked to directly indicate the extent to which a decision attribute or outcome matters to them or how good or bad they deem it to be. These values are then used in a model that calculates alignment between what matters to the user and the available decision options. |
| Discrete-choice experiments (example 38 ) | The user is asked to make a series of choices between 2 (or more) alternatives, where each alternative is characterized by attributes and their associated levels. |
| Open discussion (example 39 ) | The user discusses what matters to them in an unstructured or semistructured discussion, possibly aided by a preset or user-created list of topics. |
| Pros and cons (resource 40 ) | The user lists advantages (pros) and disadvantages (cons) of options and/or indicates the relevance (“this matters to me”) or importance (e.g., on a Likert scale) of each advantage or disadvantage. |
| Ranking (example 41 ) | The user is asked to place attributes in order of importance, relative to each other. |
| Rating scales (example 42 ) | The user indicates the importance of an attribute on a visual analog scale (e.g., paper-based visual analog scale, online slider) or Likert scale approximating a visual analog scale. If the rating is then used to calculate and show which option fits best, the method is classified as (multicriteria) decision analysis. |
| Social matching (example 43 ) | The user “observes different characters’ decisions and/or decision-making processes and identifies 1 or more characters” with whom they identify. 16 |
| Standard gamble (example 44 ) | The user indicates their choice between a) living the rest of their life in a particular health state (in the current context, a health state relevant to the health decision they are making) and b) taking a gamble between 2 possible outcomes: the probability p of living the remainder of their life in a state of optimal health and the probability 1 –p of immediate death. |
| Time tradeoff (example 44 ) | The user indicates how many remaining lifetime years in full health they would be willing to give up (i.e., “trade off”) to avoid living for the rest of their life in the health state representing the decision making option of interest. |
Multicriteria decision analysis or decision analysis is an umbrella term. It encompasses some of the other, more specific categories (e.g., discrete-choice experiments, best–worst scaling.) When applicable, we use the more specific, narrower categories. Otherwise, we use the umbrella term multicriteria decision analysis or, for brevity in figures, decision analysis. In addition, although within multicriteria decision analysis, the user may be asked to rate attributes on rating scales, what distinguishes multicriteria decision analysis from other methods such as rating scales is that the model calculates how well or poorly the options align with what matters to a user.
Methods
Our overall methods were guided by the Cochrane handbook. We report according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 45 guidelines.
Eligibility Criteria
We included published reports of comparative evaluations of explicit values clarification methods, whether they were called “values clarification methods” in the publications or not. This meant that we included trials of preference elicitation methods that had been trialed as values clarification methods (e.g., multicriteria decision analysis or discrete-choice experiments). We included evaluations using comparative methods (i.e., randomized controlled trials or randomized experiments of 1 or more values clarification methods). The comparisons could be 1 or more values clarification methods compared to a control method or compared to each other. Because we sought to understand the effects of values clarification methods, we excluded evaluations using descriptive study designs (e.g., acceptability and feasibility study, development study), observational study designs (e.g., reporting outcomes before and after use of a values clarification method), and reports of values clarification methods that did not evaluate the method independently of the patient decision aid in which it was used. Randomized experiments comparing 1 or more values clarification methods had to use distinctly different methods, meaning that more than the content or presentation of information in the values clarification method varied.
We did not apply language restrictions. We applied date restrictions to the portion of the review for which we had already conducted a systematic review (i.e., evaluations of values clarification methods that used the term values clarification).17,22 Specifically, for this subgroup, we added articles indexed or published starting in 2014 to the existing set of articles indexed or published prior to 2014 that we had already identified using the same search strategy. We applied no date restrictions to the new, expanded portion of the review (i.e., evaluations of values clarification methods that did not use the term values clarification).
Information Sources
We performed a systematic literature search in MEDLINE, EMBASE, Web of Science, the Cochrane Library, and CINAHL.
Search Strategy
We developed a draft search strategy in collaboration with an information specialist (FB; see Acknowledgments). Search strategies for each database are shown in online Appendix 1. We reviewed search strategies with all authors to ensure they were inclusive of relevant preference elicitation methods that might be used for values clarification. We conducted hand searches by reviewing articles that cited the previous version of these standards (values clarification chapter) or a previous systematic review of values clarification methods.
Study Records: Data Management
We managed data with Covidence (Melbourne, Australia), reviewing data records at regular team meetings.
Study Records: Selection Process
Two independent reviewers (SC, MM, TP, CR, CR-B) screened titles and abstracts to assess potential relevance, with a third reviewer adjudicating discrepancies and discussions of questions and points of disagreement at regular team meetings. Two independent reviewers then reviewed the full text of all articles deemed potentially relevant based on their title and abstract. Discrepancies in inclusion and exclusion at full text were adjudicated through team discussions at regular meetings until we reached consensus.
Study Records: Data Collection Process
Two independent, trained research team members (SC, MM, TP, CR, CR-B) extracted data from each article using a standardized and pilot-tested data extraction form based on a previous form 17 and adapted to this review. We resolved disagreements through discussion until consensus was reached. We contacted authors to collect any needed data that they did not report or were unable to report in their publication.
Data Items
Regarding study participants, we recorded the sample size for control and intervention groups along with basic inclusion and exclusion criteria and whether or not they were making the actual decision or if the study was hypothetical. We defined a hypothetical scenario as one in which people are asked (explicitly or implicitly) to imagine that they are in a certain situation or facing a certain decision. We defined a real scenario as one in which people are facing a decision (e.g., because they have received a diagnosis) or are members of a population likely to face the decision in the near term (e.g., parents of children eligible to receive vaccines within the coming months).
Regarding interventions, we recorded the type of explicit values clarification method as listed in Table 2. We also recorded specific characteristics of each values clarification method, namely, whether it explicitly requires the user to engage with tradeoffs, whether it explicitly shows the user the correspondence between their options and what they value, and which, if any, theoretical or conceptual framework underpins it. Where relevant, we recorded whether a variable was collected via self-report, meaning whether responses were completed by participants themselves or by independent researchers based on direct observation, including coded qualitative data.
For comparators (controls), we recorded whether the comparator was no values clarification method or an implicit method and treated both as equivalent controls. The Cochrane review of patient decision aids specifies that all patient decision aids must contain implicit values clarification methods at minimum, 14 and it is accordingly rare to have patient decision aids that do not present potential benefits and harms of options in organized ways. In other words, in the context of patient decision aids, there is no meaningful distinction between implicit methods and no values clarification. The different terminology is simply a function of how authors choose to name their control. We also recorded studies that compared different types of explicit values clarification methods to each other.
Outcomes
Whenever such data were available, we extracted data regarding values congruence (i.e., the extent to which choices aligned with stated values) as our primary outcome, as well as secondary outcomes: decision readiness (worry, decision uncertainty, decision making preparation, knowledge), decisional conflict (measured with a version of the Decisional Conflict Scale and/or its subscales 46 ), decision made, and postdecision and postimplementation health and well-being (decisional regret, longer-term health outcomes). Following data extraction by pairs of trained reviewers (SC, MM, TP, CR, CR-B), 3 authors (HOW, SCD, JJ) mapped all outcomes into broad outcome groups: worry (including perceived risk), decision uncertainty (not including decisional conflict), decisional conflict (decisional conflict scale or any subscales), decision making preparation (including self-efficacy for decision making), beliefs (including beliefs about the condition or underlying decision structure), knowledge, values (including reported utilities), shared decision making (i.e., the extent to which shared decision making occurred or not), effects on communication (including quality, length, or existence of communication), satisfaction with care, preferences (i.e., preferences expressed), decision (choice made and implemented) or decisional intent (choice intended or made and not yet implemented), values congruence, informed decision making (i.e., the extent to which someone made an evidence-informed, values-congruent, behaviorally implemented decision9,47), postdecision feelings (including satisfaction, regret), postdecision health, and user assessment of the intervention (including acceptability, satisfaction, perceived balance). We conducted meta-analyses on primary outcome values congruence and secondary outcome decisional conflict, as these outcomes had sufficient studies to do so.
Risk of Bias in Individual Studies
Independent, trained research team members assessed risk of bias for each study using methods as defined in the Cochrane handbook, section 8.5. 48 We conducted quantitative data syntheses with and without studies identified as being at high risk of bias to determine the sensitivity of overall findings to these studies.
Data Synthesis
We synthesized frequency-based results (e.g., how many values clarification methods reflect a given design) descriptively. To synthesize effects on outcomes, we pooled all experiments that evaluated a values clarification method against no values clarification method or an implicit method. For multiarmed studies in which the comparison of a decision aid with and without a values clarification method included an arm that was not relevant to our comparison of interest (e.g., an information booklet serving as a control condition in an evaluation of the decision aid), we ignored the third arm. For multiarmed studies containing 2 or more different values clarification methods and 1 arm of implicit values clarification or control, we considered each comparison of a values clarification method against implicit values clarification, meaning that each of the multiarmed studies included in this review contributed multiple comparisons to the pooled set.
To meta-analyze results for values congruence, we pooled results from 11 studies using risk differences and applying a random-effects model. Here, risk differences refers to differences between treatment and control arms regarding the risk of making a value-incongruent decision. We extracted dichotomous data indicating the frequency (i.e., number of events and sample size) of values-incongruent decisions. To meta-analyze results for decisional conflict, we pooled results using standardized mean differences applying a random-effects model. We extracted data on total scores on the Decisional Conflict Scale. We explored and reported consistency using Higgins I2, which offers a measure of statistical heterogeneity across pooled studies. Specifically, this statistic describes the percentage of total variation across studies that is due to heterogeneity rather than chance. 49 When we included multiple comparisons from a single study in a meta-analysis, we conducted sensitivity analyses by restricting meta-analyses to 1 comparison per contributing study and meta-analyzing all possible combinations using a random-effects model. This allowed us to ascertain whether the overall Higgins I2 estimate might be influenced by the inclusion of multiple study arms from similar populations. We used the Cochrane risk-of-bias tool to assess study bias along 7 domains as well as to assess an overall risk of bias. Where data permitted, we conducted subgroup meta-analyses of different types of explicit values clarification methods and of explicit values clarification methods that do and do not contain specific design features already identified in previous work, 16 namely, whether the method explicitly requires the user to engage with tradeoffs in any way, whether it explicitly provides the user with the implications of what they value, and which, if any, theoretical or conceptual framework underpins it. We used P = 0.05 as a threshold for statistical significance and conducted analyses in RevMan, version 5.4.
Results
Articles Identified
Out of 30,648 records screened at the title and abstract stage and 279 screened at the full-text stage, we identified 33 articles that met our inclusion criteria describing trials of 43 values clarification methods. Twenty-four of the articles were new articles identified in this update of the International Patient Decision Aids Standards (IPDAS). We excluded 2 of the articles previously included in the IPDAS values clarification chapter because they did not meet our revised inclusion criteria requiring randomized controlled trials and instead reported, for example, pre–post study designs. The PRISMA diagram of included articles is shown in Figure 1.
Figure 1.
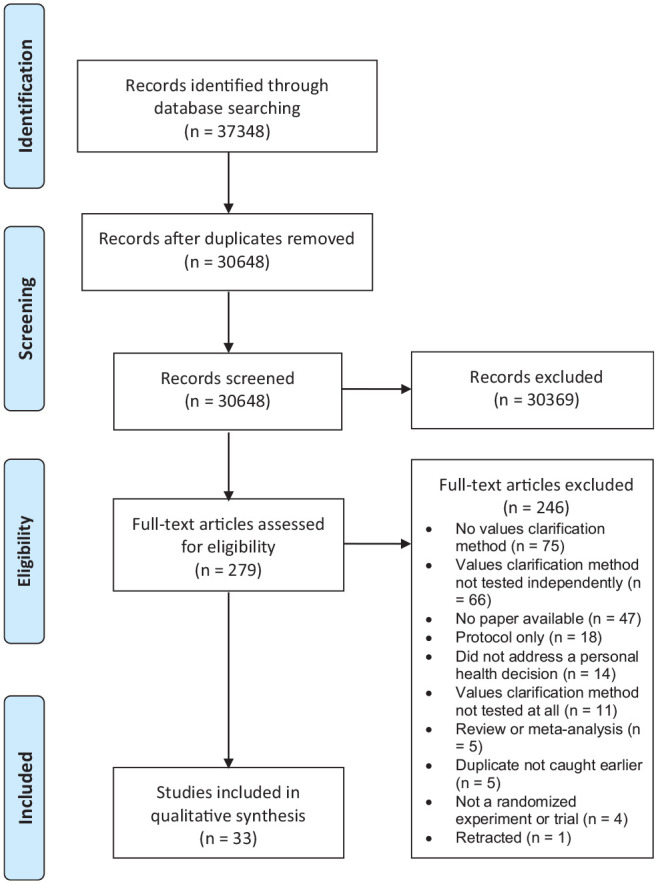
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
The decision context varied across studies. Out of the 43 included trials, 25 (58%) addressed treatment decisions, 9 (21%) screening decisions, 4 (9%) prevention, 3 (7%) genetic testing, and 2 (5%) diagnostic testing. Thirteen of the 43 trials (30%) centered on a yes/no decision to take an option or not, 18 (42%) a choice between 2 or more options, and 12 (28%) both a yes/no and a choice between 2 or more options. Most decisions (22/43, 51%) were real decisions, meaning that the person was making this decision in their actual life. The rest were hypothetical (18/43, 42%), or it was not entirely clear whether the decision was real or hypothetical (3/43, 3%). The most commonly reported outcomes were decisional conflict and/or its subscales (29/43, 67%), decision and/or decisional intentions (22/43, 51%), knowledge (13/43, 30%), and values congruence (12/43, 28%).
As shown in the overview of included studies in Table 3, there was substantial diversity in the types of values clarification methods used. Decision analysis or multicriteria decision analysis was the most commonly trialed method. Full study details are available in online Appendix 2.
Table 3.
Study Details
| Type(s) of Values Clarification Method(s) | Study | Population a | Decision | Summary of Findings b |
|---|---|---|---|---|
| Adaptive conjoint analysis | de Achaval et al., 2012 50 | n = 208 people with knee osteoarthritis | Whether to receive medication and therapy or total knee arthroplasty | Values clarification method decreased decisional conflict and required more intense cognitive involvement. |
| Adaptive conjoint analysis | Fraenkel et al., 2007 51 | n = 87, age at least 60 years old, self-report of pain involving 1 or both knees on most days of the month | Choice between 5 treatments for knee pain | Values clarification method increased self-confidence in and preparation for shared decision making and increased arthritis self-efficacy. |
| Adaptive conjoint analysis | Hess et al., 2015 52 | n = 374 women aged 18 years or older with abnormal uterine bleeding and potential candidates for either surgical or medical treatment | Whether or not to be treated for abnormal uterine bleeding and, if yes, which treatment to undertake | Values clarification method did not reduce decision regret or improve treatment satisfaction. |
| Adaptive conjoint analysis | Hutyra et al., 2019 53 | n = 200 people between 18 and 35 years of age at risk for experiencing a first-time anterior shoulder dislocation | Operative or nonoperative treatment for first-time anterior shoulder dislocation | Values clarification method increased alignment between patients’ treatment decisions and evidence-based recommendations. |
| Adaptive conjoint analysis | Jayadevappa et al., 2015 54 | n = 743 people with newly diagnosed localized prostate cancer | Choice between 6 options for early stage prostate cancer | Values clarification method improved satisfaction with care, satisfaction with decision, reduced regrets, and aligned treatment choice with risk category. |
| Allocation of points | Witteman et al., 2020 55 | n = 817 adults asked to imagine they had been diagnosed with colon cancer | Choice between 2 hypothetical surgeries for colon cancer | Values clarification method (strategy 6b in article) increased values congruence and reduced decisional conflict. |
| Analytical hierarchy process | Myers, 2003 56 | n = 199 men aged 50–69 years with no personal history of prostate cancer/benign prostate hyperplasia | Whether or not to be screened for prostate cancer | Values clarification method decreased rates of prostate cancer screening. Race/ethnicity analyses showed African American men increased screening while white men decreased screening. |
| Analytical hierarchy process | Myers et al., 2005 57 | n = 242 African American men, 40–69 years of age and no history of prostate cancer | Whether or not to be screened for prostate cancer and, if yes, choice of method/extent of screening | Values clarification method increased prostate cancer screening. |
| Best–worst scaling | Shirk et al., 2017 58 | n = 122 men with incident localized prostate cancer | Choice between 3 options for incident localized prostate cancer | Values clarification method decreased decisional conflict. |
| Decision analysis c | Bekker et al., 2004 59 | n = 106 pregnant women receiving a screen-positive maternal serum screening result | Whether or not to have a prenatal diagnosis for Down syndrome | Values clarification method helped women make more informed prenatal diagnosis decisions. |
| Decision analysis c | Clancy et al., 1988 60 | n = 1,280 resident and faculty physicians unvaccinated against hepatitis B | Choice between 3 options to manage risk of hepatitis B | Values clarification method resulted in greater action taking (screening or vaccination). |
| Decision analysis c | Feldman-Stewart et al., 2012 61 | n = 156 people with newly diagnosed prostate cancer | Choice between more than 5 main options for early stage prostate cancer | Values clarification method increased preparation for decision making and decreased decision regret. Decisional conflict decreased with and without values clarification method. |
| Decision analysis c | Hopkin et al., 2019 62 | n = 349 adults asked to imagine that they had to choose a statin | Choice between 5 commonly used statins | Values clarification method reduced decisional conflict and increased levels of preparation for decision making. |
| Decision analysis c | Montgomery et al., 2003 63 | n = 217 adults aged 30–80 years with newly diagnosed hypertension | Whether or not to start drug therapy for hypertension | Values clarification method increased knowledge and reduced total decisional conflict by significantly reducing scores on uninformed, unclear values and unsupported subscales and somewhat reducing scores on uncertainty subscale. Values clarification method did not influence scores on decision quality subscale, nor did it change state anxiety, decision intention, or ultimate decision. |
| Decision analysis c | Montgomery et al., 2007 64 | n = 742 pregnant women with 1 previous lower-segment caesarean section | Choice of planned mode of delivery | Values clarification method reduced decisional conflict and increased frequency of having a vaginal birth. |
| Decision analysis c | Witteman et al., 2015 65 | n = 407 parents who make medical decisions for at least 1 child aged 6 months to 18 years and whose child had not yet received the flu vaccine | Whether their child would receive a vaccine against influenza this flu season | Values clarification method had no effect on values congruence. Values clarification method combined with best practices in risk communication increased intentions to vaccinate, particularly among participants who had not had their children vaccinated against influenza in the past 5 years. |
| Decision analysis c | Witteman et al., 202055,d | n = 1,731 adults asked to imagine they had been diagnosed with colon cancer deciding between 2 treatment options | Choice between 2 hypothetical surgeries for colon cancer | Values clarification method (strategies 2a, 2a + 2b, 6c, 6b + 6c in article) increased values congruence and reduced decisional conflict when this was measured (strategies 6c, 6b + 6c in article). |
| Discrete choice experiment | Brenner et al., 2014 66 | n = 615 people between the ages of 50 and 75 years at average risk for colorectal cancer | Whether or not to be screened for colorectal cancer, and, if yes, which screening test to use | Values clarification method influenced choice of most important screening test attribute but did not affect unlabeled test preference, values clarity, or intent to be screened. |
| Discrete choice experiment | Pignone et al., 2012 67 | n = 104 adults aged 48–75 years at average risk for colon cancer | Whether or not to be screened for colorectal cancer and, if yes, which screening test to use | Values clarification method influenced choice of most important attribute but did not affect values clarity, intent to be screened, or choice of unlabeled screening test. |
| Discrete choice experiment | Pignone et al., 2013 68 | n = 604 men aged 50–70 years at average risk of prostate cancer | Whether or not to be screened for prostate cancer | Values clarification method slightly reduced choice of dying as the most important attribute and increased unlabeled PSA-like screening option but did not influence intent to be screened. |
| Open discussion | Au et al., 2012 69 | n = 306 people with chronic obstructive pulmonary disease | Preferences for end-of-life care | Values clarification method helped identify what mattered to patients regarding end-of-life care and communication. Quality of communication improved. |
| Open discussion | Epstein et al., 2018 70 | n = 99 people with advanced gastrointestinal cancer | Choice between options for end-of-life care | Values clarification method improved communication about future medical cancer care but had no effect on decisional conflict or well-being and increased distress. |
| Open discussion | Kennedy et al., 2002 71 | n = 894 women with uncomplicated menorrhagia | Choice between treatment options for menorrhagia | Values clarification method resulted in minimal improvements in self-reported health status, lower use of a more invasive treatment, higher patient satisfaction, more frequent clinician perceptions of “longer than usual” consultations, and lower overall costs. Providing information alone did not affect treatment choices. |
| Open discussion | Lerman et al., 1997 72 | n = 700 women aged 18–75 years who had had at least 1 first-degree relative with breast and/or ovarian cancer | Whether or not to provide a blood sample for BRCA1 testing in the future | Values clarification method increased the perceived importance of the limitations and risk of BRCA1 testing and decreased the perceived importance of the benefits of BRCA1 testing. No effect of values clarification method on intent to test. |
| Open discussion | Matheis-Kraft et al., 1997 73 | n = 60 women over age 70 years with at least 1 family member or friend who might act as their proxy to make decisions about life-sustaining treatment | Preferences for care in case of decisional incapacity | Values clarification of method’s effectiveness or lack thereof depended on which statistic (κ or percent agreement) was used to measure concordance between women and proxies. |
| Pros and cons | Abhyankar et al., 2010 74 | n = 30 healthy women asked to imagine having been diagnosed with breast cancer, undergoing lumpectomy, and getting a suggestion for chemotherapy by their doctor | Choice between having standard adjuvant chemotherapy or taking part in a clinical trial testing a new chemotherapy for early stage breast cancer | Values clarification method resulted in more use of personal values when evaluating attributes of options, somewhat less ambivalence, and less uncertainty and did not change preferred option. |
| Pros and cons | O’Connor et al., 1999 75 | n = 201 women aged 50–69 years who had never used hormone therapy | Whether or not to take hormone replacement therapy after menopause | Values clarification method had no effect on clarity of values, values congruence, total decisional conflict, other subscales of the Decisional Conflict Scale, or acceptability of intervention. |
| Pros and cons | Paquin et al., 2021 76 | n = 1,000 people aged 18–44 years who were pregnant or whose partner was pregnant or planning to become pregnant in the next 2 years | Whether or not to use genomic sequencing to identify genetic variants in one’s child | Values clarification method decreased parental beliefs against genomic sequencing. |
| Pros and cons | Peinado et al., 2020 15 | n = 1,000 people aged 18–44 years who were pregnant or whose partner was pregnant or planning to become pregnant in the next 2 years | Whether or not to enroll their newborn child in a medical research study that would involve screening for genetic conditions | Values clarification method decreased decisional regret and increased clarity of personal values but had no effect on overall decisional conflict or on intent to have one’s child tested. |
| Pros and cons | Witteman et al., 2020 55 | n = 772 adults asked to imagine they had been diagnosed with colon cancer | Choice between 2 hypothetical surgeries for colon cancer | Values clarification method (strategy 4b in article) reduced decisional conflict but did not change values congruence. |
| Rating scales | Garvelink et al., 201477,d | n = 271 healthy women | Whether or not to undergo fertility-preserving procedures prior to cancer treatment | Values clarification method had no effect on knowledge or decisional conflict. |
| Rating scales | Kuppermann et al., 2014 78 | n = 710 pregnant women who had not yet undergone screening or diagnostic testing for fetal aneuploidy in the current pregnancy | Whether or not to have any screening or diagnostic testing for fetal aneuploidy; if screening or testing is desired, whether to start with screening or with invasive diagnostic testing; and which specific screening and/or diagnostic test(s) to undergo | Values clarification method increased patient knowledge and resulted in less invasive prenatal test use and more informed choices. Values clarification method did not change decisional conflict or decisional regret. |
| Rating scales (with and without decision-analytic summary) | Feldman-Stewart et al., 2006 79 | n = 90 male volunteers asked to imagine that they had just been diagnosed with early stage prostate cancer | Choice between 4 options for early stage prostate cancer | Participants preferred values clarification method with decision-analytic summary over values clarification method without summary and no values clarification method. |
| Rating scales | Witteman et al., 2020 55 | n = 785 adults asked to imagine they had been diagnosed with colon cancer | Choice between 2 hypothetical surgeries for colon cancer | Values clarification method (strategy 6a in article) reduced decisional conflict but did not change values congruence. |
| Rating scales + ranking | Brenner et al., 2014 66 | n = 614 people between the ages of 50 and 75 years at average risk for colorectal cancer | Whether or not to be screened for colorectal cancer, and, if yes, what screening test to use | Values clarification method increased the importance placed on risk reduction as an important attribute but did not affect unlabeled test preference, values clarity, or intent to be screened. |
| Rating scales + ranking | Pignone et al., 2012 67 | n = 104 adults aged 48–75 years at average risk for colon cancer | Whether or not to be screened for colorectal cancer, and, if yes, what screening test to use | Values clarification method influenced choice of most important attribute but did not affect values clarity, intent to be screened, or choice of screening test. |
| Rating scales + ranking | Pignone et al., 2013 68 | n = 609 men aged 50–70 years at average risk of prostate cancer | Whether or not to be screened for prostate cancer | Values clarification method increased the importance of dying (attribute importance) but did not influence intent to be screened. |
| Rating scales + ranking | Sheridan et al., 2010 80 | n = 137 men aged 45–80 years with no history of cardiovascular disease | Whether or not to initiate behaviors to prevent coronary heart disease and, if so, which behaviors | Values clarification method had no effect. Decisional conflict, perceived values congruence, and self-efficacy for health behaviors improved with and without values clarification. Behavioral intentions did not change. |
| Time tradeoff + rating scales | Frosch et al., 2008 81 | n = 611 men older than 50 years | Whether or not to be screened for prostate cancer | Values clarification method increased cancer knowledge scores and decreased decisional conflict. |
PSA, prostate-specific antigen.
n is given for the study as a whole. See supplementary appendix for further details about each study.
Outcomes are in bold.
Decision analysis or multicriteria decision analysis is an umbrella term. It encompasses some of the other, more specific categories (e.g., discrete-choice experiments, best–worst scaling). Throughout the article, when applicable, we use the more specific, narrower categories. Otherwise, we use the umbrella term multicriteria decision analysis or, for brevity in figures, decision analysis.
Quality Assessment
Overall study quality was acceptable, with the majority of studies at low risk of bias on most elements. Eight studies were deemed to be at high risk of bias on 1 element, with the majority in Blinding of Participants and Personnel (Performance Bias). Eighteen additional studies were deemed unclear on this element. Blinding of Outcome Assessment (Detection Bias) was the next most common source of potential bias, with 1 study at high risk of bias and 20 more unclear. Full details of risk of bias assessments are available in online Appendix 3.
Values Congruence
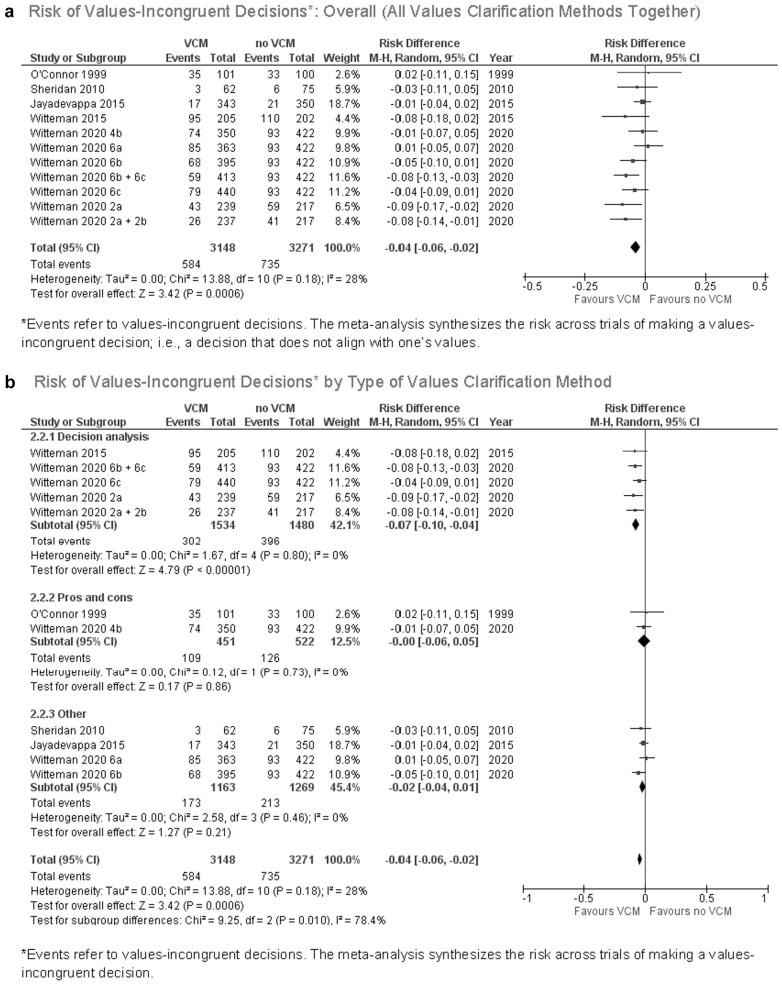
As shown in Figure 2a, included explicit values clarification methods, as a group, increased values congruence, meaning people making decisions that aligned with their stated values. Eleven out of 43 trials (26%) reported the number of people who made values-congruent or values-incongruent decisions. The pooled risk difference of making a values-incongruent decision when using one of the trialed values clarification methods was –0.04 (95% confidence interval [CI], –0.06 to –0.02; P < 0.001). The I 2 of 28% indicates a low level of statistical heterogeneity. 82 This estimate was robust to the inclusion and exclusion of multiple comparisons from a single study (see online Appendix 3, Suppl. Figures S10–S20).
Figure 2.
(a) Risk of values-incongruent decisions: overall (all values clarification methods together). (b) Risk of values-incongruent decisions by type of values clarification method.
Figure 2b shows a statistically significant subgroup difference by type of values clarification method. The results suggest that decision analysis is more likely to encourage values-congruent decisions compared to other explicit values clarification methods within this set of trials (χ2 = 9.25, P = 0.01). The results show no significant subgroup differences by whether the method explicitly requires the user to engage with tradeoffs in any way, whether it explicitly provides the user with the implications of what they value, or whether the method is underpinned by a formal theoretical or conceptual framework (see online Appendix 3). There were no studies in this analysis with a high risk of bias.
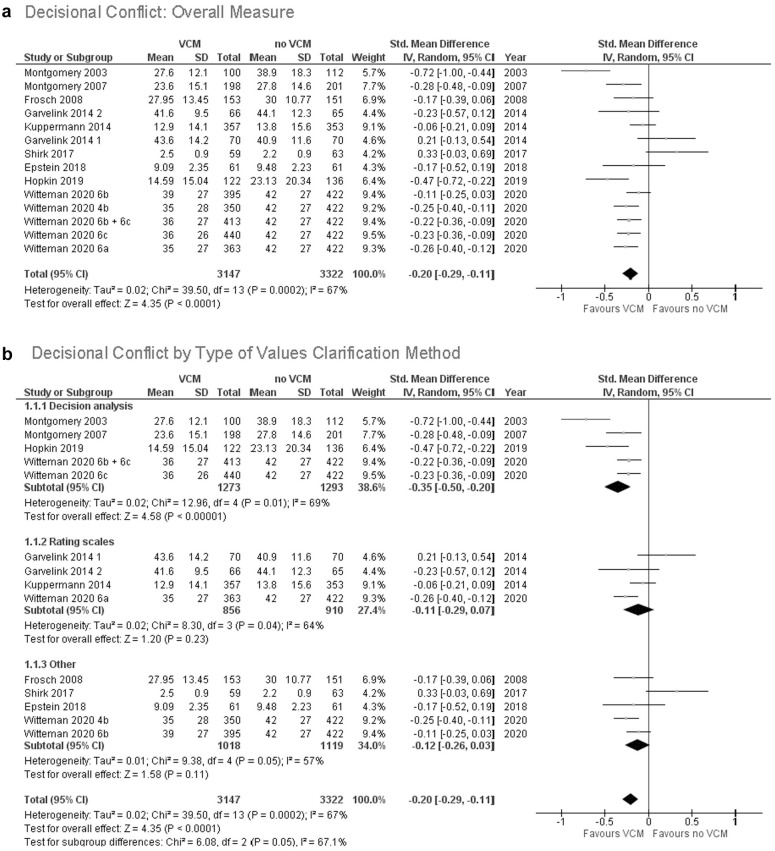
Decisional Conflict
As shown in Figure 3a, explicit values clarification methods decrease decisional conflict. For the 14 of 43 (33%) trials for which we had complete data, the pooled standardized mean difference for decisional conflict was –0.20 (95% CI, –0.29 to –0.11; P < 0.001). The I 2 of 67% represents moderate to high statistical heterogeneity. This estimate was similar with inclusion and exclusion of multiple comparisons from a single study (see online Appendix 3, Suppl. Figures S22–S26). Figure 3b shows there was no significant subgroup difference by type of values clarification method (χ2 = 6.08, P = 0.05). We found no significant subgroup differences by tradeoffs, implications, theory, or risk of bias (see online Appendix 3).
Figure 3.
(a) Decisional conflict: overall measure. (b) Decisional conflict by type of values clarification method.
Head-to-Head Evaluations of Values Clarification Methods
The 5 studies that compared values clarification methods to each other reported findings that align with the findings of our meta-analyses. Methods that provided users with explicit feedback regarding how the decision options align with their stated values led to somewhat better outcomes, including greater values congruence. 55 When asked to compare methods to each other, study participants also preferred a values clarification method that explicitly showed them how the decision options align with their stated values. 79 Different values clarification methods yielded different patterns of attribute importance.66–68 Brief summaries of each study are available in online Appendix 3.
Discussion
Overall, our systematic review and meta-analyses confirm that explicit values clarification methods improve decision outcomes, notably by increasing values congruence and decreasing decisional conflict. Patient decision aids should include an explicit values clarification method.
While the best explicit values clarification method may depend on context—for example, urgent v. routine care or the extent to which a decision has a clear set of decision attributes—our analyses suggest that patient decision aid developers may wish to consider methods that draw on multicriteria decision analysis. The apparent advantages of such methods shown in our analyses may reflect similarities between the process and the outcome. In other words, increased values congruence yielded by decision-analytic methods may be a function of the ways in which such methods transparently show people how their options align with their stated values. We also caution that when these methods use prespecified attributes, there might not be the flexibility for users to add new attributes, highlighting the importance of research to inform attribute selection. We acknowledge that some researchers have argued that health professionals having an unhurried, high-quality conversation with patients may be a preferred approach for at least some patients, especially when decision attributes are many and varied. However, in this systematic review, trials of Open Discussion values clarification methods did not demonstrate strong results, suggesting that such an ideal may be difficult to achieve.
To advance further knowledge on the merits and pitfalls of different values clarification methods, we recommend that authors of future trials of values clarification methods report 4 outcomes: decisional conflict, decision or decision intention, values congruence, and decisional regret. When possible, authors should make use of validated scales that have good psychometric properties and are commonly reported, as this facilitates evidence synthesis.
Decisional conflict should be assessed before people make the decision, using a version of the Decisional Conflict Scale.46,83 Decisions or decision intentions should be assessed when the decision is made.
Values congruence should be assessed once the decision is made. We acknowledge that including values congruence as an outcome brings both measurement and conceptual issues. Measurement issues exist because there are disagreements about how to measure what matters to people (or, indeed, whether it is conceptually possible to do so) and compare such measures to what people choose. 84 Values congruence should not be measured using the values clarity subscale of the Decisional Conflict Scale, as this subscale measures perceived values clarity, not values congruence. 55 Further research is required to determine whether measuring values congruence might introduce bias or otherwise negatively influence decision making.
Decisional regret should be assessed with a version of the Decisional Regret Scale85,86 after people make the decision, ideally with a sufficiently long delay so that longer-term effects can be captured. This scale, whose items include, “I would go for the same choice if I had to do it over again,” assesses how people feel about the decision itself, not the decision process. An included study in this review showed that a values clarification method reduced decisional regret but only after a year had passed following implementation of the decision. 61
For all 4 measures, authors should clearly report sample mean and sample standard deviation for continuous measures, numbers in each category for categorical measures, and sample size per study arm in all cases. Finally, we recommend that patient decision aid developers explain the rationale for their choice of values clarification method.
Our study has 4 main limitations. First, the included data were of moderate quality. Although this review includes many robust trials, the included studies often measured different outcomes or the same outcomes in different ways, there were missing data in some studies, some studies had high risk of bias (often because it was not possible to prevent study participants from ascertaining the study arm to which they were assigned), and some of our meta-analyses had high heterogeneity. Together, these issues suggest a degree of caution in our conclusions. Second, we did not distinguish between subtypes of values clarification methods. For example, different adaptive conjoint analysis exercises may be very different from each other, as might open discussions or many other values clarification methods we grouped together, particularly those we grouped under the broad umbrella term of multicriteria decision analysis. Indeed, the values clarification methods used and trialed may simply reflect authors’ interests and expertise. The selection may also reflect views about whether it is preferable to invite users to explicitly consider individual attributes (e.g., rating scales or multicriteria decision analysis) or to consider options more holistically (e.g., discrete-choice experiments or adaptive conjoint analysis). Given the breadth of methods available, further comparative effectiveness research is needed to conclusively determine the superiority of any given method. Third, although assessment of values generally occurred following provision of information about options and attributes, we were unable to determine whether all instances of improved values congruence reflected informed values, as not all trials measured knowledge. Fourth and finally, our primary findings were heavily influenced by studies conducted with relatively homogeneous populations making hypothetical decisions. Although our sensitivity analyses suggested no differences between studies in real and hypothetical contexts, we nonetheless believe further study is needed in more diverse populations making real decisions before drawing firmer conclusions.
Our study also has 3 main strengths. First, we catalog definitions and resources regarding values clarification methods, as well as recommended outcomes to report in studies. In doing so, we hope to offer more clarity and structure to a literature that can be confusing to navigate, particularly for those who are newer to developing patient decision aids. Second, we begin to answer a core question that commonly arises when developing a patient decision aid: when including a values clarification method, which type of method should one use? Third and finally, we used rigorous methods and an expansive, systematic search. By conducting a systematic review, we reduced our likelihood of missing relevant studies. By including meta-analyses, we offer stronger findings and recommendations than would be possible without pooling data across multiple studies.
In conclusion, particularly in contexts in which people may make health decisions unaligned with what matters to them, patient decision aids should include an explicit values clarification method. Patient decision aid developers may wish to consider the potential advantages of multicriteria decision analysis. Future research should further investigate which methods lead to the best outcomes across or within particular decisions, populations, and settings. Authors of randomized controlled trials of explicit values clarification methods should report decisional conflict, decision made, values congruence, and decisional regret.
Supplemental Material
Supplemental material, sj-doc-1-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making
Supplemental material, sj-pdf-1-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making
Supplemental material, sj-pdf-2-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making
Supplemental material, sj-xlsx-1-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making
Acknowledgments
We thank Frédéric Bergeron, MLIS, for assistance with search strategy, Caroline Beaudoin for assistance in resolving article counts, and all authors of the original articles who generously gave their time to provide missing data when we were unable to extract the data needed from their papers.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided in part by the Canadian Institutes of Health Research (CIHR) FDN-148426 (principal investigator: HOW). HOW receives salary support from Tier 2 Canada Research Chair in Human-Centred Digital Health and received salary support during this study from a Fonds de Recherche du Québec-Santé (FRQS) Research Scholar Junior 2 Career Award. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the article.
ORCID iDs: Holly O. Witteman  https://orcid.org/0000-0003-4192-0682
https://orcid.org/0000-0003-4192-0682
Ruth Ndjaboue  https://orcid.org/0000-0002-4716-6505
https://orcid.org/0000-0002-4716-6505
Arwen Pieterse  https://orcid.org/0000-0001-6395-0052
https://orcid.org/0000-0001-6395-0052
Thierry Provencher  https://orcid.org/0000-0003-2182-7835
https://orcid.org/0000-0003-2182-7835
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making website at http://journals.sagepub.com/home/mdm.
Contributor Information
Holly O. Witteman, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; VITAM Research Centre, Quebec City, Quebec, Canada; CHU de Québec Research Centre, Quebec City, Quebec, Canada.
Ruth Ndjaboue, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; VITAM Research Centre, Quebec City, Quebec, Canada.
Gratianne Vaisson, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; CHU de Québec Research Centre, Quebec City, Quebec, Canada.
Selma Chipenda Dansokho, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Bob Arnold, UPMC Palliative and Supportive Institute, Division of General Internal Medicine, Section of Palliative Care and Medical Ethics, University of Pittsburgh, Pittsburgh, PA, USA.
John F. P. Bridges, Department of Biomedical Informatics, The Ohio State University College of Medicine, Columbus, OH, USA
Sandrine Comeau, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Angela Fagerlin, Department of Population Health Sciences, University of Utah School of Medicine, Salt Lake City, UT, USA.
Teresa Gavaruzzi, Department of Developmental Psychology and Socialization, University of Padova, Padova, Italy.
Melina Marcoux, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Arwen Pieterse, Leiden University Medical Center, Leiden, The Netherlands.
Michael Pignone, Departments of Internal Medicine and Population Health, Dell Medical School, University of Texas, Austin, TX, USA.
Thierry Provencher, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Charles Racine, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Dean Regier, School of Population and Public Health, University of British Columbia, Vancouver, British Columbia, Canada.
Charlotte Rochefort-Brihay, Department of Family and Emergency Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Praveen Thokala, School of Health and Related Research, University of Sheffield, Sheffield, UK.
Marieke Weernink, Municipal Health Services (GGD), Enschede, The Netherlands.
Douglas B. White, Program on Ethics and Decision Making in Critical Illness, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Celia E. Wills, College of Nursing, Center on Healthy Aging, Self-Management and Complex Care, The Ohio State University, Columbus, OH, USA
Jesse Jansen, Department of Family Medicine/CAPHRI, Maastricht University, Maastricht, The Netherlands.
References
- 1. Barrett B, McKenna P. Communicating benefits and risks of screening for prostate, colon, and breast cancer. Fam Med. 2011;43:248–53. [PubMed] [Google Scholar]
- 2. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572. [DOI] [PubMed] [Google Scholar]
- 3. Llewellyn-Thomas HA, Crump RT. Decision support for patients: values clarification and preference elicitation. Med Care Res Rev. 2013;70:50S–79S. [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann TC, Bakhit M, Durand M-A, et al. Basing information on comprehensive, critically appraised, and up-to-date syntheses of the scientific evidence: an update from the International Patient Decision Aid Standards [published online March 4, 2021]. Med Decis Making. [DOI] [PubMed] [Google Scholar]
- 5. Muscat DM, Smith J, Mac O, et al. Addressing health literacy in patient decision aids: an update from the International Patient Decision Aid Standards [published online May 29, 2021]. Med Decis Making. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonner C, Trevena LJ, Gaissmaier W, et al. Current best practice for presenting probabilities in patient decision aids: fundamental principles [published online March 4, 2021]. Med Decis Making. [DOI] [PubMed] [Google Scholar]
- 7. Trevena LJ, Bonner C, Okan Y, et al. Current challenges when using numbers in patient decision aids: advanced concepts [published online March 4, 2021]. Med Decis Making. [DOI] [PubMed] [Google Scholar]
- 8. Thompson R, Paskins Z, Main BG, et al. Addressing conflicts of interest in health and medicine: current evidence and implications for patient decision aid development [published online May 8, 2021]. Med Decis Making. [DOI] [PubMed] [Google Scholar]
- 9. Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect. 2001;4:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Llewellyn-Thomas HA. Patients’ health-care decision making: a framework for descriptive and experimental investigations. Med Decis Making. 1995;15:101–6. [DOI] [PubMed] [Google Scholar]
- 11. Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff. 2013;32:276–84. [DOI] [PubMed] [Google Scholar]
- 12. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312:1295–6. [DOI] [PubMed] [Google Scholar]
- 13. Munro S, Stacey D, Lewis KB, et al. Choosing treatment and screening options congruent with values: do decision aids help? Sub-analysis of a systematic review. Patient Educ Couns. 2016;99:491–500. [DOI] [PubMed] [Google Scholar]
- 14. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peinado S, Paquin RS, Rini C, et al. Values clarification and parental decision making about newborn genomic sequencing. Health Psychol. 2020;39(4):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Witteman HO, Scherer LD, Gavaruzzi T, et al. Design features of explicit values clarification methods: a systematic review. Med Decis Making. 2016;36:453–71. [DOI] [PubMed] [Google Scholar]
- 17. Witteman HO, Gavaruzzi T, Scherer LD, et al. Effects of design features of explicit values clarification methods: a systematic review. Med Decis Making. 2016;36:760–6. [DOI] [PubMed] [Google Scholar]
- 18. Bridges JFP, Brett Hauber A, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–13. [DOI] [PubMed] [Google Scholar]
- 19. Ho M, Saha A, McCleary KK, et al. A framework for incorporating patient preferences regarding benefits and risks into regulatory assessment of medical technologies. Value Health. 2016;19:746–50. [DOI] [PubMed] [Google Scholar]
- 20. Kristensen FB, Husereau D, Huić M, et al. Identifying the need for good practices in health technology assessment: summary of the ISPOR HTA Council Working Group Report on Good Practices in HTA. Value Health. 2019;22:13–20. [DOI] [PubMed] [Google Scholar]
- 21. O’Connor A, Llewellyn-Thomas H, Dolan J, et al. Section D: clarifying and expressing values. In: O’Connor A, Llewellyn-Thomas H, Stacey D, eds. IPDAS Collaboration Background Document. International Patient Decision Aids Standards (IPDAS) Collaboration; 2005. p 17–23. Available from: http://ipdas.ohri.ca/IPDAS_Background.pdf [Google Scholar]
- 22. Fagerlin A, Pignone M, Abhyankar P, et al. Clarifying values: an updated review. BMC Med Inform Decis Making. 2013;13(suppl 2):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charles C, Gafni A. The vexing problem of defining the meaning, role and measurement of values in treatment decision-making. J Comp Eff Res. 2014;3:197–209. [DOI] [PubMed] [Google Scholar]
- 24. Lim C, Berry ABL, Hirsch T, et al. Understanding what is most important to individuals with multiple chronic conditions: a qualitative study of patients’ perspectives. J Gen Intern Med. 2017;32:1278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rocque R, Chipenda Dansokho S, Grad R, et al. What matters to patients and families: a content and process framework for clarifying preferences, concerns, and values. Med Decis Making. 2020;40(6):722–34. [DOI] [PubMed] [Google Scholar]
- 26. Food and Drug Administration. Patient preference information—voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling guidance for industry, Food and Drug Administration staff, and other stakeholders. Available from: https://www.fda.gov/media/92593/download
- 27. Pieterse AH, de Vries M, Kunneman M, et al. Theory-informed design of values clarification methods: a cognitive psychological perspective on patient health-related decision making. Soc Sci Med. 2013;77:156–63. [DOI] [PubMed] [Google Scholar]
- 28. de Vries M, Fagerlin A, Witteman HO, et al. Combining deliberation and intuition in patient decision support. Patient Educ Couns. 2013;91:154–60. [DOI] [PubMed] [Google Scholar]
- 29. Fischhoff B, Barnato AE. Value awareness: a new goal for end-of-life decision making. MDM Policy Pract. 2019;4(1):2381468318817523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ali S, Ronaldson S. Ordinal preference elicitation methods in health economics and health services research: using discrete choice experiments and ranking methods. Br Med Bull. 2012;103:21–44. [DOI] [PubMed] [Google Scholar]
- 31. Whitty JA, Lancsar E, Rixon K, et al. A systematic review of stated preference studies reporting public preferences for healthcare priority setting. Patient. 2014;7:365–86. [DOI] [PubMed] [Google Scholar]
- 32. Pieterse AH, Berkers F, Baas-Thijssen MCM, et al. Adaptive conjoint analysis as individual preference assessment tool: feasibility through the internet and reliability of preferences. Patient Educ Couns. 2010;78:224–33. [DOI] [PubMed] [Google Scholar]
- 33. Bansback N, Li LC, Lynd L, et al. Development and preliminary user testing of the DCIDA (dynamic computer interactive decision application) for ‘nudging’ patients towards high quality decisions. BMC Med Inform Decis Making. 2014;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dolan JG, Boohaker E, Allison J, et al. Patients’ preferences and priorities regarding colorectal cancer screening. Med Decis Making. 2013;33:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ungar WJ, Hadioonzadeh A, Najafzadeh M, et al. Quantifying preferences for asthma control in parents and adolescents using best–worst scaling. Respir Med. 2014;108:842–51. [DOI] [PubMed] [Google Scholar]
- 36. Thokala P, Devlin N, Marsh K, et al. Multiple criteria decision analysis for health care decision making—an introduction: report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19:1–13. [DOI] [PubMed] [Google Scholar]
- 37. Regier DA, Peacock S. Theoretical foundations of MCDA. In: Marsh K, Goetghebeur M, Thokala P, et al. eds. Multi-Criteria Decision Analysis to Support Healthcare Decisions. Cham, Switzerland: Springer International; 2017. p 9–28. [Google Scholar]
- 38. Hazlewood GS, Marshall DA, Barber CEH, et al. Using a discrete-choice experiment in a decision aid to nudge patients towards value-concordant treatment choices in rheumatoid arthritis: a proof-of-concept study. Patient Prefer Adherence. 2020;14:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weir KR, Bonner C, McCaffery K, et al. Pharmacists and patients sharing decisions about medicines: Development and feasibility of a conversation guide. Res Social Adm Pharm. 2019;15:682–90. [DOI] [PubMed] [Google Scholar]
- 40. Ottawa Hospital Research Institute. Ottawa personal decision guides—patient decision aids. Available from: https://decisionaid.ohri.ca/decguide.html
- 41. Masya LM, Young JM, Solomon MJ, et al. Preferences for outcomes of treatment for rectal cancer: patient and clinician utilities and their application in an interactive computer-based decision aid. Dis Colon Rectum. 2009;52:1994–2002. [DOI] [PubMed] [Google Scholar]
- 42. Heckerling PS, Verp MS, Albert N. Patient or physician preferences for decision analysis: the prenatal genetic testing decision. Med Decis Making. 1999;19:66–77. [DOI] [PubMed] [Google Scholar]
- 43. Jibaja-Weiss ML, Volk RJ. Utilizing computerized entertainment education in the development of decision aids for lower literate and naive computer users. J Health Commun. 2007;12:681–97. [DOI] [PubMed] [Google Scholar]
- 44. Stiggelbout AM, Kiebert GM, Kievit J, et al. Utility assessment in cancer patients: adjustment of time tradeoff scores for the utility of life years and comparison with standard gamble scores. Med Decis Making. 1994;14:82–90. [DOI] [PubMed] [Google Scholar]
- 45. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O’Connor AM. User Manual—Decisional Conflict Scale. Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf
- 47. Michie S, Dormandy E, Marteau TM. The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns. 2002;48:87–91. [DOI] [PubMed] [Google Scholar]
- 48. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews for Interventions. Available from: https://handbook-5-1.cochrane.org/front_page.htm
- 49. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Achaval S, Fraenkel L, Volk RJ, et al. Impact of educational and patient decision aids on decisional conflict associated with total knee arthroplasty. Arthritis Care Res. 2012;64:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fraenkel L, Rabidou N, Wittink D, et al. Improving informed decision-making for patients with knee pain. J Rheumatol. 2007;34:1894–8. [PubMed] [Google Scholar]
- 52. Hess LM, Litwiller A, Byron J, et al. Preference elicitation tool for abnormal uterine bleeding treatment: a randomized controlled trial. Patient. 2015;8:217–27. [DOI] [PubMed] [Google Scholar]
- 53. Hutyra CA, Smiley S, Taylor DC, et al. Efficacy of a preference-based decision tool on treatment decisions for a first-time anterior shoulder dislocation: a randomized controlled trial of at-risk patients. Med Decis Making. 2019;39:253–63. [DOI] [PubMed] [Google Scholar]
- 54. Jayadevappa R, Chhatre S, Gallo JJ, et al. Treatment preference and patient centered prostate cancer care: design and rationale. Contemp Clin Trials. 2015;45:296–301. [DOI] [PubMed] [Google Scholar]
- 55. Witteman HO, Julien A-S, Ndjaboue R, et al. What helps people make values-congruent medical decisions? Eleven strategies tested across 6 studies. Med Decis Making. 2020;40:266–78. [DOI] [PubMed] [Google Scholar]
- 56. Myers RE. Value-Based Decision-Making in Prostate Cancer Early Detection. DAMD17-98-1-8641. Philadelphia, PA: Thomas Jefferson University; 2003. [Google Scholar]
- 57. Myers RE, Daskalakis C, Cocroft J, et al. Preparing African-American men in community primary care practices to decide whether or not to have prostate cancer screening. J Natl Med Assoc. 2005;97:1143–54. [PMC free article] [PubMed] [Google Scholar]
- 58. Shirk JD, Crespi CM, Saucedo JD, et al. Does patient preference measurement in decision aids improve decisional conflict? A randomized trial in men with prostate cancer. Patient. 2017;10(6):785–98. [DOI] [PubMed] [Google Scholar]
- 59. Bekker HL, Hewison J, Thornton JG. Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomised controlled trial. Prenat Diagn. 2004;24:265–75. [DOI] [PubMed] [Google Scholar]
- 60. Clancy CM, Cebul RD, Williams SV. Guiding individual decisions: a randomized, controlled trial of decision analysis. Am J Med. 1988;84:283–8. [DOI] [PubMed] [Google Scholar]
- 61. Feldman-Stewart D, Tong C, Siemens R, et al. The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made. Med Decis Making. 2012;32:616–26. [DOI] [PubMed] [Google Scholar]
- 62. Hopkin G, Au A, Collier VJ, et al. Combining multiple treatment comparisons with personalized patient preferences: a randomized trial of an interactive platform for statin treatment selection. Med Decis Making. 2019;39:264–77. [DOI] [PubMed] [Google Scholar]
- 63. Montgomery AA, Fahey T, Peters TJ. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. Br J Gen Pract. 2003;53:446–53. [PMC free article] [PubMed] [Google Scholar]
- 64. Montgomery AA, Emmett CL, Fahey T, et al. Two decision aids for mode of delivery among women with previous caesarean section: randomized controlled trial. BMJ. 2007;334:1305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Witteman HO, Chipenda Dansokho S, Exe N, et al. Risk communication, values clarification, and vaccination decisions. Risk Anal. 2015;35:1801–19. [DOI] [PubMed] [Google Scholar]
- 66. Brenner A, Howard K, Lewis C, et al. Comparing 3 values clarification methods for colorectal cancer screening decision-making: a randomized trial in the US and Australia. J Gen Intern Med. 2014;29:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pignone MP, Brenner AT, Hawley S, et al. Conjoint analysis versus rating and ranking for values elicitation and clarification in colorectal cancer screening. J Gen Intern Med. 2012;27:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pignone MP, Howard K, Brenner AT, et al. Comparing three techniques for eliciting patient values for decision making about prostate-specific antigen screening: a randomized controlled trial. JAMA Intern Med. 2013;173:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest. 2012;141:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Epstein AS, O’Reilly EM, Shuk E, et al. A randomized trial of acceptability and effects of values-based advance care planning in outpatient oncology: person-centered oncologic care and choices. J Pain Symptom Manage. 2018;56:169–77.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kennedy ADM, Sculpher MJ, Coulter A, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. JAMA. 2002;288:2701. [DOI] [PubMed] [Google Scholar]
- 72. Lerman C, Biesecker B, Benkendorf JL, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer Inst. 1997;89:148–57. [DOI] [PubMed] [Google Scholar]
- 73. Matheis-Kraft C, Roberto KA. Influence of a values discussion on congruence between elderly women and their families on critical health care decisions. J Women Aging. 1997;9:5–22. [DOI] [PubMed] [Google Scholar]
- 74. Abhyankar P, Bekker HL, Summers BA, et al. Why values elicitation techniques enable people to make informed decisions about cancer trial participation. Health Expect. 2011;14(suppl 1):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. O’Connor AM, Wells GA, Tugwell P, et al. The effects of an ‘explicit’ values clarification exercise in a woman’s decision aid regarding postmenopausal hormone therapy. Health Expect. 1999;2(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Paquin RS, Peinado S, Lewis MA, et al. A behavior-theoretic evaluation of values clarification on parental beliefs and intentions toward genomic sequencing for newborns. Soc Sci Med. 2021;271:112037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Garvelink MM, ter Kuile MM, Stiggelbout AM, et al. Values clarification in a decision aid about fertility preservation: does it add to information provision? BMC Med Inform Decis Making. 2014;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kuppermann M, Pena S, Bishop JT, et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized clinical trial. JAMA. 2014;312:1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Feldman-Stewart D, Brennenstuhl S, Brundage MD, et al. An explicit values clarification task: development and validation. Patient Educ Couns. 2006;63:350–6. [DOI] [PubMed] [Google Scholar]
- 80. Sheridan SL, Griffith JM, Behrend L, et al. Effect of adding a values clarification exercise to a decision aid on heart disease prevention: a randomized trial. Med Decis Making. 2010;30:E28–E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Frosch D, Bhatnagar V, Tally S, et al. Internet patient decision support. Arch Intern Med. 2008;168:363–9. [DOI] [PubMed] [Google Scholar]
- 82. Deeks JJ, Higgins JPT, Altman DG, et al. Analysing data and undertaking meta-analyses: 10.10.2 Identifying and measuring heterogeneity. In: Higgins J, Thomas J, Chandler J, et al. eds. Cochrane Handbook for Systematic Reviews of Interventions (version 6.2). Cochrane. Available from: http://ipdas.ohri.ca/IPDAS_Background.pdf [Google Scholar]
- 83. Ottawa Hospital Research Institute. Decisional conflict scale—evaluation measures—patient decision aids. Available from: https://decisionaid.ohri.ca/eval_dcs.html
- 84. Winn K, Ozanne E, Sepucha K. Measuring patient-centered care: an updated systematic review of how studies define and report concordance between patients’ preferences and medical treatments. Patient Educ Couns. 2015;98:811–21. [DOI] [PubMed] [Google Scholar]
- 85. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281–92. [DOI] [PubMed] [Google Scholar]
- 86. Ottawa Hospital Research Institute. Decision regret scale—evaluation measures—patient decision aids. Available from: https://decisionaid.ohri.ca/eval_regret.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making
Supplemental material, sj-pdf-1-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making
Supplemental material, sj-pdf-2-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making
Supplemental material, sj-xlsx-1-mdm-10.1177_0272989X211037946 for Clarifying Values: An Updated and Expanded Systematic Review and Meta-Analysis by Holly O. Witteman, Ruth Ndjaboue, Gratianne Vaisson, Selma Chipenda Dansokho, Bob Arnold, John F. P. Bridges, Sandrine Comeau, Angela Fagerlin, Teresa Gavaruzzi, Melina Marcoux, Arwen Pieterse, Michael Pignone, Thierry Provencher, Charles Racine, Dean Regier, Charlotte Rochefort-Brihay, Praveen Thokala, Marieke Weernink, Douglas B. White, Celia E. Wills and Jesse Jansen in Medical Decision Making