Key Points
Question
Can a psychological treatment based on the reappraisal of primary chronic back pain as due to nondangerous central nervous system processes provide substantial and durable pain relief?
Findings
In this randomized clinical trial, 33 of 50 participants (66%) randomized to 4 weeks of pain reprocessing therapy were pain-free or nearly pain-free at posttreatment, compared with 10 of 51 participants (20%) randomized to placebo and 5 of 50 participants (10%) randomized to usual care, with gains largely maintained through 1-year follow-up. Treatment effects on pain were mediated by reduced beliefs that pain indicates tissue damage, and longitudinal functional magnetic resonance imaging showed reduced prefrontal responses to evoked back pain and increased resting prefrontal-somatosensory connectivity in patients randomized to treatment relative to patients randomized to placebo or usual care.
Meaning
Psychological treatment focused on changing beliefs about the causes and threat value of primary chronic back pain may provide substantial and durable pain relief.
This randomized clinical trial tests whether pain reprocessing therapy can provide durable and substantial relief from chronic back pain and investigates treatment mechanisms.
Abstract
Importance
Chronic back pain (CBP) is a leading cause of disability, and treatment is often ineffective. Approximately 85% of cases are primary CBP, for which peripheral etiology cannot be identified, and maintenance factors include fear, avoidance, and beliefs that pain indicates injury.
Objective
To test whether a psychological treatment (pain reprocessing therapy [PRT]) aiming to shift patients’ beliefs about the causes and threat value of pain provides substantial and durable pain relief from primary CBP and to investigate treatment mechanisms.
Design, Setting, and Participants
This randomized clinical trial with longitudinal functional magnetic resonance imaging (fMRI) and 1-year follow-up assessment was conducted in a university research setting from November 2017 to August 2018, with 1-year follow-up completed by November 2019. Clinical and fMRI data were analyzed from January 2019 to August 2020. The study compared PRT with an open-label placebo treatment and with usual care in a community sample.
Interventions
Participants randomized to PRT participated in 1 telehealth session with a physician and 8 psychological treatment sessions over 4 weeks. Treatment aimed to help patients reconceptualize their pain as due to nondangerous brain activity rather than peripheral tissue injury, using a combination of cognitive, somatic, and exposure-based techniques. Participants randomized to placebo received an open-label subcutaneous saline injection in the back; participants randomized to usual care continued their routine, ongoing care.
Main Outcomes and Measures
One-week mean back pain intensity score (0 to 10) at posttreatment, pain beliefs, and fMRI measures of evoked pain and resting connectivity.
Results
At baseline, 151 adults (54% female; mean [SD] age, 41.1 [15.6] years) reported mean (SD) pain of low to moderate severity (mean [SD] pain intensity, 4.10 [1.26] of 10; mean [SD] disability, 23.34 [10.12] of 100) and mean (SD) pain duration of 10.0 (8.9) years. Large group differences in pain were observed at posttreatment, with a mean (SD) pain score of 1.18 (1.24) in the PRT group, 2.84 (1.64) in the placebo group, and 3.13 (1.45) in the usual care group. Hedges g was −1.14 for PRT vs placebo and −1.74 for PRT vs usual care (P < .001). Of 151 total participants, 33 of 50 participants (66%) randomized to PRT were pain-free or nearly pain-free at posttreatment (reporting a pain intensity score of 0 or 1 of 10), compared with 10 of 51 participants (20%) randomized to placebo and 5 of 50 participants (10%) randomized to usual care. Treatment effects were maintained at 1-year follow-up, with a mean (SD) pain score of 1.51 (1.59) in the PRT group, 2.79 (1.78) in the placebo group, and 3.00 (1.77) in the usual care group. Hedges g was −0.70 for PRT vs placebo (P = .001) and −1.05 for PRT vs usual care (P < .001) at 1-year follow-up. Longitudinal fMRI showed (1) reduced responses to evoked back pain in the anterior midcingulate and the anterior prefrontal cortex for PRT vs placebo; (2) reduced responses in the anterior insula for PRT vs usual care; (3) increased resting connectivity from the anterior prefrontal cortex and the anterior insula to the primary somatosensory cortex for PRT vs both control groups; and (4) increased connectivity from the anterior midcingulate to the precuneus for PRT vs usual care.
Conclusions and Relevance
Psychological treatment centered on changing patients’ beliefs about the causes and threat value of pain may provide substantial and durable pain relief for people with CBP.
Trial Registration
ClinicalTrials.gov Identifier: NCT03294148.
Introduction
Chronic pain affects 20% of people in the US, with an estimated annual cost of more than $600 billion.1,2 The most common type is chronic back pain (CBP). In approximately 85% of cases, definitive peripheral causes of CBP cannot be identified, and central nervous system processes are thought to maintain pain.3,4,5,6,7 For people with this type of CBP— often referred to as primary, nonspecific, nociplastic, or centralized pain—psychological and behavioral treatments are recommended.8,9,10 Although these treatments can improve functioning, reductions in pain intensity are limited11,12 and better treatments are needed.
Advances in the neuroscience of pain13,14,15,16,17 and interoception18,19,20,21 suggest new directions for treatment development. In constructionist and active inference models, pain is a prediction about bodily harm, shaped by sensory input and context-based predictions.18,19,22,23,24,25,26 Fearful appraisals of tissue damage can cause innocuous somatosensory input to be interpreted and experienced as painful.22,24,27,28 Such constructed perceptions can become self-reinforcing: threat appraisals enhance pain, which is in turn threatening, creating positive feedback loops that maintain pain after initial injuries have healed.27,29,30,31
As pain becomes chronic, it is increasingly associated with activity in the affective and motivational systems tied to avoidance and less closely tied to systems encoding nociceptive input.14,32,33,34 Accordingly, brain regions serving allostasis and predictive control18,23—including the default mode network, somatosensory and insular cortices, amygdala, and nucleus accumbens—have been implicated in animal models13,14,15,16,17 and human studies of chronic pain22,25,32,33,35,36 and pain modulation.24,25,28,37,38,39
We developed pain reprocessing therapy (PRT) based on this understanding of primary chronic pain. Leading psychological interventions for pain typically present the causes of pain as multifaceted and aim primarily to improve functioning and secondarily to reduce pain. PRT emphasizes that the brain actively constructs primary chronic pain in the absence of tissue damage and that reappraising the causes and threat value of pain can reduce or eliminate it.
In this study, we conducted the first test of PRT. In a randomized clinical trial with 1-year follow-up, we compared PRT with both open-label placebo and usual care control conditions. We tested hypothesized mechanisms of PRT with mediation analyses and longitudinal functional magnetic resonance imaging (fMRI) during spontaneously occurring and evoked back pain. fMRI provided objective correlates of treatment effects and identified potential neurobiological treatment mechanisms.
Methods
Participants and Trial Design
The trial was preregistered on ClinicalTrials.gov (Identifier: NCT03294148) and conducted from August 2017 to November 2018, with 1-year follow-up completed by November 2019. Clinical and fMRI data were analyzed from January 2019 to August 2020, after data collection at each follow-up timepoint was complete. Participants aged 21 to 70 years with back pain for at least half the days of the last 6 months and 1-week average pain intensity score of 4 of 10 or greater at screening were recruited from the community in Boulder, Colorado. We targeted primary CBP, excluding patients with leg pain worse than back pain (eMethods in Supplement 2). Power analysis targeted 80% power (α = .05) to detect a medium effect (d = 0.62) on pain intensity at the primary end point (eMethods in Supplement 2). Participants provided written informed consent as approved by the University of Colorado Institutional Review Board. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for social and psychological intervention trials.
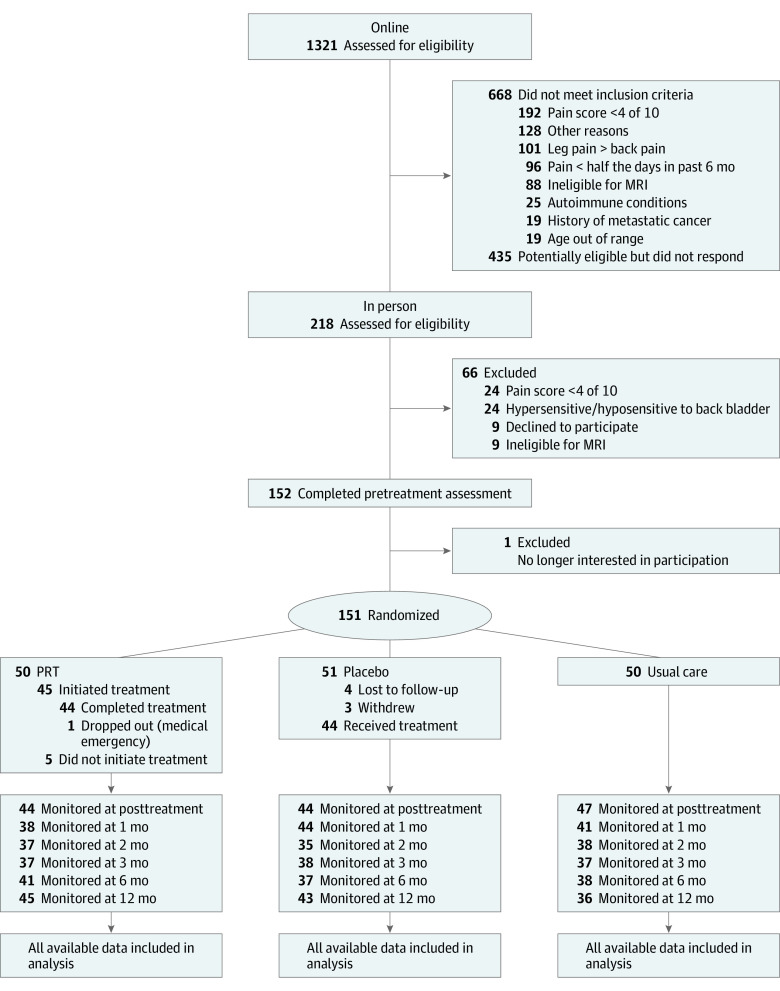
Participants completed an eligibility and consent session, followed by a baseline assessment session with fMRI. They were subsequently randomized to PRT, placebo, or usual care with equal probability, balancing on age, sex, baseline pain, and opioid use using an imbalance-minimization algorithm40 (eMethods in Supplement 2). The primary end point (posttreatment fMRI session) occurred 1 month after the baseline fMRI. Participants completed online follow-up assessments at 1, 2, 3, 6, and 12 months after the primary end point (Figure 1).
Figure 1. CONSORT Participant Flow Diagram.
MRI indicates magnetic resonance imaging.
Interventions
PRT
PRT seeks to promote patients’ reconceptualization of primary (nociplastic) chronic pain as a brain-generated false alarm. PRT shares some concepts and techniques with existing treatments for pain41,42,43,44,45,46,47,48 and with the cognitive behavioral treatment of panic disorder.66
Participants completed a 1-hour telehealth evaluation and education session with a physician (H.S.) assessing likely centralized vs peripheral contributions to pain, including a review of available preexisting spinal imaging. Assessment findings and centralized pain education were shared with the patient (eAppendix 1 in Supplement 2).
Participants then completed 8 individual 1-hour therapy sessions with a therapist with extensive PRT experience (A.G. or C.U.) twice weekly for 4 weeks. Techniques included (1) providing personalized evidence for centralized pain; (2) guided reappraisal of pain sensations while seated and while engaging in feared postures or movements; (3) techniques addressing psychosocial threats (eg, difficult emotions) potentially amplifying pain; and (4) techniques to increase positive emotions and self-compassion. PRT followed the treatment protocol found in eAppendix 2 in Supplement 2.
Treatment fidelity was assessed by independent raters coding audiorecordings of PRT sessions (eMethods and eAppendix 3 in Supplement 2). A mean (SD) of 4.93 (0.87) of 6 PRT elements were present in each session, and all sessions included at least 3 elements, indicating high treatment fidelity.
Open-label Placebo Plus Usual Care
Participants watched 2 videos describing how placebo treatments can powerfully relieve pain even when known to be inert (eg, they can automatically trigger the body’s natural healing response).49 A subcutaneous injection described as saline was administered by a physician (K.K.) at the site of greatest back pain during an empathic, validating clinical encounter at an orthopedic medical center. Open-label placebo treatments are as effective or nearly as effective as traditional (deceptive) placebos for CBP and other chronic symptoms when administered in this manner (eMethods in Supplement 2).50,51,52 Participants in this group were also asked to continue their ongoing care as usual and not start other new treatments until after the study period.
Usual Care
Participants in this group were given no additional treatment. They agreed to continue their ongoing care as usual and not start new treatments before the posttreatment assessment. After the posttreatment assessment, they were given a chronic pain workbook53 and access to http://www.unlearnyourpain.com.
Clinical Measures
The primary outcome was average pain over the last week on a numerical rating scale from 0 to 10 from the Brief Pain Inventory Short Form, assessed at the 1-month postbaseline session. We also calculated the proportion of participants reporting pain reduction of 30% or more, pain reduction of 50% or more, and a pain score of 0 or 1, indicating a pain-free or nearly pain-free state. Secondary outcomes included pain interference (Oswestry Disability Index); Patient-Reported Outcome Measurement Information System (PROMIS) short forms for depression, anxiety, anger, and sleep quality; and the Positive and Negative Affect Scale (measure details in the eMethods in Supplement 2).
We considered 3 measures of pain beliefs as potential mediators: (1) the Tampa Scale of Kinesiophobia (TSK-11), assessing belief that pain indicates injury and fear of movement; (2) the Pain Catastrophizing Scale (PCS); and (3) the Survey of Pain Attitudes Emotion subscale (SOPA-Emotion), assessing beliefs that stress and negative emotion increase pain. Adverse events were recorded when participants spontaneously reported them to study personnel. Baseline pain was computed as the average score from 2 prerandomization assessments (eligibility session and pretreatment fMRI session).
Neuroimaging Measures
Structural T1 and multiband blood oxygenation level–dependent functional imaging was conducted on a 3-T Siemens Prisma Fit MRI scanner with standard fMRI preprocessing (eMethods in Supplement 2). During fMRI, participants completed (1) an evoked back pain task with a series of randomly ordered trials distending the back to 1 of 4 intensity levels and (2) a spontaneous pain scan in which participants rested and rated ongoing pain once per minute (design details in the eMethods in Supplement 2; fMRI data quality measures shown in eFigures 6 and 7 in Supplement 2). Participants rated pain during scanning on a visual analog scale from 0 (no pain) to 100 (worst pain imaginable).
Statistical Analyses
Intent-to-treat analyses (including all randomized patients) were performed for the primary outcome with a mixed-effects model (fitlme, MATLAB 2020a), including 2 group × time interactions (PRT vs placebo × posttreatment vs pretreatment and PRT vs usual care × posttreatment vs pretreatment), covariates for age and sex, and a random intercept per participant. Treatment response rates for 30% or greater reduction in pain, 50% or greater reduction in pain, and a pain-free or nearly pain-free state at posttreatment and 1-year follow-up were based on all randomized patients; those missing data were considered nonresponders. For follow-up time points and secondary outcomes, we calculated Hedges g for the PRT vs placebo and PRT vs usual care comparisons. Follow-up time points were analyzed individually, testing group differences in change from baseline to each time points. The placebo vs usual care comparison will be reported elsewhere.
To investigate psychological treatment mechanisms, we (1) correlated pretreatment to posttreatment changes in pain intensity with pretreatment to posttreatment changes in pain beliefs (TSK-11, PCS, and SOPA-Emotion) within each group and (2) tested pretreatment to posttreatment changes in pain beliefs as mediators of treatment effects on pain at follow-up timepoints (1 through 12 months posttreatment), controlling for baseline pain. PRT vs placebo and PRT vs usual care were tested in separate models. We also tested the reverse: whether pretreatment to posttreatment pain reductions mediated treatment effects on pain beliefs at follow-up, controlling for baseline pain beliefs (eMethods in Supplement 2). Correlational and mediation analyses were not prespecified in the trial protocol.
Evoked Back Pain Analyses
An evoked back pain localizer identified brain regions positively associated with evoked back pain intensity at baseline. The localizer was conducted within a mask of regions of interest (medial prefrontal, posteromedial, insula, cingulate, and somatosensory cortices; amygdala; and nucleus accumbens; eMethods and eFigure 1 in Supplement 2; localizer task design in eFigure 8 in Supplement 2). We tested for treatment effects (group × time interactions) in the average activity of clusters positively associated with evoked back pain using a mixed-effects (random-effects) model, applying a 1-tailed threshold of P < .05 owing to directional hypotheses that PRT would reduce activity in pain-positive clusters.
Spontaneous Pain Connectivity Analyses
Evoked pain analyses identified group × time interactions in the anterior insula, anterior midcingulate (aMCC), and a prefrontal region. We submitted these 3 regions as seeds to connectivity analyses in the spontaneous pain scan. We conducted permutation tests (threshold-free cluster-enhancement; eMethods in Supplement 2) testing for group × time interactions in connectivity between these seed regions and 2 areas most often demonstrating altered connectivity in chronic pain: (1) the midline default mode network, including the medial prefrontal and posteromedial cortex, and (2) primary somatosensory cortex (S1)36,54,55,56,57,58,59 (masks in eFigure 2 in Supplement 2).
Results
We randomized 151 participants (54% female; mean [SD] age, 41.1 [15.6] years; mean [SD] CBP duration, 10.0 [8.9] years). At baseline, patients reported low to moderate pain intensity scores (mean [SD], 4.10 [1.26]) to 4.41 [1.29]) and disability (mean [SD], 23.34 [10.12] on the Oswestry Disability Index), with similar pain and demographic characteristics across groups (Table 1).
Table 1. Baseline Patient Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Pain reprocessing therapy | Placebo | Usual care | |
| Demographic characteristics | |||
| Age, mean (SD), y | 42.6 (16.2) | 39.4 (14.9) | 41.3 (15.9) |
| Sex | |||
| Female | 29 (58) | 25 (49) | 27 (54) |
| Male | 21 (42) | 26 (51) | 23 (46) |
| Education | |||
| High school or less | 0 | 0 | 0 |
| Some college | 11 (22) | 15 (29) | 15 (30) |
| College graduate | 39 (78) | 36 (71) | 35 (70) |
| Married | 26 (52) | 25 (49) | 30 (60) |
| Racea | |||
| American Indian or Alaskan Native | 0 | 0 | 1 (2) |
| Asian/Pacific Islander | 3 (6) | 2 (4) | 0 |
| Black (not of Hispanic origin) | 0 | 2 (4) | 1 (2) |
| White (not of Hispanic origin) | 46 (92) | 45 (88) | 43 (86) |
| Other or unknown | 1 (2) | 2 (4) | 5 (10) |
| Hispanic ethnicity | 0 | 2 (4) | 2 (4) |
| Employment status | |||
| Full-time (>30 h/wk) | 33 (66) | 26 (51) | 28 (56) |
| Part-time (5-30 h/wk) | 10 (20) | 12 (24) | 13 (26) |
| Unemployed/lightly employed (<5 h/wk) | 7 (14) | 13 (25) | 9 (18) |
| Subjective socioeconomic status, mean (SD), 1-1060 | 6.8 (1.8) | 6.4 (2.0) | 6.7 (1.6) |
| Exercise | |||
| Almost none | 6 (12) | 1 (2) | 4 (8) |
| 1 h/wk | 4 (8) | 7 (14) | 9 (18) |
| 3 h/wk | 17 (34) | 23 (45) | 14 (28) |
| 7 h/wk | 19 (38) | 18 (35) | 21 (42) |
| ≥14 h/wk | 4 (8) | 2 (4) | 2 (4) |
| Pain-related characteristics | |||
| Pain duration, mean (SD), y | 10.7 (9.7) | 8.9 (8.2) | 10.5 (8.9) |
| Current opioid use (yes/no) | 5 (10) | 2 (4) | 2 (4) |
| Pain in body sites besides back? | |||
| None | 5 (10) | 9 (18) | 4 (8) |
| A little | 29 (58) | 24 (47) | 28 (56) |
| A moderate amount | 11 (22) | 15 (29) | 16 (32) |
| A lot | 5 (10) | 3 (6) | 2 (4) |
Race and ethnicity were collected in accord with National Institutes of Health guidelines by multiple choice self-report.
Of 50 participants randomized to PRT, 44 (88%) completed all treatment sessions and the posttreatment assessment. Five participants dropped out prior to initiating PRT and 1 had an unrelated medical emergency. Of 51 participants randomized to placebo, 44 (86%) received the treatment, all of whom completed the posttreatment assessment. Of the 50 participants randomized to usual care, 47 (94%) completed the posttreatment assessment (Figure 1).
Twenty patients in the PRT group had preexisting spinal imaging, all of which showed at least 1 spinal anomaly (median of 4 findings per patient; eTable 1 in Supplement 2) assessed by a physician (H.S.) as not causal of pain (eMethods and eAppendix 1 in Supplement 2).61
Clinical Outcomes
Patients randomized to PRT reported substantial reductions in pain intensity at posttreatment compared with both control groups, with a mean (SD) pain score of 1.18 (1.24) in the PRT group, 2.84 (1.64) in the placebo group, and 3.13 (1.45) in the usual care group (Figure 2; Table 2). Patients in the PRT group reported a pain reduction of 1.79 (on the 0 to 10 numerical rating scale) relative to placebo (t137.63 = 6.06; P < .001; g, −1.14; 95% CI, −1.65 to −0.71) and reported a pain reduction of 2.40 relative to the usual care group (t135.69 = 8.13; P < .001; g, −1.74; 95% CI, −2.28 to −1.32). A total of 33 of 50 patients randomized to PRT (66%), corresponding to 73% of the 45 patients who initiated PRT, were pain-free or nearly pain-free at posttreatment, compared with 10 of 51 patients (20%) in the placebo group and 5 of 50 patients (10%) in the usual care group. At 1-year follow-up, effects of PRT on pain remained large relative to both control groups, with a mean (SD) pain score of 1.51 (1.59) in the PRT group, 2.79 (1.78) in the placebo group, and 3.00 (1.77) in the usual care group. Hedges g was −0.70 for PRT vs placebo (P = .001) and −1.05 for PRT vs usual care (P < .001) (Table 2; treatment response rates in eTable 2 in Supplement 2; individual patient pain trajectories in eFigure 3 in Supplement 2).
Figure 2. Clinical Outcomes.
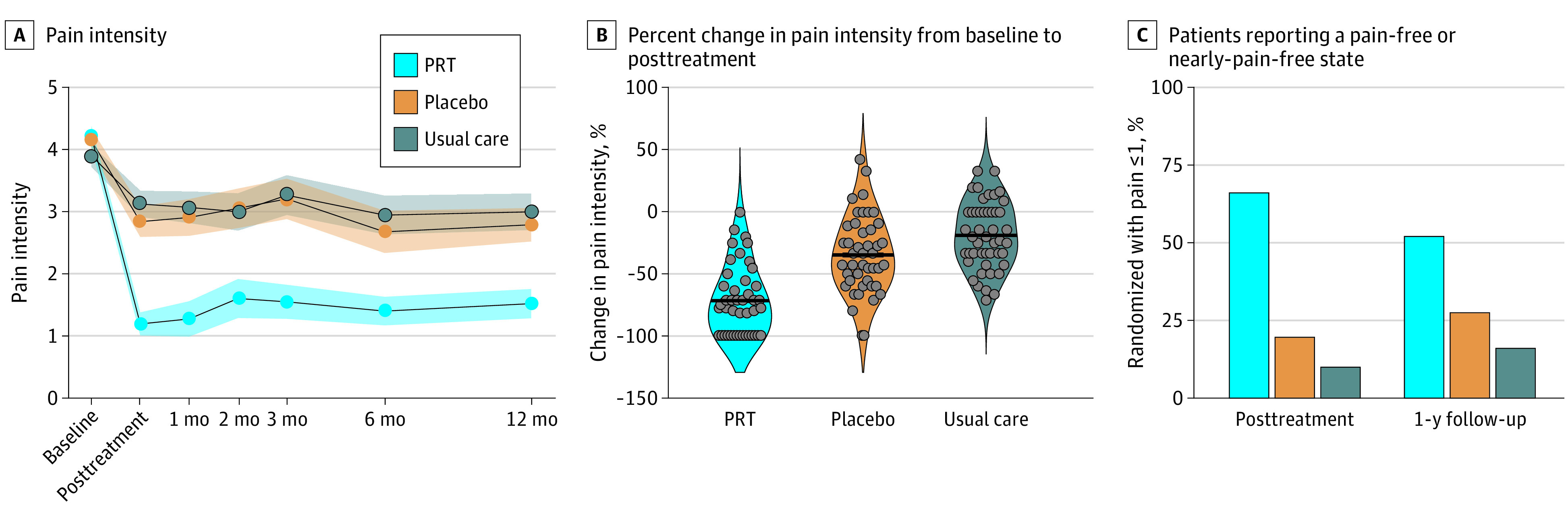
A, Shading indicates standard error. B, Dots represent individual participants; thick lines represent the group mean. C, Percentage of patients reporting pain scores of 0 or 1 of 10 (ie, pain-free or nearly pain-free) at posttreatment and at 1-year follow-up. PRT indicates pain reprocessing therapy.
Table 2. Primary and Secondary Clinical Outcomes.
| Between-group differencesa | Mean (SD) | PRT vs placebo, g (SE)b | P value | PRT vs usual care, g (SE)b | P value | ||
|---|---|---|---|---|---|---|---|
| PRT | Placebo | Usual care | |||||
| Primary outcome | |||||||
| Pain intensity (0-10) | |||||||
| Baseline | 4.22 (1.21) | 4.16 (1.33) | 3.91 (1.24) | NA | NA | NA | NA |
| Posttreatment | 1.18 (1.24) | 2.84 (1.64) | 3.13 (1.45) | −1.14 (0.24) | <.001 | −1.75 (0.24) | <.001 |
| At 1 mo | 1.26 (1.77) | 2.91 (1.97) | 3.07 (1.63) | −0.83 (0.27) | <.001 | −1.24 (0.29) | <.001 |
| At 2 mo | 1.59 (1.92) | 3.06 (1.89) | 3.00 (1.86) | −0.84 (0.28) | .001 | −1.03 (0.28) | <.001 |
| At 3 mo | 1.54 (1.68) | 3.21 (2.02) | 3.27 (1.95) | −0.93 (0.23) | <.001 | −1.35 (0.25) | <.001 |
| At 6 mo | 1.39 (1.48) | 2.68 (2.08) | 2.95 (1.93) | −0.74 (0.23) | .001 | −1.14 (0.26) | <.001 |
| At 12 mo | 1.51 (1.59) | 2.79 (1.78) | 3.00 (1.77) | −0.70 (0.21) | .001 | −1.05 (0.24) | <.001 |
| Secondary outcome | |||||||
| Oswestry Disability Index (0-100) | |||||||
| Baseline | 23.70 (10.70) | 23.06 (10.14) | 23.26 (9.67) | NA | NA | NA | NA |
| Posttreatment | 10.14 (10.63) | 19.00 (11.07) | 20.68 (10.68) | −1.30 (0.28) | <.001 | −1.70 (0.26) | <.001 |
| At 1 mo | 10.58 (14.26) | 18.68 (11.95) | 20.30 (9.04) | −1.04 (0.25) | <.001 | −1.61 (0.27) | <.001 |
| At 2 mo | 9.57 (12.86) | 19.43 (11.84) | 21.37 (11.07) | −1.30 (0.29) | <.001 | −1.55 (0.23) | <.001 |
| At 3 mo | 9.68 (13.39) | 21.42 (14.32) | 23.57 (13.36) | −1.26 (0.28) | <.001 | −1.61 (0.25) | <.001 |
| At 6 mo | 9.80 (11.94) | 18.50 (13.43) | 20.84 (11.57) | −0.96 (0.26) | <.001 | −1.3 (0.28) | <.001 |
| At 12 mo | 11.16 (13.13) | 18.52 (12.60) | 18.78 (12.59) | −0.23 | <.001 | −0.83 (0.24) | <.001 |
| PROMIS depression, raw score (8-32) | |||||||
| Baseline | 14.66 (4.39) | 13.17 (4.67) | 12.85 (4.74) | NA | NA | NA | NA |
| Posttreatment | 12.23 (4.94) | 11.75 (4.05) | 11.81 (4.45) | −0.35 (0.24) | .099 | −0.56 (0.24) | .009 |
| At 1 mo | 12.87 (5.23) | 10.64 (3.57) | 11.57 (4.61) | 0.13 (0.23) | .555 | −0.54 (0.25) | .019 |
| At 2 mo | 12.51 (4.88) | 11.11 (4.95) | 11.76 (5.17) | −0.08 (0.24) | .723 | −0.51 (0.24) | .028 |
| At 3 mo | 11.47 (4.64) | 12.45 (6.09) | 12.30 (4.51) | −0.57 (0.24) | .015 | −0.90 (0.22) | <.001 |
| At 6 mo | 12.90 (5.28) | 10.97 (4.00) | 11.84 (4.65) | −0.09 (0.24) | .701 | −0.47 (0.23) | 0.40 |
| At 12 mo | 12.53 (5.12) | 11.95 (5.86) | 12.75 (4.50) | −0.20 (0.23) | .360 | −0.62 (0.24) | .007 |
| PROMIS anger, raw score (5-25) | |||||||
| Baseline | 12.46 (3.73) | 10.97 (3.18) | 11.17 (3.18) | NA | NA | NA | NA |
| Posttreatment | 9.52 (3.91) | 9.89 (3.81) | 10.45 (3.86) | −0.62 (0.21) | .004 | −0.78 (0.21) | <.001 |
| At 1 mo | 9.50 (4.40) | 8.84 (3.27) | 10.55 (3.19) | −0.23 (0.25) | .291 | −0.91 (0.25) | <.001 |
| At 2 mo | 10.70 (4.68) | 9.37 (3.30) | 10.00 (3.92) | −0.11 (0.23) | .652 | −0.28 (0.25) | .231 |
| At 3 mo | 9.31 (4.06) | 9.87 (4.78) | 10.49 (3.52) | −0.52 (0.21) | .027 | −0.92 (0.25) | <.001 |
| At 6 mo | 9.83 (4.49) | 9.31 (2.96) | 10.51 (3.44) | −0.38 (0.25) | .099 | −0.90 (0.23) | <.001 |
| At 12 mo | 10.49 (4.15) | 9.64 (3.55) | 10.89 (3.38) | −0.16 (0.21) | .454 | −0.61 (0.22) | .008 |
| PROMIS anxiety, raw scores (8-40) | |||||||
| Baseline | 16.37 (5.88) | 15.52 (5.83) | 15.11 (6.40) | NA | NA | NA | NA |
| Posttreatment | 15.02 (6.16) | 13.89 (5.78) | 14.11 (6.99) | 0 (0.22) | 1.00 | −0.21 (0.21) | .318 |
| At 1 mo | 14.58 (6.45) | 12.25 (4.81) | 13.75 (6.78) | 0.36 (0.20) | .109 | −0.29 (0.21) | .203 |
| At 2 mo | 14.14 (7.07) | 13.23 (6.74) | 13.58 (6.75) | 0.02 (0.24) | .923 | −0.22 (0.25) | .348 |
| At 3 mo | 13.75 (6.45) | 14.50 (7.42) | 14.08 (6.42) | −0.34 (0.23) | .147 | −0.62 (0.21) | .009 |
| At 6 mo | 14.88 (7.12) | 13.00 (5.14) | 14.59 (6.90) | 0.03 (0.24) | .907 | −0.50 (0.24) | .028 |
| At 12 mo | 14.09 (6.79) | 14.07 (7.51) | 14.81 (6.94) | −0.20 (0.22) | .362 | −0.56 (0.23) | .014 |
| PROMIS sleep, raw score (8-40) | |||||||
| Baseline | 22.21 (6.54) | 22.65 (6.38) | 22.63 (6.26) | NA | NA | NA | NA |
| Posttreatment | 17.73 (6.75) | 20.50 (6.17) | 20.89 (6.02) | −0.41 (0.23) | .056 | −0.63 (0.22) | .003 |
| At 1 mo | 17.18 (6.38) | 21.02 (6.34) | 21.62 (6.45) | −0.46 (0.25) | .039 | −0.89 (0.27) | <.001 |
| At 2 mo | 17.08 (6.71) | 19.71 (6.72) | 21.74 (7.19) | −0.38 (0.24) | .112 | −0.84 (0.27) | <.001 |
| At 3 mo | 16.67 (6.67) | 20.16 (7.05) | 21.73 (6.26) | −0.44 (0.24) | .061 | −1.08 (0.24) | <.001 |
| At 6 mo | 17.85 (7.24) | 19.42 (6.22) | 21.38 (6.03) | −0.29 (0.23) | .198 | −0.85 (0.23) | <.001 |
| At 12 mo | 18.11 (7.36) | 19.95 (5.79) | 21.19 (6.73) | −0.23 (0.22) | .272 | −0.60 (0.25) | .009 |
Abbreviations: NA, not applicable; PROMIS, Patient-Reported Outcome Measurement Information System; PRT, pain reprocessing therapy.
Effect sizes show the group difference in change from baseline (group × time interaction), including all available data at the follow-up time point and corresponding baseline data for effect size computation.
Hedges g and SE estimated with bootstrapping procedure.
Analyses of secondary outcomes at posttreatment revealed significant reductions in disability and anger for PRT vs both controls (g, −0.62 to −1.7; P < .005) and improvements in sleep (g, −0.56; P = .009) and depression (g, −0.63; P = .003) relative to usual care (Table 2). Treatment gains on secondary outcomes were largely maintained at 1-year follow-up (Table 2). Significant PRT vs control effects were observed at posttreatment for positive affect (Positive and Negative Affect Schedule; g for PRT vs placebo, 0.63, g for PRT vs usual care, 0.59; P < .005; eTable 3 in Supplement 2) but not for negative affect or alcohol, cannabis, or opioid use (eTable 3 in Supplement 2). Treatment satisfaction was high among participants in the PRT group (eTable 4 in Supplement 2).
Mediation Analyses
Pretreatment to posttreatment reductions in TSK-11 and pain intensity scores were correlated among participants in the PRT group (r42 = 0.44; P = .003; eFigure 4 in Supplement 2). This correlation was not significant for the placebo condition (r42 = 0.16; P = .29) or usual care condition (r45 = 0.27; P = .07). Pretreatment to posttreatment changes in PCS and SOPA-Emotion scores did not correlate with pain reductions within any group.
Pretreatment to posttreatment reductions in TSK-11 scores mediated PRT vs placebo and PRT vs usual care effects on pain intensity at most follow-up time points (eFigure 4 and eTables 5 and 6 in Supplement 2). The reverse was also true: pretreatment to posttreatment pain reductions mediated PRT vs placebo and PRT vs usual care effects on TSK-11 at follow-up. Pretreatment to posttreatment changes in PCS and SOPA-Emotion did not mediate PRT vs control effects at any follow-up time point. Treatment effects on TSK-11 were very large at posttreatment (g for PRT vs placebo, −1.90; g for PRT vs usual care,−1.67; P < .001).
Neither age nor sex moderated the treatment effect on pain intensity (eMethods in Supplement 2). No adverse events were reported for PRT.
Neuroimaging Outcomes
Evoked Back Pain
At baseline, increased back distention led to increased pain (mean [SD] for distention level 1, 32.15 [18.57]; distention level 2, 37.91 [20.30]; distention level 3, 46.70 [21.71]; distention level 4, 52.73 [21.78]). There was a significant effect of distention level on pain (mean [SD] β for inflation, 7.05 [5.06]; t95 = 13.64; P < .001. Individual patient-evoked pain data are shown in eFigure 5 in Supplement 2.
Patients receiving PRT reported significant pretreatment to posttreatment reductions in evoked back pain relative to placebo (β, −13.05 on a 101-point visual analog scale; t122.85 = −2.82; P = .006; g, −0.60; 95% CI, −1.06 to −0.16) and relative to usual care (β, −19.61; t79.52 = −4.03; P < .001; g, −0.99; 95% CI, −1.50 to −0.55; Figure 3A). Among patients in the PRT group, pretreatment to posttreatment reductions in evoked back pain and 1-week average back pain intensity were correlated (r32 = 0.47; P = .005).
Figure 3. Effects of Treatment on Evoked and Spontaneous Back Pain and Related Brain Function.
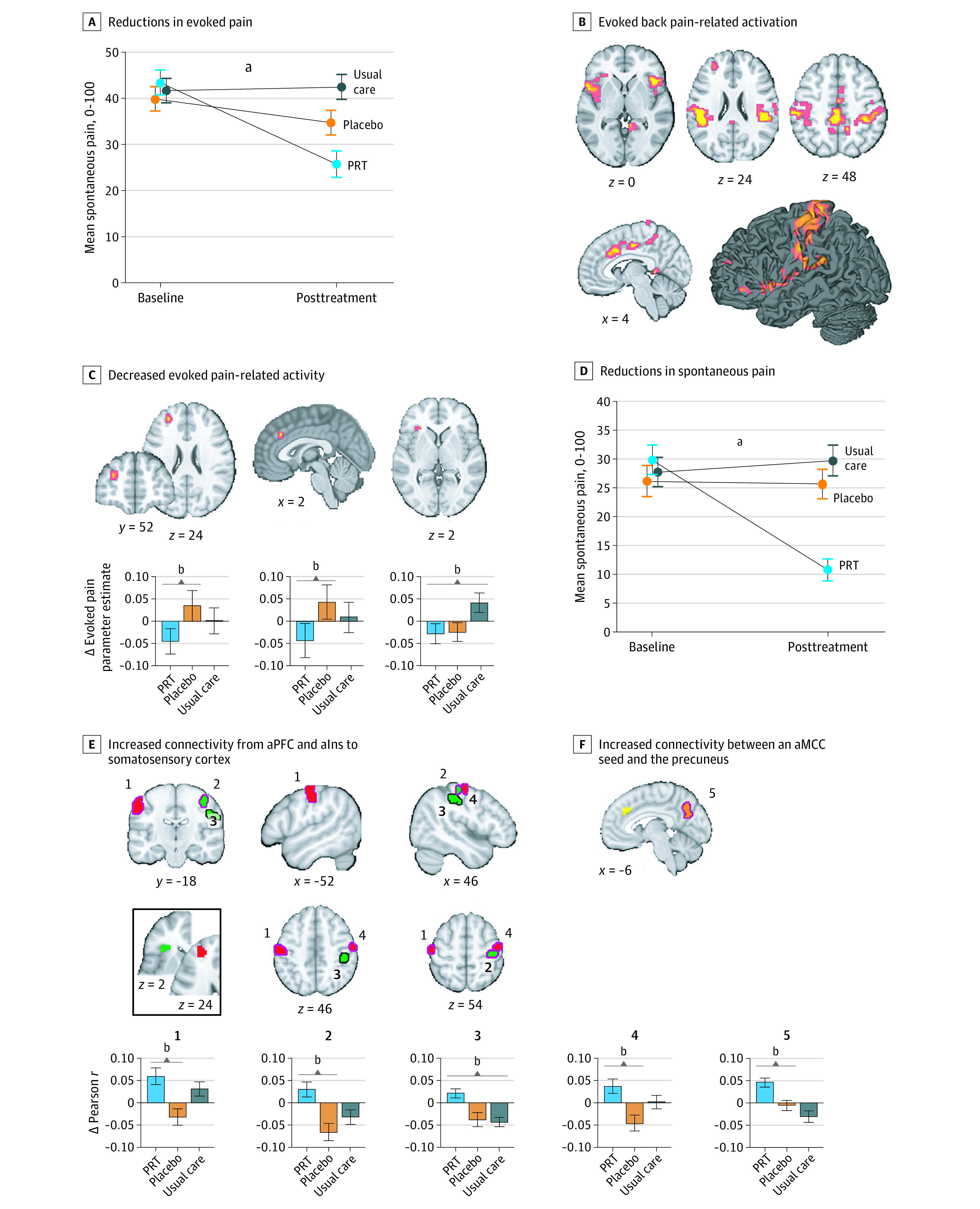
A, Error bars show standard error. B, Coordinates and statistics for activations provided in eTable 7 in Supplement 2; analyses conducted within a mask of regions of interest; eFigure 1 in Supplement 2. C, Decreased evoked pain-related activity was observed in anterior midcingulate (aMCC) and anterior prefrontal regions for PRT vs placebo and left anterior insula for PRT vs usual care. D, Error bars show standard error. E, PRT vs control conditions increased aPFC-seeded (red clusters) and aIns-seeded (green clusters) connectivity with primary somatosensory cortex (permutation test, P < .05). Inset shows seed regions, derived from evoked pain analyses; magenta outlines, PRT vs placebo; black outlines, PRT vs usual care. F, PRT vs usual care increased connectivity between an aMCC seed (yellow; derived from evoked back pain analyses) and the precuneus (orange). Connectivity analyses were conducted within primary somatosensory cortex and medial default mode network masks.
aP < .001.
bP < .05.
Localizer analyses identified 16 regions within the mask of interest positively associated with evoked pain intensity, including bilateral insula, cingulate, bilateral somatotopic back areas S1 and secondary somatosensory cortex, and prefrontal regions (Figure 3B; eFigure 1 and eTable 7 in Supplement 2). Relative to placebo, PRT reduced pain-related activity in aMCC (t133.48 = −1.73; P = .04) and the anterior prefrontal cortex (aPFC; t133.48 = −1.85; P = .03). Relative to usual care, PRT reduced pain-related activity in the left anterior insula (aIns; t120.1 = −2.34; P = .01; Figure 3C).
Spontaneous Pain
Patients receiving PRT reported reductions in spontaneous pain relative to placebo (β, −18.24 on a 101-point visual analog scale; t140.66 = −4.59; P < .001; g, −0.92; 95% CI, −1.44 to −0.47) and relative to usual care (β, −21.53; t79 = −5.26; P < .001; g, −1.11; 95% CI, −1.66 to −0.66; Figure 3D).
We submitted the aMCC, aPFC, and aIns regions exhibiting treatment effects in evoked pain analyses as connectivity seed regions in the spontaneous pain task. Within S1, PRT vs placebo and PRT vs usual care led to increased aPFC- and aIns-seeded connectivity to 4 distinct S1 subregions (permutation test COPE-MAX, 3.55-3.91; P < .05). Within the medial default mode network, PRT vs usual care increased aMCC-precuneus connectivity (permutation test COPE-MAX, 4.23; P = .01; Figure 3E; cluster coordinates and statistics in eTable 8 in Supplement 2). No group × time interactions were found for aPFC- or aIns-seeded connectivity to default mode network regions or for aMCC-seeded connectivity to S1.
Discussion
PRT yielded large reductions in CBP intensity relative to open-label placebo and usual care control conditions in a community sample, with nearly two-thirds of randomized patients and 73% of those initiating PRT reporting they were pain-free or nearly pain-free at posttreatment. Large effects of PRT on pain continued at 1-year follow-up. PRT also reduced experimentally evoked back pain and spontaneous pain during fMRI with large effect sizes, and several secondary outcomes (eg, disability and anger) also improved for PRT relative to the control groups.
PRT targets primary (nociplastic) pain by shifting patients’ beliefs about the causes and threat value of pain. It presents pain as a reversible, brain-generated phenomenon not indicative of peripheral pathology, consistent with active inference and constructionist accounts of interoception and pain.18,19,22,23,24,25,26,27 PRT builds on and extends existing psychological treatment models. Cognitive-behavioral, acceptance-based, and mindfulness-based interventions typically aim to improve functioning by decreasing pain catastrophizing, enhancing pain coping or acceptance, and promoting engagement in valued life activities.41,44,46,48,62 Exposure-based treatments share with PRT an emphasis that painful activities are not injurious,42,63,64,65 but do not emphasize reappraising pain sensations and reattributing the causes of pain. Some pain neuroscience education interventions present pain in a similar way as PRT,43 though they typically lack guided exposure and reappraisal exercises.
Large reductions in pain are rarely observed in CBP psychological treatment trials.11,12 Relatively unique components of PRT potentially contributing to the observed effects include (1) an in-depth medical and psychological assessment generating personalized evidence for centralized pain; (2) reattribution of pain to reversible learning- and affect-related brain processes rather than bodily injury; and (3) a unique combination of cognitive, somatic, and exposure-based techniques supporting pain reappraisal (eDiscussion in Supplement 2).
Correlational and mediational analysis results support changes in fear-inducing pain beliefs as a potential PRT mechanism. Effects of PRT on pain beliefs were also mediated by pain intensity reductions, perhaps because pain reductions promote beliefs in pain modifiability (eDiscussion in Supplement 2). Changes in pain beliefs are not unique to PRT, but PRT may more strongly change these beliefs compared with existing therapies (eTable 6 in Supplement 2).
These hypothesized mechanisms are consistent with extinction-based treatment approaches to anxiety disorders.42,65 For example, 85% of patients became free of panic symptoms following treatment focused on reappraising somatic symptoms as caused by nondangerous central nervous system processes (eg, false alarms).66
PRT reduced evoked pain-related activity in aPFC, aMCC, and aIns. The aPFC and adjacent dorsolateral prefrontal cortex (dlPFC) are implicated in the detection and inhibition of pain.67 aPFC reductions following PRT suggest a potential reduction of pain-related signals or decreased prioritization of pain control. The aMCC and aIns are cortical convergence zones in the construction of negative affect in pain and other domains.20,68,69,70 Cognitive pain regulation strategies, including mindful acceptance38,39 and placebo analgesia,24,25,28 have been found to reduce aMCC and aIns responses to pain, demonstrating parallels between experimental findings and our clinical findings. The aIns reductions in our study were not specific to PRT vs placebo and may reflect processes common to both these interventions.
PRT also increased aPFC and aIns connectivity to S1, aligning with previous findings that cognitive behavioral therapy for fibromyalgia57 and acupuncture for CBP55 increased aIns-S1 connectivity. Increased aPFC and aIns connectivity to S1 may reflect increased attention to somatosensory input in constructing pain.71 This is congruent with mindfulness-based treatments promoting nonreactive attention to bodily sensations, reducing catastrophizing.38,39,48,71 Yet, increased S1 connectivity has also been associated with increased clinical pain,72 and the role of S1 connectivity remains unclear.55 PRT vs usual care also increased aMCC-precuneus connectivity, with intermediate effects observed in participants receiving placebo treatment. Altered default mode connectivity has often been reported in chronic pain, although typically with heightened connectivity for patients vs controls (eDiscussion in Supplement 2).36,54,56,58,59
Limitations
This study has limitations. The study sample was relatively well educated and active and reported long-standing low to moderate pain and disability at baseline. The physician and therapists were experts in the treatment model. Future studies should test generalizability to other patient populations, therapists, and treatment contexts (eDiscussion in Supplement 2). The fMRI effect sizes were modest, with some results not surviving whole-brain correction (eMethods in Supplement 2). Future trials should test PRT efficacy relative to leading psychological and medical treatments (eDiscussion in Supplement 2).
Conclusions
Overall, our findings raise key possibilities about the nature and treatment of primary CBP. Changing fear- and avoidance-inducing beliefs about the causes and threat value of pain may provide substantial, durable pain relief for people with primary CBP.
Trial protocol
eMethods
eResults
eDiscussion
eTable 1. Spinal anomalies among participants randomized to PRT
eTable 2. Treatment response rates
eTable 3. Secondary clinical outcomes measured only at pretreatment and posttreatment
eTable 4. Treatment satisfaction and patient global impression of change
eTable 5. Mediation results
eTable 6. Values for mediators at each timepoint
eTable 7. Evoked back pain localizer results
eTable 8. Regions showing pretreatment to posttreatment connectivity changes for PRT vs placebo or PRT vs usual care
eFigure 1. Evoked back pain localizer
eFigure 2. Target masks for seed connectivity analyses
eFigure 3. Individual trajectories of pain intensity for participants in the PRT, placebo and usual care groups
eFigure 4. Effects of PRT on pain-related fear and avoidance and beliefs that pain indicates injury
eFigure 5. Evoked back pain at pretreatment
eFigure 6. High vs low thumb pressure stimulation
eFigure 7. Histogram of quality control-functional connectivity correlations for spontaneous pain scans
eFigure 8. Continuous pain regressors for 4 randomly chosen sample individuals
eAppendix 1. Initial medical pain assessment and education session
eAppendix 2. Pain reprocessing therapy description
eAppendix 3. PRT treatment fidelity checklist
eReferences
Data sharing statement.
References
- 1.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. doi: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press; 2011. doi: 10.17226/13172 [DOI] [PubMed] [Google Scholar]
- 3.Hartvigsen J, Hancock MJ, Kongsted A, et al. ; Lancet Low Back Pain Series Working Group . What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356-2367. doi: 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 4.Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736-747. doi: 10.1016/S0140-6736(16)30970-9 [DOI] [PubMed] [Google Scholar]
- 5.Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Primers. 2018;4(1):52. doi: 10.1038/s41572-018-0052-1 [DOI] [PubMed] [Google Scholar]
- 6.Henschke N, Maher CG, Refshauge KM, et al. Prevalence of and screening for serious spinal pathology in patients presenting to primary care settings with acute low back pain. Arthritis Rheum. 2009;60(10):3072-3080. doi: 10.1002/art.24853 [DOI] [PubMed] [Google Scholar]
- 7.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363-370. doi: 10.1056/NEJM200102013440508 [DOI] [PubMed] [Google Scholar]
- 8.Karshikoff B, Jensen KB, Kosek E, et al. Why sickness hurts: a central mechanism for pain induced by peripheral inflammation. Brain Behav Immun. 2016;57:38-46. doi: 10.1016/j.bbi.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 9.Nicholas M, Vlaeyen JWS, Rief W, et al. ; IASP Taskforce for the Classification of Chronic Pain . The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28-37. doi: 10.1097/j.pain.0000000000001390 [DOI] [PubMed] [Google Scholar]
- 10.Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29(1):6-19. doi: 10.1016/j.berh.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 11.Williams ACC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2020;8(8):CD007407. doi: 10.1002/14651858.CD007407.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of physicians clinical practice guideline. Ann Intern Med. 2017;166(7):493-505. doi: 10.7326/M16-2459 [DOI] [PubMed] [Google Scholar]
- 13.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3)(suppl):S2-S15. doi: 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18(1):20-30. doi: 10.1038/nrn.2016.162 [DOI] [PubMed] [Google Scholar]
- 15.Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science. 2019;363(6424):276-281. doi: 10.1126/science.aap8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale J, Zhou H, Zhang Q, et al. Scaling up cortical control inhibits pain. Cell Rep. 2018;23(5):1301-1313. doi: 10.1016/j.celrep.2018.03.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua T, Chen B, Lu D, et al. General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat Neurosci. 2020;23(7):854-868. doi: 10.1038/s41593-020-0632-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleckner IR, Zhang J, Touroutoglou A, et al. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav. 2017;1:0069. doi: 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett LF. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci. 2017;12(1):1-23. doi: 10.1093/scan/nsx060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419-429. doi: 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterling P. Allostasis: a model of predictive regulation. Physiol Behav. 2012;106(1):5-15. doi: 10.1016/j.physbeh.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 22.Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron. 2015;87(3):474-491. doi: 10.1016/j.neuron.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petzschner FH, Weber LAE, Gard T, Stephan KE. Computational psychosomatics and computational psychiatry: toward a joint framework for differential diagnosis. Biol Psychiatry. 2017;82(6):421-430. doi: 10.1016/j.biopsych.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 24.Büchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81(6):1223-1239. doi: 10.1016/j.neuron.2014.02.042 [DOI] [PubMed] [Google Scholar]
- 25.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16(11):1277-1283. doi: 10.1038/nm.2229 [DOI] [PubMed] [Google Scholar]
- 26.Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ. 2020;370:m1668. doi: 10.1136/bmj.m1668 [DOI] [PubMed] [Google Scholar]
- 27.Seymour B. Pain: a precision signal for reinforcement learning and control. Neuron. 2019;101(6):1029-1041. doi: 10.1016/j.neuron.2019.01.055 [DOI] [PubMed] [Google Scholar]
- 28.Ashar YK, Chang LJ, Wager TD. Brain mechanisms of the placebo effect: an affective appraisal account. Annu Rev Clin Psychol. 2017;13(1):73-98. doi: 10.1146/annurev-clinpsy-021815-093015 [DOI] [PubMed] [Google Scholar]
- 29.Jepma M, Koban L, van Doorn J, Jones M, Wager TD. Behavioural and neural evidence for self-reinforcing expectancy effects on pain. Nat Hum Behav. 2018;2(11):838-855. doi: 10.1038/s41562-018-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlaeyen JWS, Crombez G. Behavioral conceptualization and treatment of chronic pain. Annu Rev Clin Psychol. 2020;16:187-212. doi: 10.1146/annurev-clinpsy-050718-095744 [DOI] [PubMed] [Google Scholar]
- 31.Meulders A. Fear in the context of pain: Lessons learned from 100 years of fear conditioning research. Behav Res Ther. 2020;131:103635. doi: 10.1016/j.brat.2020.103635 [DOI] [PubMed] [Google Scholar]
- 32.Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(Pt 9):2751-2768. doi: 10.1093/brain/awt211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Napadow V, Harris RE. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of ‘centralized’ pain? Arthritis Res Ther. 2014;16(5):425. doi: 10.1186/s13075-014-0425-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014;17(11):1607-1612. doi: 10.1038/nn.3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Čeko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp. 2015;36(6):2075-2092. doi: 10.1002/hbm.22757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martucci KT, Shirer WR, Bagarinao E, et al. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network—a resting-state study from the MAPP Research Network. Pain. 2015;156(9):1755-1764. doi: 10.1097/j.pain.0000000000000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo C-W, Roy M, Buhle JT, Wager TD. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol. 2015;13(1):e1002036. doi: 10.1371/journal.pbio.1002036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kober H, Buhle J, Weber J, Ochsner KN, Wager TD. Let it be: mindful acceptance down-regulates pain and negative emotion. Soc Cogn Affect Neurosci. 2019;14(11):1147-1158. doi: 10.1093/scan/nsz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage. 2013;64(1):538-546. doi: 10.1016/j.neuroimage.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao L, Yank V, Ma J. Algorithm for balancing both continuous and categorical covariates in randomized controlled trials. Comput Methods Programs Biomed. 2012;108(3):1185-1190. doi: 10.1016/j.cmpb.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 41.Thorn BE, Eyer JC, Van Dyke BP, et al. Literacy-adapted cognitive behavioral therapy versus education for chronic pain at low-income clinics: a randomized controlled trial. Ann Intern Med. 2018;168(7):471-480. doi: 10.7326/M17-0972 [DOI] [PubMed] [Google Scholar]
- 42.Vlaeyen JWS, Morley S, Linton S, Boersma K, De Jong J. Pain-Related Fear: Exposure-Based Treatment for Chronic Pain. IASP Press; 2012. [Google Scholar]
- 43.Moseley GL, Butler DS. Fifteen years of explaining pain: the past, present, and future. J Pain. 2015;16(9):807-813. doi: 10.1016/j.jpain.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 44.McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol. 2014;69(2):178-187. doi: 10.1037/a0035623 [DOI] [PubMed] [Google Scholar]
- 45.Lumley MA, Schubiner H, Lockhart NA, et al. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. Pain. 2017;158(12):2354-2363. doi: 10.1097/j.pain.0000000000001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315(12):1240-1249. doi: 10.1001/jama.2016.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190. doi: 10.1007/BF00845519 [DOI] [PubMed] [Google Scholar]
- 48.Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82(3):448-459. doi: 10.1037/a0035798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591. doi: 10.1371/journal.pone.0015591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locher C, Frey Nascimento A, Kirsch I, Kossowsky J, Meyer A, Gaab J. Is the rationale more important than deception? a randomized controlled trial of open-label placebo analgesia. Pain. 2017;158(12):2320-2328. doi: 10.1097/j.pain.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 51.Kleine-Borgmann J, Schmidt K, Hellmann A, Bingel U. Effects of open-label placebo on pain, functional disability, and spine mobility in patients with chronic back pain: a randomized controlled trial. Pain. 2019;160(12):2891-2897. doi: 10.1097/j.pain.0000000000001683 [DOI] [PubMed] [Google Scholar]
- 52.Lembo A, Kelley JM, Nee J, et al. Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial. Pain. 2021. doi: 10.1097/j.pain.0000000000002234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schubiner H, Betzold M. Unlearn Your Pain. Mind Body Publishing; 2010. [Google Scholar]
- 54.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64(7):2398-2403. doi: 10.1002/art.34412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mawla I, Ichesco E, Zöllner HJ, et al. Greater Somatosensory Afference With Acupuncture Increases Primary Somatosensory Connectivity and Alleviates Fibromyalgia Pain via Insular γ-Aminobutyric Acid: A Randomized Neuroimaging Trial. Arthritis Rheumatol. 2021;73(7):1318-1328. doi: 10.1002/art.41620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502-511. doi: 10.1038/nrn3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazaridou A, Kim J, Cahalan CM, et al. Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin J Pain. 2017;33(3):215-221. doi: 10.1097/AJP.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J, Mawla I, Kong J, et al. Somatotopically specific primary somatosensory connectivity to salience and default mode networks encodes clinical pain. Pain. 2019;160(7):1594-1605. doi: 10.1097/j.pain.0000000000001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong J, Spaeth RB, Wey H-Y, et al. S1 is associated with chronic low back pain: a functional and structural MRI study. Mol Pain. 2013;9(1):43. doi: 10.1186/1744-8069-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586-592. doi: 10.1037/0278-6133.19.6.586 [DOI] [PubMed] [Google Scholar]
- 61.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811-816. doi: 10.3174/ajnr.A4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keefe FJ, Caldwell DS, Williams DA, et al. Pain coping skills training in the management of osteoarthritic knee pain: a comparative study. Behav Ther. 1990;21(1):49-62. doi: 10.1016/S0005-7894(05)80188-1 [DOI] [Google Scholar]
- 63.Logan DE, Carpino EA, Chiang G, et al. A day-hospital approach to treatment of pediatric complex regional pain syndrome: initial functional outcomes. Clin J Pain. 2012;28(9):766-774. doi: 10.1097/AJP.0b013e3182457619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherry DD, Wallace CA, Kelley C, Kidder M, Sapp L. Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin J Pain. 1999;15(3):218-223. doi: 10.1097/00002508-199909000-00009 [DOI] [PubMed] [Google Scholar]
- 65.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46(1):5-27. doi: 10.1016/j.brat.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 66.Barlow DH, Craske MG, Cerny JA, Klosko JS. Behavioral treatment of panic disorder. Behav Ther. 1989;20(2):261-282. doi: 10.1016/S0005-7894(89)80073-5 [DOI] [Google Scholar]
- 67.Seminowicz DA, Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. 2017;18(9):1027-1035. doi: 10.1016/j.jpain.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Craig AD. How do you feel—now? the anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59-70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- 69.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35(3):121-143. doi: 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geuter S, Boll S, Eippert F, Büchel C. Functional dissociation of stimulus intensity encoding and predictive coding of pain in the insula. Elife. 2017;6:e24770. doi: 10.7554/eLife.24770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med. 2012;35(6):591-602. doi: 10.1007/s10865-011-9391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J, Loggia ML, Cahalan CM, et al. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67(5):1395-1405. doi: 10.1002/art.39043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods
eResults
eDiscussion
eTable 1. Spinal anomalies among participants randomized to PRT
eTable 2. Treatment response rates
eTable 3. Secondary clinical outcomes measured only at pretreatment and posttreatment
eTable 4. Treatment satisfaction and patient global impression of change
eTable 5. Mediation results
eTable 6. Values for mediators at each timepoint
eTable 7. Evoked back pain localizer results
eTable 8. Regions showing pretreatment to posttreatment connectivity changes for PRT vs placebo or PRT vs usual care
eFigure 1. Evoked back pain localizer
eFigure 2. Target masks for seed connectivity analyses
eFigure 3. Individual trajectories of pain intensity for participants in the PRT, placebo and usual care groups
eFigure 4. Effects of PRT on pain-related fear and avoidance and beliefs that pain indicates injury
eFigure 5. Evoked back pain at pretreatment
eFigure 6. High vs low thumb pressure stimulation
eFigure 7. Histogram of quality control-functional connectivity correlations for spontaneous pain scans
eFigure 8. Continuous pain regressors for 4 randomly chosen sample individuals
eAppendix 1. Initial medical pain assessment and education session
eAppendix 2. Pain reprocessing therapy description
eAppendix 3. PRT treatment fidelity checklist
eReferences
Data sharing statement.