Abstract
Human Bartonella infections result in diverse medical presentations, whereas many cats appear to tolerate chronic bacteremia without obvious clinical abnormalities. Eighteen specific-pathogen-free cats were inoculated with Bartonella henselae- and/or Bartonella clarridgeiae-infected cat blood and monitored for 454 days. Relapsing bacteremia did not correlate with changes in protein profiles or differences in antigenic protein recognition. Intradermal skin testing did not induce a delayed type hypersensitivity reaction to cat scratch disease skin test antigen. Thirteen cats were euthanatized at the end of the study. Despite persistent infection, clinical signs were minimal and gross necropsy results were unremarkable. Histopathology revealed peripheral lymph node hyperplasia (in all of the 13 cats), splenic follicular hyperplasia (in 9 cats), lymphocytic cholangitis/pericholangitis (in 9 cats), lymphocytic hepatitis (in 6 cats), lymphoplasmacytic myocarditis (in 8 cats), and interstitial lymphocytic nephritis (in 4 cats). Structures suggestive of Bartonella were visualized in some Warthin-Starry stained sections, and Bartonella DNA was amplified from the lymph node (from 6 of the 13 cats), liver (from 11 cats) heart (from 8 cats), kidney (from 9 cats), lung (from 2 cats), and brain (from 9 cats). This study indicates that B. henselae or B. clarridgeiae can induce chronic infection following blood transfusion in specific-pathogen-free cats and that Bartonella DNA can be detected in blood, brain, lymph node, myocardium, liver, and kidney tissues of both blood culture-positive cats and blood culture-negative cats. Detection of histologic changes in these cats supports a potential etiologic role for Bartonella species in several idiopathic disease processes in cats.
During the past decade, substantial evidence has been generated to support the role of Bartonella species as important human pathogens. Bartonellosis in people is characterized by highly variable patterns of disease, including hemolytic anemia, septicemia, endocarditis, osteolysis, bacillary angiomatosis, myositis, retinitis, encephalopathy, and lymphadenopathy (cat scratch disease [CSD]) (3, 31). Two species, Bartonella henselae and Bartonella clarridgeiae, have been cultured from the blood of cats (37, 28), and other potentially unique isolates have been reported (40). B. henselae has been directly responsible for all of the aforementioned presentations except hemolytic anemia. B. clarridgeiae infection in a cat in association with CSD was recently reported, but the spectrum of human disease associated with this novel species is unknown (23).
Prevalence surveys indicate that a remarkable number of cats throughout the world are subclinically infected with Bartonella and that these cats have the potential to act as a reservoir for human infection (4, 10, 17, 19, 45). Initial epidemiologic studies of cats seroreactive to B. henselae antigens failed to identify historical abnormalities or clinical manifestations associated with feline bartonellosis; however, two recent reports describe a positive correlation between Bartonella seroreactivity and renal disease, stomatitis, or lymphadenopathy (13, 46). Several investigators have performed transmission experiments in cats, but obvious morbidity has not been associated with acute infection (1, 11, 14, 15, 24, 38). However, cats were euthanatized (2 to 32 weeks postinoculation) for pathological evaluation in only one of these studies (15). From human studies of bartonellosis, it is known that B. henselae can invade or attach to endothelial cells, pericytes, macrophages, and neutrophils (3, 31). Although we have observed B. henselae within feline erythrocytes (21), pathogenesis studies in cats have been unsuccessful in defining the intracellular location(s) that facilitates persistent occult infection. In an attempt to determine if predictable clinical indications or postmortem findings of feline bartonellosis exist, we experimentally infected specific-pathogen-free (SPF) cats with blood from two naturally bacteremic cats that had induced CSD in their owners. Blood donor cats were infected with either B. henselae (type II) or both B. henselae (type II) and B. clarridgeiae. Observations compiled during the first 213 days of this transmission study have been reported elsewhere (24). Since it appears that acute bartonella infection fails to induce clinical manifestations in most cats, we extended the duration of our study from 213 to 454 days in order to examine the clinical and pathologic consequences of chronic bartonella infection in cats maintained in an environmentally controlled setting. Extending the observation period allowed us to further compare and contrast the diagnostic utility of serologic, microbiologic, immunologic, and molecular testing. In addition, we evaluated the antimicrobial efficacy of doxycycline and enrofloxacin for treatment of bartonella infection, the effect of iatrogenic immunosuppression, and the potential for cats to become reinfected after challenge exposure. In this report, we describe results compiled from day 213 to day 454 of the study and the postmortem findings in SPF cats chronically infected with Bartonella.
(Presented in part at the 16th Annual American College of Veterinary Internal Medicine Forum, 21 to 25 May 1998, San Diego, Calif.)
MATERIALS AND METHODS
Experimental animals.
Eighteen SPF cats that received bartonella-infected blood were previously described in a report of the first 213 days postinoculation (24). Briefly, young SPF cats (approximately 16 weeks old) that were Bartonella culture negative and seronegative were inoculated with blood or urine from cats that were bacteremic with Bartonella or with blood from uninfected SPF controls. Cats that originally received uninfected blood inoculum in the first half of the study or were previously inoculated with infected blood but failed to become bacteremic as assessed by blood culture were reinoculated intravenously (i.v.) with 10 ml of infected blood (10% acid citrate dextrose [ACD] [vol/vol]) on day 213. All cats were continuously housed in an ectoparasite-free facility and received biweekly physical examinations with concomitant monitoring of body temperature, complete blood counts, blood cultures for Bartonella bacteremia, and determination of Bartonella-specific antibodies. On day 454 of the study, 13 cats were euthanatized by barbiturate overdose (Beuthanasia-D Special; Schering-Plough, Kenilworth, N.J.) and exsanguinated by cardiocentesis. All experiments were performed in accordance with North Carolina State University, Institutional Animal Care-and-Use Committee guidelines under protocol 94-072.
Documentation of infection by blood culture and serology.
Blood cultures were performed with 1.5 ml of blood aseptically obtained by jugular phlebotomy and placed in Pediatric Isolator tubes (Wampole Laboratories, Cranbury, N.J.) as described previously (22). Blood cultures were incubated on 5% rabbit blood agar (BBL; Becton Dickinson and Co., Cockeysville, Md.) at 35°C, in 5% CO2, for up to 60 days. At one time point, cat blood agar was evaluated as an alternative growth medium. Blood from a Bartonella culture-negative, seronegative cat was drawn into ACD (10% [vol/vol]) to prevent coagulation and added (5% [vol/vol]) to Trypticase soy agar (BBL). Bartonella-specific seroreactivity was assessed by using an indirect immunofluorescence assay (IFA) (22).
Antimicrobial treatment and experimental immunosuppression.
On day 276 of the study, 16 of 18 cats were randomly assigned to receive oral treatment with either enrofloxacin (22.7 mg every 12 h [q12h]) or doxycycline (25 mg q12h) for 14 or 28 days as described elsewhere (25). Two cats were maintained as untreated, infected controls. Approximately 3 months after antimicrobial treatment was initiated (day 363), all treated cats and controls received a single intramuscular (i.m.) injection of methylprednisolone acetate (MPA) (20 mg/kg) (Depo-Medrol; Upjohn, Kalamazoo, Mich.) in the biceps femoris muscle.
Challenge exposure.
Seven days after corticosteroid administration (day 370), blood was drawn for culture, and immediately thereafter, 13 cats were challenge exposed to either homologous (same donor; n = 6) or heterologous (different donor; n = 7) infected blood inoculum. Four cats remained unchallenged, and one cat died from an incident unrelated to Bartonella infection. Challenge exposure was performed by i.v. inoculation of ACD-treated blood (10 ml) from an infected donor. Reinfection status of cats following challenge exposure was evaluated by IFA serology, blood culture, and PCR analysis of EDTA-treated blood.
Intradermal skin test.
CSD skin test antigen (gift of Andrew J. Margileth), previously determined to contain B. henselae DNA (2), was administered to 16 of the 18 experimentally-infected cats, 1 naturally-infected cat (blood donor for inoculum), and 2 Bartonella culture-negative, seronegative SPF cats. Six 0.05-ml aliquots of skin test antigen (1:1,000, 1:500, 1:100, 1:50, 1:25, and neat) were injected intradermally (i.d.) in a shaved region of the lateral thorax. Since all cats, including SPF controls, were previously immunized and received booster doses against feline panleukopenia virus (FPV), concentrated FPV antigen was administered as a positive control. Sterile saline was used as the negative control. The injection sites were examined for induration and erythema 6, 12, 24, 36, 48, 60, 72, and 96 h after administration.
SDS-PAGE and Western immunoblotting.
Bartonella isolates from seven cats that manifested recurrent bacteremia were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting. The isolates were chosen from samples collected at various time points during the 454-day experiment, and immunoreactive proteins were evaluated by using host sera collected at the same time points. Agar-grown subcultures (5 to 7 days old) were scraped from plates in phosphate-buffered saline (PBS) and centrifuged at 10,000 × g for 10 min. Whole-cell lysates of Bartonella isolates were prepared by resuspending the bacterial pellet in distilled water. Protein concentrations of the samples were determined by the bicinchoninic acid (BCA) method (Sigma Chemical Co., St. Louis, Mo.) and adjusted to approximately 3 μg/μl. Each complex protein mixture was denatured in an equal volume of sample buffer (60 mM Tris hydrochloride, 2% SDS, 5% 2-β-mercaptoethanol, 10% glycerol, and 0.00125% bromophenol blue) for 5 min in a 100°C water bath. Aliquots of the denatured sample were electrophoresed through a discontinuous SDS-polyacrylamide gel (4% stacking, 10% separating) in a Mini-Protean II apparatus (BioRad, Hercules, Calif.) at a constant current of 30 mA/gel for 1 h. Fractionated proteins were visualized by staining with Coomassie brilliant blue and then electrophoretically transferred to 0.45-μm-pore-size nitrocellulose membranes at 100 V for 1 h. Membranes were transiently stained with Ponceau S to ensure adequate transfer, blocked with 5% milk in Tris-buffered saline (TBS) for 1 h at room temperature, then washed twice for 5 min each time in TBS. Immobilized proteins were probed overnight at room temperature with cat sera diluted 1:200 in 0.05% Tween 20 in TBS (TTBS). The nitrocellulose membranes were subsequently incubated with phosphatase-labeled, whole-molecule goat anti-cat immunoglobulin G (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) and immersed in alkaline phosphatase substrate solution (BioRad) to visualize protein bands.
Identification of isolates by PCR-RFLP.
Bartonella blood culture isolates were disrupted by glass beads in a minibeadbeater (Biospec, Bartlesville, Okla.), and bacterial DNA was extracted with phenol-chloroform and precipitated with ethanol. Genotypic analysis was performed by PCR-restriction fragment length polymorphism (RFLP) of the 16S rRNA gene (restricted with DdeI and MnlI) and the 16S to 23S intergenic spacer region (restricted with HaeIII, AluI, and αTaqI) as reported for the first half of the study (24).
Gross necropsy and histopathology.
Prior to fixation of the eyes in Bouins solution, aqueous humor was aspirated and frozen at −70°C for Bartonella-specific immunoglobulin and DNA analysis (27). Bone marrow was placed in Trump’s fixative. Other tissues were examined macroscopically and fixed in 10% neutral buffered formalin for paraffin embedding. Paraffin-embedded sections (5 μm thick) were stained with hematoxylin and eosin, Giemsa, and Warthin-Starry silver stains for histological analysis.
Extraction of DNA from cerebrospinal fluid, blood, and other tissues.
Ten paraffin-embedded sections (5 μm thick) from each block of interest (prescapular lymph node, liver, left ventricle, kidney, brain, or lung) were placed in a sterile 1.8-ml microcentrifuge tube, deparaffinized in xylene, and rehydrated through a series of alcohol washes and PBS. Rehydrated tissue or 100 μl of EDTA-treated blood was washed in PBS and incubated with 0.4 ml of digestion buffer (100 mM NaCl, 10 mM Tris-HCl [pH 8.0], 25 mM EDTA [pH 8.0], 0.5% SDS, 100 μg of proteinase K/ml) overnight at 50°C. The digested material was extracted twice with Tris-buffered phenol and phenol-chloroform-isoamyl alcohol. DNA was precipitated overnight with ethanol and resuspended in 25 to 300 μl of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). DNA was extracted from cerebrospinal fluid (CSF) by using the Isoquick DNA isolation kit according to the manufacturer’s recommendations (Orca Research, Bothell, Wash.). Template DNAs, extracted from Toxoplasma gondii (gift of Michael Davidson, North Carolina State University), Chlamydia psittaci, and Chlamydia pneumoniae (gifts of Trudy Messemer, Centers for Disease Control and Prevention) with the Isoquick protocol were used as controls for nonspecific PCR amplification. Contamination control samples were not consistently used during the DNA extraction process; however, reagents were divided into aliquots in clean microcentrifuge tubes before the addition of DNA-containing material.
PCR amplification of DNA from body fluid and tissues.
Adaptations of primers derived from the 16S rRNA region by Bergmans et al. were used for PCR analysis of cat tissues (5). For the amplification of Bartonella DNA from blood, CSF, and tissue sections, the forward primer 5′ AGAGTTTGATCCTGGCTCAG 3′ (16SFmod) and the reverse primer 5′ CCGATAAATCTTTCTCCCTAA 3′ (Bh1) were used, generating a product of 185 bp. The second set of primers was used for in situ hybridization experiments and consisted of the forward primer 5′ GGCAGGCTTAACACATGCAAGTC 3′ (Bar1f) and the reverse primer 5′ GGCTCATCCATCTCCGATAAATC 3′ (Bar1r). The expected size of the product generated with these primers is 163 bp. The sensitivity limits of the first and second sets of primers were 100 pg and 10 fg of Bartonella DNA, respectively.
DNA amplification was carried out in 100-μl reaction volumes. Each reaction mixture contained 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 1% Triton X-100, 3 mM MgCl2, 200 μM deoxynucleoside triphosphates, 0.2 μM primers, 2 U of Taq DNA polymerase (Promega, Madison, Wis.), and 1 μg of DNA with a 50-μl overlay of mineral oil. To minimize nonspecific amplification, a hot-start PCR method was used. Samples were exposed for 5 min at 95°C and then cooled to 80°C for the addition of enzyme. This was followed by 35 cycles of 30 s at 95°C, 60 s at 54°C, and 45 s at 72°C, before the reaction was finished with extension for 5 min at 72°C. The PCR mixture without DNA template was included as a negative control in each amplification assay. PCR products were visualized with ethidium bromide following electrophoresis through a 2% agarose gel. Amplicons derived from blood (all cats) and other tissues (cats 3 and 17) were sequenced by the North Carolina State University DNA sequencing facility to confirm the amplification of Bartonella DNA and to distinguish between B. henselae and B. clarridgeiae PCR products.
In situ hybridization of cat tissue.
Formalin-fixed, paraffin-embedded tissue from selected cats was sectioned onto charged slides, deparaffinized, and rehydrated. To enhance probe permeability, tissue sections were treated with proteinase K (100 μg/μl) at 37°C for 15 min and washed in TBS (0.15 M NaCl, 0.015 M Tris [pH 8.0]). Denaturation was performed in sterile deionized distilled water at 85°C for 20 min, washed in cold sterile deionized distilled water for 3 min, and then air dried. The hybridization mixture was prepared in advance by mixing 100 μl of sterile deionized distilled water, 100 μl of 0.1% SDS, 100 μl of a 10-mg/ml solution of polyvinyl pyrrolidone, 400 μl of 50% dextran sulfate in 10× SET (1.5 M NaCl, 0.2 M Tris-HCl, 10 mM EDTA [pH 7.8]) and 1,000 μl of deionized formamide. The mixture was drawn through a 0.2-μm-pore-size filter, divided into 170-μl aliquots, and stored at −20°C until used. Production of single-stranded digoxigenin-labeled probe was obtained by linear amplification by using the reverse primer according to a method described by Lo et al. (29). Each 50-μl reaction mixture contained 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 1% Triton X-100, 1.5 mM MgCl2, 200 μM dA/G/C/TTP/DIG-dUTP, 0.2 μM reverse primer (Bar1r), 2 U of Taq enzyme, and 50 to 100 ng of purified B. henselae PCR product (163 bp). Amplification conditions were the same as those for the B. henselae PCR described earlier. Since the reaction is not logarithmic, a fresh aliquot of enzyme was added after the last cycle and 35 more cycles were repeated, for a total of 70 cycles of amplification. The labeled probe was purified by the addition of 2.5 μl of glycogen (20 mg/ml), 0.1 volume of 3 M sodium acetate, and 2.5 volumes of cold ethanol. The DNA was precipitated at −70°C, air dried, and resuspended in 50 μl of TE/SDS (0.1%). Twenty microliters of a 10-μg/ml solution containing digoxigenin-labeled probe and 10 μl of a 10-mg/ml solution containing salmon sperm DNA were added to 170 μl of the hybridization mixture and denatured at 95°C for 20 min in a boiling-water bath. An aliquot of the probe mixture was applied to the slide, coverslipped, and sealed with silicone glue. The probed section was denatured by placing the slide on a 95°C hot plate for 10 min, and hybridization was performed at 37°C overnight in a moist petri dish. Slides were washed three times for 5 min each time in TBS–0.5% Triton X-100 at 37°C and then washed three times for 5 min each time in 0.5% TBS at 65°C. Slides were blocked for 20 min at 37°C with 15% dried milk in AP 7.5 (0.1 M Tris-HCl, 0.1 M NaCl, 3 mM MgCl2 [pH 7.5]) containing 0.5% Triton X-100 and then washed in AP 7.5–0.5% Triton X-100. Alkaline phosphatase-conjugated anti-digoxigenin (Boehringer Mannheim, Indianapolis, Ind.) diluted 1:750 in AP 7.5–2% bovine serum albumin–0.5% Triton X-100 was applied to probed sections and incubated at 37°C for 30 min. Slides were washed three times for 5 min each time in AP 7.5–0.5% Triton X-100 and were then washed three times for 5 min each time in AP 9.0 (0.1 M Tris-HCl, 0.1 M NaCl, 0.1 M MgCl2 [pH 9.0]). Color was developed overnight at 37°C by washing slides in a solution of 12 ml of AP 9.0 with 36 μl of nitroblue tetrazolium (100 mg/ml) (Boehringer Mannheim) and 40 μl of 5-bromo-4-chloro-3-indolylphosphate (50 mg/ml) (Boehringer Mannheim). Slides were washed in TE (10 mM Tris-HCl, 0.1 mM EDTA [pH 7.4]), counterstained for 1 s with methyl green and washed with double-distilled water. After air drying, slides were dehydrated (with 80% ethanol for 1 min, 95% ethanol for 1 min, 100% ethanol twice for 1 min each time, xylene for 3 min, and Clear Rite [Richard-Allan Scientific, Kalamazoo, Mich.] for 3 min), and coverslips were applied with Permount (Fisher Scientific, Norcross, Ga.).
Statistical analysis.
The chi-square test was used to evaluate the relationship between blood culture results and the presence of Bartonella DNA in various tissues.
RESULTS
Clinical evaluation.
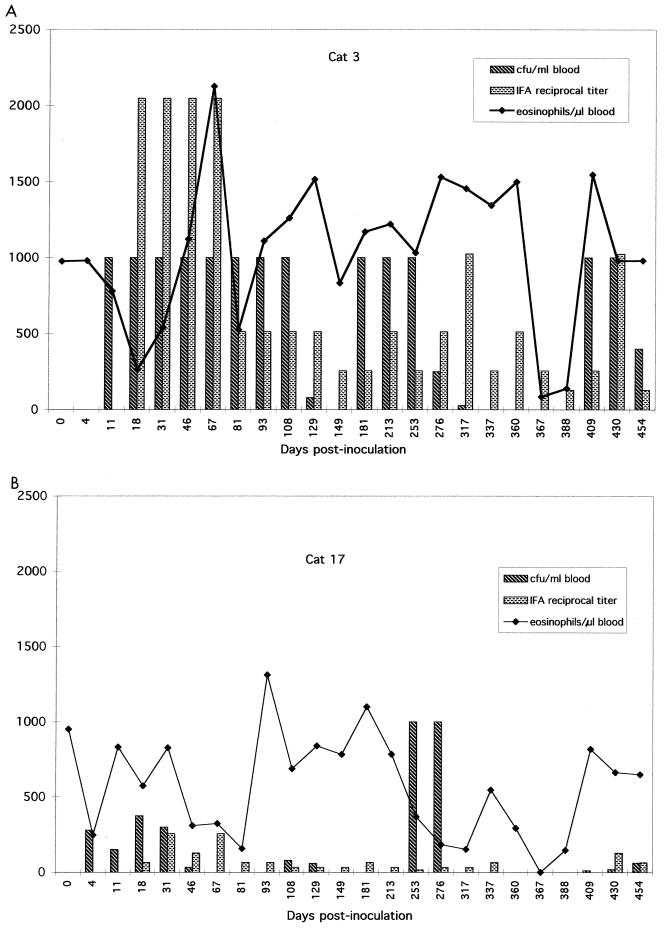
Cats were intermittently bacteremic without obvious clinical signs, except for cat 10, which developed focal motor seizures, nystagmus, and intermittent rigidity on day 252. CSF protein concentration was normal (8.8 mg/dl), but cell numbers were increased (9 monocytes, 5 lymphocytes, and no erythrocytes per μl). Neurologic abnormalities resolved spontaneously and did not recur. Culture-negative intervals occurred randomly and ranged in duration from 1 to 4 months. Reciprocal titers of Bartonella-specific immunoglobulins ranged from <16 to 1,024; however, seroreactivity was not necessarily temporally associated with culture-proven bacteremia. Although cats became febrile and anemic shortly after the initial inoculation (24), recurrent episodes of bacteremia were not accompanied by fever or anemia. As previously reported, routine blood film analysis was nonconfirmatory of bacteremia (24). The optimal sampling, fixing, and staining techniques have yet to be determined. However, even bacteremia at the level of 105 CFU/ml would be equivalent to approximately 1 bacterium per oil immersion field, and therefore bacteria might be difficult to detect by using conventional staining and microscopy. Differential cell counts remained within reference ranges, except for the count of eosinophils, which remained elevated (>750 cells/μl) throughout the study (days 0 to 454). Graphic presentations of bacteremia, seroreactivity, and eosinophilia in two cats are shown in Fig. 1. Uveitis was not observed, but several cats developed cataracts after 1 year of infection.
FIG. 1.
Graphic representation of Bartonella bacteremia levels, IFA titer, and blood eosinophil number in cats 3 (A) and 17 (B), which were siblings and treated similarly throughout the 454-day observation period. These cats were inoculated with B. henselae on day 0, received enrofloxacin for 2 weeks (cat 3) or doxycycline for 2 weeks (cat 17) starting on day 276, were injected with MPA on day 363, and were euthanatized on day 454. These cats were not challenge exposed, but were bacteremic and had Bartonella-specific antibodies in aqueous humor at sacrifice.
Blood culture isolates.
Since B. henselae and B. clarridgeiae appear to be well adapted to survival in cats and are easily isolated from cat blood, for samples collected at one time point we compared Trypticase soy agar supplemented with 5% cat blood as a growth medium to rabbit blood agar. Primary isolation did not occur faster, nor was growth following subpassage more rapid or vigorous, on cat blood as compared with rabbit blood agar. Selected Bartonella isolates were identified to the species level by PCR-RFLP analysis of the 16S rRNA gene and 16S to 23S intergenic spacer region. PCR-RFLP analysis previously revealed that one donor cat was naturally coinfected with B. henselae and B. clarridgeiae. When inoculated with blood obtained at the same sampling time, three recipients became bacteremic with B. henselae, whereas 30 and 60 days later four other recipients became bacteremic with B. clarridgeiae. Two cats remained blood culture negative but were PCR-positive for B. clarridgeiae as confirmed by DNA sequencing of amplicons derived from whole-blood samples.
Antimicrobial treatment, immunosuppression, and challenge inoculation.
Enrofloxacin or doxycycline initiated on day 276 decreased colony counts or eliminated bacteremia in some cats but not others (25). In an attempt to rule out latent infection in culture-negative cats, exogenous corticosteroid was administered to temporarily immunosuppress cats and induce recrudescence of bacteremia. Following corticosteroid administration, the leukogram reflected a typical stress response (mature neutrophilia and lymphopenia), and leukocyte numbers returned to normal within 1 month. Four days postinjection all 18 cats were blood culture negative, including 4 cats that were culture positive during the previous month. At the next time point (22 days later), blood cultures from these four cats again grew Bartonella.
Three months after receiving the first dose of enrofloxacin or doxycycline (treatment durations varied from 14 to 28 days), cats were challenged with homologous or heterologous blood inoculum. Among the experimentally infected cats, one of the seven cats that received heterologous inoculum and one of the six cats that received homologous inoculum became blood culture positive with the challenge strain. Challenge did not induce an anamnestic serologic response. Despite four successive negative blood cultures during the subsequent 3 months, the remaining abacteremic cats had postmortem changes and PCR evidence of Bartonella DNA in the blood and tissues that was representative of the challenge inoculum. As all but two treated cats were subsequently challenged, we are unable to correlate overall treatment efficacy with postmortem PCR results.
SDS-PAGE and Western immunoblotting.
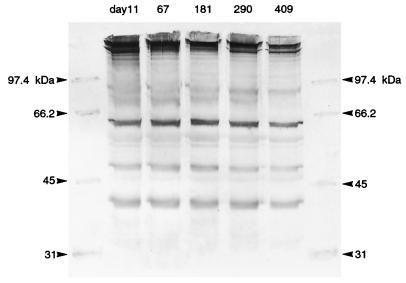
SDS-PAGE analysis was performed on 35 Bartonella isolates cultured from seven cats (five isolates per cat) at various time points during their relapsing bacteremia. Isolates were obtained between days 11 and 454. No differences were detected among the protein profiles of the five Bartonella isolates obtained from each cat over time. Two distinct patterns corresponded with previous PCR-RFLP analysis, indicating that some cats were infected with B. henselae and others were infected with B. clarridgeiae. Only B. clarridgeiae strains contained a protein migrating at approximately 42 kDa.
Western immunoblotting did not reveal substantial differences in Bartonella antigenic protein recognition during the course of infection. The number of proteins recognized and the intensity with which postinoculation serum reacted against these proteins were consistent for each cat at all time points assessed. Antibodies directed against proteins of approximately 32, 42, 48, 65, 68, and 90 kDa were detected in most cats (Fig. 2). Antibodies from all cats elicited a strong response to a protein of approximately 65 kDa, while antibodies from only cats that were bacteremic with B. clarridgeiae recognized a protein of 68 kDa. Preinoculation sera were nonreactive against immobilized Bartonella proteins.
FIG. 2.
Western immunoblot obtained by using antigen prepared from five B. henselae strains isolated from cat 3 between days 11 and 409 of the study and serum obtained on day 290. Serum obtained at any of the five time points recognized the same antigenic proteins with all isolates. Preinoculation serum was nonreactive against all isolates. Emergence of antigenic variants was not detected.
Intradermal skin testing.
Examination of intradermal test sites revealed only three weak responders. Erythema without induration was observed at the 1× CSD antigen test site of two experimentally infected cats (one cat each infected with B. clarridgeiae and with B. henselae) 18 and 36 h after inoculation. One cat that was experimentally infected but remained culture negative throughout the study had erythema and induration at the 1× FPV site 24 h after inoculation. All other cats were skin test negative for both antigens.
Postmortem evaluation and histopathology.
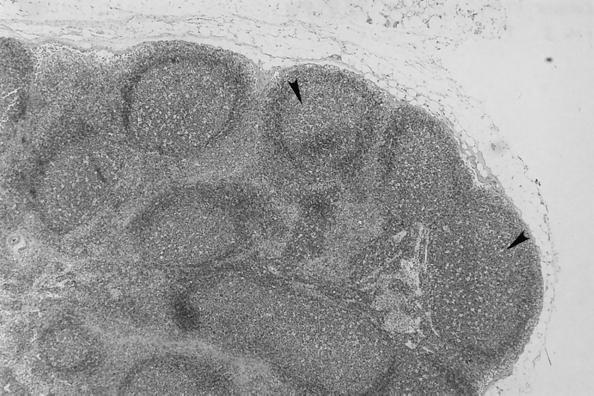
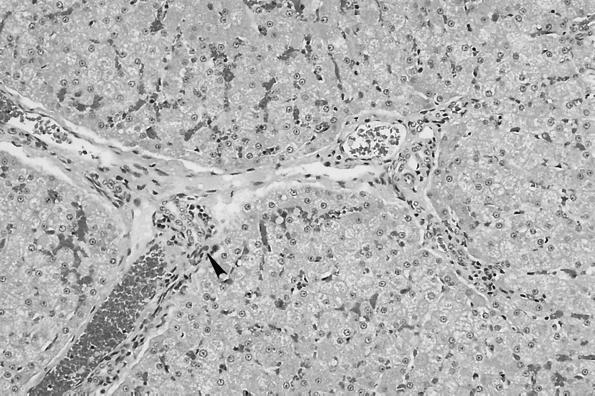
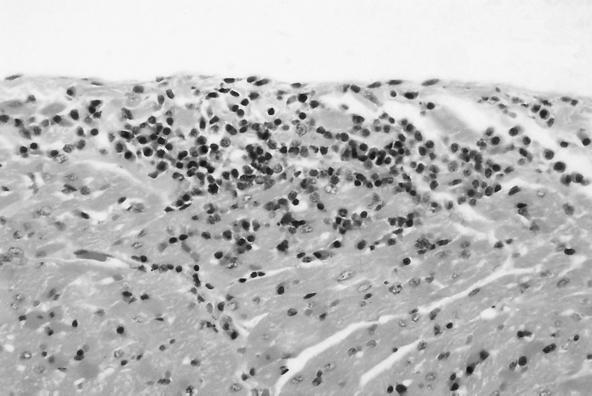
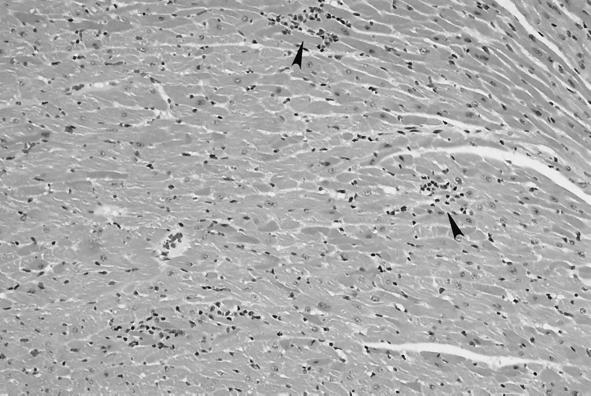
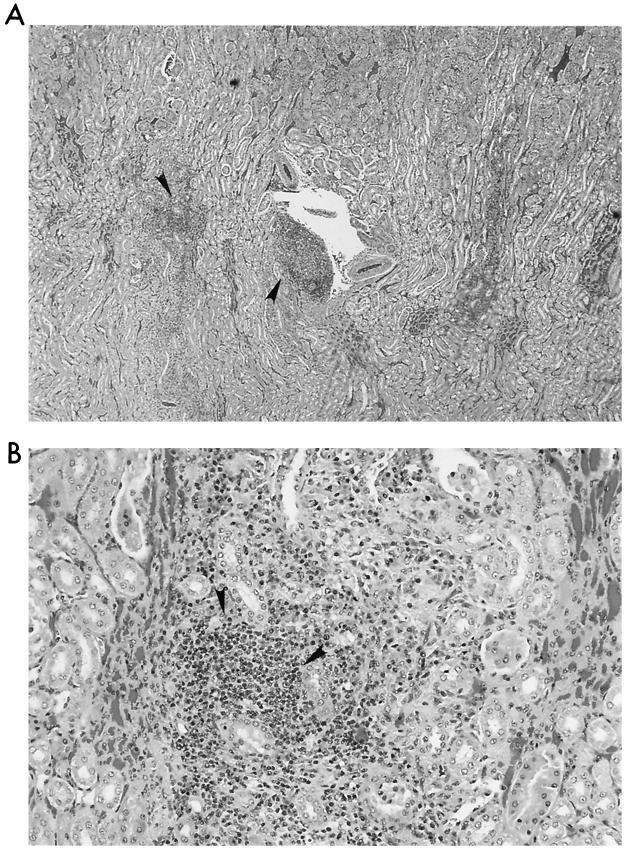
No macroscopic abnormalities were observed in experimentally infected cats at necropsy. Organs did not appear to be enlarged or misshapen; however, nonspecific microscopic pathology was present in the lymph nodes, spleen, liver, heart, and kidney. Reactive follicular hyperplasia was evident in peripheral lymph nodes of all cats; approximately half of the sections contained moderate to large germinal centers in most or all of the follicles (Fig. 3). Similarly, splenic follicular hyperplasia was observed in 9 (69%) of 13 cats. In the liver, small mononuclear accumulations replaced hepatocytes or were associated with hepatocellular necrosis in 6 (46%) of the 13 cats. Many foci were randomly scattered in lobules and occasionally located next to central veins. In addition, small to moderate numbers of lymphocytes and fewer plasma cells surrounded and often obscured the bile ducts in portal areas in (69%) of the 13 cats (Fig. 4). In the same region, structures suggestive of Bartonella were visualized by Giemsa (Fig. 5a) or Warthin-Starry silver stain (Fig. 5b). Focal aggregates of mononuclear cells were observed displacing or replacing myocardial fibers in heart tissue derived from 8 (62%) of the 13 cats (Fig. 6). Some lesions were subendocardial while others were randomly scattered throughout the myocardium (Fig. 7). The cells appeared to be a mixture of lymphocytes and plasma cells, but in some locations were hard to distinguish from proliferating satellite cells. Interstitial inflammation was present in the kidneys of 4 (31%) of the 13 cats. Lesions were primarily lymphocytic, but in one instance, plasma cells and macrophages were also observed. Inflammatory foci were small to moderate in size and located in the outer cortex or near the corticomedullary junction (Fig. 8). Tubular loss and thickened glomerular capsules were also evident in one cat. In another cat, the renal cortex was markedly compressed, secondary to hydronephrosis and presumably unrelated to Bartonella infection. As a result of compression of the renal pelvis, medullary tubules were missing and replaced by a thick fibrous band containing numerous lymphoid nodules.
FIG. 3.
Prescapular lymph node. Follicular hyperplasia is characterized by large germinal centers in all follicles. Sporadic mitotic figures are scattered in hyperplastic follicles.
FIG. 4.
Small to moderate numbers of lymphocytes and sporadic plasma cells surround and obscure bile ducts in portal areas.
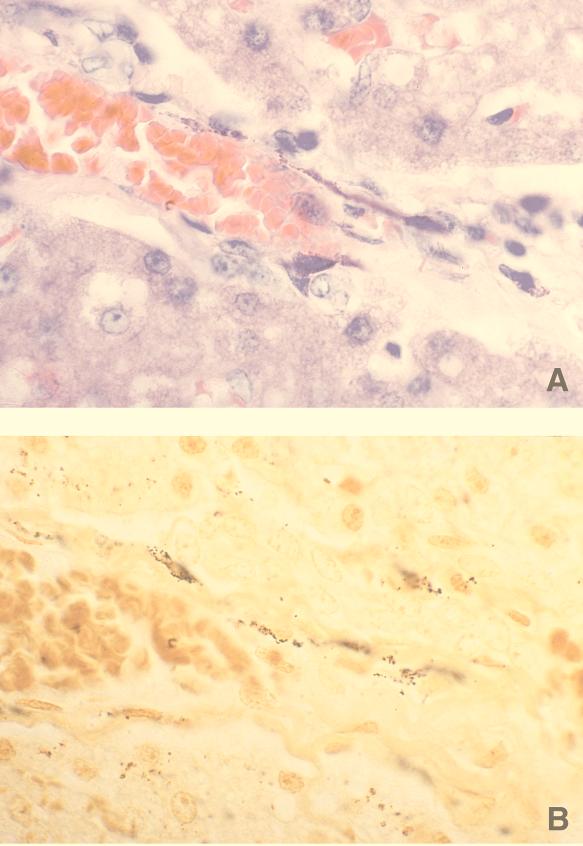
FIG. 5.
Liver. (A) Giemsa stain depicting small dark-stained structures linearly arranged along an interlobular vein. Size and morphological structure are consistent with Bartonella. (B) Warthin-Starry stain. Giemsa-positive structures shown in panel A are also positively stained with silver stain.
FIG. 6.
Heart. Focal accumulation of lymphocytes and plasma cells displacing myocardial fibers with sporadic myocardial fiber loss are seen.
FIG. 7.
Heart. Multiple foci of lymphocytes and plasma cells displacing and replacing myocardial fibers are seen.
FIG. 8.
Kidney. (A) Multiple moderate sized interstitial foci of lymphocytes and macrophages are located in the region of the corticomedullary junction. (B) Higher magnification of one inflammatory focus demonstrates that the majority of cells are lymphocytes.
In the eyes, there was mild to marked fiber clefting of the lens and mild to moderate lymphoid hyperplasia. Bartonella-specific IgG and/or Bartonella DNA were detected in the aqueous humor of some cats regardless of cataract status. Ophthalmic results are reported in detail elsewhere as a component of a larger study (27).
PCR analysis of tissues.
Although Bartonella species were cultured from only 4 of 13 cats at the time of sacrifice, PCR amplicons were obtained from blood from all cats. Insufficient DNA for PCR analysis was available from bone marrow specimens that were preserved in Trump’s solution for electron microscopy. Bartonella DNA was amplified from the brain (from 9 [69%] of the 13 cats), prescapular lymph node (6 cats [46%]), lung (2 cats [17%]), left ventricle (8 cats [62%]), liver (11 cats [85%]), and kidney (9 cats [69%]). Lung and spleen tissue obtained from one blood culture-positive and one culture-negative infected cat were positive for Bartonella DNA as assessed by using the in situ hybridization technique. Table 1 depicts the relationship between blood culture and PCR results for each cat. A significant correlation (P < 0.02) was observed between negative blood culture at the time of euthanasia and the amplification of Bartonella DNA from brain but not other tissues. CSF was collected on day 81 from one cat (cat 10) that manifested neurologic deficits and fever between days 77 and 81 of the study and again on day 252. After cytological analysis confirmed the absence of red blood cell contamination, the CSF specimen was frozen at −70°C. PCR analysis of the banked CSF from cat 10 as well as postmortem brain tissue were both positive for Bartonella DNA. In an effort to rule out nonspecific amplification, DNA extracted from T. gondii, C. psittaci, or C. pneumoniae was not amplified by using the primer pair 16SFmod and Bh1.
TABLE 1.
Postmortem PCR amplification results and Bartonella blood culture status at sacrifice (day 454)a
| Tissue | PCR result for culture-positive cat no.
|
% | PCR results for culture-negative cat no.
|
% | Total PCR positive (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 17 | 1 | 6 | 8 | 9 | 10 | 13 | 14 | 15 | 18 | ||||
| Blood | + | + | + | + | 100 | + | + | + | + | + | + | + | + | + | 100 | 100 |
| Brainb | − | − | −c | + | 25 | + | + | + | + | +d | + | − | + | + | 89 | 69 |
| Lymph node | +e | −e | +e | +e | 75 | +e | −e | −e | −e | +e | −e | −e | +e | −e | 34 | 46 |
| Lung | + | − | − | − | 25 | − | − | + | − | − | − | − | − | − | 11 | 17 |
| Left ventricle | +e | +e | + | −e | 75 | −e | − | +e | + | +e | +e | + | −e | − | 56 | 62 |
| Liver | +e | +e | −e | +e | 75 | − | + | +e | + | +e | +e | + | +e | +e | 89 | 85 |
| Kidney | + | + | + | + | 100 | + | + | −e | + | +e | − | − | +e | −e | 56 | 64 |
+, amplification of Bartonella DNA; −, no amplification of Bartonella DNA.
There was a statistically significant association between negative blood culture results and the ability to amplify Bartonella DNA from brain tissue (P < 0.02; chi-square test).
Neurologic dysfunction on day 106 but afebrile and no CSF was drawn.
CSF obtained from this cat during an episode of neurologic dysfunction (day 81) was PCR-positive; focal seizure activity was noted on day 2522, but this cat was afebrile. CSF was not saved for PCR.
Histologic lesions were observed in adjacent tissue.
DISCUSSION
Persistent bartonella infection was not associated with morbidity or mortality in chronically infected cats. Despite persistent infection, clinical signs were minimal or absent. Self-limiting neurologic dysfunction of 2 days’ duration was observed in two cats during the first half of the study, on days 77 and 115, respectively, and on a second occasion in one of these cats, at 252 days. No abscesses developed at injection sites of our cats when originally inoculated i.m. or i.v. or following subsequent i.v. challenge with infected blood. Other investigators have occasionally reported abscesses or raised, circumscribed lesions at the inoculation site following i.d. or i.d. and subcutaneous injection of laboratory-cultivated B. henselae (14, 15, 38). Ophthalmoscopy disclosed cataracts in six of eight infected cats evaluated on day 436. Although these cats were not littermates, the possibility of a hereditary component cannot be eliminated. However, cats of similar bloodlines have been used for long-term studies of ocular disease associated with T. gondii and feline immunodeficiency virus (FIV) infections at our institution without the development of cataractous lesions. Uveitis was not detected during the 454-day observation period, but lack of detection could be a function of the extended periods between ophthalmologic examinations. Most cats remained eosinophilic, despite the absence of fleas and intestinal parasites, presumably as a function of chronic Bartonella infection.
This study indicates that persistent Bartonella infection in cats can result in pathologic changes in most major organs. Gross necropsy results were unremarkable; however, tissue sections from peripheral lymph nodes, spleen, liver, heart, and kidney contained microscopic inflammatory foci. Although histologic changes were not specific for Bartonella, the distribution of lesions was consistent with systemic dissemination of a blood-borne infection. Follicular hyperplasia of lymph nodes and spleen represents a nonspecific tissue response to chronic antigenic stimulation, which would be expected to occur with protracted Bartonella infection. Although lymphadenopathy was observed earlier in the experiment (24), enlarged lymph nodes or splenomegaly was not observed during chronic infection. Ueno et al. reported an association between lymph node swelling and Bartonella seroreactivity in naturally infected cats; however, lymph node histological study was not performed (46). Lymphoid hyperplasia in CSD patients frequently progresses to development of scattered granulomas with central areas of necrosis which coalesce to form pyogranulomas. Abscesses or granulomata were not apparent in tissue sections derived from our cats. In contrast, Guptill et al. described necrotizing granulomas in the lymph nodes of one cat at 8 weeks postinoculation that were similar to what has been found in CSD patients (15). These investigators also reported splenic lymphoid hyperplasia in experimentally infected cats and observed microabscesses and neutrophilic infiltration in the lymph nodes of two cats at 2 and 4 weeks postinoculation.
Bartonella DNA was amplified from the livers of 85% of experimentally infected cats, and most tissue sections contained lymphocytic periportal infiltrates. These observations suggest that Bartonella may be involved in the pathogenesis of idiopathic hepatic disease in cats. Rarely, we observed Warthin-Starry-stained structures resembling bartonella that were localized to the vasculature of the liver (Fig. 6). However, the difficulty of demonstrating Bartonella organisms in tissues has been reported by other investigators. Brouqui et al. recently described the lack of sensitivity of Warthin-Starry staining (sensitivity, 46%; or 6 of 13 specimens) or immunohistochemistry (sensitivity, 38%, or 5 of 13 specimens) for detection of B. henselae in lymph nodes from patients with confirmed cases of CSD (7). In addition, during the development of bartonellosis in a murine model, Slater et al. failed to visualize organisms by Warthin-Starry staining of mouse tissues despite bacteremia, positive organ cultures, and identification of microscopic inflammatory nodules (44). In three acutely infected experimental cats, however, immunohistochemical techniques used by Guptill and colleagues occasionally revealed extracellular organisms in the liver and spleen 2 to 8 weeks postinoculation (15).
Previously, we reported the occurrence of bartonella endocarditis in a dog (6), and Bartonella species are known to cause cardiac disease in people (3, 31). Recently, Holmberg et al. described a human case of myocarditis in which blood and organ cultures were negative but B. henselae DNA was amplified from a thoracic lymph node (18). The etiologic agent of myocarditis in cats is often undetermined. Ventricular tissue derived from our experimentally infected cats contained variable degrees of lymphoplasmacytic inflammation, and although no distinct bacteria were observed, several heart sections contained Bartonella DNA. In light of these observations, the contribution of Bartonella infection to the progression of nonfatal myocarditis or cardiomyopathy in cats deserves further attention.
Renal disease in middle-aged and older cats is common, with chronic interstitial nephritis of undetermined cause being the most common histologic lesion associated with renal failure. Bartonella DNA was detected in the kidneys of 9 of the 13 cats. Focal interstitial inflammatory lesions were observed in kidneys from 4 of the 13 cats, and tubular and glomerular sclerosis was observed in 1 cat. Given the endemicity of Bartonella infection in cats, the lesions observed in experimentally infected cats, and detection of Bartonella DNA in the kidneys of naturally infected cats (26), these organisms may contribute to feline renal disease. In support of this possibility, Glaus et al. observed an increased frequency of renal disease or stomatitis in Bartonella seroreactive sick cats from Switzerland and southern Germany (13). Previously published data have established the infectivity of urine from people bacteremic with Bartonella quintana (9). In addition, anecdotal reports mention exposure to cat urine as the only risk factor for the subsequent development of CSD (12). From in vitro experiments, we know that B. henselae can remain viable in cat urine for at least 48 h (26). Although cats inoculated i.m. with urine sediment from bacteremic cats failed to become infected (24), this may have been due to inadequate preparation of the inoculum or other unknown factors.
During the first half of this study two cats manifested single, transient episodes of nonlocalizing neurologic signs (24). One of these cats had a brief recurrence during the chronic-phase study. These CNS abnormalities were perhaps not surprising given the wide range of neurologic manifestations that can occur in people with CSD- or human immunodeficiency virus-associated encephalopathy (12, 30). No lesions were observed in brain tissue; however, 9 of 13 brains were positive for Bartonella by PCR. In addition, banked CSF collected from one of the affected cats during a dysfunctional episode was also PCR positive. Bartonella-specific PCR has also been used to analyze brain tissue and CSF from human immunodeficiency virus-infected patients with or without neurologic disease (34, 43). Investigators found B. henselae DNA only in patients experiencing dementia. Furthermore, only PCR-positive patients were bartonella seroreactive or positive by immunohistochemistry. Of interest, the amplification of Bartonella DNA from brain tissue of infected cats was significantly associated with our inability to isolate organisms from the blood (P < 0.02). Perhaps Bartonella persists in endothelial cells within the vasculature of the brain or within a phagocytic cell type, such as microglia. In vitro, microglia derived from fetal cat brains can support B. henselae for at least 14 days (33).
Amplification of Bartonella DNA from the blood of culture-negative cats may be a function of the sensitivity limits of each diagnostic modality or may reflect amplification of DNA from dead bacteria. Although the presence of DNA does not confirm viability of organisms, inoculation of blood from culture- negative, PCR-positive animals into cats or mice can lead to bacteremia, indicating that the level of detection of blood culture is above the dose necessary to establish infection (26). Furthermore, it is doubtful that PCR amplification of Bartonella DNA from tissue sections reflects detection of circulating organisms. In our experiment, lung tissue had the lowest frequency of PCR positivity despite being a highly perfused tissue.
Both donor cats were presumed to be bacteremic with B. henselae prior to the initiation of the transmission study based on morphologic and biochemical characteristics of their isolates. However, during subsequent PCR-RFLP analysis of the DNA coding for rRNA, it was discovered that one of the two donors used in the study was coinfected with B. henselae (type II) and B. clarridgeiae, while the other donor was infected with only B. henselae (type II). B. henselae strains can be subtyped as type I or II based upon genotypic differences (5). Currently, it is not known whether these genotypic differences are predictive of virulent or pathogenic phenotypes in cats or people. Following experimental infection of cats with the Houston-1 strain of B. henselae (type I) by Regnery et al. and Greene et al., bacteremia of short duration and without relapse was reported (14, 38). Discrepancies between our results (prolonged bacteremia with relapse) and those of other investigators might also be due to our use of infected cat blood rather than laboratory-cultivated inoculum. No apparent differences in the course of infection were observed between our recipients of B. henselae-infected blood and recipients of B. henselae and B. clarridgeiae-infected blood. Gurfield et al. also reported cats naturally coinfected with B. henselae and B. clarridgeiae or B. henselae types I and II (16), but whether certain types or species are predominant over others during coinfection is unknown. Transfusion recipients of blood from our coinfected donor cat appeared to be bacteremic with either B. henselae or B. clarridgeiae. At all time points, only one species was identified in a particular cat; however, more colony picks may have revealed coinfection.
Dual infection implies a lack of cross-protectivity between some Bartonella species and subtypes. Among the SPF cats, one cat that received heterologous inoculum became blood culture positive with the heterologous strain, and PCR analysis of blood from the other heterologous recipients, although they were culture negative, amplified DNA from the heterologous strain. In a concurrent experiment, using PCR-RFLP, we documented the transmission of B. clarridgeiae to a cat that was naturally infected with B. henselae for at least 10 months and then became culture negative for 19 months prior to challenge, while housed in our isolation facility (26). These results obtained for cats originally infected experimentally or naturally support claims of incomplete immunity against different strains of Bartonella as observed by others (38, 47) and will be an important consideration for future vaccine development. To date, it is unknown whether acquired immunity to Bartonella in cats is lasting or eventually wanes. Other investigators reported that cats challenged with homologous strains have not become bacteremic but manifested an increase in IgG titer (14, 38, 47). In this study, one cat that received homologous inoculum was bacteremic postchallenge, and all other recipients of homologous inoculum were positive for the homologous strain by PCR analysis and DNA sequencing of amplicons derived from postchallenge blood specimens. However, we were unable to determine if the bacteremia represented reinfection in the bacteremic cat or exacerbation of the original infection in the PCR-positive cats or if the DNAemia was indicative of viable bacteria or reminiscent DNA from dead organisms. As data continue to accumulate, our ability to interpret the diagnostic relevance of molecular testing for Bartonella infection in cats will improve.
Given the presumed immunocompetence of our experimentally infected SPF cats, we investigated the possibility that emergence of antigenic variants could contribute to the maintenance of persistent, recurrent bacteremia. This mechanism of averting host cell immune responses has been well described in regard to infections with Borrelia hermsii (39). In our experiments, Bartonella species could be differentiated from one another by SDS-PAGE, but variability between isolates of B. henselae or B. clarridgeiae derived from the same cat over time was not evident. B. clarridgeiae but not B. henselae possesses flagella; therefore, the additional 42-kDa protein observed for lysates of B. clarridgeiae is most likely flagellin (41). Western immunoblotting failed to detect consistent differences in antigenic protein recognition against Bartonella isolates derived at various time points. The strong response elicited against a protein of approximately 65 kDa for both B. henselae- and B. clarridgeiae-infected cats probably reflects genus-specific reactivity, since a 65-kDa surface protein of B. bacilliformis is also strongly immunogenic (20). Bartonella may use multiple evasive strategies to ensure prolonged survival in cats.
Although the mechanism(s) by which bartonella cells avoid host cell antimicrobial defenses is unknown, intracellular survival may allow the organism to persist for prolonged periods in the host. The principal host cell for intracellular replication of B. henselae and B. clarridgeiae is yet to be determined, but we previously demonstrated the presence of Bartonella within erythrocytes of naturally infected cats (21), and Mehock et al. recently showed that B. henselae can enter feline erythrocytes in vitro (32). Another potential cell type for colonization is the lymphocyte as postulated by Pece et al. (35). Persistent lymphocyte infection with recurrent bacteremia could occur, since lymphocytes are long lived and recirculate between the tissues and vascular system for years. Mononuclear phagocytes also have life spans measured in months to years and could provide a mobile intracellular environment for dissemination of Bartonella to various sites. In preliminary studies, elicited peritoneal macrophages from BALB/c mice were capable of supporting viable B. henselae for at least 72 h in vitro (8).
An early reference in the medical literature mentioned that cats of various ages were refractory to the i.d. injection of fresh, purulent material obtained from CSD patients or prepared skin test antigen (36). The cats in this study were ideal subjects to assess the cell-mediated immune response to CSD skin test antigen because they were experimentally inoculated with Bartonella and their infection status was monitored for 16 months. Despite persistent bartonella infection, gross evidence of a delayed-type hypersensitivity (DTH) response to CSD antigen was infrequently observed. This is not surprising because DTH, while common in people and occasionally observed in dogs, has not been recognized in cats. Thus, skin testing cats to assess Bartonella exposure is of little clinical use since the DTH response is difficult to demonstrate grossly (42).
As observed in many cases of human infection, attaining therapeutic elimination of bartonella can be difficult (25). Furthermore, given the latent nature of feline infection and unpredictable recurrence of bacteremia, elimination is difficult to prove. In an attempt to provoke bacteremia in cats that might be latently infected after antimicrobial treatment, we administered a single immunosuppressive dose of MPA. This also allowed us to examine the effect of hypercorticoidism on seroreactivity and clinical presentation. Hypercorticoid Bartonella-infected cats did not show aggravated bacteremia or develop clinical signs following injection of MPA. Although iatrogenic immunosuppression with a single dose of MPA did not result in recrudescent bacteremia or illness, the effect of multiple doses, as might be administered therapeutically, or surgically-induced immunosuppression is unknown.
In summary, this experimental transmission study in young cats demonstrates that chronic, asymptomatic infection with B. henselae (type II) and/or B. clarridgeiae can induce histologic changes in various organs and supports a potential etiologic role for Bartonella in several idiopathic disease processes in cats. Based on these and other observations in chronically infected cats, a role for Bartonella species as a pathogenic organism in cats is slowly evolving. Although the cats in this study developed subtle lesions without overt disease manifestations, intercurrent infection, immunosuppression, environmental factors, or nutritional stress could contribute to more severe pathologic sequelae. The pathologic tissue changes we report are not specific for Bartonella, but since these cats were SPF, presumably exposed to only B. henselae and/or B. clarridgeiae, maintained in isolation, and had evidence of Bartonella DNA localized to different organs, it seems likely that Bartonella was responsible for these observations. Given the endemicity of feline bartonellosis in the natural cat population, the definition of normal histology in cats should probably be reexamined in regard to Bartonella infection studies. Furthermore, since culture, histopathology, and PCR frequently yield disparate results, this study demonstrates the importance of using a combination of techniques for the diagnosis of bartonellosis.
ACKNOWLEDGMENTS
We thank Mike Davidson for performing ophthalmic examinations on cats and Frank Geoly for reviewing stained sections of eyes. We also thank Andrew Margileth for providing CSD skin test antigen, Monica Mattmüller for preparation of paraffin-embedded tissue for histopathology and PCR analysis, and Bruno Chomel for characterizing B. henselae strains as type II.
Financial support was provided by Pfizer Animal Health, West Chester, Pa., Heska Corporation, Fort Collins, Colo., and Bayer AG, Institute of Biology, Leverkusen, Germany.
REFERENCES
- 1.Abbott R C, Chomel B B, Kasten R W, Floyd-Hawkins K A, Kikuchi Y, Koehler J E, Pedersen N C. Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immun Microbiol Infect Dis. 1997;20:41–51. doi: 10.1016/s0147-9571(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B, Kelly C, Threlkel R, Edwards K. Detection of Rochalimaea henselae in cat-scratch disease skin test antigens. J Infect Dis. 1993;168:1034–1036. doi: 10.1093/infdis/168.4.1034. [DOI] [PubMed] [Google Scholar]
- 3.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baneth G, Kordick D L, Hegarty B C, Breitschwerdt E B. Comparative seroreactivity to Bartonella henselae and Bartonella quintana among cats from Israel and North Carolina. Vet Microbiol. 1995;50:95–103. doi: 10.1016/0378-1135(96)00006-5. [DOI] [PubMed] [Google Scholar]
- 5.Bergmans A M C, Schellekens J F P, van Embden J D A, Schouls L M. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouqui P, Leipdi H, Houpikian P, Raoult D. Presented at the 13th Sesquiannual Meeting of the American Society for Rickettsiology, Champion, Pa., 21 to 24 September 1997. 1997. Warthin-Starry strain and immunohistochemistry as diagnostic tools in the lymph nodes of CSD patients, abstr. 11. [Google Scholar]
- 8.Brown, T. T., K. O. Shin, D. L. Kordick, and E. B. Breitschwerdt. 1997. Unpublished data.
- 9.Bruce D. Trench fever: final report of the war office trench fever investigation committee. J Hyg. 1921;20:258–288. doi: 10.1017/s0022172400034008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Childs J E, Olson J G, Wolf A, Cohen N, Fakile Y, Rooney J A, Bacellar F, Regnery R L. Prevalence of antibodies to Rochalimaea species (cat scratch disease agent) in cats. Vet Rec. 1995;136:519–520. doi: 10.1136/vr.136.20.519. [DOI] [PubMed] [Google Scholar]
- 11.Chomel B, Kasten R W, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield A N, Abbott R C, Pedersen N C. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels W B, MacMurray F G. Cat scratch disease: report of one hundred sixty cases. JAMA. 1954;154:1247–1251. doi: 10.1001/jama.1954.02940490011003. [DOI] [PubMed] [Google Scholar]
- 13.Glaus T, Hofmann-Lehmann R, Greene C, Glaus B, Wolfensberger C, Lutz H. Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. J Clin Microbiol. 1997;35:2883–2885. doi: 10.1128/jcm.35.11.2883-2885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene C E, McDermott M, Jameson P H. Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection. J Clin Microbiol. 1996;34:1682–1685. doi: 10.1128/jcm.34.7.1682-1685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guptill L, Slater L, Wu C, Lin T, Glickman L, Welch D, HogenEsch H. Experimental infection of young cats with the zoonotic organism Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 16.Gurfield A N, Boulouis J-J, Chomel B B, Heller R, Kasten R W, Yamamoto K, Piemont Y. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J Clin Microbiol. 1997;35:2120–2123. doi: 10.1128/jcm.35.8.2120-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heller R, Artois M, Xemar V, DeBriel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmberg M, Wesslen L, Hjelm E, Pahlson C, Lindquist O, Friman G, Regnery R. Presented at the 13th Sesquiannual Meeting of the American Society for Rickettsiology, Champion, Pa., 21 to 24 September 1997. 1997. Bartonella spp. in a 60 year-old Swedish male with myocarditis who succumbed to sudden death, abstr. 31. [Google Scholar]
- 19.Jameson P H, Greene C E, Regnery R L, Dryden M, Marks A, Brown J, Cooper J, Glaus B, Greene R. Seroprevalence of Rochalimaea henselae in pet cats throughout regions of North America. J Infect Dis. 1995;172:1145–1149. doi: 10.1093/infdis/172.4.1145. [DOI] [PubMed] [Google Scholar]
- 20.Knobloch J, Schreiber M. Bb65, a major immunoreactive protein of Bartonella bacilliformis. Am J Trop Med Hyg. 1990;43:373–379. doi: 10.4269/ajtmh.1990.43.373. [DOI] [PubMed] [Google Scholar]
- 21.Kordick D L, Breitschwerdt E B. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655–1656. doi: 10.1128/jcm.33.6.1655-1656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kordick D L, Wilson K H, Sexton D J, Hadfield T L, Berkhoff H A, Breitschwerdt E B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33:3245–3251. doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papule, fever, and lymphadenopathy (cat-scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kordick D L, Breitschwerdt E B. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res. 1997;58:492–497. [PubMed] [Google Scholar]
- 25.Kordick D L, Papich M G, Breitschwerdt E B. Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrob Agents Chemother. 1997;41:2448–2455. doi: 10.1128/aac.41.11.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kordick D L, Breitschwerdt E B. –1998. 1995. Unpublished data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lappin, M. R., W. Jensen, D. L. Kordick, K. Karem, and E. B. Breitschwerdt. Bartonella spp. antibodies and DNA in aqueous humor of cats. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 28.Lawson P A, Collins M D. Description of Bartonella clarridgeiae sp. nov. isolated from the cat of a patient with Bartonella henselae septicemia. Med Microbiol Lett. 1996;5:64–73. [Google Scholar]
- 29.Lo Y M, Yap E P H, An S F, McGee T O D, Fleming K A. Nonisotope probe generation by PCR. In: Griffin H G, Griffin A M, editors. PCR technology: current innovations. Boca Raton, Fla: CRC Press, Inc.; 1994. p. 43. [Google Scholar]
- 30.Marra C M. Neurologic complications of Bartonella henselae infection. Curr Opin Neurol. 1995;8:164–169. doi: 10.1097/00019052-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 32.Mehock J R, Greene C E, Gherardini F C, Hahn T-W, Krause D C. Bartonella henselae invasion of feline erythrocytes in vitro. Infect Immun. 1998;66:3462–3466. doi: 10.1128/iai.66.7.3462-3466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñana, K. R., E. B. Breitschwerdt, D. L. Kordick, and T. T. Brown. 1997. Unpublished data.
- 34.Patnaik M, Schwartzman W A, Peter J B. Presented at the 33rd Annual Meeting of Infectious Diseases Society of American, San Francisco, Calif., 16 to 18 September 1995. 1995. Detection of Bartonella henselae in brain tissue of patients with HIV-associated neurological disease, abstr. 18. [Google Scholar]
- 35.Pece S, Maffione A B, Petruzzelli R, Greco B, Giuliani G, Partipilo M R, Amarri S, Schettini F, Jirillo E, Fumarola D. Rochalimaea henselae organisms possess an elevated capacity of binding to peripheral blood lymphocytes from patients with cat scratch disease. Microbios. 1994;77:95–100. [PubMed] [Google Scholar]
- 36.Prier J E. Cat-scratch fever. Ann NY Acad Sci. 1958;70:650–667. doi: 10.1111/j.1749-6632.1958.tb35420.x. [DOI] [PubMed] [Google Scholar]
- 37.Regnery R, Martin M, Olson J. Naturally occurring “Rochalimaea henselae” infection in domestic cat. Lancet. 1992;340:557. doi: 10.1016/0140-6736(92)91760-6. [DOI] [PubMed] [Google Scholar]
- 38.Regnery R L, Rooney J A, Johnson A M, Nesby S L, Manzewitsch P, Beaver K, Olson J G. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714–1719. [PubMed] [Google Scholar]
- 39.Restrepo B I, Barbour A G. Antigen diversity in the bacterium Borrelia hermsii through “somatic” mutations in rearranged vmp genes. Cell. 1994;78:867–876. doi: 10.1016/s0092-8674(94)90642-4. [DOI] [PubMed] [Google Scholar]
- 40.Sandler A, Buhler C, Pelz K, von Cramm E, Bredt W. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J Clin Microbiol. 1997;35:584–587. doi: 10.1128/jcm.35.3.584-587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherer D C, DeBuron-Connors I, Minnick M F. Characterization of Bartonella bacilliformis flagella and effect of anti-flagellin antibodies on invasion of human erythrocytes. Infect Immun. 1993;61:4962–4971. doi: 10.1128/iai.61.12.4962-4971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz R D, Adams L S. Immunologic methods for the detection of humoral and cellular immunity. Vet Clin N Am. 1978;8:721–753. doi: 10.1016/s0091-0279(78)50110-x. [DOI] [PubMed] [Google Scholar]
- 43.Sevall J S, Mam V, Patnaik M, Peter J B. PCR detection of R. henselae DNA in CSF of patients with HIV encephalopathy. Neurology. 1993;43:A371. . (Abstract 820P.) [Google Scholar]
- 44.Slater L N, Robinson A M, Min K W, Greenfield R A. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Rochalimaea henselae bacteremia in mice: a basis for potential models of bacillary angiomatosis and peliosis, and of cat-scratch disease, abstr. 1598; p. 411. [Google Scholar]
- 45.Ueno H, Muramatsu Y, Chomel B B, Hohdatsu T, Koyama H, Morita C. Seroepidemiological survey of Bartonella (Rochalimaea) henselae in domestic cats in Japan. Microbiol Immunol. 1995;39:339–341. doi: 10.1111/j.1348-0421.1995.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 46.Ueno H, Hohdatsu T, Muramatsu Y, Koyama H, Morita C. Does coinfection of B. henselae and FIV induce clinical disorders in cats? Microbiol Immunol. 1996;40:617–620. doi: 10.1111/j.1348-0421.1996.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto K, Chomel B B, Kasten R W, Chang C-C, Tseggai T, Decker P R, Mackowiak M, Floyd-Hawkins K A, Pedersen N C. Homologous protection but lack of heterologous protection by various species and type of Bartonella in specific pathogen free cats. Vet Immunol Immunopathol. 1998;65:191–204. doi: 10.1016/s0165-2427(98)00154-8. [DOI] [PubMed] [Google Scholar]