Key Points
Question
What is the temporal association between episodes of atrial fibrillation (AF) and ischemic stroke in patients with cardiac implantable electronic devices (CIEDs)?
Findings
In this case-crossover study including 891 patients with CIEDs and ischemic stroke, multihour AF episodes raised the odds of stroke 3.7-fold for up to 30 days. The risk of stroke was highest within 5 days of the AF episode and decreased rapidly thereafter.
Meaning
In patients with CIEDs, multihour episodes of AF were temporally associated with a transient increase in the risk of stroke; these findings support a study of time-delimited anticoagulation in patients with infrequent multihour episodes of AF.
This case-crossover study evaluates the temporal association between episodes of atrial fibrillation and stroke in patients with cardiac implantable electronic devices.
Abstract
Importance
Understanding the temporal association between atrial fibrillation (AF) and ischemic stroke informs our understanding of the AF-stroke mechanism and treatment of paroxysmal AF.
Objective
To define the temporal association between episodes of AF and stroke in patients with cardiac implantable electronic devices (CIEDs).
Design, Setting, and Participants
In this case-crossover study, data from a large national electronic health record database were linked with a single-vendor database of heart rhythm records of patients with CIEDs capable of continuous heart rhythm monitoring. Patients with CIEDs who sustained an ischemic stroke who also had 120 days of continuous remote rhythm monitoring prestroke were included. Data were collected from January 2007 to March 2017, and data were analyzed from November 2019 to June 2020.
Exposure
AF for 5.5 hours or more on any given day during days 1 to 30 vs days 91 to 120 prestroke.
Main Outcomes and Measures
Odds ratio for stroke comparing AF during days 1 to 30 vs 91 to 120 prestroke. This analysis was planned prior to the study.
Results
From 466 635 patients included in both the Optum electronic health record and CareLink databases, 891 patients with CIEDs and ischemic stroke with continuous monitoring in the 120 days prestroke were identified. Of 891 included patients, 575 (64.5%) were male, and the median (interquartile range) age was 76 (67-82) years. The vast majority of patients with stroke had either no AF meeting the threshold duration of 5.5 hours or more in both the case and control periods (682 of 891 [76.5%]) or AF of 5.5 hours or more in both periods (143 of 891 [16.0%]). For those not meeting the 5.5-hour AF threshold in either period, there was no or very little AF throughout the 120 days prestroke. A total of 66 patients had informative, discordant arrhythmic states, with 52 having AF of 5.5 hours or more in the case period vs 14 in the control period (odds ratio [OR], 3.71; 95% CI, 2.06-6.70). Stroke risk was increased most in days 1 to 5 following an AF episode (OR, 5.00; 95% CI, 2.62-9.55). AF greater than 23 hours on a given day was associated with the clearest increase in stroke risk (OR, 5.00; 95% CI, 2.08-12.01).
Conclusions and Relevance
In this large cohort of patients with CIEDs and continuous rhythm monitoring prior to ischemic stroke, excess stroke risk above baseline was highest within 5 days of an episode of AF of 5.5 hours or more in duration and diminished rapidly thereafter. Our findings are consistent with the traditional view that AF is directly and transiently associated with ischemic stroke. These results provide support for trials of time-delimited anticoagulation for patients with infrequent multihour episodes of AF and rigorous, continuous rhythm monitoring.
Introduction
Studies of subclinical atrial fibrillation (SCAF) detected by cardiac implantable electronic devices (CIEDs) document elevated stroke risk among patients with multihour episodes of AF, though at a rate lower than that of clinically detected AF.1,2,3 A curious finding of such studies is that most ischemic strokes experienced by patients with SCAF are not preceded by a recent episode of AF.4,5 These results have raised the question whether AF is a causal risk factor for stroke or simply a risk marker for other causes of stroke or, perhaps, both a risk factor and a risk marker.6,7,8,9 A corollary of this uncertainty is whether long-term anticoagulation is needed in patients with CIEDs who have episodes of SCAF.10 A major weakness of the CIED-based studies of SCAF and stroke has been small numbers of stroke events, compromising statistical power. This was previously addressed using a case-crossover analysis of linked data sets from the US Veterans Administration hospitalization claims records and Medtronic’s CareLink database of continuous heart rhythm records. This study reported a sharp rise in stroke risk within 30 days of onset of a multihour episode of AF.11 But even this analysis included only a small number of informative cases, ie, patients with discordant AF burden in the case and control time periods. The current study uses the very large Optum deidentified electronic health record (EHR) database, also linked to the CareLink heart rhythm database, to validate and extend the previous study’s findings.
Methods
Assembly of Patients With Ischemic Stroke
The patient cohort was assembled by linking data from January 2007 to March 2017 in the Optum deidentified EHR database with the Medtronic CareLink database of heart rhythm records from patients with CIEDs capable of continuous heart rhythm monitoring, including transvenous devices with atrial leads and insertable cardiac monitors. The linked databases have been described previously.12 The linkage resulted in 466 635 individuals matched in the data sets. Patients hospitalized with acute ischemic stroke were included if they had a primary hospital discharge diagnosis including one of the following International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10 codes: 433.X, 434.X, 436.X, I63.X, I65.X, and I66.X. In a sensitivity analysis, we restricted our code set to the more specific stroke codes of ICD-9 codes 433.X1 and 434.X1 and ICD-10 code I63.X.13 In addition, the hospitalization had to include a further treatment or test corroborating the diagnosis of stroke, such as magnetic resonance imaging (eTable 1 in the Supplement). Only a patient’s first stroke during the study period was included. Our analyses were restricted to patients with stroke having at least 120 days of continuous heart rhythm recording prestroke (eFigure 1 in the Supplement). Patient clinical features were ascertained via ICD-9 and ICD-10 codes listed in the EHR prior to the patient’s stroke. Patients were only included in the analysis if they also had medications recorded in the EHR database. Patients were considered to be taking anticoagulants if there was a prescription recorded for warfarin or a non–vitamin K oral anticoagulant within 210 days prior to the patient’s stroke. The institutional review board at Northwestern University determined that the study was not human research and provided an exemption for this analysis.
Measurement of AF Burden
We obtained CIED remote monitoring data from the device manufacturer (CareLink Data Warehousing and Analytics Service, Medtronic). Previous studies have shown that the detection algorithms used in Medtronic CIEDs are capable of quantifying AF duration with more than 95% accuracy.14 The Medtronic CareLink remote monitoring platform records the total duration of AF episodes during a 24-hour calendar day.
Statistical Analysis
Case-crossover studies are designed to assess the short-term association of a time-varying exposure (here, AF) with an outcome (here, ischemic stroke).15 Each patient serves as their own control, where exposure is contrasted between the case period immediately preceding the patient’s stroke and a control period, typically preceding the case period. The control period should be distant enough in time that the occurrence of the time-varying exposure should not be highly correlated with the exposure in the case period and close enough in time that the patient’s long-term risk factors for stroke are the same as in the case period. Patients whose exposure history is concordant, ie, the same in both the case and control periods, are noninformative, conferring no information about the temporal exposure-outcome association. In contrast, patients with discordant exposure histories are informative. For patients with discordant heart rhythm patterns, we compared case vs control periods using matched analyses for dichotomous outcomes, generating odds ratios (ORs) with 95% CIs and P values. Conditional logistic regression adjusted for oral anticoagulant use. In our primary analysis, we assessed the OR for stroke of AF burden of 5.5 hours or more per day, with days 1 to 30 before the stroke serving as our case period and days 91 to 120 prestroke serving as our control period. The threshold of 5.5 hours or more per day was associated with elevated stroke risk in the TRENDS study.1 To assess how long after an episode of AF the risk of stroke was increased, we ascertained days with 5.5 hours or more of AF during mutually exclusive 5-day periods prestroke (ie, days 1 to 5, 6 to 10, 11 to 15, 16 to 20, 21 to 25, and 26 to 30 days prestroke) and compared these results with comparable 5-day periods during the control period, ie, days 1 to 5 with days 91 to 95 prestroke, days 6 to 10 with days 96 to 100, and so on. For any given patient, only the most proximate day meeting the 5.5-hour threshold was counted. Because of uncertainty in the timing of anticoagulant treatment, we assumed that patients with an anticoagulant prescription listed in their EHR were taking anticoagulants in both the case and control periods. We assessed whether the odds ratio in our primary analysis differed in patients taking versus not taking oral anticoagulants. The statistical significance of this difference was assessed by the 2-tailed P value for the interaction term in a conditional logistic regression model. Significance was set at P < .05. All analyses were performed with SAS software version 9.4 (SAS Institute).
Results
We included 891 eligible patients with ischemic stroke. Their median (interquartile range [IQR]) age was 76 (67-82) years, 575 (64.5%) were male, 464 (52.1%) had a prior stroke or transient ischemic attack, and 426 (47.8%) carried a clinical diagnosis of AF. The mean (SD) CHA2DS2-VASc score (congestive heart failure, hypertension, age of 75 years or older [doubled], diabetes, stroke/transient ischemic attack/thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], age of 65 to 75 years, sex category [female]) was 4.9 (2.1). A total of 535 patients (60.0%) had received cardiac defibrillators, 242 (27.2%) pacemakers, and 114 (12.8%) insertable cardiac monitors, and 188 (21.1%) were taking oral anticoagulants (Table 1).
Table 1. Clinical Characteristics of Eligible Patients.
| Characteristic | No. (%) |
|---|---|
| Total, No. | 891 |
| Age, median (IQR), y | 76 (67-82) |
| ≥75 | 474 (53.2) |
| ≥65 | 719 (80.7) |
| Sex | |
| Male | 575 (64.5) |
| Female | 316 (35.5) |
| Device type | |
| ICM | 114 (12.8) |
| IPG | 223 (25.0) |
| ICD | 296 (33.2) |
| CRT-P | 19 (2.1) |
| CRT-D | 239 (26.8) |
| CHA2DS2-VASc score | |
| Mean (SD) | 4.9 (2.1) |
| 0 | 11 (1.2) |
| 1 | 50 (5.6) |
| 2 | 71 (8.0) |
| 3-4 | 202 (22.7) |
| ≥5 | 557 (62.5) |
| Hypertension | 715 (80.2) |
| Heart failure | 504 (56.6) |
| Stroke/TIA | 464 (52.1) |
| Diabetes | 345 (38.7) |
| Myocardial infarction | 306 (34.3) |
| Atrial fibrillation | 426 (47.8) |
| Oral anticoagulation | 188 (21.1) |
Abbreviations: CHA2DS2-VASc; congestive heart failure, hypertension, age of 75 years or older (doubled), diabetes, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age of 65 to 75 years, sex category (female); CRT-D, cardiac resynchronization therapy–defibrillator; CRT-P, cardiac resynchronization therapy–pacemaker; ICD, implantable cardioverter-defibrillator; ICM, insertable cardiac monitor; IPG, implantable pulse generator; IQR, interquartile range; TIA, transient ischemic attack.
We assessed the presence of 5.5 hours or more of AF on any given day as the threshold for our primary analysis. A total of 825 patients (92.6%) had noninformative AF rhythm records, ie, the pattern was the same in both 30-day case and control periods. A total of 682 patients (76.5%) had no periods of AF meeting our daily AF threshold in either period; this included 621 patients who had no AF at all using the de minimis standard of 6 minutes or more of AF on at least 1 day. A total of 143 patients (16.0%) had at least 1 day with 5.5 hours or more of AF in both periods. However, 66 patients (7.4%) had informative, discordant rhythm patterns: 52 (5.8%) had at least 1 day with 5.5 hours or more of AF in the 30 days immediately preceding their stroke but no days in the control period, while 14 (1.6%) had the reverse pattern, resulting in an unadjusted OR of 3.71 (95% CI, 2.06-6.70). To gain precision, we repeated the analysis including three 30-day control periods in the analysis using days 31 to 60, 61 to 90, and 91 to 120 prestroke; the resulting OR for this 3:1 matched analysis was 2.95 (95% CI, 1.83-4.76), consistent with the 1:1 analysis.
eFigure 2 in the Supplement displays the AF patterns of all 66 patients with discordant rhythm patterns using the 5.5-hour per day threshold, including the 52 patients with AF only in the case period and the 14 with AF only in the control period. Among those patients with AF in the case period, the prestroke burden was high, with a median (IQR) maximum daily burden of 24.0 (13.2-24.0) hours. The median (IQR) total hours in AF for these patients in the 30-day case period was 116.7 (29.1-395.5) hours. Most episodes were proximate to the day of stroke, with 23 of 52 (44%) having an AF episode of 5.5 hours or more on the day preceding the stroke. Many of these patients had multiple days of long episodes of AF in the prestroke period, with 12 (23%) having persistent AF for the full 30-day interval. By contrast, there was very little AF in their control periods (days 91 to 120). For those patients with discordant rhythm patterns with AF in the control period, the burden of AF was modestly lower than for patients with discordant AF patterns in the case period, with a median (IQR) maximum daily burden of 19.7 (8.2-24.0) hours in the 30-day control period and a median (IQR) total of 59.5 (23.5-189.0) hours during the entire control period. A random set of the rhythm patterns of 10 patients with AF in both periods and 10 patients without any 5.5-hour AF episodes in either period is available in eFigure 3 in the Supplement. For patients with AF in both periods, AF was often continuous throughout the 120 days prestroke. For those not meeting the 5.5-hour AF threshold in either period, there was no or very little AF throughout the 120 days prestroke.
We anticipated that the effect of temporally proximate AF might be muted among patients taking anticoagulants. Among patients not taking oral anticoagulants in both the case and control periods, the OR was 7.80 (95% CI, 3.07-19.79); among patients taking oral anticoagulants in both periods, the OR was 1.44 (95% CI, 0.62-3.38; P for interaction = .009) (Table 2).
Table 2. Association of Temporally Proximate Atrial Fibrillation (AF) With Risk of Stroke Stratified by Use of Anticoagulants: Comparing Occurrence of AF Above the Threshold 5.5 Hours or More on Any Day on Days 1 to 30 vs 91 to 120 Prestroke.
| Measure | Patients, No. | |||
|---|---|---|---|---|
| Not taking anticoagulation | Taking anticoagulation | |||
| Case period with AF | Case period with no AF | Case period with AF | Case period with no AF | |
| Control period with AF | 89 | 5a | 54 | 9a |
| Control period with no AF | 39a | 570 | 13a | 112 |
| Odds ratio (95% CI) | 7.80 (3.07-19.8) | 1.44 (0.62-3.38) | ||
Patients with discordant rhythm records.
The association of proximate AF with risk of stroke was essentially the same in patients 75 years and older (OR, 3.63; 95% CI, 1.66-7.93) and in those younger than 75 years (OR, 3.83; 95% CI, 1.56-9.41). We further stratified our analysis by the following characteristics: male vs female sex; history of stroke or transient ischemic attack vs no history; prior diagnosis of AF vs no prior diagnosis; CHA2DS2-VASc score of 3 or less vs 4 or more; and by type of cardiac implantable device (eFigure 4 in the Supplement). There is a suggestion that the association might be greater in those without a history of AF and in women, but in all strata, the point estimate ORs were substantially greater than 1.0.
We repeated our analysis including only patients with the more specific ischemic stroke ICD-9 codes of 433.x1 and 434.x1 and ICD-10 code of I63. This reduced the number of patients with discordant rhythm patterns to 48, including 36 patients with at least 1 day with 5.5 hours or more of AF in the case period and 12 with at least 1 day with 5.5 hours or more of AF in the control period, for an OR of 3.00 (95% CI, 1.56-5.77), consistent with our analysis using a broader set of stroke ICD-9 and ICD-10 codes.
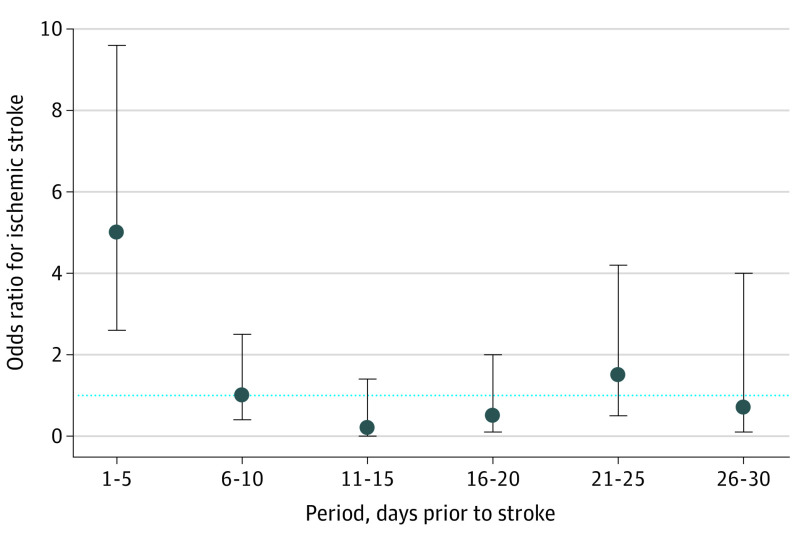
We explored the association of mutually exclusive sequential 5-day periods containing AF of 5.5 hours or more in a given day with risk of stroke. The 682 patients with no qualifying AF episode in either the 30-day case or control periods were excluded. The remaining 209 patients with at least 1 day with a qualifying AF episode in either the 30-day case or control periods formed the basis of this analysis. Among patients with discordant 5-day rhythm histories, the overwhelming majority had 5.5 hours or more of AF on days immediately prior (ie, days 1 to 5) to their stroke, resulting in an OR of 5.00 (95% CI, 2.62-9.55). The point estimates of ORs for stroke were near 1.0 for AF (5.5 hours or more on a given day) during 5-day periods more distant than days 1 to 5, although the confidence intervals were wide (Figure) (eTable 2 in the Supplement).
Figure. Odds Ratios for Ischemic Stroke for Sequential, Nonoverlapping 5-Day Intervals Containing at Least 1 Day With 5.5 Hours or More of Atrial Fibrillation.
Five-day intervals proximate to the stroke were the case periods and were compared with corresponding matched within-patient 5-day control periods beginning 90 days earlier (days 1 to 5 prestroke were compared with days 91 to 95, days 6 to 10 were compared with days 96-100, and so on). Error bars indicate 95% CIs. The horizontal dotted line is the odds ratio of 1.0, ie, reflecting no association. Detailed results are provided in eTable 2 in the Supplement.
To assess the association between increasing daily AF burden and stroke risk, we repeated our 1:1 case vs control period analysis (days 1 to 30 vs days 91 to 120), varying the maximal daily hours of AF according to the mutually exclusive categories given in Table 3. The 23-hour AF threshold conferred a clear increase in stroke risk (OR, 5.00; 95% CI, 2.08-12.01). The shorter thresholds were indeterminate, reflecting the fact that few strokes were preceded by days with 23 hours or less of AF.
Table 3. Association of Temporally Proximate Atrial Fibrillation (AF) With Risk of Stroke: Exploring the Association of Mutually Exclusive Cumulative Amounts of AF on Any Given Day During the 30-Day Case Period vs the 30-Day Control Perioda.
| AF threshold | Case period with AF | Case period with no AF | Odds ratio (95% CI) |
|---|---|---|---|
| 0.5 to 1 h | |||
| Control period with AF | 1 | 3b | 3.33 (0.92-12.11) |
| Control period with no AF | 10b | 638 | |
| 1 to 3 h | |||
| Control period with AF | 2 | 5b | 1.60 (0.52-4.89) |
| Control period with no AF | 8b | 638 | |
| 3 to 6 h | |||
| Control period with AF | 2 | 2b | 2.00 (0.37-10.92) |
| Control period with no AF | 4b | 638 | |
| 6 to 23 h | |||
| Control period with AF | 11 | 4b | 2.75 (0.88-8.64) |
| Control period with no AF | 11b | 638 | |
| >23 h | |||
| Control period with AF | 115 | 6b | 5.00 (2.08-12.01) |
| Control period with no AF | 30b | 638 |
In these analyses, the category of no AF (n = 638) corresponds to a total of less than 0.5 hours of AF on a given day. A total of 39 patients were excluded from this analysis because their AF burden fell into different positive AF categories in their case and control periods (eg, 1 hour in the case period and 4 hours in the control period).
Patients with discordant rhythm records.
Discussion
We assessed the temporal association between episodes of AF and short-term risk of ischemic stroke. From data sets linking health care and heart rhythm records of patients with CIEDs, we identified 891 patients who sustained an ischemic stroke and had 120 days of continuous heart rhythm recordings prestroke. For each patient, we compared heart rhythms in the 30 days immediately prestroke with those during the control period of 91 to 120 days prestroke. As in other studies of patients with CIEDs, most patients had little to no AF.1,16,17 However, using an AF burden threshold of 5.5 hours on any given day, 66 patients had discordant informative heart rhythm patterns, with 52 experiencing AF episodes only in the 30-day period prestroke, resulting in an OR of 3.71 (95% CI, 2.06-6.70). In our analysis, more than 23 hours of AF on any day in the 30-day case period conferred the highest OR for stroke, a finding consistent with a follow-up analysis of the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT).18 We also found the highest stroke risk occurred within 5 days of an AF episode. This finding was similar to the earlier, smaller case-crossover study performed almost exclusively in male patients with cardioverter-defibrillators,11 but the current study found a more dramatic decline in stroke risk posed by more distant AF episodes. The temporal association between AF episodes and stroke risk was seen across strata of multiple clinical features. Finally, we observed that AF episodes were not associated with increased stroke risk in patients taking anticoagulants, consistent with the stroke-suppressing effect of anticoagulants on AF-related stroke. In all, our results strongly support the hypothesis that multihour episodes of AF substantially elevate the baseline risk of ischemic stroke in this population and that this risk diminishes to background risk rapidly following the AF episode.
The traditionally accepted mechanism by which AF causes ischemic stroke is that AF leads to less forceful blood flow from the atria, leading to thrombus formation in the left atrium, particularly in the left atrial appendage.19 Thrombus transit into the cerebral circulation then produces ischemic stroke. For many years, there has been concern that AF is more a risk marker than a causal risk factor, although these mechanisms are not mutually exclusive.6,20 More recently, the risk marker perspective has been bolstered by evidence of a link between AF and atrial myopathy.7,21 In several studies of long-term heart rhythm monitoring in patients with SCAF, most ischemic strokes were not preceded by episodes of AF.4,5,22 Such observations have been adduced to support the hypothesis that ischemic stroke may be more associated with an underlying atrial myopathy than with SCAF itself in patients with CIEDs.7 These SCAF studies have been limited by small numbers of stroke events. The current study addresses this limitation by linking the large Optum deidentified database to the CareLink database, resulting in 891 total stroke events in patients with CIEDs and continuous heart rhythm monitoring prestroke. The 66 patients with stroke with discordant rhythm histories allow a well-powered case-crossover analysis.
Limitations
Our study was subject to notable limitations. As an observational study, there is the potential for residual confounding. This concern is substantially reduced by the case-crossover design, where each patient is their own control, with the result that chronic confounding conditions, eg, hypertension, were likely the same in both study periods. Our assessment of stroke depended on ICD-9 and ICD-10 codes. These stroke code sets have been validated in other studies.13,23,24 In addition, we required that each patient also have at least one of a set of tests or treatments consistent with an acute stroke. When we used a more specific set of stroke codes, our results remained largely the same. Our ICD-9 and ICD-10 codes identified ischemic stroke but not embolic stroke specifically, and we did not have access to the clinical details of the stroke events, limiting our assessment of mechanism.25 Findings from patients with CIEDs may not generalize to the broader population of patients. However, the Danish Loop study in a more general, elderly population found patterns of SCAF on insertable cardiac monitors consistent with the findings of studies of SCAF in patients with CIEDs.16,17 Our case-crossover design provides an efficient measure of relative risk of stroke shortly after periods of AF. There may be concern that our analysis used only the 66 patients with discordant rhythm patterns of a total of 891 patients with stroke. As with standard case-control studies, our self-controlled analysis applies to the entire cohort, giving rise to the 891 stroke events.26,27 Also, as with case-control studies, our study provides an estimate of the relative effect of AF burden, ie, the OR, but cannot directly provide absolute risks of stroke. It also cannot determine whether patients with SCAF are at somewhat increased risk of stroke during periods of sinus rhythm.
Conclusions
Among patients with CIEDs with discordant AF patterns, we found that AF raised the 30-day risk of ischemic stroke more than 3-fold. This risk was highest within 5 days of a multihour episode of AF and diminished rapidly thereafter. These effects were not seen in patients taking anticoagulants, suggesting that ischemic strokes in patients taking anticoagulants resulted from non-AF mechanisms. Our results are consistent with the traditional view that AF may be a causal risk factor for ischemic stroke in patients with CIEDs rather than a simple risk marker, although these attributes may not be mutually exclusive. In addition, our findings indicate that stroke risk is a function of AF duration and frequency. It would be premature to base anticoagulation decisions in patients with SCAF on our results. But our findings provide support for a randomized trial of intermittent, time-delimited anticoagulation covering only multihour AF episodes vs continuous anticoagulation in patients with infrequent paroxysmal AF. The time-delimited strategy could only be safely implemented in patients who have rigorous, continuous heart rhythm monitoring and access to potent, fast-acting anticoagulants.
eTable 1. Current Procedural Terminology (CPT) corroborating a diagnosis of stroke.
eTable 2. AF patterns in paired 5-day case and control periods prior to an ischemic stroke, matched 1:1 within patients.
eFigure 1. Assembly of 891 patients with CIEDs who sustained an ischemic stroke and also had 120 days of continuous heart rhythm recording prestroke.
eFigure 2. AF records for all patients with stroke with discordant patterns in days 1 to 30 and 91 to 120 prestroke.
eFigure 3. Atrial fibrillation (AF) records for a random sample of patients with stroke with concordant patterns in days 1 to 30 and 91 to 120 prestroke.
eFigure 4. Odds of ischemic stroke within 30 days of a qualifying atrial fibrillation (AF) episode vs 91 to 120 days after a qualifying AF episode.
References
- 1.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474-480. doi: 10.1161/CIRCEP.109.849638 [DOI] [PubMed] [Google Scholar]
- 2.Healey JS, Connolly SJ, Gold MR, et al. ; ASSERT Investigators . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120-129. doi: 10.1056/NEJMoa1105575 [DOI] [PubMed] [Google Scholar]
- 3.Bertaglia E, Blank B, Blomström-Lundqvist C, et al. Atrial high-rate episodes: prevalence, stroke risk, implications for management, and clinical gaps in evidence. Europace. 2019;21(10):1459-1467. doi: 10.1093/europace/euz172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daoud EG, Glotzer TV, Wyse DG, et al. ; TRENDS Investigators . Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011;8(9):1416-1423. doi: 10.1016/j.hrthm.2011.04.022 [DOI] [PubMed] [Google Scholar]
- 5.Brambatti M, Connolly SJ, Gold MR, et al. ; ASSERT Investigators . Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129(21):2094-2099. doi: 10.1161/CIRCULATIONAHA.113.007825 [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Go AS, Desvigne-Nickens P, et al. Research priorities in atrial fibrillation screening: a report from a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2021;143(4):372-388. doi: 10.1161/CIRCULATIONAHA.120.047633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(3):895-900. doi: 10.1161/STROKEAHA.115.012004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellano JM, Chinitz J, Willner J, Fuster V. Mechanisms of stroke in atrial fibrillation. Card Electrophysiol Clin. 2014;6(1):5-15. doi: 10.1016/j.ccep.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 9.Calenda BW, Fuster V, Halperin JL, Granger CB. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016;13(9):549-559. doi: 10.1038/nrcardio.2016.106 [DOI] [PubMed] [Google Scholar]
- 10.Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137-145. doi: 10.1016/j.ahj.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 11.Turakhia MP, Ziegler PD, Schmitt SK, et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8(5):1040-1047. doi: 10.1161/CIRCEP.114.003057 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140(20):1639-1646. doi: 10.1161/CIRCULATIONAHA.119.041303 [DOI] [PubMed] [Google Scholar]
- 13.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36(8):1776-1781. doi: 10.1161/01.STR.0000174293.17959.a1 [DOI] [PubMed] [Google Scholar]
- 14.Purerfellner H, Gillis AM, Holbrook R, Hettrick DA. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol. 2004;27(7):983-992. doi: 10.1111/j.1540-8159.2004.00569.x [DOI] [PubMed] [Google Scholar]
- 15.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193-221. doi: 10.1146/annurev.publhealth.21.1.193 [DOI] [PubMed] [Google Scholar]
- 16.Healey JS, Alings M, Ha A, et al. ; ASSERT-II Investigators . Subclinical atrial fibrillation in older patients. Circulation. 2017;136(14):1276-1283. doi: 10.1161/CIRCULATIONAHA.117.028845 [DOI] [PubMed] [Google Scholar]
- 17.Diederichsen SZ, Haugan KJ, Brandes A, et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol. 2019;74(22):2771-2781. doi: 10.1016/j.jacc.2019.09.050 [DOI] [PubMed] [Google Scholar]
- 18.Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38(17):1339-1344. doi: 10.1093/eurheartj/ehx042 [DOI] [PubMed] [Google Scholar]
- 19.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373(9658):155-166. doi: 10.1016/S0140-6736(09)60040-4 [DOI] [PubMed] [Google Scholar]
- 20.Chesebro JH, Fuster V, Halperin JL. Atrial fibrillation—risk marker for stroke. N Engl J Med. 1990;323(22):1556-1558. doi: 10.1056/NEJM199011293232209 [DOI] [PubMed] [Google Scholar]
- 21.King JB, Azadani PN, Suksaranjit P, et al. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(11):1311-1321. doi: 10.1016/j.jacc.2017.07.758 [DOI] [PubMed] [Google Scholar]
- 22.Camen S, Ojeda FM, Niiranen T, et al. Temporal relations between atrial fibrillation and ischaemic stroke and their prognostic impact on mortality. Europace. 2020;22(4):522-529. doi: 10.1093/europace/euz312 [DOI] [PubMed] [Google Scholar]
- 23.Thigpen JL, Dillon C, Forster KB, et al. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8(1):8-14. doi: 10.1161/CIRCOUTCOMES.113.000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao RJR, Andrade JG, Deyell MW, Jackson H, McAlister FA, Hawkins NM. Sensitivity, specificity, positive and negative predictive values of identifying atrial fibrillation using administrative data: a systematic review and meta-analysis. Clin Epidemiol. 2019;11:753-767. doi: 10.2147/CLEP.S206267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 26.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014;43(5):1645-1655. doi: 10.1093/ije/dyu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mostofsky E, Coull BA, Mittleman MA. Analysis of observational self-matched data to examine acute triggers of outcome events with abrupt onset. Epidemiology. 2018;29(6):804-816. doi: 10.1097/EDE.0000000000000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Current Procedural Terminology (CPT) corroborating a diagnosis of stroke.
eTable 2. AF patterns in paired 5-day case and control periods prior to an ischemic stroke, matched 1:1 within patients.
eFigure 1. Assembly of 891 patients with CIEDs who sustained an ischemic stroke and also had 120 days of continuous heart rhythm recording prestroke.
eFigure 2. AF records for all patients with stroke with discordant patterns in days 1 to 30 and 91 to 120 prestroke.
eFigure 3. Atrial fibrillation (AF) records for a random sample of patients with stroke with concordant patterns in days 1 to 30 and 91 to 120 prestroke.
eFigure 4. Odds of ischemic stroke within 30 days of a qualifying atrial fibrillation (AF) episode vs 91 to 120 days after a qualifying AF episode.