Key Points
Question
What are the clinicopathologic features of persistent chemotherapy-induced alopecia (pCIA) in breast cancer survivors?
Findings
Most patients in this case series presented with either diffuse alopecia or hair thinning in the distribution of female pattern hair loss, with trichoscopic and histopathologic features indistinguishable from those of androgenetic alopecia. A few patients presented with inflammatory scarring alopecia.
Meaning
This study suggests that hair follicle miniaturization (the hallmark of androgenetic alopecia) is a prominent feature in pCIA; recognized androgenetic alopecia treatments, including minoxidil and antiandrogen therapy, can also improve hair density for patients with pCIA.
Abstract
Importance
Alopecia induced by classic chemotherapy affects up to 65% of patients and is usually reversible. However, there are increasing reports of persistent chemotherapy-induced alopecia (pCIA), especially for patients treated with taxane-containing chemotherapy regimens.
Objective
To analyze the clinicopathologic characteristics and response to treatment of patients with pCIA after chemotherapy for breast cancer.
Design, Setting, and Participants
In this case series, a retrospective evaluation was performed of patients with a diagnosis of pCIA after chemotherapy for breast cancer in 4 specialist hair clinics from November 1, 2011, to February 29, 2020.
Main Outcomes and Measures
Clinical, trichoscopic, and histopathologic characteristics and treatment outcomes were analyzed. For patients who presented with diffuse alopecia or diffuse rarefaction of hair over the midfrontal scalp with widening of the central part line and preservation of the frontal hairline, the Sinclair scale (grades 1-5, where 1 indicates normal hair density and 5 indicates the most severe stage of hair loss, with little or no hair in the centroparietal region) was used to assess severity.
Results
One hundred patients (99 women [99%]; mean age at presentation, 54.0 years [range, 29.0-74.1 years]) were included. Most patients had diffuse nonscarring alopecia (n = 39), female pattern hair loss (n = 55), or male pattern hair loss (n = 6). Six patients developed cicatricial alopecia. Taxane-containing regimens were used for most patients (92 [92%]) and were associated with more severe alopecia than regimens that did not contain taxanes (median Sinclair grade, 4 [IQR, 3-5] vs 2 [IQR, 2-2.5]; P < .001). A total of 76 of 86 patients (88%) had trichoscopic signs indistinguishable from those of androgenetic alopecia. Of 18 patients who had biopsies, 14 had androgenetic alopecia–like features, 2 had cicatricial alopecia, and 2 had features of both. Both topical and oral minoxidil, sometimes combined with antiandrogen therapy, were associated with an improvement in hair density (median Sinclair grade, 4 [IQR, 3-5] before treatment vs 3 [IQR, 2-4] after treatment; P < .001).
Conclusions and Relevance
This case series outlines previously unreported features of pCIA in patients with breast cancer, including a trichoscopic description. Cosmetically significant regrowth was achieved for a significant proportion of patients with topical or systemic treatments, suggesting that pCIA may be at least partly reversible.
This case series analyzes the clinicopathologic characteristics and response to treatment of patients with persistent chemotherapy-induced alopecia after chemotherapy for breast cancer.
Introduction
Breast cancer is the most common malignant neoplasm affecting women worldwide, comprising 25.4% of all new cancers diagnosed in 2018.1 Death rates from breast cancer have decreased considerably over time owing to refinement in treatment regimens, including the use of adjuvant chemotherapy and endocrine therapy.2 However, adverse effects are significant, with hair loss affecting up to 65% of patients receiving classic chemotherapy regimens3; 58% of patients receiving chemotherapy consider hair loss to be the most troubling adverse event, with 8% reporting they would decline treatment because of this adverse effect.3
Taxane-based chemotherapy regimens are currently regarded as standard first-line therapy for breast cancer in the UK.4 Chemotherapy-induced alopecia (CIA), as well as nail and cutaneous changes, occur in up to 89% of patients being treated with taxane-based regimens.5 Typically, hair loss begins 1 to 3 weeks after initiation of treatment and is usually fully reversible, with complete hair regrowth within 3 to 6 months of treatment cessation.3,6,7,8,9
Persistent CIA (pCIA; also referred to as permanent CIA), defined as absent or incomplete hair regrowth 6 months or more after completion of treatment, has been increasingly identified.8,10,11,12,13,14,15,16,17,18,19 Initial reports of pCIA involved high-dose conditioning chemotherapy with busulfan and cyclophosphamide for hematopoietic stem cell transplantation.6,9,10,13,16,19,20 Subsequently, other agents, including melphalan, thiotepa, carboplatin, etoposide, docetaxel, and paclitaxel, have been implicated,6,9,10,13,16,18,19,20 with taxanes up to 8 times more likely to be associated with persistent alopecia.12
Although scalp cooling has been shown to prevent pCIA,15 there is, to date, no consistently effective treatment for this undesirable adverse effect, to our knowledge. The objective of the present study was to describe the clinical, trichoscopic, and histopathologic characteristics and response to treatment of patients with pCIA after chemotherapy for breast cancer.
Methods
Study Design
This retrospective case series incorporated data from November 1, 2011, to February 29, 2020. Cases were collected from specialist hair clinics based at the Departments of Dermatology, Salford Royal National Health Service Foundation Trust (Greater Manchester, UK) and Queen Elizabeth University Hospital (Glasgow, UK); Sinclair Dermatology (Melbourne, Australia); and Hair and Nail Medicine (Bonn, Germany). Patients referred with persistent alopecia after undergoing standard chemotherapy for breast cancer were included. Exemption from ethics approval and waiver of informed consent were granted by the National Health Service Health Research Authority as the study does not meet the definition of research with human participants as outlined in the Department of Health and Social Care Eligibility Criteria.
Clinicopathologic Evaluation
Persistent CIA was defined as absent or incomplete hair regrowth 6 months or more after chemotherapy, with the diagnosis confirmed by a dermatologist with expertise in hair and scalp disorders. The clinical pattern of hair loss from the scalp, eyebrows, and eyelashes was evaluated. For patients who presented with uniform hair thinning on the entire scalp (diffuse alopecia [DA]) or diffuse rarefaction of hair over the midfrontal scalp with widening of the central part line and preservation of the frontal hairline (clinically indistinguishable from female pattern hair loss [FPHL]), we used the Sinclair scale (grades 1-5, where 1 indicates normal hair density and 5 indicates the most severe stage of hair loss, with little or no hair in the centroparietal region) to assess severity.21 For any patient, man or woman, who presented with alopecia resembling male pattern hair loss (MPHL), severity was measured using the Hamilton-Norwood classification (types I-VII, where I indicates no significant hair loss or recession of the anterior hairline and VII indicates the most severe stage of hair loss, with only a band of hair around the back and sides of the head).22 Although these classifications were not specifically developed for pCIA, they allowed for a useful assessment of hair loss severity in the present study patients. Trichoscopy was performed using a handheld dermatoscope (10-fold magnification). Biopsies were performed for a small number of patients to confirm the diagnosis or exclude significant inflammation or scarring. Horizontal and vertical hematoxylin-eosin–stained sections from 4-mm punch biopsy specimens were examined. The numbers of terminal and vellus follicles and hairs in different stages of the hair cycle were evaluated.
Response to Treatment
Response to treatment was assessed only for those who had completed 6 months or more of therapy with topical minoxidil, low-dose oral minoxidil (LDOM), and/or antiandrogens. The corresponding Sinclair grades (DA and FPHL types) or Hamilton-Norwood types (MPHL type) after treatment were assessed. Adverse effects were also recorded.
Statistical Analysis
Data were summarized using descriptive statistics. The Mann-Whitney test was used to compare Sinclair grades between patients who received taxane-containing regimens or endocrine therapy and those who did not. The Wilcoxon signed rank test was used to compare Sinclair grades before and after treatment. A 2-sided P < .05 was considered statistically significant. Data entry and analyses were performed using IBM SPSS Statistics, version 24.0 (IBM Corp).
Results
One hundred patients with a diagnosis of pCIA (99 women [99%] and 1 man [1%]; mean age at presentation, 54.0 years [range, 29.5-74.1 years]) were seen at 4 hair clinics (Table 1). Most denied any premorbid hair and scalp conditions, but 5 patients had preexisting FPHL and 1 had scalp psoriasis.
Table 1. Baseline Characteristics of Patients With Persistent Chemotherapy-Induced Alopecia.
| Baseline characteristic | No. (%) (N = 100) |
|---|---|
| Age, mean (range), y | 54.0 (29.5-74.1) |
| Sex | |
| Male | 1 (1) |
| Female | 99 (99) |
| Race and ethnicity | |
| Afro-Caribbean | 2 (2) |
| European | 91 (91) |
| Middle Eastern | 1 (1) |
| South Asian (eg, Indian or Sri Lankan) | 6 (6) |
| Preexisting scalp and hair conditions | |
| Female pattern hair loss | 5 (5) |
| Psoriasis | 1 (1) |
| Taxane-based chemotherapy | |
| Docetaxela | 84 (84) |
| Paclitaxelb | 8 (8) |
| Nontaxane chemotherapy regimens | |
| Fluorouracil, epirubicin, and cyclophosphamide | 4 (4) |
| Cyclophosphamide, methotrexate, and fluorouracil | 3 (3) |
| Epirubicin and cyclophosphamide | 1 (1) |
| Tamoxifen | 49 (49) |
| Aromatase inhibitors | |
| Anastrozole | 19 (19) |
| Letrozole | 15 (15) |
| Exemestane | 9 (9) |
| Goserelin | 4 (4) |
| Targeted therapy | |
| Trastuzumab | 19 (19) |
| Trastuzumab and pertuzumab | 2 (2) |
| Scalp cooling | 6 (6) |
| Laboratory investigationsc | |
| Normal results | 46 (46) |
| Low | |
| Vitamin D | 16 (16) |
| Zinc | 8 (8) |
| Ferritin | 6 (6) |
| Folate | 1 (1) |
| Hormonal abnormalitiesd | 8 (8) |
Mostly in combination with fluorouracil, epirubicin, and cyclophosphamide.
In combination with epirubicin and cyclophosphamide or doxorubicin and cyclophospamide.
Included complete blood cell count; renal and liver function tests; serum ferritin, zinc, vitamin D, and thyroid-stimulating hormone; and a hormone profile (unbound androgen index, total testosterone, unbound testosterone, dehydroepiandrosterone sulfate, sex hormone binding globulin, and prolactin).
Included high total testosterone (n = 3), high unbound androgen index (n = 1), high dehydroepiandrosterone sulfate (n = 1), high prolactin (n = 1), low total testosterone (n = 1), and low sex hormone binding globulin (n = 1).
All patients had a history of invasive ductal or lobular carcinoma of the breast diagnosed between 2004 and 2017. Oncologic management included breast surgery, sentinel lymph node biopsy with or without axillary dissection, radiotherapy, chemotherapy, endocrine therapy, and targeted therapy. For 92 patients, the chemotherapy protocol included docetaxel (n = 84) or paclitaxel (n = 8) (Table 1). Twenty-six patients had hormone receptor–negative breast cancer and were therefore not candidates for adjuvant endocrine therapy. Only 6 patients had prophylactic scalp hypothermia. Blood tests were performed for 74 patients, with vitamin D deficiency the most common biochemical abnormality (n = 16).
Clinical characteristics are summarized in eTable 1 in the Supplement. Thirty-nine patients presented with globally reduced hair density that also involved the occipital area (DA), whereas 55 patients had thinning of the centroparietal scalp hair in an FPHL distribution (median Sinclair grade, 4 [IQR, 2.5-5]) (Figure 1). Five female patients had bitemporal recession or balding of the crown in an MPHL distribution. The only male patient in the cohort had extensive baldness resembling MPHL Hamilton-Norwood type VII. Women exposed to a taxane had more pronounced hair loss than those who were not (median Sinclair grade, 4 [IQR, 3-5] vs 2 [IQR, 2-2.5]; P < .001). Six patients developed cicatricial alopecia with clinical and/or histologic features consistent with lichen planopilaris, fibrosing alopecia in a pattern distribution, or frontal fibrosing alopecia. Ancillary features included persistent thinning of eyebrows (n = 50) and eyelashes (n = 32).
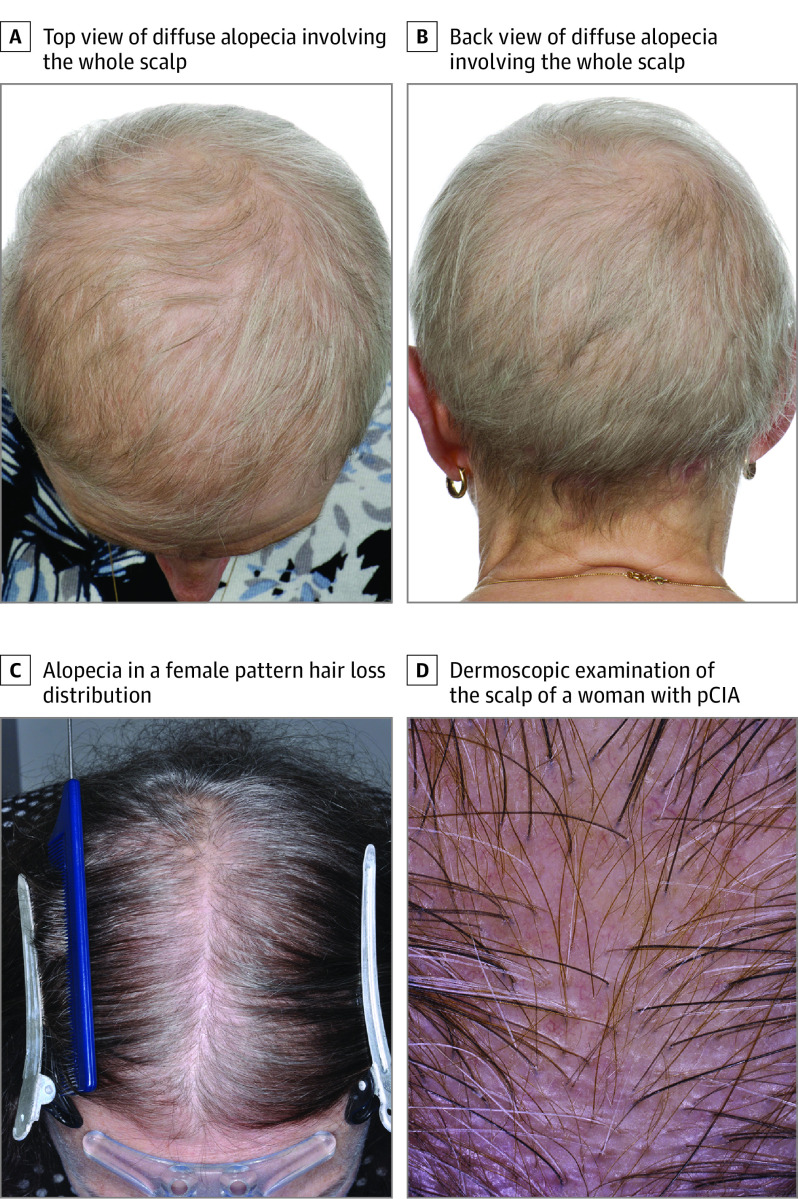
Figure 1. Patterns of Hair Loss in Patients With Persistent Chemotherapy-Induced Alopecia (pCIA).
A, Diffuse alopecia involving the whole scalp (top view). B, Diffuse alopecia involving the whole scalp (back view). C, Alopecia in a female pattern hair loss distribution. D, Dermoscopic examination of the scalp of a woman with pCIA showing hair shaft diameter variability, vellus hairs, and increased single-hair follicular units but no loss of follicular ostia.
Of 68 patients who received hormone therapy, 28 (41%) presented with DA and 40 (59%) presented with FPHL. The pattern of hair loss did not differ significantly for 26 patients with hormone receptor–negative breast cancer (who did not receive endocrine therapy), with DA in 11 patients (42%) and FPHL in 15 patients (58%) (P = .92). Hair loss severity did not differ between patients who received endocrine therapy and those who did not (median Sinclair grade, 4 [IQR, 2.5-5] for patients who received endocrine therapy vs 3.75 [IQR, 3-4.5] for patients who did not receive endocrine therapy; P = .90). The 6 patients with cicatricial alopecia were not included in the analysis. Subgroup analysis of the 92 patients treated with taxane-containing regimens did not show a statistically significant difference between patients with hormone receptor–positive breast cancer and patients with hormone receptor–negative breast cancer in the pattern of hair loss (DA in 40% [25 of 62] and FPHL in 60% [37 of 62] vs DA in 40% [10 of 25] and FPHL in 60% [15 of 25]; P = .98) or severity of hair loss (median Sinclair grade, 4 [IQR, 3-5] vs 4 [IQR, 3-4.5]; P = .53).
A trichoscopic examination was performed for 90 patients. The most common features were hair shaft diameter variability (76 of 86 [88%]), increased vellus hairs (72 of 85 [85%]), and predominant single-hair follicular units (32 of 62 [52%]). Perifollicular erythema and/or scaling was present in 8 patients, and follicular ostia were absent in 6 patients. Other features included empty follicular ostia (n = 10) and focal atrichia (n = 9).
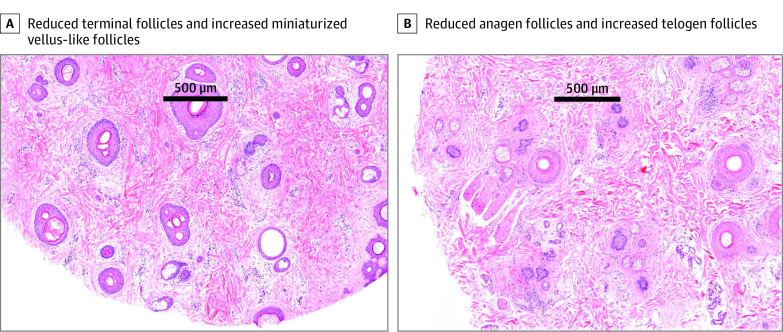
Eighteen patients had scalp biopsies. Fourteen patients had nonscarring alopecia detected on biopsy specimens, but hair counts were not reported in 3 cases (eTable 2 in the Supplement). All 14 cases showed androgenetic alopecia (AGA)–like features with decreased terminal hairs (mean [SD] density, 19 [8] hairs/mm2), increased vellus hairs (mean [SD] terminal to vellus ratio, 1.78 [0.72]), and fibrous streamers (Figure 2). Two patients had cicatricial alopecia, and 2 patients had features of both cicatricial alopecia and AGA. Additional features included increased telogen hairs (mean [SD] anagen to telogen ratio, 3.70 [4.19]), mild perifollicular inflammation (n = 3), fibrosis (n = 2), and reduced sebaceous glands (n = 2). Four patients with suspected lichen planopilaris or fibrosing alopecia in a pattern distribution had supporting features detected on biopsy specimens, with 3 showing active inflammation (lymphocytic infiltrate around the distal hair follicle [HF]). Frontal fibrosing alopecia was diagnosed clinically in 2 cases.
Figure 2. Horizontal Sections of Scalp Biopsies of Patients With Persistent Chemotherapy-Induced Alopecia.
A, Reduced terminal follicles and increased miniaturized, vellus-like follicles. B, Reduced anagen follicles and increased telogen follicles (hematoxylin-eosin, original magnification ×40).
Forty-nine patients (49%) completed 6 months or more of treatment and were eligible for assessment of effectiveness. An increase in hair density was observed after a mean (SD) treatment duration of 25 (24) months (median Sinclair grade, 4 [IQR, 3-5] before treatment vs 3 [IQR, 2-4] after treatment; P < .001) (Figure 3). This statistically significant difference in pretreatment and posttreatment Sinclair grades was maintained in the following 3 subgroups: topical minoxidil (2% or 5% concentration), LDOM monotherapy (0.5-10 mg/d), and LDOM combined with an antiandrogen (spironolactone [25-100 mg/d], flutamide [50-75 mg/d], or bicalutamide [10 mg/d]) (Table 2).
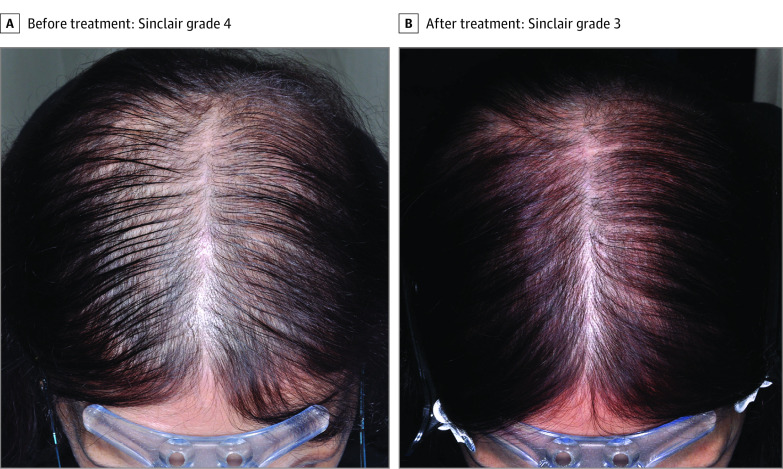
Figure 3. Persistent Chemotherapy-Induced Alopecia Before and After Treatment.
A, Persistent chemotherapy-induced alopecia in a female pattern hair loss distribution in a 58-year-old woman, Sinclair grade 4, before treatment with low-dose oral minoxidil, spironolactone, and flutamide. B, Significant hair regrowth to Sinclair grade 3 after 12 months of treatment.
Table 2. Pretreatment and Posttreatment Sinclair Grades for Patients With Persistent Chemotherapy-Induced Alopecia (DA and FPHL Only) Who Received 6 Months or More of Treatment.
| Treatment | No. of patients | Mean (SD) duration of treatment, mo | Sinclair grade treatment, median (IQR) | P value | |
|---|---|---|---|---|---|
| Before | After | ||||
| Topical minoxidil | 21 | 17 (17) | 4 (3-5) | 3 (2.5-4) | .002 |
| LDOM | 18 | 29 (28) | 4 (2-5) | 2.5 (2-4) | .002 |
| LDOM and antiandrogena | 10 | 33 (25) | 5 (2-5) | 3 (2-5) | .03 |
| Any treatment | 49 | 25 (24) | 4 (3-5) | 3 (2-4) | <.001 |
Abbreviations: DA, diffuse alopecia; FPHL, female pattern hair loss; LDOM, low-dose oral minoxidil.
Antiandrogen: spironolactone, flutamide, or bicalutamide.
Other treatment modalities included platelet-rich plasma injections (n = 2; ineffective) and hair transplant (n = 1). Four of 6 patients who developed scarring alopecia were treated with a superpotent topical corticosteroid with or without doxycycline. Eleven patients with eyebrow hypotrichosis were managed with bimatoprost, 0.03%, an ophthalmic solution applied directly to the affected areas. Of those, eyebrow hair density improved in 2 patients, 2 patients had no response, and the remainder were lost to follow-up.
Of the 6 patients who had scalp cooling, 5 presented with hair loss in an FPHL distribution (median Sinclair grade, 3 [IQR, 2-3]), whereas 1 developed lichen planopilaris. One patient with FPHL experienced an improvement in hair density (from Sinclair grade 2 to 1) after 13 months of LDOM therapy, whereas another had no change in hair density (Sinclair grade 2) after 6 months of LDOM therapy.
Adverse effects were uncommon and generally mild. One patient reported hypertrichosis with topical minoxidil. Adverse events from LDOM included hypertrichosis (n = 14), fluid retention (n = 3), postural hypotension (n = 2), palpitations (n = 1), dyspnea (n = 1), and chest pain (n = 1) and were managed with dose reduction or treatment discontinuation in 3 cases. Of the patients receiving spironolactone, 1 reported lethargy, resulting in cessation, and another experienced a transient elevation in liver transaminases. Flutamide treatment was stopped in 1 patient owing to nausea and abdominal pain. No adverse effects of bicalutamide were reported.
Discussion
Long-standing alopecia after chemotherapy has been reported in 14% of childhood cancer survivors and 10% to 30% of adult breast cancer survivors.15,23 Persistent hair loss in the setting of breast cancer has been reported mostly after taxane-based and cyclophosphamide-based chemotherapy.12,16,23 Unfortunately, pCIA is rarely included as an adverse event in clinical trials and is commonly underrecognized by oncologists.16,23
Hair loss is a common adverse effect of chemotherapy because these agents target not only rapidly dividing neoplastic cells but also highly proliferative matrix keratinocytes in anagen hair bulbs. Murine models for CIA using cyclophosphamide have identified 2 distinct dose-dependent pathways of chemotherapy-induced HF damage. Mild-to-moderate HF damage (usually with a lower dose of chemotherapy) is associated with dystrophic anagen, where the hair is shed and the HF undergoes primary recovery in the same anagen phase with generation of a defective hair shaft. The HF subsequently progresses through catagen and telogen to enter secondary recovery, during which a new normal anagen hair is generated. If the HF encounters a more severe insult (eg, a higher dose of chemotherapy), it undergoes dystrophic catagen, with abrupt anagen arrest and premature catagen, followed by an abnormally shortened telogen phase. In dystrophic catagen, the HF immediately progresses to secondary recovery by premature induction of a new anagen phase. Consequently, the dystrophic catagen response results in a more rapid recovery than dystrophic anagen.24,25,26 In humans, anagen effluvium begins shortly after initiation of chemotherapy, although a proportion of HFs undergo anagen-to-telogen conversion with subsequent shedding (ie, telogen effluvium).27 The shedding pattern may be determined by the cycle stage of individual HFs when the insult occurs.27
In most patients, hair fully regrows after chemotherapy. However, the persistent hair loss seen in patients with pCIA may be explained by irreversible damage to HF stem cells (HFSCs), which are slow-cycling quiescent cells usually resistant to chemotherapy.3,6,8,10,14,18,26 Although, to our knowledge, HFSC damage has not yet been documented in pCIA samples, normal human HFs cultured ex vivo with docetaxel or paclitaxel show massive mitotic defects and apoptosis in not only bulb matrix keratinocytes but also in bulge stem cells that express keratin 15, suggesting that taxanes have the potential to inflict HFSC damage.28 Furthermore, a recent genetic association study identified a variant located in the ABCB1 gene in patients with persistent alopecia after docetaxel-based chemotherapy for breast cancer.29 The ABCB1 gene, which is expressed in human HFSCs,30 encodes the membrane transporter P-glycoprotein, also known as multidrug resistance protein 1, which mediates the efflux of anticancer drugs and other xenobiotics from cells.31 Thus, the genetic variant identified by Núñez-Torres et al29 may result in impaired elimination of taxanes by HFSCs and, consequently, their intracellular accumulation. It has been shown that cumulative doses of docetaxel greater than 400 mg/m2 are more likely to be associated with severe alopecia requiring a wig 18 months after chemotherapy.15 These data suggest that exposure to a high concentration of taxanes in carriers of the risk allele may result in HFSC destruction and, therefore, persistent hair loss.29
Another theory proposed to explain pCIA is that chemotherapy “unmasks” previously unrecognized FPHL. Against this hypothesis is that most of the present cohort did not report premorbid alopecia, with many in fact reporting high prechemotherapy hair densities. However, objective premorbid hair density data are currently lacking to support or refute this theory.
Our findings are similar to those reported by Freites-Martinez et al,32 who identified an AGA-like pattern in 59% of patients with pCIA and DA in 41% of patients with pCIA. The inclusion of taxanes in adjuvant chemotherapy regimens was associated with statistically more severe alopecia in the present patient cohort. Although pCIA is known to mimic FPHL phenotypically in a significant number of breast cancer survivors, trichoscopic features have rarely been described.18,23 The present study found that trichoscopic features of pCIA were indistinguishable from those of AGA, including hair shaft diameter variability, increased vellus hairs, and predominant single-hair follicular units. The histologic features of pCIA presenting as nonscarring hair loss were similar to those described in AGA, with prominent HF miniaturization, characterized by a reduced terminal to vellus ratio. Furthermore, we noted significantly increased telogen follicles, with a reduction in the anagen to telogen ratio similar to that described in 2 previous studies.11,33
The predominant phenotypes of pCIA show prominent vellus hairs both clinically and histologically, suggesting that terminal HFs undergo miniaturization. The heterogeneity in fiber size between different HFs, but not within individual hair fibers, suggests that miniaturization occurs abruptly during the transition between hair cycle stages, rather than during the anagen phase.34 Theoretically, as proposed in AGA, the ability of HFSCs to give rise to progenitor cells may be impaired either directly or indirectly through reduced dermal papilla signaling during the telogen-to-anagen transition.34,35,36 Furthermore, paclitaxel induces apoptosis of dermal papilla cells (whose number correlates with the size and shape of the hair shaft)34,35 in a concentration-dependent manner, suggesting a potential mechanism for pCIA-associated miniaturization.37
A few patients presented with inflammatory scarring alopecia with clinical and histologic features supporting the diagnosis of lichen planopilaris or its variants (frontal fibrosing alopecia and fibrosing alopecia in a pattern distribution). Collapse of the HF bulge immune privilege and TH1-biased inflammation resulting in epithelial HFSC destruction and epithelial-mesenchymal transition of bulge cells are fundamental to lichen planopilaris pathogenesis.38 It is not inconceivable that chemotherapy-induced HFSC damage could trigger these processes in a small number of susceptible individuals.
Evidence for therapeutic agents that stimulate hair growth in pCIA is lacking, to our knowledge. Moderate to significant improvement has been demonstrated in up to 67% of women with pCIA treated with topical minoxidil or spironolactone.23,32 Low-dose oral minoxidil has recently emerged as an effective and safe treatment for AGA.39,40,41,42 In a large study of LDOM safety, systemic adverse effects occurred in only 5.5% of patients; in all cases, these adverse effects were minor and resolved with dose adjustment or treatment discontinuation.43 However, to our knowledge, there is only 1 report of cosmetically significant regrowth in a breast cancer survivor with persistent alopecia treated with LDOM.19 In the present case series, an improvement in hair density was observed in most of the patients treated with topical minoxidil or LDOM, with a more favorable outcome seen with LDOM with or without antiandrogens. One major benefit of the present study is the sensitivity and consistency of the grading scales used. As with FPHL, it is likely that the improvement in hair density is mainly due to early stimulation of telogen or kenogen HFs to reinitiate anagen rather than reversal of miniaturization.44 Despite a hypothetical risk of stimulation of hormone receptor–positive tumors, spironolactone has not been associated with increased recurrence in breast cancer survivors.45 In the present case series, topical prostaglandins, which have been used successfully to treat idiopathic eyebrow hypotrichosis,46,47 promoted regrowth in a number of patients with persistent chemotherapy-induced eyebrow hair loss.
Limitations
The present study has certain limitations, including the retrospective design, relatively small sample size, and selected nature of the study population, limiting the generalizability of our findings. Furthermore, we cannot be certain that the patients had normal prechemotherapy hair densities. Accurately phenotyping patients with breast cancer prior to chemotherapy is challenging because it would require assessment after the cancer diagnosis and before the first course of chemotherapy—a small time window of approximately 2 weeks. Therefore, we suggest that the next step in pCIA research should be a prospective study in which a baseline evaluation of hair density with supporting trichoscopy is performed among patients awaiting chemotherapy. Finally, it needs to be acknowledged that most of the patients in this case series received endocrine therapy, which is independently associated with hair loss. However, several observations suggest that chemotherapy was the predominant cause of hair loss in our cohort, namely: (1) there was no statistically significant difference in the pattern or severity of alopecia between patients who were treated with endocrine therapy and those who were not; (2) 26% of patients had hormone receptor–negative breast cancer and therefore did not receive tamoxifen or aromatase inhibitors but still developed pCIA; (3) endocrine therapy–induced alopecia typically develops 16.8 months after treatment initiation, whereas in our case series, patients’ hair never recovered completely after chemotherapy; and (4) a significant proportion of patients (39%) had DA, which is atypical for endocrine therapy–induced alopecia.48,49
Conclusions
To our knowledge, the present study represents the largest series of breast cancer survivors with pCIA with clinical, trichoscopic, and histopathologic characteristics and evaluation of therapeutic outcomes. Our findings suggest that pCIA is more severe in patients who receive a taxane as part of their chemotherapy regimen. A significant proportion of this cohort experienced an improvement in hair density with treatment, suggesting that pCIA may be at least partly reversible. We suggest that pCIA should be renamed “persistent CIA,” rather than “permanent CIA,” to reflect these findings. Ultimately preventing HF damage in the first place, through the wider use of hair-protective strategies (eg, scalp hypothermia) and the development of novel approaches (eg, cyclin-dependent kinase 4/6 inhibitors),28 holds the most promise for curtailing this distressing adverse effect of chemotherapy.
eTable 1. Clinical Features of 100 Patients With Persistent Chemotherapy-Induced Alopecia
eTable 2. Hair Counts Assessed in Horizontal Sections of 4-mm Punch Biopsies of the Scalp in Patients With Persistent Chemotherapy-Induced Alopecia
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.McPherson K, Steel CM, Dixon JM. ABC of breast diseases: breast cancer—epidemiology, risk factors, and genetics. BMJ. 2000;321(7261):624-628. doi: 10.1136/bmj.321.7261.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubio-Gonzalez B, Juhász M, Fortman J, Mesinkovska NA. Pathogenesis and treatment options for chemotherapy-induced alopecia: a systematic review. Int J Dermatol. 2018;57(12):1417-1424. doi: 10.1111/ijd.13906 [DOI] [PubMed] [Google Scholar]
- 4.Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A. Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2007;11(40):1-144. doi: 10.3310/hta11400 [DOI] [PubMed] [Google Scholar]
- 5.Marks DH, Qureshi A, Friedman A. Evaluation of prevention interventions for taxane-induced dermatologic adverse events: a systematic review. JAMA Dermatol. 2018;154(12):1465-1472. doi: 10.1001/jamadermatol.2018.3465 [DOI] [PubMed] [Google Scholar]
- 6.Choi M, Kim MS, Park SY, et al. Clinical characteristics of chemotherapy-induced alopecia in childhood. J Am Acad Dermatol. 2014;70(3):499-505. doi: 10.1016/j.jaad.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 7.Chon SY, Champion RW, Geddes ER, Rashid RM. Chemotherapy-induced alopecia. J Am Acad Dermatol. 2012;67(1):e37-e47. doi: 10.1016/j.jaad.2011.02.026 [DOI] [PubMed] [Google Scholar]
- 8.Freites-Martinez A, Shapiro J, Goldfarb S, et al. Hair disorders in patients with cancer. J Am Acad Dermatol. 2019;80(5):1179-1196. doi: 10.1016/j.jaad.2018.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24(4):432-442. doi: 10.1111/j.1529-8019.2011.01430.x [DOI] [PubMed] [Google Scholar]
- 10.Champagne C, Taylor M, Farrant P. Permanent chemotherapy-induced nonscarring alopecia and premature ovarian failure. Clin Exp Dermatol. 2015;40(5):589-590. doi: 10.1111/ced.12596 [DOI] [PubMed] [Google Scholar]
- 11.Fonia A, Cota C, Setterfield JF, Goldberg LJ, Fenton DA, Stefanato CM. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76(5):948-957. doi: 10.1016/j.jaad.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 12.Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study. Oncologist. 2019;24(3):414-420. doi: 10.1634/theoncologist.2018-0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim GM, Kim S, Park HS, et al. Chemotherapy-induced irreversible alopecia in early breast cancer patients. Breast Cancer Res Treat. 2017;163(3):527-533. doi: 10.1007/s10549-017-4204-x [DOI] [PubMed] [Google Scholar]
- 14.Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23(11):2879-2884. doi: 10.1093/annonc/mds095 [DOI] [PubMed] [Google Scholar]
- 15.Martín M, de la Torre-Montero JC, López-Tarruella S, et al. Persistent major alopecia following adjuvant docetaxel for breast cancer: incidence, characteristics, and prevention with scalp cooling. Breast Cancer Res Treat. 2018;171(3):627-634. doi: 10.1007/s10549-018-4855-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palamaras I, Misciali C, Vincenzi C, Robles WS, Tosti A. Permanent chemotherapy-induced alopecia: a review. J Am Acad Dermatol. 2011;64(3):604-606. doi: 10.1016/j.jaad.2010.03.020 [DOI] [PubMed] [Google Scholar]
- 17.Prevezas C, Matard B, Pinquier L, Reygagne P. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160(4):883-885. doi: 10.1111/j.1365-2133.2009.09043.x [DOI] [PubMed] [Google Scholar]
- 18.Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63(2):333-336. doi: 10.1016/j.jaad.2009.06.063 [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57(4):e130-e132. doi: 10.1111/ajd.12350 [DOI] [PubMed] [Google Scholar]
- 20.Basilio FM, Brenner FM, Werner B, Rastelli GJ. Clinical and histological study of permanent alopecia after bone marrow transplantation. An Bras Dermatol. 2015;90(6):814-821. doi: 10.1590/abd1806-4841.20154013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair R, Jolley D, Mallari R, Magee J. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51(2):189-199. doi: 10.1016/S0190-9622(03)00045-8 [DOI] [PubMed] [Google Scholar]
- 22.Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68(11):1359-1365. doi: 10.1097/00007611-197511000-00009 [DOI] [PubMed] [Google Scholar]
- 23.Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80(5):1199-1213. doi: 10.1016/j.jaad.2018.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodó E, Tobin DJ, Kamenisch Y, et al. Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am J Pathol. 2007;171(4):1153-1167. doi: 10.2353/ajpath.2007.061164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paus R, Handjiski B, Eichmüller S, Czarnetzki BM. Chemotherapy-induced alopecia in mice: induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol. 1994;144(4):719-734. [PMC free article] [PubMed] [Google Scholar]
- 26.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14(2):e50-e59. doi: 10.1016/S1470-2045(12)70553-3 [DOI] [PubMed] [Google Scholar]
- 27.Kanti V, Nuwayhid R, Lindner J, et al. Analysis of quantitative changes in hair growth during treatment with chemotherapy or tamoxifen in patients with breast cancer: a cohort study. Br J Dermatol. 2014;170(3):643-650. doi: 10.1111/bjd.12716 [DOI] [PubMed] [Google Scholar]
- 28.Purba TS, Ng’andu K, Brunken L, et al. CDK4/6 inhibition mitigates stem cell damage in a novel model for taxane-induced alopecia. EMBO Mol Med. 2019;11(10):e11031. doi: 10.15252/emmm.201911031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Núñez-Torres R, Martín M, García-Sáenz JÁ, et al. Association between ABCB1 genetic variants and persistent chemotherapy-induced alopecia in women with breast cancer. JAMA Dermatol. 2020;156(9):987-991. doi: 10.1001/jamadermatol.2020.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haslam IS, El-Chami C, Faruqi H, Shahmalak A, O’Neill CA, Paus R. Differential expression and functionality of ATP-binding cassette transporters in the human hair follicle. Br J Dermatol. 2015;172(6):1562-1572. doi: 10.1111/bjd.13549 [DOI] [PubMed] [Google Scholar]
- 31.Gregers J, Gréen H, Christensen IJ, et al. Polymorphisms in the ABCB1 gene and effect on outcome and toxicity in childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2015;15(4):372-379. doi: 10.1038/tpj.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155(6):724-728. doi: 10.1001/jamadermatol.2018.5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miteva M, Misciali C, Fanti PA, Vincenzi C, Romanelli P, Tosti A. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33(4):345-350. doi: 10.1097/DAD.0b013e3181fcfc25 [DOI] [PubMed] [Google Scholar]
- 34.Pantelireis N, Higgins CA. A bald statement—current approaches to manipulate miniaturisation focus only on promoting hair growth. Exp Dermatol. 2018;27(9):959-965. doi: 10.1111/exd.13690 [DOI] [PubMed] [Google Scholar]
- 35.Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140(8):1676-1683. doi: 10.1242/dev.090662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garza LA, Yang CC, Zhao T, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121(2):613-622. doi: 10.1172/JCI44478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen PH, Wang CY, Hsia CW, et al. Impact of taxol on dermal papilla cells—a proteomics and bioinformatics analysis. J Proteomics. 2011;74(12):2760-2773. doi: 10.1016/j.jprot.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 38.Harries MJ, Jimenez F, Izeta A, et al. Lichen planopilaris and frontal fibrosing alopecia as model epithelial stem cell diseases. Trends Mol Med. 2018;24(5):435-448. doi: 10.1016/j.molmed.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 39.Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019;81(2):648-649. doi: 10.1016/j.jaad.2019.04.054 [DOI] [PubMed] [Google Scholar]
- 40.Ramos PM, Sinclair RD, Kasprzak M, Miot HA. Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: a randomized clinical trial. J Am Acad Dermatol. 2020;82(1):252-253. doi: 10.1016/j.jaad.2019.08.060 [DOI] [PubMed] [Google Scholar]
- 41.Sharma AN, Michelle L, Juhasz M, Muller Ramos P, Atanaskova Mesinkovska N. Low-dose oral minoxidil as treatment for non-scarring alopecia: a systematic review. Int J Dermatol. 2020;59(8):1013-1019. doi: 10.1111/ijd.14933 [DOI] [PubMed] [Google Scholar]
- 42.Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57(1):104-109. doi: 10.1111/ijd.13838 [DOI] [PubMed] [Google Scholar]
- 43.Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84(6):1644-1651. doi: 10.1016/j.jaad.2021.02.054 [DOI] [PubMed] [Google Scholar]
- 44.Hugh Rushton D, Norris MJ, Van Neste D. Hair regrowth in male and female pattern hair loss does not involve the conversion of vellus hair to terminal hair. Exp Dermatol. 2016;25(6):482-484. doi: 10.1111/exd.12945 [DOI] [PubMed] [Google Scholar]
- 45.Wei C, Bovonratwet P, Gu A, Moawad G, Silverberg JI, Friedman AJ. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83(4):1021-1027. doi: 10.1016/j.jaad.2020.05.081 [DOI] [PubMed] [Google Scholar]
- 46.Carruthers J, Beer K, Carruthers A, et al. Bimatoprost 0.03% for the treatment of eyebrow hypotrichosis. Dermatol Surg. 2016;42(5):608-617. doi: 10.1097/DSS.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suwanchatchai W, Tanglertsampan C, Pengsalae N, Makornwattana M. Efficacy and safety of bimatoprost 0.03% versus minoxidil 3% in enhancement of eyebrows: a randomized, double-blind, split-face comparative study. J Dermatol. 2012;39(10):865-866. doi: 10.1111/j.1346-8138.2012.01579.x [DOI] [PubMed] [Google Scholar]
- 48.Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy–induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154(6):670-675. doi: 10.1001/jamadermatol.2018.0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saggar V, Wu S, Dickler MN, Lacouture ME. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18(10):1126-1134. doi: 10.1634/theoncologist.2013-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Clinical Features of 100 Patients With Persistent Chemotherapy-Induced Alopecia
eTable 2. Hair Counts Assessed in Horizontal Sections of 4-mm Punch Biopsies of the Scalp in Patients With Persistent Chemotherapy-Induced Alopecia