Key Points
Question
Does the combination of vasopressin and methylprednisolone administered during in-hospital cardiac arrest improve return of spontaneous circulation?
Findings
In this randomized trial that included 501 patients with in-hospital cardiac arrest in Denmark, the proportion of patients who achieved return of spontaneous circulation was 42% in the vasopressin and methylprednisolone group and 33% in the placebo group, a difference that was statistically significant.
Meaning
Among patients with in-hospital cardiac arrest, administration of vasopressin and methylprednisolone compared with placebo significantly increased the likelihood of return of spontaneous circulation, but it is uncertain whether there is benefit or harm for long-term survival.
Abstract
Importance
Previous trials have suggested that vasopressin and methylprednisolone administered during in-hospital cardiac arrest might improve outcomes.
Objective
To determine whether the combination of vasopressin and methylprednisolone administered during in-hospital cardiac arrest improves return of spontaneous circulation.
Design, Setting, and Participants
Multicenter, randomized, double-blind, placebo-controlled trial conducted at 10 hospitals in Denmark. A total of 512 adult patients with in-hospital cardiac arrest were included between October 15, 2018, and January 21, 2021. The last 90-day follow-up was on April 21, 2021.
Intervention
Patients were randomized to receive a combination of vasopressin and methylprednisolone (n = 245) or placebo (n = 267). The first dose of vasopressin (20 IU) and methylprednisolone (40 mg), or corresponding placebo, was administered after the first dose of epinephrine. Additional doses of vasopressin or corresponding placebo were administered after each additional dose of epinephrine for a maximum of 4 doses.
Main Outcomes and Measures
The primary outcome was return of spontaneous circulation. Secondary outcomes included survival and favorable neurologic outcome at 30 days (Cerebral Performance Category score of 1 or 2).
Results
Among 512 patients who were randomized, 501 met all inclusion and no exclusion criteria and were included in the analysis (mean [SD] age, 71 [13] years; 322 men [64%]). One hundred of 237 patients (42%) in the vasopressin and methylprednisolone group and 86 of 264 patients (33%) in the placebo group achieved return of spontaneous circulation (risk ratio, 1.30 [95% CI, 1.03-1.63]; risk difference, 9.6% [95% CI, 1.1%-18.0%]; P = .03). At 30 days, 23 patients (9.7%) in the intervention group and 31 patients (12%) in the placebo group were alive (risk ratio, 0.83 [95% CI, 0.50-1.37]; risk difference: −2.0% [95% CI, −7.5% to 3.5%]; P = .48). A favorable neurologic outcome was observed in 18 patients (7.6%) in the intervention group and 20 patients (7.6%) in the placebo group at 30 days (risk ratio, 1.00 [95% CI, 0.55-1.83]; risk difference, 0.0% [95% CI, −4.7% to 4.9%]; P > .99). In patients with return of spontaneous circulation, hyperglycemia occurred in 77 (77%) in the intervention group and 63 (73%) in the placebo group. Hypernatremia occurred in 28 (28%) and 27 (31%), in the intervention and placebo groups, respectively.
Conclusions and Relevance
Among patients with in-hospital cardiac arrest, administration of vasopressin and methylprednisolone, compared with placebo, significantly increased the likelihood of return of spontaneous circulation. However, there is uncertainty whether this treatment results in benefit or harm for long-term survival.
Trial Registration
ClinicalTrials.gov Identifier: NCT03640949
This randomized trial compares the effectiveness of the combination of vasopressin and methylprednisolone vs placebo administered during in-hospital cardiac arrest on return of spontaneous circulation.
Introduction
In-hospital cardiac arrest occurs in approximately 2000 patients each year in Denmark and 300 000 patients each year in the United States.1,2 Outcomes remain poor, with only approximately 25% surviving to hospital discharge in 2017 in the United States.3 Despite this low survival, there has been limited research focused on improving outcomes for this patient population.3
Similar to the out-of-hospital setting, treatment of in-hospital cardiac arrest focuses on early recognition, basic life support (eg, chest compressions and ventilations), advanced life support (eg, defibrillation and drugs), and subsequent post–cardiac arrest care. Most recommendations for treatment of in-hospital cardiac arrest are extrapolated from the out-of-hospital setting. Drugs currently used during in-hospital cardiac arrest, when appropriate, includes epinephrine and amiodarone or lidocaine.4,5
In 2 randomized, double-blind trials, published in 2009 and 2013, Mentzelopoulos et al6,7 compared the addition of vasopressin (20 IU for each dose of epinephrine) and 1 dose of glucocorticoids (40 mg of methylprednisolone) during cardiac arrest with placebo. Both trials, which had a combined sample size of 368 patients, showed a large improvement in outcomes.6,7 For example, the most recent and largest of the trials found that survival with a favorable neurologic outcome occurred in 18 of 130 patients (14%) in the intervention group compared with 7 of 138 patients (5%) in the placebo group, a finding that was statistically significant.7 Despite these findings, the current United States and European guidelines8,9 for treatment of cardiac arrest do not recommend the use of vasopressin and glucocorticoids, reflecting lack of clinical trial data that confirmed the findings of Mentzelopoulos et al.
The Vasopressin and Methylprednisolone for In-Hospital Cardiac Arrest (VAM-IHCA) trial was designed to test whether vasopressin and glucocorticoids can improve return of spontaneous circulation for patients with in-hospital cardiac arrest.
Methods
Trial Design and Oversight
This trial was an investigator-initiated, multicenter, randomized, placebo-controlled, parallel group, double-blind, superiority trial of vasopressin and methylprednisolone during adult in-hospital cardiac arrest.10 The protocol, which is provided in Supplement 1, was written by the steering committee and approved by the regional ethics committee and the Danish Medicines Agency. Minor differences between the article and the protocol are described in eAppendix 1 in Supplement 2. An independent data monitoring committee oversaw the trial. Oral and subsequent written informed consent was temporarily obtained from a physician independent of the trial until the patient regained capacity or a surrogate became available according to Danish legislation (additional details are provided in the protocol in Supplement 1). Patients or surrogates provided consent for all patients who survived.
Patients
Patients were included from 10 hospitals in Denmark, including 4 large university hospitals. Adult patients (age ≥18 years) were eligible for the trial if they had an in-hospital cardiac arrest and received at least 1 dose of epinephrine during the cardiac arrest. Patients with a cardiac arrest that started outside the hospital were not included. Exclusion criteria included a clearly documented do-not-resuscitate order prior to the cardiac arrest, prior enrollment in the trial, invasive mechanical circulatory support (extracorporeal circulation or left ventricular assist device) at the time of the cardiac arrest, and known or suspected pregnancy at the time of the cardiac arrest.
Randomization
The study kits, and therefore the patients, were randomized in a 1:1 ratio via a random number generator to either vasopressin and methylprednisolone or placebo in blocks with random sizes of 2, 4, or 6. The randomization was stratified according to site.
Intervention
The trial drugs consisted of 40 mg of methylprednisolone (Solu-Medrol, Pfizer) and 20 IU of vasopressin (Empressin, Amomed Pharma GmbH) given as soon as possible after the first dose of epinephrine. Additional doses of vasopressin (20 IU) were administered after each epinephrine dose for a maximum of 4 doses (80 IU). Placebo consisted of 9 mg/mL of sodium chloride from identical ampoules. The trial drugs were placed in a blinded study kit, which was brought to the cardiac arrest by a dedicated member of the clinical cardiac arrest team. The trial was double-blind, with patients, investigators, clinicians, and outcome assessors unaware of the allocated treatment.
Outcomes
The primary outcome was return of spontaneous circulation, which was defined as spontaneous circulation with no further need for chest compressions sustained for at least 20 minutes.11
Key secondary outcomes included survival at 30 days and survival at 30 days with a favorable neurologic outcome, which was defined as a Cerebral Performance Category score of 1 or 2. The Cerebral Performance Category score is a 5-point scale assessing neurologic outcomes after brain damage, with higher scores indicating worse outcomes.12 Additional outcomes, as described below, were considered tertiary outcomes.
Neurologic outcome was also assessed using the modified Rankin Scale, which is a 7-point scale, with higher scores indicating worse outcomes.13 A score of 0 to 3 was considered a favorable outcome. At 30 days, health-related quality of life was assessed using the EuroQol 5 Dimension 5 Level (EQ-5D-5L) and indexed based on Danish data.14,15 The results from the EQ-5D-5L are reported both as the numeric value directly assessed by the patient and as the indexed value. The numeric value is reported on a scale from 0 to 100, with higher scores indicating a better health-related quality of life, while the indexed value can also be negative. Outcomes were assessed in person if the patient was still in the hospital and otherwise by a telephone interview. If the patient was not able to participate, a relative or clinical personnel provided the assessment. Similar outcomes were assessed at 90 days, 180 days, and 1 year. Results for the 30- and 90-day follow-up are provided here, while data on longer-term outcomes are still being collected.
As a measure of organ dysfunction after return of spontaneous circulation, we collected data on the Sequential Organ Failure Assessment (SOFA) score at 24, 48, and 72 hours after the cardiac arrest as well as vasopressor- and ventilator-free days within the first 14 days. The SOFA score ranges from 0 to 24, with higher scores indicating worse organ dysfunction.16 Predefined potential adverse events, including hyperglycemia, hypernatremia, infections, gastrointestinal bleeding, and mesenteric and peripheral ischemia, were also collected, with a full list and definitions provided in the protocol in Supplement 1.
Sample Size Calculation
The sample size was based on the primary outcome of return of spontaneous circulation. Based on unpublished preliminary data from the participating hospitals, we assumed that 45% of patients in the placebo group would achieve return of spontaneous circulation. We assumed an absolute difference of 13% between the placebo and intervention group, corresponding to 58% of patients achieving return of spontaneous circulation in the intervention group. This effect estimate is consistent with the Mentzelopoulos et al6,7 trials. With these estimates, an α of .05, and the use of the χ2 test, a total of 492 patients were required to have 80% power to detect a statistically significant difference between groups.
Statistical Analysis
Patients were analyzed according to their randomized assignment. The analyses only included patients receiving the first dose of either of the trial drugs and meeting all inclusion criteria and no exclusion criteria.17
Binary data are presented as counts and percentages, and differences between groups are presented as both risk differences and risk ratios with 95% CIs. CIs were estimated using the method described by Miettinen and Nurmimen.18 Continuous data are presented as means with SDs or medians with first and third quartiles, depending on the distribution of the data. Differences between groups in continuous outcomes are presented as mean differences with 95% CIs obtained from generalized linear models with robust errors. As a sensitivity analysis, the risk ratio for the primary outcome was estimated while adjusting for site and strong prognostic factors, specifically age, whether the cardiac arrest was witnessed, and the initial rhythm, as covariates.19,20 Modified Poisson regression was used for this analysis.21
Two-sided P values, obtained from Fisher exact test, are reported for the primary and key secondary outcomes. A P value of less than .05 was considered significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes should be interpreted as exploratory. Five subgroup analyses, all prespecified in the protocol, were performed according to the first documented rhythm, witnessed status, patient age, time from cardiac arrest to trial drug administration, and time from epinephrine administration to administration of the trial drug.
All analyses were performed in SAS version 9.4 (SAS Institute).
Results
Patient Characteristics
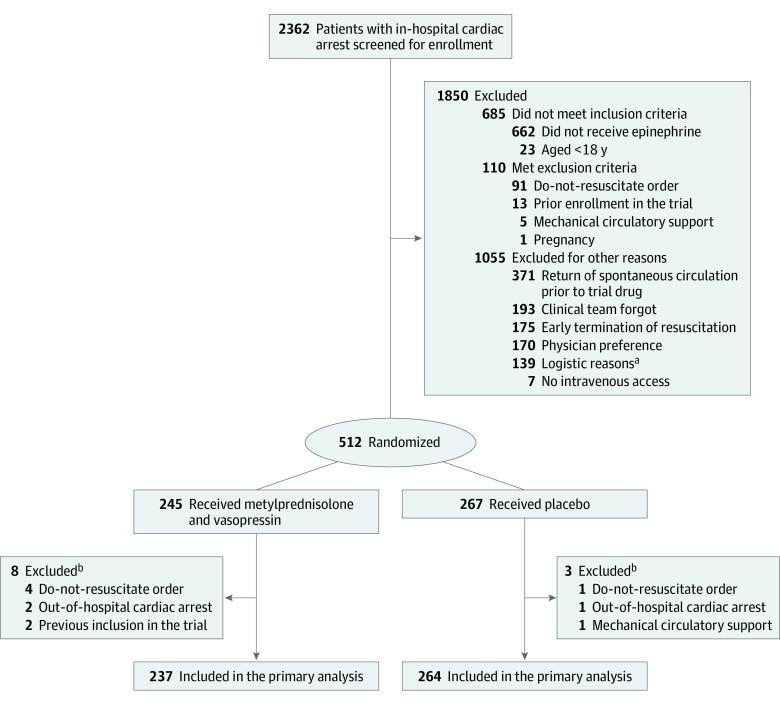
From October 15, 2018, to January 21, 2021, 512 patients were randomized and received the trial drugs (Figure 1; eTable 1 in Supplement 2), with the last 90-day follow-up occurring on April 21, 2021. Eleven patients were excluded because they did not meet all inclusion criteria or met at least 1 exclusion criterion, leaving 501 patients for the analysis (237 in the intervention group and 264 in the placebo group). There was no loss to follow-up.
Figure 1. Screening and Randomization in the VAM-IHCA Trial of Methylprednisolone and Vasopressin for In-Hospital Cardiac Arrest.
aLogistic reasons included not enough personnel (n = 61), no study drug available (n = 45), inability to obtain surrogate consent (n = 1), and other (n = 32), which included patients isolated with COVID-19.
bPatients who were excluded after receiving the trial drugs had inclusion/exclusion criteria not known at the time of the cardiac arrest.
Baseline characteristics were similar between the 2 groups (Table 1; eTable 2 in Supplement 2). The mean (SD) age was 71 (13) years and 322 (64%) were men. Most of the cardiac arrests (66%) occurred among patients who were receiving care in standard medical or surgical units and presented with an initial nonshockable rhythm (90%). The median time from the cardiac arrest to epinephrine and trial drug administration was 5 minutes (first and third quartiles: 3, 8) and 8 minutes (first and third quartiles: 6, 12), respectively. Non–trial-related interventions during and after the cardiac arrest were generally similar between groups (eTables 3 and 4 in Supplement 2). There were 14 patients (3%) who did not receive the methylprednisolone/placebo trial drug but received vasopressin/placebo and 8 patients (2%) who did not receive the vasopressin/placebo trial drug but received methylprednisolone/placebo (eTable 5 in Supplement 2). Of those receiving vasopressin/placebo, 139 (28%), 145 (29%), 81 (16%), and 128 (26%) received 1, 2, 3, and 4 doses, respectively (eTable 5 in Supplement 2).
Table 1. Baseline Characteristics According to Treatment Assignmenta.
| Characteristic | No. (%) | |
|---|---|---|
| Vasopressin and methylprednisolone (n = 237) | Placebo (n = 264) | |
| Patient characteristics | ||
| Age, y | 71 (13) | 70 (12) |
| Sex | ||
| Male | 148 (62) | 174 (66) |
| Female | 89 (38) | 90 (34) |
| BMIb | 26 (23-31) | 26 (23-31) |
| Medical historyc | ||
| Arterial hypertension | 148 (62) | 167 (63) |
| Coronary artery disease | 76 (32) | 92 (35) |
| Atrial fibrillation | 69 (29) | 66 (25) |
| Diabetes | 69 (29) | 78 (30) |
| Pulmonary disease | 67 (28) | 82 (31) |
| Cancer | 55 (23) | 49 (19) |
| Kidney disease | 54 (23) | 49 (19) |
| Chronic heart failure | 47 (20) | 56 (21) |
| Stroke | 46 (19) | 40 (15) |
| Venous thromboembolism | 15 (6) | 14 (5) |
| Liver disease | 8 (3) | 11 (4) |
| Dementia | 5 (2) | 3 (1) |
| Any glucocorticoids prior to hospital admission | 34 (14) | 30 (11) |
| Interventions prior to cardiac arrest | ||
| Kidney replacement therapy | 25 (11) | 20 (8) |
| Invasive mechanical ventilation | 20 (8) | 30 (11) |
| Vasopressor infusion | 13 (5) | 23 (9) |
| Cardiac arrest characteristics | ||
| Location | ||
| Hospital ward | 163 (69) | 168 (64) |
| Intensive care unit | 23 (10) | 18 (7) |
| Emergency department | 19 (8) | 38 (14) |
| Cardiac catherization laboratory | 12 (5) | 23 (9) |
| Operating room | 4 (2) | 3 (1) |
| Otherd | 16 (7) | 14 (5) |
| Monitored | 87 (37) | 121 (46) |
| Witnessed | 168 (71) | 202 (77) |
| Initial rhythm | ||
| Pulseless electrical activity | 134 (57) | 138 (52) |
| Asystole | 82 (35) | 95 (36) |
| Ventricular fibrillation | 17 (7) | 22 (8) |
| Ventricular tachycardia | 4 (2) | 9 (3) |
| Time from cardiac arrest recognition to | ||
| Epinephrine administration, min | 5 (3-7) | 5 (3-8) |
| Drug administration, min | 8 (6-12) | 9 (6-12) |
Abbreviation: BMI, body mass index.
Continuous variables are presented as means with SDs or medians with first and third quartiles and categorical variables as counts and percentages.
Data not available on 13 patients in the intervention group and 10 patients in the placebo group. Calculated as weight in kilograms divided by height in meters squared.
Definitions are provided in eAppendix 2 in Supplement 2. Medical history was based on review of the electronical medical record.
Other includes multiple different locations including the radiology department, the dialysis department, the psychiatric department, and outside departments (eg, hospital entrance).
Primary Outcome
There were 100 patients (42%) in the intervention group and 86 patients (33%) in the placebo group who achieved return of spontaneous circulation, corresponding to a risk ratio of 1.30 (95% CI, 1.03-1.63; risk difference, 9.6% [95% CI, 1.1%-18.0%]; P = .03; Table 2). The risk ratio was slightly higher when adjusting for site and prognostic factors (risk ratio, 1.38 [95% CI, 1.10-1.72]). Results were generally consistent across predefined subgroups (Figure 2). The median time to return of spontaneous circulation was 16 minutes (first and third quartiles: 12, 25) in the intervention group and 18 minutes (first and third quartiles: 11, 31) in the placebo group.
Table 2. Outcomes According to Treatment Assignmenta.
| Vasopressin and methylprednisolone (n = 237) | Placebo (n = 264) | Difference, % (95% CI)b | Risk ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Return of spontaneous circulation | 100 (42) | 86 (33) | 9.6 (1.1 to 18.0) | 1.30 (1.03 to 1.63) | .03 |
| Secondary outcomes | |||||
| 30-d Outcomes | |||||
| Survival | 23 (9.7) | 31 (12) | −2.0 (−7.5 to 3.5) | 0.83 (0.50 to 1.37) | .48 |
| Favorable neurologic outcome (CPC 1-2)c | 18 (7.6) | 20 (7.6) | 0.0 (−4.7 to 4.9) | 1.00 (0.55 to 1.83) | >.99 |
| Favorable neurologic outcome (mRS 0-3)d | 11 (4.6) | 19 (7.2) | −2.6 (−6.9 to 1.7) | 0.64 (0.32 to 1.31) | |
| EQ-5D-5Le | 62 (15) | 56 (23) | 6 (−4 to 17) | ||
| EQ-5D-5L–Indexe | 45 (37) | 40 (33) | 5 (−14 to 24) | ||
| 90-d Outcomes | |||||
| Survival | 20 (8.4) | 24 (9.1) | −0.7 (−5.7 to 4.5) | 0.93 (0.53 to 1.62) | |
| Favorable neurologic outcome (CPC 1-2)c | 18 (7.6) | 20 (7.6) | 0.0 (−4.7 to 4.9) | 1.00 (0.55 to 1.83) | |
| Favorable neurologic outcome (mRS 0-3)d | 15 (6.3) | 20 (7.6) | −1.3 (−5.8 to 3.4) | 0.84 (0.44 to 1.58) | |
| EQ-5D-5Le | 70 (18) | 69 (18) | 1 (−9 to 11) | ||
| EQ-5D-5L–indexe | 69 (32) | 72 (26) | −3 (−20 to 14) | ||
Abbreviations: CPC, Cerebral Performance Category; EQ-5D-5L, EuroQol 5 Dimension 5 Level; mRS, modified Rankin Scale.
Continuous variables are presented as means with SDs and categorical variables as counts and percentages.
Risk difference for binary outcomes and mean difference for continuous outcomes.
Cerebral Performance Category is a 5-point scale assessing neurologic outcomes after brain damage, with higher scores indicating worse outcomes. A score of 1 or 2 is considered a favorable outcome.
The mRS is a 7-point scale with higher scores indicating worse outcomes. A score of 0 to 3 is considered a favorable outcome.
The results from the EQ-5D-5L are reported both as the numeric value directly assessed by the patient and as the indexed value. The numeric value is reported on a scale from 0 to 100, with higher scores indicating a better health-related quality of life, while the indexed value can also be negative.
Figure 2. Subgroups Results for Return of Spontaneous Circulation.
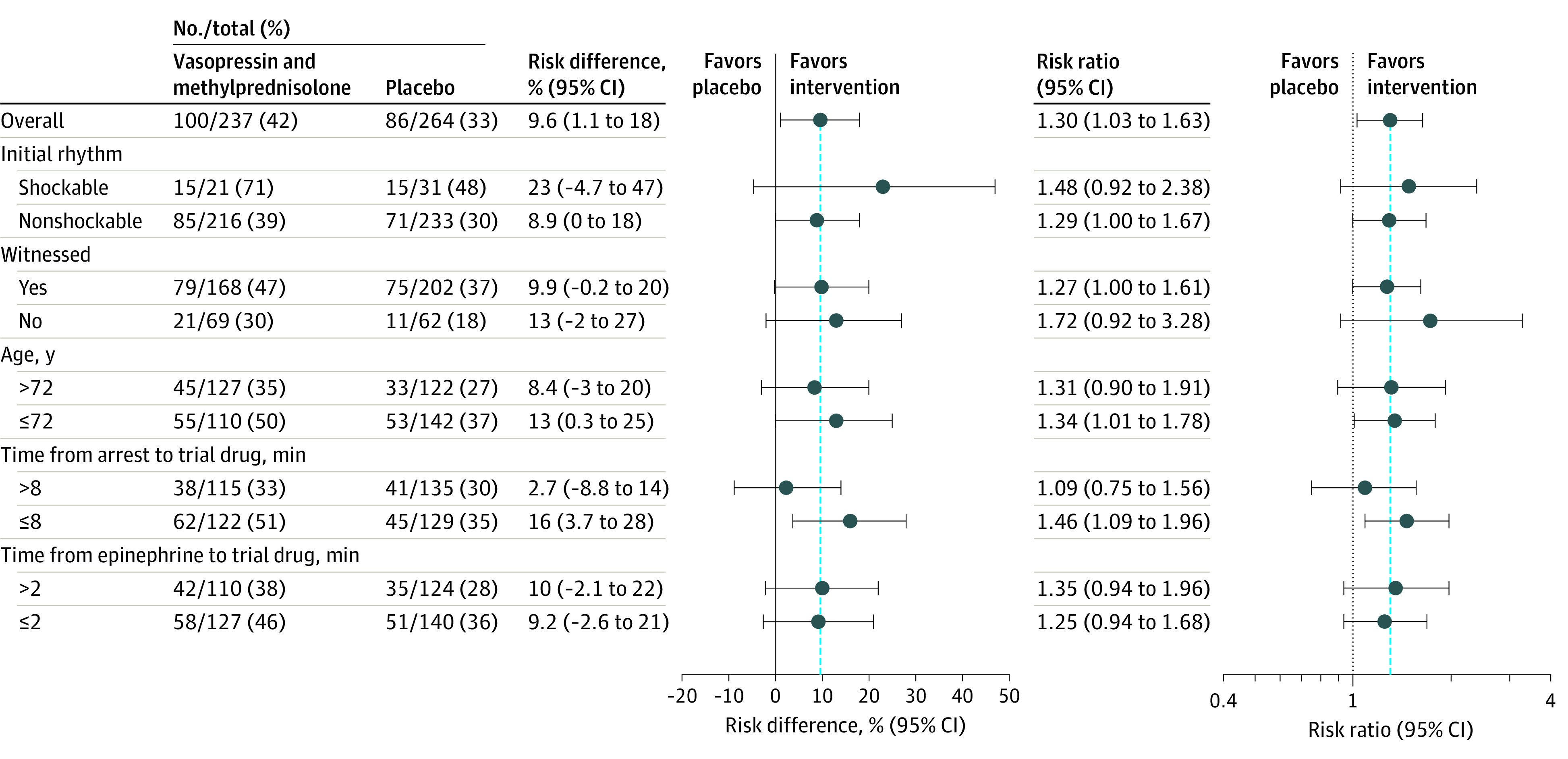
Subgroup results are presented for 5 predefined subgroups. Continuous variables were dichotomized at the median. The time of the cardiac arrest corresponds to the recognition of the cardiac arrest. The blue dashed lines indicate overall effect.
Secondary Outcomes
At 30 days, 23 patients (9.7%) in the intervention group and 31 patients (12%) in the placebo group were alive (risk ratio, 0.83 [95% CI, 0.50-1.37]; risk difference, −2.0% [95% CI, −7.5% to 3.5%]; P = .48). A favorable neurologic outcome, based on the Cerebral Performance Category score, was observed in 18 patients (7.6%) in the intervention group and 20 patients (7.6%) in the placebo group at 30 days (risk ratio, 1.00 [95% CI, 0.55-1.83]; risk difference, 0.0% [95% CI, −4.7% to 4.9%]; P > .99). Results for survival and favorable neurologic outcome were generally consistent across predefined subgroups (eFigures 1 and 2 in Supplement 2).
Tertiary Outcomes
A favorable neurologic outcome at 30 days, based on the modified Rankin Scale score, was observed in 11 patients (4.6%) in the intervention group and 19 patients (7.2%) in the placebo group (risk ratio, 0.64 [95% CI, 0.32-1.31]; risk difference, −2.6% [95% CI, −6.9% to 1.7%]). Health-related quality of life did not differ between groups at 30 days (Table 2).
Outcomes at 90 days are presented in Table 2 and eFigure 3 in Supplement 2 and showed no statistically significant difference between groups.
Post–cardiac arrest organ dysfunction, as assessed by the SOFA score, were not statistically significantly different between groups, as were the number of vasopressor- and ventilator-free days (eTable 6 in Supplement 2). Additional details about the outcomes are reported in eTables 7, 8, and 9 in Supplement 2.
Adverse Events
Predefined potential adverse events are reported in eTable 10 in Supplement 2. In patients with return of spontaneous circulation, hyperglycemia occurred in 77 (77%) in the intervention group and 63 (73%) in the placebo group. Hypernatremia occurred in 28 (28%) and 27 (31%) in the intervention and placebo groups, respectively.
Discussion
In this trial, the combination of vasopressin and methylprednisolone compared with placebo administered during in-hospital cardiac arrest resulted in a statistically significant improvement in the primary outcome of return of spontaneous circulation. There were no differences in the secondary outcomes including survival and favorable neurologic outcome at 30 and 90 days.
The administration of vasopressin, which is also known as antidiuretic hormone, results in vasoconstriction. Because of this effect, vasopressin has been recommended as a second-line vasoactive agent in the setting of septic shock.22 Vasoconstriction is also of interest in relation to cardiac arrest, because an increase in arterial blood pressure may increase the coronary perfusion pressure and thereby the chance of return of spontaneous circulation,23,24 a prerequisite for longer-term survival. Despite these potential beneficial effects, trials of vasopressin in primarily out-of-hospital cardiac arrest have failed to show an improvement in outcomes, perhaps partly due to the late administration of drugs in this setting.25 Corticosteroids, another drug often used in the setting of septic shock due to a reduction in vasopressor requirements,26 exerts a wide range of functions in the body, including regulation of metabolism, inflammation, and cell proliferation. Studies in patients with cardiac arrest have demonstrated that levels of cortisol are higher in patients who have been resuscitated when compared with patients who have not been resuscitated,27 which may illustrate an impaired endocrine response in nonsurvivors. This is supported by animal studies where the administration of hydrocortisone during cardiac arrest increased return of spontaneous circulation.28 Data on glucocorticoid administration during human cardiac arrest are limited, and small studies have shown conflicting results.29
In the 2 trials by Mentzelopoulos et al,6,7 the investigators found improvements in return of spontaneous circulation as well as an improvement in survival to hospital discharge. The current trial found an improvement in return of spontaneous circulation with a risk ratio of 1.30, which is consistent with the previous trials’ findings.6,7 However, contrary to the previous trials, there was no improvement in survival. In the current trial, the point estimate for survival suggested harm, while the confidence included both clinically relevant harm and benefit. There are a number of possible explanations for the difference in results between the current and the previous trials. First, the previous trials included the administration of post–cardiac arrest hydrocortisone to patients with circulatory shock in the intervention group.6,7 Second, in the previous trials, research personnel administered the trial interventions, whereas it was administered by the clinical personnel in the current trial. Thus, while the current approach is more consistent with clinical practice, it might have resulted in a delay in the administration of the trial drugs compared with the previous trials. Third, there are important differences in patient characteristics. Patients in the previous trials were younger and more often had a cardiac arrest that was witnessed, occurring in the intensive care unit, and with an initial rhythm of asystole.6,7 Survival in the control group was higher in the current trial despite a lower proportion of patients with return of spontaneous circulation.6,7
Trials within cardiac arrest have found that intracardiac arrest pharmacological interventions can increase return of spontaneous circulation with little or no clear improvement in long-term outcomes.4,5 In the current trial, there was an absolute increase of 9.6% in return of spontaneous circulation. Other than epinephrine, this effect is larger than what previously has been shown for any other pharmacological intracardiac arrest intervention. Return of spontaneous circulation is the principal goal of cardiopulmonary resuscitation and a prerequisite for longer-term survival. The mechanistic goal of vasopressin and methylprednisolone is to increase return of spontaneous circulation and it is possible that other interventions, including post–cardiac arrest interventions, are needed to translate this effect into improvements in longer-term outcomes.
The current trial has strengths. Within the context of in-hospital cardiac arrest,3 the trial was large and included more patients than the 2 previous trials combined. The trial was multicenter and included hospitals of various sizes. Long-term outcomes, including quality of life, were obtained from all patients with no loss to follow-up.
Limitations
The trial has several limitations. First, a large proportion of patients, who were potentially eligible, were not included (Figure 1). While this has no influence on the trial’s internal validity, it could affect generalizability.
Second, while the median time to drug delivery was only 8 minutes, the drug was delivered relatively late in some patients. This could influence the results but is likely a reflection of clinical practice.
Third, the trial was powered to the primary outcome of return of spontaneous circulation. Given the much lower proportion of patients with survival and favorable neurologic outcome, the trial was not powered for these outcomes. The results cannot exclude potential benefit or harm of the intervention on these outcomes.
Fourth, while overall survival might appear low in the current trial (8%-9%), this is likely a reflection of the inclusion criteria, which required administration of at least 1 dose of epinephrine. Outcomes for cardiac arrest in Denmark are generally favorable compared with other countries.2,3
Conclusions
Among patients with in-hospital cardiac arrest, administration of vasopressin and methylprednisolone, compared with placebo, significantly increased the likelihood of return of spontaneous circulation. However, there is uncertainty whether this treatment results in benefit or harm for long-term survival.
Trial Protocol
eAppendix 1. Discrepancies Between the Manuscript and the Protocol
eAppendix 2. Definitions of Past Medical History
eFigure 1. Subgroup Results for 30-Day Survival
eFigure 2. Subgroup Results for 30-Day Favorable Neurologic Outcome
eFigure 3. Kaplan-Meier Curve for 90-Day Survival According to Treatment Assignment
eTable 1. Inclusions per Site
eTable 2. Additional Baseline Characteristics According to Treatment Assignment
eTable 3. Cardiac Arrest Interventions
eTable 4. Post-Cardiac Arrest Characteristics in Those Surviving at Least 24 Hours
eTable 5. Trial Drug and Protocol Deviations
eTable 6. Organ Dysfunction After Return of Spontaneous Circulation
eTable 7. Hospital Disposition and Cause of Death
eTable 8. Neurologic Outcomes
eTable 9. EQ-5D-5L Subcategories
eTable 10. Pre-defined Potential Adverse Events
Data Sharing Statement
References
- 1.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation Investigators . Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12(7):e005580. doi: 10.1161/CIRCOUTCOMES.119.005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen LW, Holmberg MJ, Løfgren B, Kirkegaard H, Granfeldt A. Adult in-hospital cardiac arrest in Denmark. Resuscitation. 2019;140:31-36. doi: 10.1016/j.resuscitation.2019.04.046 [DOI] [PubMed] [Google Scholar]
- 3.Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: a review. JAMA. 2019;321(12):1200-1210. doi: 10.1001/jama.2019.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins GD, Ji C, Deakin CD, et al. ; PARAMEDIC2 Collaborators . A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379(8):711-721. doi: 10.1056/NEJMoa1806842 [DOI] [PubMed] [Google Scholar]
- 5.Kudenchuk PJ, Brown SP, Daya M, et al. ; Resuscitation Outcomes Consortium Investigators . Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374(18):1711-1722. doi: 10.1056/NEJMoa1514204 [DOI] [PubMed] [Google Scholar]
- 6.Mentzelopoulos SD, Zakynthinos SG, Tzoufi M, et al. Vasopressin, epinephrine, and corticosteroids for in-hospital cardiac arrest. Arch Intern Med. 2009;169(1):15-24. doi: 10.1001/archinternmed.2008.509 [DOI] [PubMed] [Google Scholar]
- 7.Mentzelopoulos SD, Malachias S, Chamos C, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310(3):270-279. doi: 10.1001/jama.2013.7832 [DOI] [PubMed] [Google Scholar]
- 8.Panchal AR, Bartos JA, Cabanas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366-S468. [DOI] [PubMed] [Google Scholar]
- 9.Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council Guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115-151. doi: 10.1016/j.resuscitation.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 10.Andersen L, Sindberg B, Holmberg M, et al. Vasopressin and Methylprednisolone for In-Hospital Cardiac Arrest: Protocol for a Randomized, Double-Blind, Placebo-Controlled Trial. Resuscitation Plus; 2021;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan JP, Berg RA, Andersen LW, et al. ; Utstein Collaborators . Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: a consensus report from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Resuscitation. 2019;144:166-177. doi: 10.1016/j.resuscitation.2019.08.021 [DOI] [PubMed] [Google Scholar]
- 12.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480-484. doi: 10.1016/S0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 13.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607. doi: 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 14.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen CE, Sørensen SS, Gudex C, Jensen MB, Pedersen KM, Ehlers LH. The Danish EQ-5D-5L value set: a hybrid model using cTTO and DCE data. Appl Health Econ Health Policy. 2021;19(4):579-591. doi: 10.1007/s40258-021-00639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 17.Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652-654. doi: 10.1136/bmj.325.7365.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213-226. doi: 10.1002/sim.4780040211 [DOI] [PubMed] [Google Scholar]
- 19.Fernando SM, Tran A, Cheng W, et al. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: systematic review and meta-analysis. BMJ. 2019;367:l6373. doi: 10.1136/bmj.l6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome: when, why, and how? BMC Med Res Methodol. 2014;14:20. doi: 10.1186/1471-2288-14-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 22.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486-552. doi: 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 23.Miano TA, Crouch MA. Evolving role of vasopressin in the treatment of cardiac arrest. Pharmacotherapy. 2006;26(6):828-839. doi: 10.1592/phco.26.6.828 [DOI] [PubMed] [Google Scholar]
- 24.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106-1113. doi: 10.1001/jama.1990.03440080084029 [DOI] [PubMed] [Google Scholar]
- 25.Mentzelopoulos SD, Zakynthinos SG, Siempos I, Malachias S, Ulmer H, Wenzel V. Vasopressin for cardiac arrest: meta-analysis of randomized controlled trials. Resuscitation. 2012;83(1):32-39. doi: 10.1016/j.resuscitation.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 26.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. Cochrane Database Syst Rev. 2015;(12):CD002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindner KH, Strohmenger HU, Ensinger H, Hetzel WD, Ahnefeld FW, Georgieff M. Stress hormone response during and after cardiopulmonary resuscitation. Anesthesiology. 1992;77(4):662-668. doi: 10.1097/00000542-199210000-00008 [DOI] [PubMed] [Google Scholar]
- 28.Smithline H, Rivers E, Appleton T, Nowak R. Corticosteroid supplementation during cardiac arrest in rats. Resuscitation. 1993;25(3):257-264. doi: 10.1016/0300-9572(93)90123-8 [DOI] [PubMed] [Google Scholar]
- 29.Varvarousi G, Stefaniotou A, Varvaroussis D, Xanthos T. Glucocorticoids as an emerging pharmacologic agent for cardiopulmonary resuscitation. Cardiovasc Drugs Ther. 2014;28(5):477-488. doi: 10.1007/s10557-014-6547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Discrepancies Between the Manuscript and the Protocol
eAppendix 2. Definitions of Past Medical History
eFigure 1. Subgroup Results for 30-Day Survival
eFigure 2. Subgroup Results for 30-Day Favorable Neurologic Outcome
eFigure 3. Kaplan-Meier Curve for 90-Day Survival According to Treatment Assignment
eTable 1. Inclusions per Site
eTable 2. Additional Baseline Characteristics According to Treatment Assignment
eTable 3. Cardiac Arrest Interventions
eTable 4. Post-Cardiac Arrest Characteristics in Those Surviving at Least 24 Hours
eTable 5. Trial Drug and Protocol Deviations
eTable 6. Organ Dysfunction After Return of Spontaneous Circulation
eTable 7. Hospital Disposition and Cause of Death
eTable 8. Neurologic Outcomes
eTable 9. EQ-5D-5L Subcategories
eTable 10. Pre-defined Potential Adverse Events
Data Sharing Statement