Abstract
Aim:
Atherosclerosis involves vascular endothelial damage and lipid metabolism disorder, which is closely related to the occurrence and development of diabetic kidney disease (DKD). However, studies on non-high albuminuria DKD (NHADKD) with an albumin to creatinine ratio (ACR) <30 mg/g are rare. This study is to investigate the relationship between atherogenic factors and the occurrence of NHADKD.
Methods:
Serum lipid indicators, lipoprotein-associated phospholipase A2 (Lip-PLA2) and homocysteine levels were measured in 1116 subjects to analyze their relationship with NHADKD.
Results:
Among all subjects, Lip-PLA2 had the closest but relatively weak correlation with ACR (r = 0.297, p < 0.001) and only homocysteine was moderately correlated with eGFR (r = −0.465, p < 0.001). However, in patients with NHADKD, these atherosclerotic factors were weakly correlated or uncorrelated with eGFR (max. |r| = 0.247). Stratified risk analysis showed that when ACR was <10 mg/g, homocysteine [OR = 6.97(4.07–11.95)], total cholesterol (total-Chol) [OR = 6.04(3.03–12.04)], and high-density lipoprotein cholesterol (HDL-Chol) [OR = 5.09(2.99–8.64)] were risk factors for NHADKD. There was no significant difference of OR between these three factors (Z = 0.430–1.044, all p > 0.05). When ACR was ⩾10mg/g, homocysteine [OR = 17.26(9.67–30.82)] and total-Chol [OR = 5.63(2.95–10.76)] were risk factors for NHADKD, and ORhomocysteine was significantly higher than ORtotal-Chol (Z = 3.023, p < 0.05).
Conclusions:
The occurrence of NHADKD may be related to the levels of homocysteine, total-Chol, HDL-Chol, and Lip-PLA2 in blood. Among them, homocysteine may be most closely related to NHADKD.
Keywords: On-high albuminuria diabetic kidney disease, dyslipidemia, homocysteine, lipoprotein-associated phospholipase A2
Introduction
Diabetic kidney disease (DKD) is the most common microvascular complication in patients with diabetes mellitus (DM) and one of the major causes of mortality in DM patients.1 According to the American Diabetes Association guidelines, the albumin to creatinine ratio (ACR) and/or estimated glomerular filtration rate (eGFR) should be regularly detected in DM patients, in order to confirm whether kidney injury occurs.2 In general, there is albuminuria and/or eGFR decline during and after kidney injury in DM patients, and in most patients, albuminuria present earlier than eGFR decline.3 Therefore, albuminuria is used as the preferred indicator for screening kidney injury in DM patients. According to the Clinical Practice Guideline of Chronic Kidney Disease (CKD) in Kidney Disease: Improving Global Outcomes (KDIGO) 2012 (hereinafter referred to as 2012 CKD Guideline),4 when the ACR of DM patients is ⩾30 mg/g for more than 3 months, kidney function injury can be confirmed. However, as early as 1994, Tsalamandris et al.5 had found that some DKD patients showed normal albuminuria but only kidney dysfunction with GFR decline. For the first time, they proposed the concept of diabetes nephropathy without albuminuria, which is now often referred to as normoalbuminuric diabetic kidney disease (NADKD).
In the past, NADKD was defined as ACR < 30 mg/g or albumin excretion rate (AER) < 30 mg/day. In 2009, the National Kidney Foundation (NKF) and the US Food and Drug Administration (FDA) redefined albuminuria into four levels6: (1) very high albuminuria (ACR > 300 mg/g) (formerly termed macroalbuminuria); (2) high albuminuria (ACR between 30 and 300 mg/g) (formerly termed microalbuminuria); (3) low albuminuria (ACR between 10 and 29 mg/g) (formerly termed normoalbuminuria); (4) normoalbuminuria (ACR < 10 mg/g). According to this latest definition, the term NADKD actually contains both low albuminuria (ACR 10–29 mg/g) and normoalbuminuria (ACR < 10 mg/g). We suppose the term non-high albuminuria DKD (NHADKD) is more accurate for cases with ACR < 30 mg/g or AER < 30 mg/day. Thus, we used the new term NHADKD in this study.
Based on current studies on DM patients, the incidence of NHADKD varies greatly in different regions from 20% to 50%,7–9 and occurs more frequently in type 2 DM,10 women,10,11 and the elderly.11,12 However, the pathogenesis of NHADKD has not been fully clarified so far. Previous studies have confirmed that the occurrence and development of DKD is closely related to dyslipidemia.13 Due to the structural abnormalities and / or dysfunction of insulin in DM patients, dyslipidemia is very common.14 At present, the blood lipid indicators used to observe or detect dyslipidemia in clinically mainly include triglyceride, total cholesterol (total-Chol), high-density lipoprotein cholesterol (HDL-Chol), low-density lipoprotein cholesterol (LDL-Chol), apolipoprotein A1 (ApoA1) and apolipoprotein B (ApoB). These lipid indicators were commonly used to assess the risk of atherogenesis.15 In addition, homocysteine and lipoprotein-associated phospholipase A2 (Lip-PLA2) are emerging indicators for evaluating atherosclerosis in recent years, and can both reflect changes in atherogenic vascular damage and inflammation, and could aggravate lipid metabolism.15 These indicators have been included as the evaluation items of blood lipid health and risk of cardiovascular disease in the American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease.16 Studies have also shown that the occurrence of DKD is partly due to an increase in atherosclerotic lipid components during lipid metabolism, which results in kidney microvascular lesions.14 It has been found that the increase of plasma Lip-PLA2 level is related to occurrence and development of DKD.17 The occurrence of NHADKD may be also related to abnormal lipid metabolism, vascular damage and inflammation.
Therefore, this study investigated the relationship among blood lipid indicators, Lip-PLA2, homocysteine and the occurrence of NHADKD in DM patients. The possible predictive value of lipid metabolism indicators, Lip-PLA2 and homocysteine in NHADKD of DM was also analyzed and discussed.
Methods and materials
Ethics
This study was approved by the Ethics Committee of Mianyang Central Hospital (approval no. S2014048 and S2018085) (Supplement 1 and 2). All subjects signed informed consent.
Subjects
All patients were from Endocrinology Department of Mianyang Central Hospital DM from January 2017 to December 2018. According to the 2012 CKD Guideline, a total of 2287 patients with a history of DM more than 5 years were follow up with monthly ACR and eGFR levels for three consecutive months. Among them, 915 patients with ACR < 30 mg/g for three consecutive months were included in this study, including 38 patients with type 1 DM and 877 patients with type 2 DM. According to the eGFR results, this group of DM patients was subdivided into simple DM patients (simple DM group, n = 751, eGFR > 60 ml/min/1.73 m2) and NHADKD patients (NHADKD group, n = 164, eGFR ⩽ 60 ml/min/1.73 m2).
Inclusion criteria for simple DM patients: (1) they were diagnosed with DM according to the American Diabetes Association (ADA) “Standards of Medical Care in Diabetes,”18,19 as well as with fasting glucose ⩾ 7.0 mmol/L or HbA1c ⩾ 6.5% or random glucose ⩾ 11.1 mmol/L or 2 h glucose ⩾ 11.1 mmol/L during oral glucose tolerance test; (2) during the regular follow-up, there were no three consecutive eGFR ⩽ 60 ml/min/1.73 m2; (3) ACR < 30 mg/g; (4) they were without any microvascular complications, which were diagnosed according to Foot Care of Standards of Medical Care in Diabetes.2,18
Inclusion criteria for NHADKD patients: (1) the diagnosis of DM was the same as that of simple DM; (2) ACR < 30mg/g; (3) during the regular follow-up, eGFR was ⩽60 ml/min/1.73 m2 for three consecutive times.
Exclusion criteria: (1) DM patients with a history of less than 5 years; (2) ACR ⩾ 30 mg/g; (3) patients treated with potentially nephrotoxic drugs or with diseases that may affect urinary albumin secretion and GFR; (4) patients with known non-DKD kidney disease; (5) menstrual women as well as pregnant women were also excluded.
Meanwhile, 201 healthy controls (Control group) with normal liver and kidney function and negative results for blood and urine routine tests were selected by random sampling. Their alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were both <40 U/L. Creatinine (Cr) was <97 μmol/L for males or <71 μmol/L for females. Cystatin C (CysC) was <1.09 mg/L, eGFR was >60 ml/min/1.73 m2, ACR was <10 mg/g, and fasting glucose was <6.0 mmol/L.
Sample collection
Blood samples (5 mL) were collected from each subject. After centrifugation at 1400 × g for 10 min, the serum was isolated. Urine samples were also collected.
Determination of serum kidney function and atherogenic indicators
Serum Cr was measured with sarcosine oxidase method; triglyceride was measured with glycerol phosphate oxidase method; total-Chol was measured with cholesterol oxidase method; Lip-PLA2 was measured with 4-nitrophenol formation rate method; homocysteine was measured with methyltransferases coupling adenosylhomocysteinase cycle method; CysC, HDL-Chol, LDL-Chol, ApoA1 and ApoB were measured with immunoturbidimetric method. The above indexes were measured on the LST008 type biochemical analyzer (HITACHI, Japan). All reagents were provided by Sichuan Maker Biotechnology Co., Ltd., China. According to the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) guidelines for management of dyslipidemia,15,16 the blood lipid was defined as abnormal when total-Chol ⩾ 5.2 mmol/L (200 mg/dL), or triglyceride ⩾ 1.7 mmol/L (150 mg/dL), or HDL-Chol < 1.0 mmol/L (40 mg/dL), or LDL-Chol ⩾ 3.4 mmol/L (130 mg/dL). According to the criteria, patients with dyslipidemia were screened out. eGFR is calculated using the CKD-EPI CysC-Cr equation20: eGFR = 135 × min (Cr/κ, 1)α × max (Cr/κ, 1)−0.601 × min (CysC/0.8, 1)−0.375 × max (CysC/0.8, 1)−0.711 × 0.995Age × 0.969 (if female), where κ = 62 μmol/L (female)/80 μmol/L (male), α = −0.248 (female)/−0.207 (male).
Determination of urine ACR
Urinary albumin and urinary Cr reagents were provided by Chongqing Biostec Co., Ltd, China. A modified immunoturbidimetric method was used for detecting urinary albumin. The sarcosine oxidase method was used to determine urinary Cr. These assays were performed on a type A25 special protein analyzer (BioSystems, Spain). ACR was calculated according to the following formula: ACR (mg/g) = Urinary albumin (mg/L)/Urinary Cr (g/L).
Statistical analysis
Statistical analysis was performed using SPSS 19.0 and MedCalc 18.2. Measurement data of non-normal distribution were expressed as M (P25, P75) and compared using independent sample Kruskal-Wallis test or Jonckheere-Terpstra test and their multiple comparisons method. Count data were expressed as n (%) and were compared using a four-table or contingency table χ2 test. The relationship between atherogenic indicators and ACR or eGFR was analyzed by Spearman correlation. When p < 0.05, the absolute value of the correlation coefficient (|r|) < 0.2 indicates weak correlation; 0.2 ⩽ |r| < 0.4 indicates mild correlation; 0.4 ⩽ |r| < 0.6 indicates moderate correlation; 0.6 ⩽ |r| < 0.8 indicates strong correlation; |r| ⩾ 0.8 indicates extremely strong correlation. Patients were stratified according to the reference upper limit of ACR recommended by NKF and FDA (ACR < 10 mg/g). The abnormal rate of observed atherogenic indicators and the risk of NHADKD were analyzed by cross-table stratified risk analysis. The risk of NHADKD was indicated by odds ratio (OR). Comparison between OR of two paired data was conducted using Z test. The difference was statistically significant with p < 0.05.
Results
Clinical characteristics of subjects
The clinical data of patients were shown in Table 1. There were 164 cases with NHADKD, accounting for 17.9% (164/915) of all DM patients. The ACR distribution and eGFR staging of the simple DM and NHADKD subjects were statistically different (p < 0.001). In the simple DM and NHADKD disease groups, the prevalence of secondary cardiovascular disease, retinopathy, and hypertension was statistically different (all p < 0.001), but the prevalence of dyslipidemia was not statistically different (p > 0.05).
Table 1.
Subject’s basic clinical characteristics.
| Observation indicators | Control (n = 201) | Simple DM (n = 751) | NHADKD (n = 164) | χ2 | p |
|---|---|---|---|---|---|
| Sex (male/female) | 118/83 | 423/328 | 87/77 | 1.177 | 0.555 |
| Age (years) | 50.9 ± 11.1 | 49.3 ± 10.7 | 50.7 ± 11.1 | 2.532 | 0.080 |
| Cardiovascular diseases (n (%)) | — | 121 (16.1) | 65 (39.6) | 44.545 | <0.001 |
| Retinopathy (n (%)) | — | 0 (0.0) | 22 (13.4) | 97.588 | <0.001 |
| Hypertension (n (%)) | — | 56 (7.5) | 33 (20.1) | 23.169 | <0.001 |
| Dyslipidemia (n (%))* | — | 442 (58.9) | 108 (65.8) | 2.466 | 0.116 |
| ACR (mg/gCr) | 6.18 (4.82, 8.34) | 8.86 (6.75, 13.24) | 11.36 (8.18, 14.45) | 154.976 | <0.001 |
| Cr (μmol/L) | 59.3 (51.1, 66.4) | 60.4 (48.0, 72.4) | 93.2 (80.7, 113.2) | 297.835 | <0.001 |
| CysC (mg/L) | 0.81 (0.72, 0.88) | 0.87 (0.77, 1.01) | 1.54 (1.35, 1.71) | 394.405 | <0.001 |
| eGFR (mL/min/1.72 m2) | 91.1 (83.8, 102.2) | 96.5 (80.0, 107.9) | 47.1 (43.3, 56.9) | 390.907 | <0.001 |
| ACR distribution (n (%)) | |||||
| <10 mg/gCr | — | 448 | 69 | 16.909 | <0.001 |
| ⩾10 mg/gCr | — | 303 | 95 | ||
| GFR staging | |||||
| G1 | — | 486 (64.7) | 0 (0.0) | 656.218 | <0.001 |
| G2 | — | 220 (29.3) | 9 (5.5) | ||
| G3a | — | 45 (6.0) | 85 (51.8) | ||
| G3b | — | 0 (0.0) | 62 (37.8) | ||
| G4 | — | 0 (0.0) | 8 (4.9) | ||
Note. The difference in age was compared by one-way ANOVA, and in ACR and eGFR was compared by Kruskal-Wallis test, and for the others by chi-square test.
Simple DM: simple diabetes mellitus (ACR < 30 mg/gCr); NHADKD: non-high albuminuria diabetic kidney disease; ACR: albumin to creatinine ratio; Cr: creatinine; CysC: cystatin C; eGFR: estimated glomerular filtration rate.
Dyslipidemia was defined as any of the total-Chol, triglyceride, HDL-Chol, and/or LDL-Chol level that exceeds the corresponding limit recommended by the AACE 2017 Guidelines.15,16
“—” Means control subjects are not involved.
Level of atherogenic factors of subjects
Jonckheere-Terpstra test and its multiple comparisons (Figure 1) showed that there was no statistical difference among three groups in levels of ApoA1 (z = 0.424, p = 0.672) and ApoB (z = 0.949, p = 0.343), but levels of total-Chol, triglyceride, HDL-Chol, LDL-Chol, homocysteine and Lip-PLA2 were all statistically different (all p < 0.001). Among these atherosclerotic indicators, total-Chol, triglyceride, LDL-Chol, homocysteine, and Lip-PLA2 in the NHADKD group and the simple DM group were significantly higher than those in the Control group (z = 4.449–15.098, all p < 0.001), while HDL -Chol was significantly lower than Control group (z = −7.591 and −4.031, both p < 0.001). There was no statistical difference in triglyceride (z = 1.743, p = 0.122), LDL-Chol (z = −1.001, p = 0.842) and Lip-PLA2 (z = −2.988, p = 0.078) between NHADKD and simple DM groups. However, total-Chol (z = 5.555, p < 0.001) and homocysteine (z = 14.378, p < 0.001) in NHADKD group were significantly higher while HDL-Chol (z = −4.691, p < 0.001) was significantly lower than those in simple DM group. Among the observed atherosclerotic indicators, only the serum levels of total-Chol, HDL-Chol and homocysteine demonstrated significant changes with disease progression (Control→simple DM→NHADKD).
Figure 1.
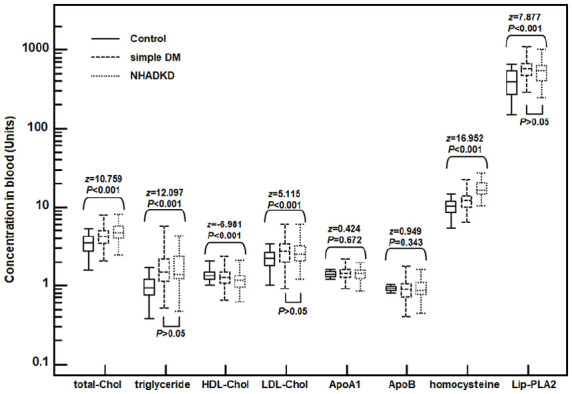
Serum levels of eight common atherogenic factors in patients.
Note. Control: healthy controls; simple DM: simple diabetes mellitus (ACR < 30 mg/g); NHADKD: non-high albuminuria diabetic kidney disease. Units: total-Chol, triglyceride, HDL-Chol, LDL-Chol were measured in mmol/L, ApoA1 and ApoB in g/L, homocysteine in μmol/L, and Lip-PLA2 in U/L. Among these observed atherosclerotic indicators, only the serum total-Chol, HDL-Chol, and homocysteine levels demonstrated significant changes with disease progression (control→simple DM→NHADKD).
Relationship between atherosclerosis index and ACR in all subjects
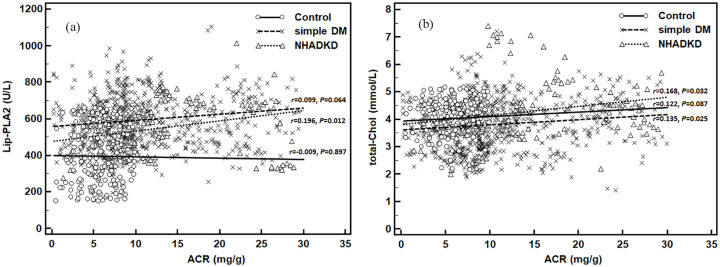
For all subjects, Spearman correlation analysis showed that total-Chol (r = = 0.233, p < 0.001) and Lip-PLA2 (r = 0.297, p < 0.001) had a mild correlation with ACR (Table 2). Triglyceride, LDL-Chol, ApoB, and homocysteine (|r| = 0.106–0.195, all p < 0.05) had a very weak correlation with ACR, while HDL-Chol (r = 0.032, p = 0.290) and ApoA1 (r = 0.044, p = 0.141) were not related with ACR. When we further performed subgroup analysis on factors with |r| > 0.2 (Lip-PLA2 and total-Chol), we found that they all showed a weaker correlation. For Lip-PLA2, only NHADKD had weak positive correlation (r = 0.196, p = 0.012), while control (r = 0.009, p = 0.897) and simple DM (r = 0.099, p = 0.064) had no correlation (Figure 2(a)). For total-Chol, NHADKD (r = 0.168, p = 0.032) and simple DM (r = 0.135, p = 0.025) had weak positive correlation, while Control (r = 0.122, p = 0.087) had no correlation (Figure 2(b)). Partial correlation analysis showed that Lip-PLA2 (rp = 0.199, p < 0.001), total-Chol (rp = 0.128, p < 0.001), triglyceride (rp = 0.086, p = 0.004) and homocysteine (rp = 0.083, p = 0.006) were partial correlated to ACR. So, among the observed atherosclerotic indicators, increased serum Lip-PLA2 level was most closely but only mildly correlated with increased urinary ACR levels.
Table 2.
Correlation between each atherogenic factor and ACR level.
| Observed indicators | r | 95%CI | p |
|---|---|---|---|
| Total-Chol | 0.233 | 0.177 to 0.288 | <0.001 |
| Triglyceride | 0.181 | 0.124 to 0.237 | <0.001 |
| HDL-Chol | 0.032 | −0.027 to 0.092 | 0.290 |
| LDL-Chol | 0.195 | 0.138 to 0.251 | <0.001 |
| ApoA1 | 0.044 | −0.015 to 0.103 | 0.141 |
| ApoB | 0.111 | 0.053 to 0.169 | <0.001 |
| Homocysteine | 0.106 | 0.048 to 0.164 | <0.001 |
| Lip-PLA2 | 0.297 | 0.242 to 0.349 | <0.001 |
Note. Total-Chol: total cholesterol; HDL-Chol: high-density lipoprotein cholesterol; LDL-Chol: low-density lipoprotein cholesterol; ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; Lip-PLA2: lipoprotein-associated phospholipase A2.
Among these observed atherosclerotic indicators, increased serum Lip-PLA2 level was most closely but only mildly correlated with increased urinary ACR levels (r = 0.297, p < 0.001).
Figure 2.
Correlation between Lip-PLA2/total-Chol and ACR levels in different groups.
Note. Control: healthy controls; simple DM: simple diabetes mellitus (ACR < 30 mg/g); NHADKD: non-high albuminuria diabetic kidney disease. (a): Correlation between Lip-PLA2 and ACR; (b): Correlation between total-Chol and ACR. Increased serum Lip-PLA2 or total-Chol level was weakly correlated with increased urinary ACR levels in different groups.
The relationship between the atherosclerosis index and eGFR in all subjects
As analyzed by Spearman correlation, there was a moderate correlation between homocysteine and eGFR (r = −0.465, p < 0.001) (Table 3). Levels of triglyceride, HDL-Chol, LDL-Chol, and Lip-PLA2 (|r| = 0.112–0.147, all p < 0.05) had very weak correlation with eGFR, while total-Chol (r = −0.003, p = 0.931), ApoA1 (r = 0.024, p = 0.423) and ApoB (r = 0.014, p = 0.653) were not related with eGFR. Additionally, homocysteine showed mild correlation with eGFR in simple DM group (r = −0.335, p < 0.001) and in NHADKD group (r = −0.247, p = 0.001) (Figure 3). In the NHADKD group, HDL-Chol (r = −0.227, p = 0.003) and Lip-PLA2 (r = 0.222, p = 0.004) showed a mild correlation with eGFR. Partial correlation analysis showed that all observed indexes were partially correlated to eGFR except triglyceride (rp = −0.018, p = 0.550), and homocysteine had the highest partial correlation coefficient with eGFR (rp = 0.482, p < 0.001). Among the observed atherosclerosis indicators, homocysteine may be an important indicator of kidney function injury in NHADKD patients.
Table 3.
Correlation between each atherogenic factor and eGFR level.
| Observed indicators | r | 95%CI | p |
|---|---|---|---|
| Total-Chol | −0.003 | −0.006 to 0.006 | 0.931 |
| Triglyceride | −0.112 | −0.170 to −0.054 | <0.001 |
| HDL-Chol | 0.120 | 0.006 to 0.178 | <0.001 |
| LDL-Chol | 0.136 | 0.078 to 0.193 | <0.001 |
| ApoA1 | 0.024 | −0.035 to 0.083 | 0.423 |
| ApoB | −0.014 | −0.045 to 0.072 | 0.653 |
| Homocysteine | −0.465 | −0.510 to −0.418 | <0.001 |
| Lip-PLA2 | 0.147 | 0.099 to 0.204 | <0.001 |
Note. total-Chol: total cholesterol; HDL-Chol: high-density lipoprotein cholesterol; LDL-Chol: low-density lipoprotein cholesterol; ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; Lip-PLA2: lipoprotein-associated phospholipase A2.
Among these observed atherosclerotic indicators, homocysteine showed a moderate correlation with eGFR (r = −0.465, p < 0.001) and the others showed a weak correlation with eGFR.
Figure 3.
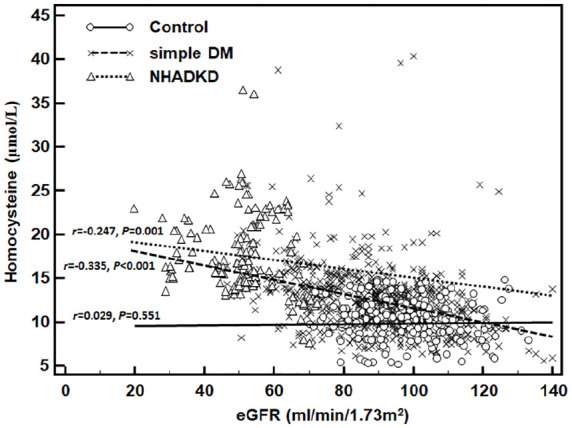
Correlation between homocysteine and eGFR levels in different groups.
Note. Control: healthy controls; simple DM: simple diabetes mellitus (ACR < 30 mg/g); NHADKD: non-high albuminuria diabetic kidney disease. Homocysteine showed a mild correlation with eGFR in simple DM group (r = −0.335, p < 0.001) or in NHADKD group (r = −0.247, p = 0.001).
Stratified risk analysis of atherosclerosis indexes on NHADKD
For all patients (751 simple DM patients and 164 NHADKD patients), if total-Chol ⩾ 5.2 mmol/L, triglyceride ⩾ 1.7 mmol/L, HDL-Chol < 1.0 mmol/L, LDL-Chol ⩾ 3.4 mmol/L, ApoA1 < 1.20 g/L, ApoB > 1.10 g/L, homocysteine ⩾ 15 μmol/L (that is, the level decided by hyperhomocysteinemia21) and Lip-PLA2 ⩾ 659 U/L (that is, the reference upper limit of Lip-PLA2 activity measurement), the levels of corresponding indicators were defined as abnormal results. Then, we used ACR of 10 mg/g (that is, the reference upper limit recommended by KIDGO) as the cutoff point to evaluate the effect of the abnormal rate of each atherosclerosis index on the risk of NHADKD. The results showed that, of the eight atherosclerosis indicators, only the increased total-Chol (OR = 5.80, p < 0.001), decreased HDL-Chol (OR = 2.43, p < 0.001), and increased homocysteine (OR = 11.43, p < 0.001) were the risk factors for all NHADKD patients (Table 4). Then all subjects were stratified with ACR: if ACR < 10 mg/g, the increased total-Chol (OR = 6.04, all p < 0.001) and increased homocysteine (OR = 6.97, p < 0.001) and decreased HDL-Chol (OR = 5.09, p < 0.001) were risk factors for NHADKD, but their OR values were not statistically different between each other (Z = 0.430–1.044, all p > 0.05); if ACR ⩾ 10 mg/g, only increased total-Chol (OR = 5.63, p < 0.001) and increased homocysteine (OR = 17.26, p < 0.001) were risk factors for NHADKD. The ORhomocysteine was significantly higher than ORtotal-Chol (Z = 3.023, p < 0.05). Thus, homocysteine may be a more useful indicator than other atherosclerosis indicators for predicting the occurrence of NHADKD.
Table 4.
Risk of stratification of NHADKD by abnormal rates of eight atherogenic factors.
| Abnormal lipid changes | n/N (%)▲ | Hierarchical risk analysis | ||||
|---|---|---|---|---|---|---|
| Simple DM | NHADKD | OR | 95%CI | z | p | |
| Increased total-Chol | ||||||
| ACR < 10 mg/gCr | 23/448 (5.1) | 17/69 (24.6) | 6.04 | 3.03–12.04 | 5.110 | <0.001 |
| ACR ⩾ 10 mg/gCr | 19/303 (6.3) | 26/95 (27.4) | 5.63 | 2.95–10.76 | 5.233 | <0.001 |
| Total | 42/751 (5.6) | 43/164 (26.2) | 5.80 | 3.61–9.33 | 7.521 | <0.001 |
| Increased triglyceride | ||||||
| ACR < 10 mg/gCr | 202/448 (45.1) | 39/69 (56.5) | 1.58 | 0.95–2.64 | 1.762 | 0.078 |
| ACR ⩾ 10 mg/gCr | 118/303 (38.9) | 34/95 (35.8) | 0.87 | 0.54–1.41 | 0.552 | 0.581 |
| Total | 320/751 (42.6) | 73/164 (44.5) | 1.15 | 0.82–1.63 | 0.446 | 0.656 |
| Decreased HDL-Chol | ||||||
| ACR < 10 mg/gCr | 83/448 (18.5) | 37/69 (53.6) | 5.09 | 2.99–8.64 | 6.016 | <0.001 |
| ACR ⩾ 10 mg/gCr | 39/303 (12.9) | 10/95 (10.5) | 0.80 | 0.38–1.66 | 0.606 | 0.545 |
| Total | 122/751 (16.2) | 47/164 (28.7) | 2.43 | 1.63–3.63 | 3.658 | <0.001 |
| Increased LDL-Chol | ||||||
| ACR < 10 mg/gCr | 89/448 (19.9) | 7/69 (10.1) | 0.46 | 0.20–1.03 | 1.891 | 0.059 |
| ACR ⩾ 10 mg/gCr | 83/303 (27.4) | 20/95 (21.1) | 0.71 | 0.41–1.23 | 1.227 | 0.220 |
| Total | 172/751 (22.9) | 27/164 (16.5) | 0.61 | 0.38–1.01 | 1.802 | 0.072 |
| Decreased ApoA1 | ||||||
| ACR < 10 mg/gCr | 74/448 (16.5) | 11/69 (15.9) | 0.96 | 0.48–1.91 | 0.120 | 0.905 |
| ACR ⩾ 10 mg/gCr | 43/303 (14.2) | 20/95 (21.1) | 1.61 | 0.89–2.91 | 1.589 | 0.112 |
| Total | 117/751 (15.6) | 31/164 (18.9) | 1.55 | 0.99–2.43 | 1.533 | 0.060 |
| Increased ApoB | ||||||
| ACR < 10 mg/gCr | 141/448 (31.5) | 29/69 (42.0) | 1.58 | 0.94–2.65 | 1.727 | 0.084 |
| ACR ⩾ 10 mg/gCr | 127/303 (41.9) | 42/95 (44.2) | 1.10 | 0.69–1.75 | 0.395 | 0.693 |
| Total | 268/751 (35.7) | 71/164 (43.3) | 1.38 | 0.98–1.94 | 1.823 | 0.068 |
| Increased homocysteine | ||||||
| ACR < 10 mg/gCr | 74/448 (16.5) | 40/69 (58.0) | 6.97 | 4.07–11.95 | 7.059 | <0.001 |
| ACR ⩾ 10 mg/gCr | 57/303 (18.8) | 76/95 (80.0) | 17.26 | 9.67–30.82 | 9.636 | <0.001 |
| Total | 131/751 (17.4) | 116/164 (70.7) | 11.43 | 7.78–16.82 | 12.387 | <0.001 |
| Increased Lip-PLA2 | ||||||
| ACR < 10 mg/gCr | 108/448 (24.1) | 10/69 (14.5) | 0.53 | 0.26–1.08 | 1.748 | 0.081 |
| ACR ⩾ 10 mg/gCr | 100/303 (33.0) | 33/95 (34.7) | 1.08 | 0.67–1.76 | 0.313 | 0.755 |
| Total | 208/751 (27.7) | 43/164 (26.2) | 0.84 | 0.57–1.24 | 0.384 | 0.701 |
Note. ▲n is the number of abnormal change for each indicator in this group after ACR stratification, and N is the total number of patients in this group after ACR stratification. Simple DM: simple diabetes mellitus (ACR < 30 mg/gCr); NHADKD: non-high albuminuria diabetic kidney disease; ACR: albumin to creatinine ratio; eGFR: estimated glomerular filtration rate; total-Chol: total cholesterol; HDL-Chol: high-density lipoprotein cholesterol; LDL-Chol: low-density lipoprotein cholesterol; ApoA1: apolipoprotein A1; ApoB: apolipoprotein B; Lip-PLA2: lipoprotein-associated phospholipase A2.
Discussion
The pathogenesis of NHADKD is unclear. Previous studies have shown that atherogenic dyslipidemia in DM has always play an important role in the occurrence and development of DKD, which mainly includes increased triglyceride-rich lipoproteins and small dense LDL, as well as decreased cholesterol-rich HDL (HDL2-cholesterol, a subcomponent of HDL-cholesterol).22–24 These lipid components are deposited in the kidney microvasculature and can cause chronic atherosclerotic damage, which induces DKD.14,25 However, the detection of these lipid components is complicated and difficult for clinical laboratories to conduct routinely. Relatively, total cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol, ApoA1, and ApoB are easy to detect and can reflect the metabolic changes of atherogenic dyslipidemia. Thus, they are considered as the main evaluation indicators of blood lipid health management.15,16 Therefore, to explore the pathogenesis of NHADKD and whether its pathogenesis is related with dyslipidemia, we should firstly start with these common lipid metabolism indicators.
However, in recent years, clinical pathological studies have found that NHADKD has some significant pathological characteristics different from DKD with obviously increased urinary albumin,26,27 which are tubulointerstitial and vascular lesions observed by kidney biopsy. Thus, kidney tubular injury may play an important role in the occurrence of NHADKD. It needs further investigation whether the kidney tubular injury is a chronic atherosclerotic injury caused by dyslipidemia. In this study, in addition to the common serum lipid metabolism indicators, we also included the recently recognized indicators of atherosclerosis, homocysteine and Lip-PLA2. Unfortunately, the six common lipid metabolism indicators (triglyceride, total cholesterol, HDL-cholesterol, LDL-cholesterol, ApoA1 and ApoB) were almost all weakly or irrelevantly related to ACR (except total cholesterol) and eGFR. Surprisingly, homocysteine had the highest correlation coefficient with eGFR, while Lip-PLA2 had the highest correlation coefficient with ACR.
Homocysteine is a metabolite produced by methionine metabolism and is a cytotoxic sulfur-containing amino acid.28 Hyperhomocysteinemia can lead to kidney function injury in DM patients via two mechanisms.29–34 On the one hand, hyperhomocysteinemia can directly lead to glomerular and tubular cell damage through reducing adenylate levels, enhancing local oxidative stress responses, causing endoplasmic reticulum stress responses, promoting protein homocysteinylation, inhibiting DNA methylation, and stimulating the synthesis and activation of Nod-like receptor protein 3 inflammasome, etc.29–31 On the other hand, hyperhomocysteinemia can activate oxidative stress to cause vascular endothelial damage, induce the expression of cell adhesion molecules and chemokines, as well as affect platelet aggregation and coagulation factor activity, etc. Thus, these changes can induce the occurrence of atherosclerosis, and eventually cause glomerulosclerotic damage.31–34 The results of this study found that homocysteine was more closely related to eGFR decline of patients than the lipid metabolism indicators. Subsequent stratified risk analysis also confirmed that high homocysteine was a more important risk factor for predicting the occurrence of NHADKD. In addition, a recent animal study found that during the development of DKD, lipid and methionine metabolism may have phase difference, that is homocysteine-methionine metabolism was activated in the early stage, and fatty acid metabolism changed significantly in the later stage.35 Furthermore, we found that the correlation between homocysteine and eGFR was lower in NHADKD patients than in simple DM patients. Therefore, we speculate that high homocysteine may play an important role in the occurrence of NHADKD, and at earlier stages of the disease its direct cytotoxic injury may be more interesting than its atherosclerotic injury; however, at later stages of the disease hyperhomocysteinemia may aggravate lipid abnormality, and its atherosclerotic injury may be the main cause for the development of NHADKD.
We also assessed the relationship between Lip-PLA2 and kidney function injury in patients with NHADKD. For more than a decade, Lip-PLA2 has also received much attention as an emerging atherosclerotic factor.36 Lip-PLA2, also known as platelet activating factor acetylhydrolase, is a member of the phospholipase A2 superfamily. It is often used as a predictor of cardiovascular events.37 Recent animal tests and a clinical trial of longitudinal disease progression study have confirmed38,39 that Lip-PLA2 can cause the occurrence of diabetic retinopathy, one of the other major microangiopathy in patients with DM. The mechanism is that Lip-PLA2 and its major enzymatic product, lysophosphatidylcholine (a type of lysolecithin), is involved in blood-retinal barrier damage. But this mechanism is not specific. We speculate that Lip-PLA2 can also cause the same or similar damage to the hematuria barrier of glomerulus. This study revealed that among six lipid metabolism indicators and Lip-PLA2 and homocysteine, the correlation coefficient between Lip-PLA2 and ACR in patients with NHADKD was the highest. The increased ACR can indirectly reflect the degree of hematuria barrier damage. Thus, our results indirectly confirm that Lip-PLA2 may also damage hematuria barrier. Unfortunately, the risk analysis showed that Lip-PLA2 did not have the ability to predict the occurrence of NHADKD, but Lip-PLA2 was weakly correlated with eGFR in NHADKD patients. It is speculated that Lip-PLA2 may not play a prominent role in the initiation of NHADKD, but it may have a certain promotion effect in NHADKD development. In addition, a study found that Lip-PLA2 secretion was greatly affected by discrepant lipid profiles in the different individuals with DM,40 which may also be one of the reasons why Lip-PLA2 is not suitable for predicting the occurrence of NDHDKD.
This study has some limitations. For example, the results of this study were limited to the relationship between dyslipidemia and NHADKD in DM patients with ACR < 30 mg/g. The relationship may be different in DM patients with ACR > 30 mg/g. In addition, only DM patients with continuous ACR < 30 mg/g during the 3-month follow-up were included. Patients with a transient increase of ACR over 30 mg/g during the follow-up, who theoretically met the inclusion criteria according to 2012 CKD Guideline, were excluded. This ensured that the patients with NHADKD were included correctly but may cause some bias in case selection. Further studies are warranted.
In conclusion, the occurrence of NHADKD may have a different pathogenesis than DKD with apparent albuminuria. Lipid abnormalities, hyperhomocysteinemia and Lip-PLA2 increasing have a certain role in promoting the occurrence and development of NHADKD. Compared with the lipid abnormalities, hyperhomocysteinemia may play a more important role in promoting the occurrence of NHADKD. Lip-PLA2 may be involved in the glomerular filtration membrane damage and NHADKD disease progression, and promote the increase of protein filtration in DM patients. Therefore, our findings may have important guiding significance for diagnosis and treatment of NHADKD.
Supplemental Material
Supplemental material, sj-pdf-1-dvr-10.1177_1479164121992524 for Vascular inflammation, atherosclerosis, and lipid metabolism and the occurrence of non-high albuminuria diabetic kidney disease: A cross-sectional study by Yuwei Yang, Peng Xu, Yan Liu, Xiaohong Chen, Yiyang He and Jiafu Feng in Diabetes & Vascular Disease Research
Supplemental material, sj-pdf-2-dvr-10.1177_1479164121992524 for Vascular inflammation, atherosclerosis, and lipid metabolism and the occurrence of non-high albuminuria diabetic kidney disease: A cross-sectional study by Yuwei Yang, Peng Xu, Yan Liu, Xiaohong Chen, Yiyang He and Jiafu Feng in Diabetes & Vascular Disease Research
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science & Technology Department of Sichuan Province, China (2015SZ0117 and 2019YJ0701).
ORCID iD: Yuwei Yang  https://orcid.org/0000-0002-0908-1228
https://orcid.org/0000-0002-0908-1228
Supplemental material: Supplemental material for this article is available online.
References
- 1.Klimontov VV, Korbut AI.Normoalbuminuric chronic kidney disease in diabetes. Ter Arkh 2018; 90(10): 94–98. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care 2020; 43(Suppl 1): S135–S51. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis2019; 75: 84–104. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 150. [DOI] [PubMed] [Google Scholar]
- 5.Tsalamandris C, Allen TJ, Gilbert RE, et al. Progressive decline in renal function in diabetic patients with and without albuminuria. Diabetes 1994;43(5):649–655. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis2009;54(2):205–226. [DOI] [PubMed] [Google Scholar]
- 7.Mottl AK, Kwon KS, Mauer M, et al. Normoalbuminuric diabetic kidney disease in the U.S. population. J Diabetes Complicat 2013;27(2):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YH, Sheu WH, Lee IT.Effects of retinopathy and chronic kidney disease on long-term mortality in type 2 diabetic inpatients with normal urinary albumin or protein: a retrospective cohort study. BMJ Open 2018; 8(7): e021655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liyanage P, Lekamwasam S, Weerarathna TP, et al. Prevalence of normoalbuminuric renal insufficiency and associated clinical factors in adult onset diabetes. BMC Nephrol 2018; 19(1): 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macisaac RJ, Jerums G.Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 2011; 20(3): 246–257. [DOI] [PubMed] [Google Scholar]
- 11.Penno G, Solini A, Orsi E, et al. Non-albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: the Renal Insufficiency and Cardiovascular Events (RIACE) Italian multicentre study. Diabetologia 2018; 61(11): 2277-2289. [DOI] [PubMed] [Google Scholar]
- 12.Boronat M, Garcia-Canton C, Quevedo V, et al. Non-albuminuric renal disease among subjects with advanced stages of chronic kidney failure related to type 2 diabetes mellitus. Ren Fail 2014; 36(2): 166–170. [DOI] [PubMed] [Google Scholar]
- 13.Katsiki N, Anagnostis P, Kotsa K, et al. Obesity, metabolic syndrome and the risk of microvascular complications in patients with diabetes mellitus. Curr Pharm Des 2019; 25(18): 2051–2059. [DOI] [PubMed] [Google Scholar]
- 14.Patti AM, Giglio RV, Papanas N, et al. Future perspectives of the pharmacological management of diabetic dyslipidemia. Expert Rev Clin Pharmacol 2019; 12(2): 129–143. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger PS.American Association of Clinical Endocri-nologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr 2018; 31(3): 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract 2017; 23(Suppl 2): 1–87. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Li TT, Zhou W, et al. Lipoprotein-associated phospholipase A2 is a risk factor for diabetic kidney disease. Diabetes Res Clin Pract 2019; 150: 194–201. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes 2016; 34(1): 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 2020; 43(Suppl 1): S14–S31. [DOI] [PubMed] [Google Scholar]
- 20.Chi XH, Li GP, Wang QS, et al. CKD-EPI creatinine-cystatin C glomerular filtration rate estimation equation seems more suitable for Chinese patients with chronic kidney disease than other equations. BMC Nephrol 2017; 18: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momin M, Jia J, Fan F, et al. Relationship between plasma homocysteine level and lipid profiles in a community-based Chinese population. Lipids Health Dis 2017; 16(1): 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo GT, De Cosmo S, Viazzi F, et al. Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: the AMD annals initiative. Diabetes Care 2016; 39(12): 2278–2287. [DOI] [PubMed] [Google Scholar]
- 23.Radcliffe NJ, Seah JM, Clarke M, et al. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Invest 2017; 8(1): 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitrofanova A, Sosa MA, Fornoni A.Lipid mediators of insulin signaling in diabetic kidney disease. Am J Physiol Renal Physiol 2019; 317(5): F1241–F1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filippatos TD, Florentin M, Georgoula M, et al. Pharmacological management of diabetic dyslipidemia. Expert Rev Clin Pharmacol 2017; 10(2): 187–200. [DOI] [PubMed] [Google Scholar]
- 26.Hwang S, Park J, Kim J, et al. Tissue expression of tubular injury markers is associated with renal function decline in diabetic nephropathy. J Diabetes Complications 2017; 31(12): 1704–1709. [DOI] [PubMed] [Google Scholar]
- 27.Ekinci EI, Jerums G, Skene A, et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care 2013; 36(11): 3620–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannibal L, Blom HJ.Homocysteine and disease: causal associations or epiphenomenons? Mol Aspects Med 2017; 53: 36–42. [DOI] [PubMed] [Google Scholar]
- 29.Karmin O, Siow YL.Metabolic imbalance of homocysteine and hydrogen sulfide in kidney disease. Curr Med Chem 2018; 25(3): 367–377. [DOI] [PubMed] [Google Scholar]
- 30.Perna AF, Ingrosso D.Homocysteine and chronic kidney disease: an ongoing narrative. J Nephrol 2019; 32(5): 673–675. [DOI] [PubMed] [Google Scholar]
- 31.Ostrakhovitch EA, Tabibzadeh S.Homocysteine in chronic kidney disease. Adv Clin Chem 2015; 72: 77–106. [DOI] [PubMed] [Google Scholar]
- 32.Kubota Y, Alonso A, Heckbert SR, et al. Homocysteine and incident atrial fibrillation: the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Heart Lung Circ 2019; 28(4): 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duygu H.Is there any link between homocysteine and atherosclerosis? J Geriatr Cardiol 2017; 14(3): 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sreckovic B, Sreckovic VD, Soldatovic I, et al. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab Syndr 2017; 11(3): 179–182. [DOI] [PubMed] [Google Scholar]
- 35.Magalhaes P, Mischak H, Zurbig P.Urinary proteomics using capillary electrophoresis coupled to mass spectrometry for diagnosis and prognosis in kidney diseases. Curr Opin Nephrol Hypertens 2016; 25(6): 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tousoulis D, Papageorgiou N, Androulakis E, et al. Lp-PLA2: a novel marker of atherosclerosis: to treat or not to treat? Int J Cardiol 2013; 165(2): 213–216. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Liu Y, Wang S, et al. Association between Lp-PLA2 and coronary heart disease in Chinese patients. J Int Med Res 2017; 45(1): 159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canning P, Kenny BA, Prise V, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc Natl Acad Sci U.S.A 2016; 113(26): 7213–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui MK, Kennedy G, Carr F, et al. Lp-PLA2 activity is associated with increased risk of diabetic retinopathy: a longitudinal disease progression study. Diabetologia 2018; 61(6): 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackisch L, Kumsaiyai W, Moore JD, et al. Differential expression of Lp-PLA2 in obesity and type 2 diabetes and the influence of lipids. Diabetologia 2018; 61(5): 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dvr-10.1177_1479164121992524 for Vascular inflammation, atherosclerosis, and lipid metabolism and the occurrence of non-high albuminuria diabetic kidney disease: A cross-sectional study by Yuwei Yang, Peng Xu, Yan Liu, Xiaohong Chen, Yiyang He and Jiafu Feng in Diabetes & Vascular Disease Research
Supplemental material, sj-pdf-2-dvr-10.1177_1479164121992524 for Vascular inflammation, atherosclerosis, and lipid metabolism and the occurrence of non-high albuminuria diabetic kidney disease: A cross-sectional study by Yuwei Yang, Peng Xu, Yan Liu, Xiaohong Chen, Yiyang He and Jiafu Feng in Diabetes & Vascular Disease Research