Abstract
In this study, the level of cell damage were analyzed immunohistochemically to clarify the association between nodular gastritis and undifferentiated gastric cancer. Thirty patients of nodular gastritis were enrolled as the nodular gastritis group. Thirty patients of non-nodular gastritis were enrolled as the control group. They were evaluated according to the updated Sydney system and used for immunohistochemical staining (p53, Ki-67, E-cadherin, and 8-OHdG). The scores based on the updated Sydney system were significantly higher in the nodular group than in the non-nodular group for histologically assessed inflammation and activity in the gastric corpus (1.91 ± 0.77 vs 1.58 ± 0.60, p = 0.049, 0.83 ± 0.81 vs 0.44 ± 0.64, p = 0.032). On immunostaining, the detection of E-cadherin was lower in the nodular group for both the antrum (1.0 ± 0.62 vs 1.47 ± 0.85, p = 0.047) and the corpus (1.16 ± 0.81 vs 1.48 ± 0.71, p = 0.043) and the p53 labeling index of the gastric corpus was higher in the nodular group than in the non-nodular group (3.06 ± 1.94 vs 2.03 ± 1.99, p = 0.015). Nodular gastritis showed significant severe inflammation and immunohistochemical cell damage compared with non-nodular gastritis. These findings may play an important role in the oncogenesis of undifferentiated gastric cancer in nodular gastritis.
Keywords: Helicobacter pylori, nodular gastritis, undifferentiated gastric cancer, E-cadherin, p53
Introduction
The strong relationship between gastric cancer (GC) and Helicobacter pylori (H. pylori) infection is clear,(1,2) and the International Agency for Research on Cancer (IARC) which is a part of the World Health Organization (WHO) have stated that H. pylori is involved in 90% of all non-cardiac gastric cancers.(3) Moreover, numerous reports have indicated that gastric cancer is inhibited by H. pylori eradication.(4–7) Although it has been noted that H. pylori infection is mainly associated with differentiated gastric cancer, it has also been reported to be involved in undifferentiated gastric cancer.(8)
Nodular gastritis is H. pylori gastritis in which elevations ranging in size from nodular to granular are seen virtually uniformly, mainly in the gastric antrum. First reported by Eastham et al.(9) in 1988, it occurs most commonly in children and young female patients.(10) Nodular elevations approximately 3 mm in size are endoscopically observed to be distributed in a roughly uniform manner, mainly in the antrum. The histopathological characteristics of nodular gastritis are hyperplasia of the crypt epithelium and lymphoid follicle formation. The findings are known to improve with H. pylori eradication.
Recently, cases have been seen in which gastric cancer, particularly undifferentiated gastric cancer, has occurred concomitantly with nodular gastritis. Consequently, it has drawn attention as the origin of gastric cancer in young persons.(11) Although nodular gastritis is often seen between the gastric antrum and angle, a characteristic of gastric cancer that occurs concomitantly with nodular gastritis has been reported to be that it is undifferentiated gastric cancer that develops in the gastric corpus. This is in contrast with differentiated gastric cancer, which develops from typical atrophic gastritis and often occurs between the lower gastric corpus and antrum in elderly men.(12) Because it occurs frequently in young persons and carries a poor prognosis, undifferentiated gastric cancer that occurs in association with nodular gastritis is a condition that demands particular attention, even as the rate of H. pylori infection is in decline, and understanding its pathophysiology and pathogenesis is a matter of urgency. In this investigation, the level of cell damage and the characteristics of cell turnover in nodular gastritis as compared with non-nodular gastritis were analyzed immunohistologically. The purpose of the study was to elucidate the oncogenic mechanism of nodular gastritis.
Materials and Methods
Subjects
Thirty patients of nodular gastritis who underwent gastrointestinal endoscopy in Oita University Hospital and Arita Gastrointestinal Hospital between January 2011 and December 2016 were enrolled as the nodular gastritis group. Thirty patients who underwent upper gastrointestinal endoscopy at the same institutions and in the same periods and were endoscopically diagnosed as having non-nodular gastritis were enrolled as the control group (the non-nodular gastritis group).
When at least two of the rapid urease test (RUT), culture test, and histological test were positive, the patient was diagnosed as having a current H. pylori infection. All 60 subjects enrolled were positive on all tests. Therefore, these subjects were diagnosed as having present H. pylori infections.
The study protocol was approved by an institutional review board of Oita University, Faculty of medicine (987). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for their being included in the study.
Endoscopic evaluation
Endoscopy was performed using an electroscope (model PQ-260, HQ-290, or others; Olympus, Tokyo, Japan). Nodular gastritis was evaluated by endoscopic observation. Endoscopic atrophy was defined using an endoscopic-atrophic-border scale previously reported by Kimura and Takemoto.(13) Endoscopic atrophy grades were scored as C0: 0, C1: 1, C2: 2, C3: 3, O1: 4, O2: 5, and O3: 6, respectively, with 0 representing absence of atrophy and 6 indicating severe atrophy.
Histology
Biopsy samples were obtained from the greater curvature of the antrum and the greater curvature of the corpus. These samples of gastric mucosa were fixed in buffered formalin for 24 h, embedded in paraffin, and cut in 4-μm-thick sections. Each sample was stained with hematoxylin and eosin and with May-Giemsa stain. The gastric mucosa was evaluated according to the updated Sydney system.(14) The degree of inflammation, neutrophil activity, atrophy, and intestinal metaplasia were evaluated according to the updated Sydney system and classified into 4 grades: 0, ‘normal’; 1, ‘mild’; 2, ‘moderate’; and 3, ‘marked’.
Immunohistochemistry
All samples were used for immunohistochemical staining. From a paraffin-embedded block, samples were cut into 4-μm-thick sections. Serial paraffin sections were washed in 1/15 mol/L phosphate buffered saline (PBS, pH 7.4) three times for 5 min, then pre-incubated in normal rabbit serum (1:10 in PBS) for 20 min. Next, these sections were incubated with primary antibodies for 16 h at 4°C, followed by exposure to avidin-biotin complex. Sections were immersed in 0.05 mol/L Tris-HCl buffer containing 0.02% 3,3'-diaminobenzidine tetrahydrochloride and 0.005% H2O2. Nuclei were counterstained with hematoxylin. The primary antibodies were mouse monoclonal antibodies that bind p53 (clone DO-7, Dako Japan, Tokyo, Japan), Ki-67 (Dako Japan), epithelial cadherin (E-cadherin; Dako Japan), and 8-hydroxy-2'-deoxyguanosine (8-OHdG; JaICA, Shizuoka, Japan). Control sections incubated with normal mouse IgG instead of the primary antibody showed no non-specific staining.
Ki-67-positive cells were counted, and labeling indices were calculated. At least 500 gastric epithelial cells were counted for each specimen. Immunohistochemical detection of p53, 8-OHdG, and E-cadherin was scored as 0 for no staining, 1 for scattered cells to 33% staining, 2 for 33% to 66% staining, and 3 for diffuse staining in nearly all cells.
Statistical analysis
Statistical analyses were performed using SPSS software (SPSS Statistics 22; IBM, Armonk, NY), and data are expressed as means ± SD. The chi-squared test and Fisher’s exact test were used to compare clinicopathological factors between groups. Student’s t test was used to compare groups by age and histochemical characteristics. P values less than 0.05 were considered significant.
Results
Endoscopic and histological findings
In the nodular gastritis group, endoscopy showed uniform granular elevations in the antrum (Fig. 1A). In the non-nodular gastritis group, endoscopy showed atrophic changes in the antrum (Fig. 1B). Because endoscopic atrophy level was matched, there was no significant difference in the degree of endoscopic atrophy between nodular and non-nodular gastritis group.
Fig. 1.

Endoscopic findings (white light). (A) Nodular gastritis (left: corpus, right: antrum). (B) Non-nodular gastritis (left: corpus, right: antrum). Endoscopic finding showed uniform granular elevations in the antrum of the nodular gastritis and atrophic changes in the antrum of the non-nodular gastritis.
The histopathological appearance indicated that lymphoid follicle was formed in the lamina propria of the antrum in the nodular gastritis group and showed distinct inflammation of antrum in both the nodular and non-nodular groups (Fig. 2A and B). The histological appearance of the greater curvature of the gastric corpus did not show atrophy in either group (Fig. 2). However, it gave an impression that inflammation and activity was stronger in the nodular gastritis group than in the non-nodular gastritis group. (Fig. 2).
Fig. 2.
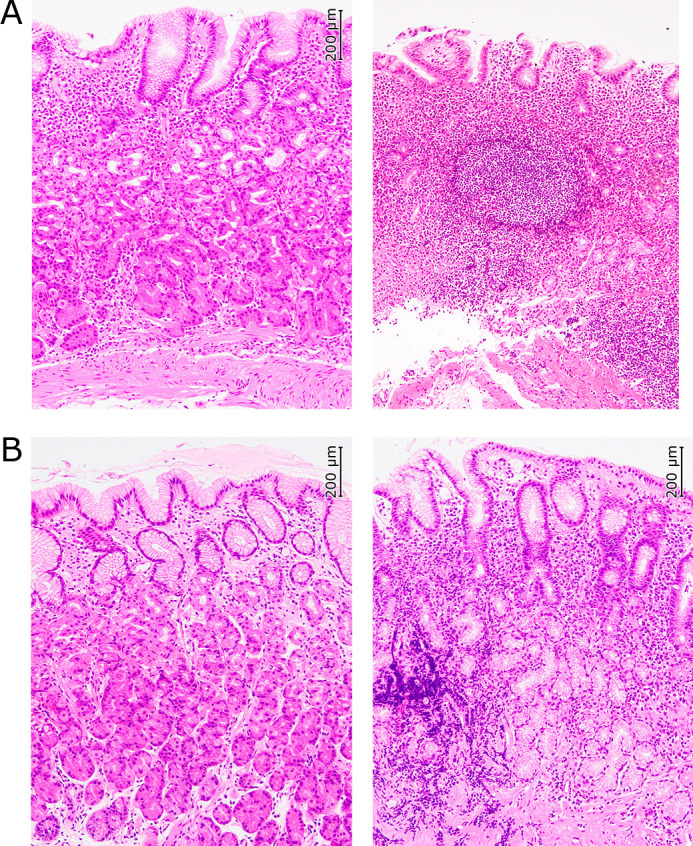
Histological findings (HE ×100). (A) Nodular gastritis (left: corpus, right: antrum). (B) Non-nodular gastritis (left: corpus, right: antrum). Lymphoid follicle was formed in the lamina propria of the antrum of the nodular gastritis. Histological findings of the antrum showed distinct inflammation in both the nodular and non-nodular gastritis.
Histological differences between nodular and non-nodular gastritis
There were no significant differences between the nodular and non-nodular gastritis groups in age and sex (Table 1). The gastritis scores based on the updated Sydney system were significantly higher in the nodular gastritis group than in the non-nodular gastritis group for histologically assessed inflammation and activity in the corpus (1.91 ± 0.77 vs 1.58 ± 0.60, p = 0.049, 0.83 ± 0.81 vs 0.44 ± 0.64, p = 0.032) and significantly lower for activity in the antrum (0.74 ± 0.77 vs 1.11 ± 0.57, p = 0.026). The intestinal metaplasia score for the gastric corpus was 0 in both groups (Table 2).
Table 1.
Comparison of background between nodular gastritis and non-nodular gastritis
| Nodular gastritis |
Non-nodular gastritis |
p vlaue | |
|---|---|---|---|
| n | 35 | 36 | |
| Age | 38.69 ± 12.37 | 40.42 ± 11.49 | 0.549 |
| Sex | 9:26 | 10:26 | 0.752 |
Statistical analysis was used Student’s t test and chi-squared test.
Table 2.
Comparison of updated Sydney system score between nodular gastritis and non-nodular gastritis
| Nodular gastritis |
Non-nodular gastritis |
p vlaue | |
|---|---|---|---|
| Updated Sydney system score | |||
| Antrum | |||
| Inflammation | 2.51 ± 0.60 | 2.39 ± 0.49 | 0.345 |
| Activity | 0.74 ± 0.77 | 1.11 ± 0.57 | 0.026 |
| Atrophy | 0.86 ± 0.59 | 0.97 ± 0.37 | 0.336 |
| Intestinal metaplasia | 0.14 ± 0.35 | 0.17 ± 0.37 | 0.785 |
| Corpus | |||
| Inflammation | 1.91 ± 0.77 | 1.58 ± 0.60 | 0.049 |
| Activity | 0.83 ± 0.81 | 0.44 ± 0.64 | 0.032 |
| Atrophy | 0.17 ± 0.45 | 0.11 ± 0.31 | 0.518 |
| Intestinal metaplasia | 0 | 0 | — |
Statistical analysis was used Student’s t test.
Immunohistochemical findings in nodular and non-nodular gastritis
On immunostaining, positive findings for Ki-67 were seen in nuclei of the glandular neck region (Fig. 3). The Ki-67 labeling index in the nodular and non-nodular groups, respectively, was 24.1 ± 15.0 vs 21.5 ± 15.6 (p = 0.245) for the antrum and 14.5 ± 12.1 vs 13.0 ± 13.1 (p = 0.300) for the corpus. Thus, the differences between the groups were not significant (Table 3).
Fig. 3.

Immunostaining Ki-67 of gastric antrum (×200). (left: nodular gastritis, right: non-nodular gastritis). Ki-67 were seen in nuclei of the glandular neck region in both groups. The differences between the groups were not significant.
Table 3.
Immunohistochemical detection of Ki-67, p53, E-cadherin and 8-OHdG
| Nodular gastritis |
Non-nodular gastritis |
p value | |
|---|---|---|---|
| Number of case | 35 | 36 | |
| Greater curvature of the antrum | |||
| Ki-67 labeling index | 24.05 ± 14.98 | 21.53 ± 15.61 | 0.245 |
| p53 (DO-7) labeling index | 4.14 ± 2.78 | 3.33 ± 2.32 | 0.094 |
| E-cadherin | 1.00 ± 0.62 | 1.47 ± 0.85 | 0.0047 |
| 8-OHdG | 1.77 ± 0.69 | 1.86 ± 0.68 | 0.291 |
| Greater curvature of the corpus | |||
| Ki-67 labeling index | 14.55 ± 12.14 | 12.98 ± 13.09 | 0.3 |
| p53 (DO-7) labeling index | 3.06 ± 1.94 | 2.03 ± 1.99 | 0.015 |
| E-cadherin | 1.16 ± 0.81 | 1.48 ± 0.71 | 0.0427 |
| 8-OHdG | 1.60 ± 0.65 | 1.64 ± 0.59 | 0.397 |
E-cadherin, 8-OHdG: scored as: 0 for no staining, 1 for scattered cells to 33% staining, 2 for 33% to 66% staining, and 3 for diffuse staining in nearly all cells. Average ± SD. Statistical analysis was used Student’s t test.
Strongly positive findings for E-cadherin were obtained near glandular epithelial cells in many patients (Fig. 4). The frequency of E-cadherin expression in the nodular and non-nodular gastritis groups, respectively, was 1.0 ± 0.62 vs 1.47 ± 0.85 (p = 0.047) for the antrum and 1.16 ± 0.81 vs 1.48 ± 0.71 (p = 0.043) for the corpus; the frequency was significantly lower in the nodular gastritis group for both the antrum and the corpus (Table 3).
Fig. 4.

Immunostaining E-cadherin of gastric corpus (×200). (left: nodular gastritis, right: non-nodular gastritis). Expression of E-cadherin was strongly recognized especially in the superficial epithelium in the non-nodular gastritis. In the nodular gastritis, the expression was weaker.
As for Ki-67, positive findings for p53 were obtained for nuclei of the glandular neck region, although there were fewer such findings than for Ki-67 (Fig. 5). Comparison of the p53 labeling indices for the groups showed no significant difference in the antrum (4.14 ± 2.78 vs 3.33 ± 2.32, p = 0.094). For the gastric corpus, however, the index was significantly higher in the nodular gastritis group than in the non-nodular gastritis group (3.06 ± 1.94 vs 2.03 ± 1.99, p = 0.015) (Table 3).
Fig. 5.

Immunostaining p53 of gastric corpus (×200). (left: nodular gastritis, right: non-nodular gastritis). In the nodular gastritis, positive cells were seen sporadically in the glandular neck region. In the non-nodular gastritis, they were not significant.
Immunohistology tests for 8-OHdG showed numerous positive findings for nuclei centering on the glandular neck region (Fig. 6). However, examination of the frequency of 8-OHdG expression showed no significant difference between the 2 groups for the antrum (1.77 ± 0.69 vs 1.86 ± 0.68, p = 0.291) or the corpus (1.60 ± 0.65 vs 1.64 ± 0.59, p = 0.397) (Table 3).
Fig. 6.

Immunostaining 8-OHdG of gastric corpus (×200) (left: nodular gastritis, right: non-nodular gastritis). In both groups, the nucleus of the glandular neck were stained. There was no significant difference in expression between the two groups.
Discussion
Damage to the gastric mucosa of some form has been implicated in the development of gastric cancer. H. pylori infection is the main cause of gastric cancer,(1,2) and progression of chronic active gastritis, atrophic gastritis, and intestinal metaplasia, which are associated with H. pylori infection, increases the risk of gastric cancer.(15)
Nodular gastritis consists of inflammatory changes in the gastric mucosa as a result of H. pylori infection, with elevations seen that are consistent with lymphoid follicles in the lamina propria.(10) Recent reports have shown that nodular gastritis serves as the origin of undifferentiated gastric cancer.(11,12) This suggests that the pathology of the gastric mucosal damage present in nodular gastritis is regarded as different from that present in non-nodular gastritis. However, there have been no previous immunohistological investigations of the gastric mucosal dynamics of nodular gastritis. The present study is the first to describe the differences in immunohistological cell dynamics and level of cellular damage between nodular and non-nodular gastritis.
The results showed the scores for histological inflammation and activity in the gastric corpus to be significantly higher in the nodular gastritis group than in the non-nodular gastritis group. H. pylori infection induces macrophages and neutrophils, which in turn induce cytokines, particularly interleukin-8 (IL-8). These processes have been identified as playing a role in cell damage.(16–18) This suggests that damage to mucosal cells in the greater curvature of the corpus is more severe with nodular gastritis than with typical H. pylori-positive gastritis in which nodules are not seen. There have been several previous reports of the increase in gastric cancer risk associated with inflammation and activity.(2) These results also confirm the potential for an increased risk of cancer in the greater curvature of the corpus in association with severe inflammation. Consequently, the results of the present study suggest that the risk of cancer in the mucosa of the greater curvature of the corpus is high with nodular gastritis. Moreover, the finding that undifferentiated gastric cancer occurs frequently in the greater curvature of the corpus in the context of nodular gastritis is also consistent with the results of the present study.(12)
In addition, the results showed the score for p53 expression to be high in the corpus in the nodular gastritis group. The role of p53 is to inhibit cell turnover, and it is also known to be a tumor suppressor gene.(19) In response to cellular DNA damage, it induces the expression of proteins such as MDM2, thereby halting cell division and inducing apoptosis.(19) Malignancies cause a mutation of wild-type p53, resulting in the loss of normal p53 activity. The mutant type of the p53 protein that increases as a result has a long half-life, and its expression in tumors is seen immunohistologically.(20,21) Previous reports have described increased p53 expression and p53 point mutations in H. pylori chronic gastritis.(22–25) This shows that there may be more of an increase in wild-type p53 expression as a result of DNA damage than expression of the mutant type of p53 in gastritis tissue. The increase in p53 protein seen in the gastric corpus mucosa with nodular gastritis as compared with non-nodular gastritis in the present study may suggest that more severe DNA damage is present in nodular gastritis mucosa than in non-nodular gastritis tissue. Mutations of the p53 gene are commonly seen in undifferentiated gastric cancer. The results of the present study showing increased p53 may also be consistent with an increased risk of undifferentiated gastric cancer with nodular gastritis.
The observation of decreased E-cadherin expression in the nodular gastritis group also suggests a relationship to increased gastric cancer risk. E-cadherin is a glycoprotein involved in cellular adhesion and is present on the surface of many cells. It is thought that E-cadherin protein deficiency resulting from mutation of the CDH1 gene, which encodes E-cadherin, may lead to dysfunction involving cell membrane permeability, and that this could result in tumor cell invasion.(26) Consequently, the possibility of E-cadherin deficiency in gastric cancer has also been suggested.(26) A congenital deficiency has been noted in hereditary diffuse gastric cancer (HDGC) syndrome.(27) Decreased E-cadherin expression in mucosa in which intestinal metaplasia had occurred was reported in non-neoplastic cases independent of the presence of H. pylori infection.(28)
The present results showed significantly lower immunohistological expression of E-cadherin with nodular gastritis than with non-nodular gastritis. Although tumor tissue was not examined in this investigation, the results suggest that E-cadherin may undergo changes prior to tumor progression, as reported by Zullo et al.(28)
Therefore, the findings suggested that, with nodular gastritis, E-cadherin protein expression may, from the gastritis stage, already be affected by cell damage resulting from a significant and intense infiltration of neutrophils, and that this may result in progression to undifferentiated gastric cancer with strong invasive tendencies. In conclusion, as compared with non-nodular H. pylori gastritis, inflammation and activity were more severe, p53 protein expression was greater immunohistologically, and E-cadherin expression was lower with nodular gastritis of the gastric corpus. The findings suggest that these factors may play an important role in the oncogenesis of undifferentiated gastric cancer of the gastric corpus in nodular gastritis. Consequently, they suggest a need for more careful endoscopic screening of nodular gastritis. Further investigation with a larger number of patients is needed to clarify the carcinogenic mechanism of nodular gastritis.
Author Contributions
KO: wrote the paper, acquisition of data, analysis and interpretation of data, statistical analysis. MK: study concept and design, paper modification, acquisition of data, analysis and interpretation of data. KM and TO: acquisition of data, analysis and interpretation of data. HA, RO, KF, OM, YH, YW, and MF: acquisition of data. KM: drafting of the manuscript, obtained funding, administrative, technical, or material support, study supervision.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP18K07946.
We thank the members of Department of Gastroenterology of Oita University; Ms. Yoko Kudo and Ms. Kanako Ito for immunostaining.
Abbreviations
- E-cadherin
epithelial cadherin
- HDGC
hereditary diffuse gastric cancer
- IL-8
interleukin-8
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- RUT
rapid urease test
Conflict of Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998; 114: 1169–1179. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 3.Herrero R, Park JY, Forman D. The fight against gastric cancer - the IARC Working Group report. Best Pract Res Clin Gastroenterol 2014; 28: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014; 348: g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Take S, Mizuno M, Ishiki K, et al. Seventeen-year effects of eradicating Helicobacter pylori on the prevention of gastric cancer in patients with peptic ulcer; a prospective cohort study. J Gastroenterol 2015; 50: 638–644. [DOI] [PubMed] [Google Scholar]
- 6.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008; 372: 392–397. [DOI] [PubMed] [Google Scholar]
- 7.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016; 150: 1113–1124.e5. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi S, Nakajima T, Kobayashi O, et al. Effect of age on the relationship between gastric cancer and Helicobacter pylori. Tokyo Research Group of Prevention for Gastric Cancer. Jpn J Cancer Res 2000; 91: 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastham EJ, Elliott TS, Berkeley D, Jones DM. Campylobacter pylori infection in children. J Infect 1988; 16: 77–79. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto M, Haruma K, Yoshihara M, et al. Nodular gastritis in adults is caused by Helicobacter pylori infection. Dig Dis Sci 2003; 48: 968–975. [DOI] [PubMed] [Google Scholar]
- 11.Kamada T, Tanaka AKI, Yamanaka Y, et al. Nodular gastritis with Helicobacter pylori infection is strongly associated with diffuse-type gastric cancer in young patients. Dig Endosc 2007; 19: 180–184. [Google Scholar]
- 12.Kamada T, Hata J, Tanaka A, et al. Nodular gastritis and gastric cancer. Dig Endosc 2006; 18: 79–83. [Google Scholar]
- 13.Kimura K. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 13: 87–97. [Google Scholar]
- 14.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima N, Kozu K, Kobayashi S. The expression of IGF-1R in Helicobacter pylori-infected intestinal metaplasia and gastric cancer. J Clin Biochem Nutr 2016; 59: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology 2004; 126: 1030–1043. [DOI] [PubMed] [Google Scholar]
- 17.Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Helicobacter pylori water soluble surface proteins prime human neutrophils for enhanced production of reactive oxygen species and stimulate chemokine production. J Clin Pathol 2003; 56: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KE, Khoi PN, Xia Y, et al. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol 2013; 19: 8192–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine AJ, Finlay CA, Hinds PW. P53 is a tumor suppressor gene. Cell 2004; 116 (2 Suppl): S67–S69. [DOI] [PubMed] [Google Scholar]
- 20.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991; 253: 49–53. [DOI] [PubMed] [Google Scholar]
- 21.Hinds PW, Finlay CA, Quartin RS, et al. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the “hot spot” mutant phenotypes. Cell Growth Differ 1990; 1: 571–580. [PubMed] [Google Scholar]
- 22.Satoh K, Kihira K, Kawata H, et al. p53 expression in the gastric mucosa before and after eradication of Helicobacter pylori. Helicobacter 2001; 6: 31–36. [DOI] [PubMed] [Google Scholar]
- 23.Kodama M, Fujioka T, Murakami K, et al. Eradication of Helicobacter pylori reduced the immunohistochemical detection of p53 and MDM2 in gastric mucosa. J Gastroenterol Hepatol 2005; 20: 941–946. [DOI] [PubMed] [Google Scholar]
- 24.Kodama M, Murakami K, Okimoto T, Sato R, Watanabe K, Fujioka T. Expression of mutant type-p53 products in H pylori-associated chronic gastritis. World J Gastroenterol 2007; 13: 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami K, Mitomi H, Yamashita K, Tanabe S, Saigenji K, Okayasu I. p53, but not c-Ki-ras, mutation and down-regulation of p21WAF1/CIP1 and cyclin D1 are associated with malignant transformation in gastric hyperplastic polyps. Am J Clin Pathol 2001; 115: 224–234. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy S. CDH1 (E-cadherin) mutation and gastric cancer: genetics, molecular mechanisms and guidelines for management. Cancer Manag Res 2019; 11: 10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998; 392: 402–405. [DOI] [PubMed] [Google Scholar]
- 28.Zullo A, Romiti A, Borrini F, et al. Alteration of E-cadherin expression in gastric mucosa: role of intestinal metaplasia and Helicobacter pylori infection. Anticancer Res 2004; 24: 1603–1607. [PubMed] [Google Scholar]


