Abstract

The silver nanoparticles (AgNPs) using the rhizome extract of Rhodiola rosea have been reported. However, their antioxidant activity and whether the biogenic AgNPs could be used to catalyze the reduction of hazardous dye or used as fluorescence enhancers are unknown. This study focused on the facile green synthesis of silver nanoparticles using the rhizome aqueous extract of R. rosea (G-AgNPs). We then studied their antioxidant activity and catalytic degradation of hazardous dye Direct Orange 26 (DO26) and Direct Blue 15 (DB15). Their effects on fluorescein’s fluorescent properties were also evaluated. The chemical AgNPs (C-AgNPs) were synthesized by reducing solid sodium borohydride (NaBH4), and its above activities were compared with those of G-AgNPs. The formation of G-AgNPs was confirmed by the appearance of brownish-gray color and the surface plasmon resonance (SPR) peak at 437 nm. The biogenic AgNPs were approximately 10 nm in size with a regular spherical shape identified from transmission electron microscopy (TEM) analysis. G-AgNPs exhibited significantly improved 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity than butylated hydroxytoluene (BHT) and C-AgNPs (p < 0.05). The biogenic G-AgNPs were also found to function as an effective green catalyst in reducing DO26 and DB15 by NaBH4, which is superior to C-AgNPs. Furthermore, G-AgNPs showed better fluorescence enhancement activity than C-AgNPs, and the concentration required was lower. When the concentration of the G-AgNP solution was 64 nmol/L, the fluorescence intensity reached the maximum of 5460, with the fluorescence enhancement efficiency of 3.39, and the fluorescence activity was stable within 48 h. This study shows the efficacy of biogenic AgNPs in catalyzing the reduction of hazardous dye DO26 and DB15. Biogenic AgNPs could also be used as fluorescence enhancers in low concentrations.
1. Introduction
Nanotechnology has gained much attention in recent decades due to its immense contribution in diverse fields such as sensors, catalysts, optics, medicine, and energy. Among the nanoparticles, silver nanoparticles (AgNPs) are of great interest to researchers due to their unique properties like good electrical conductivity, antimicrobial activity, photoelectrochemical activity, anti-biofilms, and enzymatic activity.1 AgNPs have been synthesized by diverse methods, including chemical reduction, impregnation, laser ablation, ultrasonic radiation, and explosion.2 Since the potent reducing and capping agents frequently used by the chemical methods are toxic to humans and the environment, there is a need to synthesize AgNPs in more economical and eco-friendly manners. The biosynthesis of AgNPs using plant extracts as the reducing and capping agents is one of the most widely used green methods. This approach has been adopted in recent years because it is simple, cost-effective, and environmentally benign. Diverse plants such as Corylus avellana,1aGivotia moluccana,3Nigella arvensis,4Lippia nodiflora,5Piper nigrum,6 green tea,7 grape seed,8Zingiber officinale,9Terminalia cuneata,10 and Calendula officinalis(11) have been successfully used to synthesize AgNPs.
Many industrial dyes are currently discharged into China’s environment, leading to severe water and environmental pollution.12 The estimated annual dye output exceeds 100 000 tons, and about 10% is discharged into the environment. Dye effluents are characterized by bright color, high organic content, complex composition, and low biodegradability.13 Among the 4000 kinds of synthetic organic dyes, azo dyes are the most widely used, and the toxicity of diazo direct dyes is the highest.14 Consequently, azo-dye wastewater is a major concern; it is necessary to develop new, more efficient technologies to mineralize these non-biodegradable compounds.
Plant Rhodiola rosea belongs to the Rhodiola genus, Crassulaceae family. They grow on mountains in northern Asia, North America, and Europe and are wildly used in traditional medicine due to their anti-inflammatory, antioxidant and antidepressant activities.15R. rosea dry roots contain 140 different organic compounds and are rich in many antioxidant polyphenolic compounds such as phenylpropanoids, flavonoids, tannins, cinnamyl alcohol, tyrosol, phenyletanoids, and proanthocyanins.16 In addition, both ethanol and aqueous extracts of R. rosea have been shown to have antioxidant activity.17
Fluorescein (FL) has been widely used in DNA hybridization and biomolecule detections due to its high fluorescence quantum yield. However, a high FL concentration is prone to fluorescence self-quenching, so FL should be controlled in a lower concentration range when used as a fluorescent marker. However, it is challenging to detect FL because of its low fluorescence intensity. By introducing metal nanostructures into the FL system, the fluorescence can be enhanced using the strong local field generated by nanostructures.18
The R. rosea extract-mediated green synthesis of silver nanoparticles is typically aided by nanosilica carriers.19 The anti-biofilm effects, for example, inhibition of Pseudomonas aeruginosa and Escherichia coli biofilms, of gold and silver nanoparticles synthesized by the R. rosea rhizome extracts have been reported.19,20 However, the simpler synthesis method of AgNPs mediated by R. rosea deserves further investigation. Whether the biogenic AgNPs could be used to catalyze the reduction of hazardous dye or as fluorescence enhancers is unknown.
Therefore, in this study, two kinds of silver nanoparticles were synthesized: one using R. rosea rhizome’s aqueous extract as a green stabilizing-reducing agent (G-AgNPs) and the other using NaBH4 as the chemical reduction agent (C-AgNPs). Their antioxidant activities were compared using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, and their potential catalytic reduction activities were compared using two diazo direct dyes, Direct Orange 26 (DO26) and Direct Blue 15 (DB15). Finally, their effects on fluorescent properties of fluorescein were also evaluated.
2. Materials and Methods
2.1. Chemicals
All chemicals used were of reagent grade. Silver nitrate (AgNO3), sodium borohydride (NaBH4), butylated hydroxytoluene (BHT), sodium citrate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and chromatographic grade acetonitrile were purchased from Aladdin Industrial Corporation. Rhodiola rosea rhizome was purchased from Anguo Yaoyuan Co., Ltd. Fluorescein, Direct Orange 26, and Direct Blue 15 were purchased from Hubei Qifei Co., Ltd. Deionized water was prepared by a Molecular ultrapure water system with an electrical resistivity of 18.25 MΩ·cm.
2.2. Preparation of R. rosea Extract
R. rosea extract was prepared using a reported method with minor modifications.21 Dry R. rosea rhizome was ground into powder (FW177 mill, Taisite Co., Tianjin, China) and screened by a 40-mesh sieve. Then, 5.0 g of the powder was extracted with 100 mL of deionized water at 100 °C for 30 min. Next, the extract was filtered using a Whatman no. 2 paper. Finally, the filtrate volume was adjusted to 100 mL with deionized water and used as a reducing source for the synthesis of AgNPs. This extraction process was repeated three times.
2.3. Green Synthesis of Colloidal AgNPs Using R. rosea
The green synthesis of colloidal AgNPs using R. rosea was conducted using a previously reported method with minor modifications.8 First, 100 mL of silver nitrate solution (1.0 mmol/L) was added to 10 mL of the prepared R. rosea rhizome extract solution. Then, the mixtures were heated at 40 °C for 20 min with magnetic stirring at 500 rpm/min (C-MAG HS7 digital magnetic stirrer, IKA Co., Staufen, Germany). The synthesized colloidal AgNPs are named G-AgNPs.
2.4. Chemical Synthesis of Colloidal AgNPs Using NaBH4
The chemical synthesis of colloidal AgNPs using NaBH4 was adapted from a reported method with minor modifications.18b First, 25 mL of AgNO3 with a concentration of 2.0 mmol/L and 20 mL of 0.5 mmol/L sodium citrate were mixed and stirred vigorously for 15 min (500 rpm/min); then, 1.2 ml of 10 mmol/L NaBH4 was injected slowly into the above solution and stirred vigorously for another 20 min (500 rpm/min). Finally, the mixtures’ volumes were brought to 50 mL with deionized water and preserved at 4 °C in the dark. The synthesized colloidal AgNPs are named C-AgNPs.
2.5. Characterization of AgNPs
2.5.1. Ultraviolet–Visible Spectrophotometry
An ultraviolet–visible spectrophotometer (UV–vis) (Mapada UV-3200, Shanghai) was used to confirm metal ions’ reduction into metal nanoparticles. For UV–vis spectroscopy studies, 0.50 mL of AgNPs was placed in a cuvette and diluted to 3.00 mL by adding deionized water. The surface plasmon resonance peak was observed between 400 and 500 nm in the visible region. The absorbance was measured in the wavelength range between 200 and 800 nm.
2.5.2. HR-TEM
Transmission electron microscopy (TEM) was performed on a Tecnai G2F20STWIN system (Thermo Fisher, Amsterdam, Netherlands) to evaluate the size and morphology at an accelerating voltage of 200 kV.
2.6. Evaluation of the Antioxidant Activity of AgNPs
The antioxidant activity of AgNPs was determined by the DPPH radical scavenging method with minor modifications.22 First, 3 mL of DPPH–ethanol solution (40 mg/L) and various volumes (20, 40, 60, 80, 100, and 200 μL) of G-AgNPs, C-AgNPs, and BHT at the concentration of 1 × 10–3 mol/L were added into the centrifuge tube and then incubated for 1 h with oscillation at room temperature in the dark. The absorbance of the supernatant at 517 nm was determined. Ethanol solution was used as a blank control, and BHT was used as a standard antioxidant. Antioxidant activity was measured by the inhibition rate I% = (1 – A1/A0) × 100. In the formula, I is the inhibition ratio, A0 is the absorbance of DPPH–ethanol solution, and A1 is the absorbance of the sample solution. All experiments were repeated three times.
2.7. Evaluation of the Catalytic Activity of AgNPs
The catalytic activity of AgNPs was determined by a published method with minor modifications.10 The catalytic activity of synthesized G-AgNPs (or G-AgNPs) was determined based on the reductive degradation of DO26 and DB15 in the presence of NaBH4. One milliliter of DO26 or DB15 (50 mg/L) was mixed with 2.0 mL of NaBH4 (0.1 mol/L) and 0.2 mL of AgNPs solution. The performance was monitored by recording the UV–vis spectra variation over time (0, 3, 6, 9, 12, 15, and 18 min). The control was also conducted without AgNPs. The degradation rate for 18 min and the retention rates for 0 min and 18 min were calculated. The degradation rate calculation formula was (1 – A1/A0) × 100, and the retention rate calculation formula was A1/A2 × 100, where A0, A1, and A2 were the absorbance values of 0 min, 18 min, and DO26 (50 mg/L) solution at 495 nm, DB15 (50 mg/L) solution at 603 nm, respectively. A pseudo-first-order reaction was assumed for the concentration of DO26 or DB15 to evaluate the reaction kinetics. The integrated form of the first-order reaction is expressed as follows: ln At/A0 = −kt, where At is the absorbance at time t, A0 is the absorbance at time 0, and k is the rate constant.
2.8. Determination of the Dye DO26 Degradation Products Formaldehyde and Aniline
The determination of formaldehyde was accomplished by the high-performance liquid chromatography (HPLC) system (1260, Agilent Co., Santa Clara). It was eluted through a reversed-phase C18 column (Zorbax 300 extend-C18, 4.6 × 100 mm (3.5 μm), Agilent Co., Santa Clara). The mobile phase was water/acetonitrile, 45:55, at a flow rate of 1 mL/min, and UV detection was performed at 413 nm. The column temperature was at 40 °C, and the injection volume was 20 μL. The GC/MS analysis of aniline was performed on a Thermo Trace gas chromatograph (Thermo Finnigan, Miami, FL) coupled with Ultral ITQ 900 mass spectrometer (Thermo Finnigan, Miami, FL) under the following analytical conditions: DB-WAX column (15 m × 0.53 mm × 0.25 μm film thickness); helium (0.5 mL/min); programmed temperature 50–220 °C (7 °C/min); injector temperature (200 °C); and interface (230 °C).8
2.9. Fluorescent Properties of Fluorescein in the Presence of AgNPs
The fluorescent properties of fluorescein in the presence of AgNPs were determined by a reported method with minor modifications.18b In a series of 15 mL centrifuge tubes, 4 mL of FL aqueous solution at a concentration of 2.5 × 10–5 mol/L was added. Then, AgNPs with different volumes were added, respectively. Ten milliliters of the mixture was taken and further diluted 25 times in deionized water after blending. The concentration of FL in the mixed solution was constant at 4 × 10–7 mol/L, and the concentrations of AgNPs were 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024, and 2048 nmol/L. The fluorescence and absorption spectra were measured after 1 h in the dark. The fluorescence intensity was determined at 0, 2, 4, 6, 8, 10, 12, 24, 36, and 48 h, with the R. rosea AgNPs concentrations of 32 and 64 nmol/L. The absorption spectrum was determined by a Mapada UV-3200 UV–vis spectrophotometer. The fluorescence spectrum was determined by a Hitachi F4500 fluorescence spectrophotometer. All fluorescence tests were performed at room temperature, and the excitation slit and emission slit were maintained at 5 nm. The excitation wavelength was 489 nm, and the emission wavelength scanning range was 400–600 nm.
2.10. Statistical Analysis
Data are expressed as the mean value ± standard deviation (SD) and analyzed using a one-way analysis of variance (ANOVA). Duncan’s honestly significant difference (HSD) was used to determine the significant differences between treatments. All statistical procedures were conducted using SPSS 22.0 for Windows. A significance level of p < 0.05 was used.
3. Results and Discussion
3.1. Spectroscopic and Structural Characterization of Synthesized AgNPs
After adding the rhizome extract of R. rosea to the Ag+ solution, a visible color change was noticed from colorless and transparent to a dark gray colloidal solution (Figure 1). The color change indicates the formation of colloidal silver particles, confirmed by UV–vis spectroscopy (Figure 2). The R. rosea rhizome was extracted with water. Also, the extracted products were characterized by UV–vis spectrum, as shown in Figure 2. This extraction process was repeated three times, and the result shows consistent repeatability. Figure 2 shows the absence of an SPR peak for the R. rosea rhizome extract, but a sharp SPR peak for AgNPs appeared around 437 nm. The shape was also visualized and confirmed by the TEM images (Figure 3). The synthesized G-AgNPs are approximately 10 nm with a uniform distribution and a regular spherical shape. AgNPs were surrounded by the organic compounds present in the R. rosea rhizome extract solution (Figure 3a,b). Thus, the stability of colloidal AgNPs could be maintained for several months (data not shown). However, the synthesized C-AgNPs showed an SPR peak at around 400 nm (Figure 2) and a diameter of about 20 nm with a cluster distribution from TEM analysis (Figure 3c,d). In this study, the R. rosea AgNPs were synthesized at a lower temperature with a shorter time and lower silver nitrate concentration using R. rosea rhizome extract as reducing and capping agents. The results show this new method is simpler and more cost-effective than the existing methods.19,20
Figure 1.
Schematic depiction of the AgNPs preparation using R. rosea rhizome.
Figure 2.

UV–vis spectra of the R. rosea rhizome extract solution and synthesized G-AgNPs and C-AgNPs.
Figure 3.
TEM images of the synthesized silver nanoparticles under different magnifications: (a) G-AgNPs at 100 nm; (b) G-AgNPs at 5 nm; (c) C-AgNPs at 200 nm; and (d) C-AgNPs at 10 nm.
3.2. Evaluation of the Antioxidant Activity of AgNPs
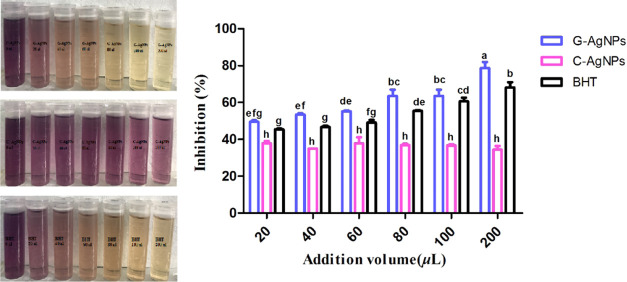
In this study, BHT was used as the control, and the DPPH free radical inhibition rate was used to compare the antioxidant activity of G-AgNPs and C-AgNPs. G-AgNPs significantly improved (p < 0.05) the DPPH free radical scavenging activity than BHT and C-AgNPs (Figure 4). The antioxidant capacity was enhanced with an increasing amount of G-AgNPs. The antioxidant activity of G-AgNPs was significantly higher (p < 0.05) than that of 40, 60, 80, or 200 μL of BHT. The scavenging efficiency of C-AgNPs on DPPH free radical was significantly lower (p < 0.05) than that of G-AgNPs and BHT at 20--200 μL. The scavenging efficiency was only 34.5–37.9%, with no significant difference between 20 and 200 μL additives (p > 0.05). The excellent antioxidant activity of G-AgNPs most likely is attributed to the antioxidant polyphenolic compounds such as phenylpropanoids, flavonoids, tannins, cinnamyl alcohol, and tyrosol in the colloidal G-AgNPs.
Figure 4.
Antioxidant activity of G-AgNPs and C-AgNPs by DPPH radical scavenging. Bars with different letters are significantly different (ANOVA and Duncan’s HSD, p < 0.05).
3.3. Catalytic Activity of Colloidal AgNPs on the Reduction of Direct Orange 26 by NaBH4
DO26 is an azo dye used to dye cellulosic yarns and fabrics, but it is non-biodegradable using conventional activated sludge processes. The synthesized colloidal AgNPs were directly used without further centrifugation to compare the catalytic abilities for the degradation of DO26.8 DO26 shows absorption maxima at 495 nm, and thus the absorbance value at 495 nm was selected to monitor the degradation of the dye. The DO26 solution’s absorption peak at 495 nm decreased with the increasing reaction time. It can also be seen from Figure 5b–d that with the decrease in the absorption peak at 495 nm, a new absorption peak appeared at 280 nm. The newly generated absorption peak at 280 nm in the catalytic reduction reaction may be related to the aniline produced by the reduction fracture of two −N-groups in the DO26 dye structure. Moreover, the absorption peak at 280 nm showed an increasing trend with the prolongation of the reaction time, indicating that more aniline was formed in the catalytic degradation process.8 The mechanism of nanometer silver’s catalytic degradation of DO26 is shown in Figure 6. It can be seen that aniline and formaldehyde are two key byproducts after the degradation of DO26. Aniline and formaldehyde were detected when the solutions were examined using gas chromatography–mass spectrometry (GC–MS) and HPLC (Figure 7). The appearance of aniline and formaldehyde supports the degradation mechanism of DO26, depicted in Figure 6.
Figure 5.
Reductive degradation of Direct Orange 26 using different catalysts: (a) comparison of the color change of mixtures, (b) G-AgNPs, (c) C-AgNPs, and (d) no AgNPs.
Figure 6.
Proposed catalytic action of AgNPs on the reduction of Direct Orange 26.
Figure 7.
Detection of aniline (a) and formaldehyde (b) produced by reductive degradation of Direct Orange 26 using AgNPs.
The rate constant and percentage dye degradation of DO26 at different times are summarized in Table 1. Both G-AgNPs and C-AgNPs could be a suitable catalyst for dye degradation; G-AgNPs is more effective than C-AgNPs. The first-order rate constants for these three compounds are in the order of G-AgNPs, C-AgNPs, and control. After 18 min of reaction, the degradation rates of DO26 by addition of G-AgNPs and C-AgNPs reached 72.8 and 40.5%, respectively. The retention rates after 18 min of reaction with G-AgNPs and C-AgNPs reached 10.8 and 4.7%, respectively. However, the retention rate and the degradation rate are reduced to 25.4 and 21.1% with the absence of AgNPs after the same time, respectively.
Table 1. Summary of Rate Constant and Percentage Dye Degradation of DO26.
| absorbance (λ = 495 nm) |
retention rate (100%) |
||||||
|---|---|---|---|---|---|---|---|
| catalyst | DO26 (50 mg/L) | 0 min | 18 min | degradation rate (100%) | 0 min | 18 min | first-order rate constant (min–1) |
| G-AgNPs | 1.1538 | 0.458 | 0.1246 | 72.79 | 39.69 | 10.80 | 0.0818 |
| C-AgNPs | 0.0906 | 0.0539 | 40.51 | 7.85 | 4.67 | 0.0359 | |
| control | 0.3718 | 0.2934 | 21.09 | 32.22 | 25.43 | 0.0138 | |
3.4. Catalytic Activity of Colloidal AgNPs on the Reduction of Direct Blue 15 by NaBH4
To compare the catalytic abilities of the DB15 degradation, the synthesized colloidal AgNPs were also directly used without further centrifugation. DB15 shows absorption maxima at 603 nm, and thus the absorbance value at 603 nm was selected to monitor the degradation of the dye. The absorption peak of DB15 at 603 nm continued to decrease with the extension of the reaction time. The rate constant and percentage dye degradation of DB15 at different times are summarized in Table 2. Both G-AgNPs and C-AgNPs could be suitable catalysts for dye degradation, but G-AgNPs are more effective than C-AgNPs. The first-order rate constants for these three compounds are in the order of G-AgNPs, C-AgNPs, and control. The degradation rates of DB15 reached 82.3 and 37.9% 18 min after the addition of G-AgNPs and C-AgNPs, respectively. The retention rates reached 10.8 and 4.0% 18 min after the addition of G-AgNPs and C-AgNPs, respectively. However, the retention rate and the degradation rate were reduced to 27.0 and 21.3% without AgNPs, respectively.
Table 2. Summary of Rate Constant and Percentage Dye Degradation of DB15.
| absorbance (λ = 603 nm) |
retention rate (100%) |
||||||
|---|---|---|---|---|---|---|---|
| catalyst | DB15 (50 mg/L) | 0 min | 18 min | degradation rate (100%) | 0 min | 18 min | first-order rate constant (min–1) |
| G-AgNPs | 0.7623 | 0.1704 | 0.0301 | 82.34 | 22.35 | 3.95 | 0.128 |
| C-AgNPs | 0.1326 | 0.0823 | 37.93 | 17.39 | 10.80 | 0.0374 | |
| control | 0.2615 | 0.2058 | 21.30 | 34.30 | 27.00 | 0.0165 | |
With the addition of colloidal AgNPs to the mixtures containing DO26 (or DB15) and NaBH4, the color of the dye solution changed immediately from a deep orange-red (or dark blue) to very light yellow with many bubbles. However, the dye solution without AgNPs showed no noticeable color change, which indicates that DO26 (or DB15) was not effectively degraded by NaBH4 alone (Figures 5 and 8 and Tables 1 and 2). The proposed mechanism of a nanometer silver’s catalytic degradation of DB15 is shown in Figure 9.
Figure 8.
Reductive degradation of Direct Blue 15 using different catalysts: (a) dye color change comparison, (b) G-AgNPs, (c) C-AgNPs, and (d) No AgNPs.
Figure 9.
Proposed catalytic action of AgNPs on the reduction of DB15.
The reduction of DO26 and DB15 can be explained in terms of the Langmuir–Hinshelwood model.10,23 NaBH4 acts as an electron donor and hydrogen supplier during the reduction process, changing the whole solution’s pH value.24 Thus, the surface charge of AgNPs becomes positive. The BH4– and DO26 (or DB15) were simultaneously adsorbed on a positively charged AgNPs surface. The catalytic reduction then begins by transferring electrons from the donor BH4– to DO26 (or DB15), where the AgNPs receive electrons from the BH4– ions and pass them on to the dye molecules. In the presence of AgNPs, a large amount of hydrogen provided by NaBH4 can lead to the hydrogenation of azo dyes.
3.5. Fluorescent Properties of Fluorescein in the Presence of AgNPs
The SPR peaks of the synthesized G-AgNPs and C-AgNPs were 437 and 400 nm, respectively. After the addition of fluorescein, the characteristic peak of the plasma resonance of silver nanoparticles remained unchanged. After the agglomeration of silver nanoparticles, a prominent absorption peak usually appears between 500 and 700 nm. However, no distinct absorption peak of silver nanoparticles was found in the absorption spectrum, indicating that silver nanoparticles’ agglomeration did not occur. Therefore, the characteristic absorption peak (475 nm) of the FL solution with AgNPs was red shifted. When the G-AgNPs concentrations were between 4 and 512 nmol/L, the peak positions all red shifted to 488 nm (Figure 10a). When the C-AgNPs concentrations were between 1 and 2048 nmol/L, the peak positions all red shifted to 485–489 nm (Figure 10b).
Figure 10.
Absorption spectra of the FL solution containing different amounts of G-AgNPs (a) and C-AgNPs (b).
FL molecules exist as anionic molecules in an aqueous solution. There is a strong interaction between the group COO– and silver nanoparticles, forming the FLn–Ag complex on the silver nanoparticles’ surface. Simultaneously, the strong local field generated by the surface plasma of AgNPs also affected the surface of adjacent FL molecules. The physical interaction of the local surface field and the chemical interaction between the surface molecules are the leading causes for the red-shift of the FL resonance absorption.
In this study, introducing low concentrations of G-AgNPs and C-AgNPs enhanced the FL fluorescence, which was mainly related to the following three factors. First, FL coated on silver nanoparticles’ surface makes the distance between FL fluorophore and silver nanoparticles close to the effective energy-transfer distance. Second, the local fields generated by silver nanoparticles are superimposed through the FL bridge, and the local fields are enhanced. Third, the formation of FLn–Ag complexes enhances light absorption. Under the combined action of these three factors, the excitation light energy collected by nanometer silver is transmitted to the FL molecule through a strong local field. This strong local field far exceeded the nonradiation transition energy transfer process, making the fluorescence enhancement effect greater than the quenching effect. Thus, the fluorescence enhancement effect is produced. When the concentration of G-AgNPs exceeds 512 nmol/L, the presence of a large number of silver nanoparticles, on the one hand, will inhibit the surface plasmon resonance of nanoparticles and reduce the local field. On the other hand, the collision quenching effect is strengthened. The overall result is a decrease in fluorescence intensity.
Figure 11a,b shows the emission spectra of G-AgNPs and C-AgNPs with various concentrations in the FL solution (excitation wavelength 489 nm). Figure 11c shows the relationship between the relative fluorescence enhancement efficiency and the concentration of synthetic AgNPs. Fluorescence enhancement efficiency refers to the ratio of FL containing silver nanoparticles to pure FL. As shown in Figure 11, the FL fluorescence intensity gradually increased with nanosilver solution. When the G-AgNP concentration was 64 nmol/L, the fluorescence intensity reached the maximum of 5460 (the FL concentration in the system was 4 × 10–7 mol/L), the fluorescence enhancement efficiency was 3.39. Subsequently, the fluorescence intensity began to decrease with the increase in the silver nanoparticle concentration. Compared with the fluorescence spectra of pure FL solution, the fluorescence spectra of FL solution with nanosilver did not change substantially, indicating that the formation of the FLn–Ag complex did not destroy the fluorophore in FL molecules. When the G-AgNPs concentration was between 1 and 512 nmol/L, the fluorescence enhancement efficiency was 1.07–3.39 (Figure 11a,c). Thus, G-AgNPs were used as fluorescence enhancers in the system. When the concentration was 1024 and 2048 nmol/L, the fluorescence intensity decreased, and the fluorescence enhancement efficiency was 0.84 and 0.72, respectively (Figure 11c). In this silver nanoparticle system, silver nanoparticles were used as quenching agents. Moreover, C-AgNPs were used as fluorescence enhancers in the system at higher concentrations. When the C-AgNPs concentration was 256 nmol/L, the fluorescence intensity reached the maximum of 3044 and the fluorescence enhancement efficiency was only 1.89 (Figure 11b,c).
Figure 11.

Emission spectra of the FL solution containing different amounts of (a) G-AgNPs and (b) C-AgNPs and comparison of the relative fluorescence enhancement efficiency (c).
As the fluorescence intensity reached the maximum when the G-AgNPs concentrations were 64 and 32 nmol/L (Figure 11), the fluorescence stability was conducted using these two concentrations. Figures 12 and 13 show that the fluorescence intensity stability of 64 nmol/L G-AgNPs was better than that of 32 nmol/L G-AgNPs. At different periods, the fluorescence intensity was significantly different from 0 h (p < 0.05). When the concentrations of G-AgNPs were 64 and 32 nmol/L, the fluorescence intensities at 48 h and 0 h were 91.80 and 61.51%, respectively.
Figure 12.
Emission spectra of the solution of FL containing 64 nmol/L G-AgNPs (a) and 32 nmol/L G-AgNPs (b) at different times.
Figure 13.
Fluorescence intensity of the FL solution containing 64 nmol/L G-AgNPs (a) and 32 nmol/L G-AgNPs (b) at different times. Bars with different letters are significantly different (ANOVA and Duncan’s HSD, p < 0.05).
4. Conclusions
This study has described a simple green synthesis of silver nanoparticles (G-AgNPs) using the rhizome extract of R. rosea, which acted both as a reducing and a capping agent. The efficacy of biogenic G-AgNPs has significantly improved for DPPH free radical scavenging activity than that of BHT or C-AgNPs (p < 0.05). The biogenic G-AgNPs were also found to function as an effective green catalyst, superior to C-AgNPs, in reducing DO26 and DB15 by NaBH4. In addition, G-AgNPs showed better fluorescence enhancement activity than C-AgNPs. The concentration required was lower, and the fluorescence activity was stable within 48 h. This study demonstrates the ability of biogenic G-AgNPs as an excellent catalyst in the degradation of DO26 and DB15, and they could be used as fluorescence enhancers in low concentrations.
Acknowledgments
The authors would like to express sincere thanks to Xingang Kong and Xiaohua Guo for instrumental analyses and Lina Meng, Tingting Gao, Jinhong Fu, and Xiaolong Duan for data determination.
This work was supported by the Key Project of Agricultural Science and Technology of Shaanxi Province (2021NY-154), the Postdoctoral Program in Shaanxi University of Technology (SLGBH16-04), and the Research Project of Shaanxi Provincial Education Department (20JS023).
The authors declare no competing financial interest.
References
- a Korkmaz N.; Ceylan Y.; Taslimi P.; Karadağ A.; Bülbül A. S.; Şen F. Biogenic nano silver: Synthesis, characterization, antibacterial, antibiofilms, and enzymatic activity. Adv. Powder Technol. 2020, 31, 2942–2950. 10.1016/j.apt.2020.05.020. [DOI] [Google Scholar]; b Schröfel A.; Kratošová G.; Šafařík I.; Šafaříková M.; Raška I.; Shor L. M. Applications of biosynthesized metallic nanoparticles: a review. Acta Biomater. 2014, 10, 4023–4042. 10.1016/j.actbio.2014.05.022. [DOI] [PubMed] [Google Scholar]; c Linnet J.; Walther A. R.; Albrektsen O.; Tavares L.; Eriksen R. L.; Jensen P. B. W.; Osadnik A.; Hassing S.; Lützen A.; Kjelstrup-Hansen J. Enhanced photoresponsivity in organic field effect transistors by silver nanoparticles. Org. Electron. 2017, 46, 270–275. 10.1016/j.orgel.2017.04.019. [DOI] [Google Scholar]; d Nayak D.; Ashe S.; Rauta P. R.; Kumari M.; Nayak B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng., C 2016, 58, 44–52. 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]; e Xu S.; Chen S.; Zhang F.; Jiao C.; Song J.; Chen Y.; Lin H.; Gotoh Y.; Morikawa H. Preparation and controlled coating of hydroxyl-modified silver nanoparticles on silk fibers through intermolecular interaction-induced self-assembly. Mater. Des. 2016, 95, 107–118. 10.1016/j.matdes.2016.01.104. [DOI] [Google Scholar]; f Seralathan J.; Stevenson P.; Subramaniam S.; Raghavan R.; Pemaiah B.; Sivasubramanian A.; Veerappan A. Spectroscopy investigation on chemo-catalytic, free radical scavenging and bactericidal properties of biogenic silver nanoparticles synthesized using Salicornia brachiata aqueous extract. Spectrochim. Acta, Part A 2014, 118, 349–355. 10.1016/j.saa.2013.08.114. [DOI] [PubMed] [Google Scholar]
- a Navaladian S.; Viswanathan B.; ViswanathR P.; Varadarajan T. K. Thermal decomposition as route for silver nanoparticles. Nanoscale Res. Lett. 2007, 2, 44–48. 10.1007/s11671-006-9028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mafuné F.; Jun-ya K.; Yoshihiro T.; Tamotsu K.; Hisahiro S. Structure and stability of silver nanoparticles in aqueous solution produced by laser ablation. J. Phys. Chem. B 2000, 104, 8333–8337. 10.1021/jp001803b. [DOI] [Google Scholar]; c Khaydarov R. A.; Khaydarov R. R.; Gapurova O.; Estrin Y.; Scheper T. Electrochemical method for the synthesis of silver nanoparticles. J. Nanopart. Res. 2009, 11, 1193–1200. 10.1007/s11051-008-9513-x. [DOI] [Google Scholar]; d Wang H.; Xueliang Q.; Jianguo C.; Shiyuan D. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf., A 2005, 256, 111–115. 10.1016/j.colsurfa.2004.12.058. [DOI] [Google Scholar]; e He C.; Liu L.; Fang Z.; Li J.; Guo J.; Wei J. Formation and characterization of silver nanoparticles in aqueous solution via ultrasonic irradiation. Ultrason. Sonochem. 2014, 21, 542–8. 10.1016/j.ultsonch.2013.09.003. [DOI] [PubMed] [Google Scholar]; f Pathrose B.; Nampoori V. P. N.; Radhakrishnan P.; Sahira H.; Mujeeb A. Effect of femtosecond laser ablated silver nanoparticles in the thermo-optic properties of basic fuchsin dye. Optik 2016, 127, 3684–3687. 10.1016/j.ijleo.2016.01.016. [DOI] [Google Scholar]; g Lerner M. I.; Alexander V P.; Elena A G.; Natalya V S.; Aleksandr S L.; Sergey G P. Structures of binary metallic nanoparticles produced by electrical explosion of two wires from immiscible elements. Powder Technol. 2016, 288, 371–378. 10.1016/j.powtec.2015.11.037. [DOI] [Google Scholar]; h Hassabo A. G.; Nada A. A.; Ibrahim H. M.; Abou-Zeid N. Y. Impregnation of silver nanoparticles into polysaccharide substrates and their properties. Carbohydr. Polym. 2015, 122, 343–350. 10.1016/j.carbpol.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Sana S. S.; Lakshman Kumar D. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater. Lett. 2018, 226, 47–51. 10.1016/j.matlet.2018.05.009. [DOI] [Google Scholar]
- Chahardoli A.; Karimi N.; Fattahi A. Nigella arvensis leaf extract mediated green synthesis of silver nanoparticles: Their characteristic properties and biological efficacy. Adv. Powder Technol. 2018, 29, 202–210. 10.1016/j.apt.2017.11.003. [DOI] [Google Scholar]
- Sudha A.; Jeyakanthan J.; Srinivasan P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour.-Effic. Technol. 2017, 3, 506–515. 10.1016/j.reffit.2017.07.002. [DOI] [Google Scholar]
- Mohapatra B.; Kuriakose S.; Mohapatra S. Rapid green synthesis of silver nanoparticles and nanorods using Piper nigrum extract. J. Alloys Compd. 2015, 637, 119–126. 10.1016/j.jallcom.2015.02.206. [DOI] [Google Scholar]
- Rolim W. R.; Milena T P.; de Araújo Lima B.; Letícia S F.; Fanny N C.; Juliana S B.; Tiago R.; Marcelo B.; Amedea B S. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66–74. 10.1016/j.apsusc.2018.08.203. [DOI] [Google Scholar]
- Ping Y.; Zhang J.; Xing T.; Chen G.; Tao R.; Choo K.-H. Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of Direct Orange 26. J. Ind. Eng. Chem. 2018, 58, 74–79. 10.1016/j.jiec.2017.09.009. [DOI] [Google Scholar]
- Vijaya J. J.; Jayaprakash N.; Kombaiah K.; Kaviyarasu K.; John Kennedy L.; Jothi Ramalingam R.; Al-Lohedan H. A.; Ma V. M.; Maaza M. Bioreduction potentials of dried root of Zingiber officinale for a simple green synthesis of silver nanoparticles: Antibacterial studies. J. Photochem. Photobiol., B 2017, 177, 62–68. 10.1016/j.jphotobiol.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Edison T. N. J. I.; Yong Rok L.; Mathur G. Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochim. Acta, Part A 2016, 161, 122–129. 10.1016/j.saa.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Baghizadeh A.; Ranjbar S.; Gupta V. K.; Asif M.; Pourseyedi S.; Karimi M. J.; Mohammadinejad R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015, 207, 159–163. 10.1016/j.molliq.2015.03.029. [DOI] [Google Scholar]
- a Khamparia S.; Jaspal D. K. Adsorption in combination with ozonation for the treatment of textile waste water: a critical review. Front. Environ. Sci. Eng. 2017, 11, 8 10.1007/s11783-017-0899-5. [DOI] [Google Scholar]; b Yang B.; Gao Y.; Yan D.; Xu H.; Wang J. Degradation Characteristics of Color Index Direct Blue 15 Dye Using Iron-Carbon Micro-Electrolysis Coupled with H(2)O(2). Int. J. Environ. Res. Public Health 2018, 15, 1523 10.3390/ijerph15071523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naje A. S.; Chelliapan S.; Zakaria Z.; Ajeel M. A.; Alaba P. A. A review of electrocoagulation technology for the treatment of textile wastewater. Rev. Chem. Eng. 2017, 33, 263–292. 10.1515/revce-2016-0019. [DOI] [Google Scholar]
- Robinson T.; Mcmullan G.; Marchant R.; Nigam P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. 10.1016/S0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Chiang H. M.; Chen H. C.; Wu C. S.; Wu P. Y.; Wen K. C. Rhodiola plants: Chemistry and biological activity. J. Food Drug Anal. 2015, 23, 359–369. 10.1016/j.jfda.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Panossian A.; Wikman G.; Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]; b Adamczak A.; Gryszczynska A.; Buchwald W. Biometric and phytochemical variability of roseroot (Rhodiola rosea L.) from field cultivation. Herba Pol. 2014, 60, 7–17. 10.2478/hepo-2014-0001. [DOI] [Google Scholar]
- a Yeh-Shuen C.; Hua-Chian L.; Chin-Feng C. Tyrosinase inhibitory effect and antioxidative activities of fermented and ethanol extracts of Rhodiola rosea and Lonicera japonica. Sci. World J. 2013, 2013, 612739 10.1155/2013/612739. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Peschel W.; Kump A.; Zomborszki Z. P.; et al. Phenylpropenoid content in high-altitude cultivated Rhodiola rosea L. provenances according to plant part,harvest season and age. Ind. Crops Prod. 2018, 111, 446–456. 10.1016/j.indcrop.2017.10.007. [DOI] [Google Scholar]; c Marchev A. S.; Dimitrova P.; Koycheva I. K.; et al. Altered expression of TRAIL on mouse T cells via ERK phosphorylation by Rhodiola rosea L. and its marker compounds. Food Chem. Toxicol. 2017, 108, 419–428. 10.1016/j.fct.2017.02.009. [DOI] [PubMed] [Google Scholar]; d Marchev A. S.; Ina Y.; Ivanka K K.; et al. Phytochemical variations of Rhodiola rosea L. wild -grown in Bulgaria. Phytochem. Lett. 2017, 20, 386–390. 10.1016/j.phytol.2016.12.030. [DOI] [Google Scholar]
- a Gill R.; Lijin T.; Walter R C S.; Eric C Le R.; Amerongen Hv.; Vinod SubramaniamGill R. Silver nanoparticle aggregates as highly efficient plasmonic antennas for fluorescence enhancement. J. Phys. Chem. C 2012, 116, 16687–16693. 10.1021/jp305720q. [DOI] [Google Scholar]; b Wang Y.; Zhou J.; Wang T. Effects of silver nanoparticles on fluorescent properties of fluorescein. Spectrosc. Spectral Anal. 2007, 27, 1555–1559. [Google Scholar]
- Pawliszak P.; Malina D.; Sobczak-Kupiec A. Rhodiola rosea extract mediated green synthesis of silver nanoparticles supported by nanosilica carrier. Mater. Chem. Phys. 2019, 234, 390–402. 10.1016/j.matchemphys.2019.05.027. [DOI] [Google Scholar]
- Singh P.; Pandit S.; Beshay M.; Mokkapati V.; Garnaes J.; Olsson M. E.; Sultan A.; Mackevica A.; Mateiu R. V.; Lutken H.; Daugaard A. E.; Baun I.; Mijakovic I. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif. Cells, Nanomed., Biotechnol. 2018, 46, S886–S899. 10.1080/21691401.2018.1518909. [DOI] [PubMed] [Google Scholar]
- Singh P.; Kim Y. J.; Wang C.; et al. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells, Nanomed., Biotechnol. 2016, 44, 1150–1157. 10.3109/21691401.2015.1011809. [DOI] [PubMed] [Google Scholar]
- Hamelian M.; Varmira K.; Veisi H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol., B 2018, 184, 71–79. 10.1016/j.jphotobiol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Wunder S.; Frank P.; Yan L.; Yu M.; Matthias B. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. 10.1021/jp101125j. [DOI] [Google Scholar]
- Dong Z.; Xuanduong L.; Yansheng L.; Chunxu D.; Jiantai M. Metal organic framework derived magnetic porous carbon composite supported gold and palladium nanoparticles as highly efficient and recyclable catalysts for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. J. Mater. Chem. A 2014, 2, 18775–18785. 10.1039/C4TA04010D. [DOI] [Google Scholar]