Abstract
Macamides are characteristically found in maca (Lepidium meyenii Walper). Fatty acid derivatives are also an important type of constituent in maca, since they not only relate to the biosynthesis of macamides in the postharvest process but also possess some bioactivities. To study their comprehensive profiles in maca tubers processed via the air-drying method, ultraperformance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) analyses were performed to identify macamide and fatty acid molecules. Their contents in maca tubers that were processed via air drying and freeze drying, respectively, were further quantified using high-performance liquid chromatography (HPLC) analyses comparing with eight macamide and three fatty acid reference standards. A total of 19 macamides (including four novel ones) and 16 fatty acid derivatives (two found in maca for the first time) were identified. Quantification analyses results showed the eight macamides with contents ranging from 31.39 to 1163.19 μg/g (on dry tuber), and fatty acids from 18.71 to 181.99 μg/g in the air-dried maca, but there were only three macamides and one fatty acid detected with very low contents (3.97–34.36 μg/g) in the freeze-dried maca. The results demonstrated that the air-drying method can increase the accumulations of macamides and fatty acids in the metabolism of maca in the postharvest process. The biosynthesis of two types of macamides, i.e., N-benzyl-oxo-octadecadienamides and N-benzyl-oxo-octadecatrienamides, was further elucidated in detail. These results provide more valuable insights into the phytochemicals of maca, which is helpful to explain its health benefits.
1. Introduction
Maca (Lepidium meyenii Walpers), being native to Peruvian Central Andes, is an important traditional plant in South America. In the past 2000 years, it was also called Peruvian ginseng and used as a functional food for strengthening body and improving sexual performance.1 Modern studies demonstrated that Maca is a safe food containing numerous nutrients, such as dietary fiber, mineral elements, essential amino acids, vitamins, etc.2 Modern phytochemical and pharmacological studies demonstrated that some types of unique constituents in maca, such as macamides (benzylamides of long-chain fatty acids) and macaenes (unsaturated long-chain fatty acid derivatives), are responsible for its various bioactivities.2,3 Maca tuber was recommended as a functional food by Food and Agriculture Organization (FAO) in 1992 and has been getting more popular in many countries since then.4 In the early 21st century, Maca was successfully transplanted and cultivated in some regions in Yunnan province and Tibet Autonomous Region of China, where the altitude of these regions is similar to that of Peruvian Central Andes. According to the color of its tubers, there are three major types of maca: black, purple, and yellow. The phytochemicals and health benefits were different between these types of maca, based on their color and cultivation origins.5−7
Because maca is extensively used for functional foods and dietary supplements worldwide, it is significant to comprehensively evaluate the quality, bioactivity, and authentication of maca material, including those cultivated in China. There were several studies focusing on the comprehensive quality evaluation of maca using a variety of analytical technologies, including liquid chromatography-tandem mass spectrometry (LC-MS/MS), inductively coupled plasma-mass spectrometry, and Fourier transform infrared spectroscopy.8−11 Determination of macamides, glucosinolates, and fatty acid derivatives using these technologies was efficiently used for the quality assessment and process control of maca.12,13 LC-MS/MS could be the best to determine the phytochemical profiles of Maca and authenticate the raw materials combined with multivariate statistical analysis.6,10
Macamides are recognized as a type of characteristic marker for quality evaluation of maca. Maca products contained abundant macamides that can significantly enhance semen quality and serum hormones, such as luteinizing hormone, estradiol, testosterone, free thyroxin, etc.,14 suggesting the importance of macamide content for Maca product quality. However, some studies demonstrated that macamide formation is directly related to postharvest processing, including drying process, storage, cleaning, etc.15−17 Our previous study results also showed that the drying process, especially lyophilization and steaming has a huge impact on the accumulation of macamides and suggested that air drying and oven drying are the appropriate postharvest processing for maca.18 Chen and his colleagues further optimized the postharvest conditions, indicating that drying temperature, sample forms, and storage period are the key factors for the accumulation of macamides.15 Given this fact, authentication of maca source based on macamides requires considering the influence of the drying process and storage.
We have preliminarily hypothesized in our previous publication12 that the formation of macamides in the postharvest drying process is related to macaenes and other long-chain fatty acid derivatives. It was explained that macamides are synthesized via the reaction of benzylamine or its substitutes and long-chain fatty acids catalyzed by enzymes during the drying process. Long-chain fatty acids are the precursors of macamides. Thus, the aims of this study are to clarify the macamide and fatty acid derivative profiles in maca tubers, which were processed via the air-drying method using ultraperformance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS), to quantify their contents in maca tubers, which were processed via air-drying and freeze-drying methods, using high-performance liquid chromatography (HPLC), and to further elucidate the biosynthetic pathways of some macamides, thus to provide more valuable insights of the phytochemicals of maca being helpful to explain its health benefits.
2. Materials and Methods
2.1. Chemicals
There were 11 reference standards, including that (Table 1) of compounds 14, 15, 18, 21, and 22, isolated from maca by column chromatography and preparative HPLC in our laboratory. The reference standards of 12, 17, 19, 26, 29, and 33 were synthesized in our laboratory. Their purity was above 98% as tested using HPLC. Their identities were confirmed using the spectroscopic methods nuclear magnetic resonance (NMR) and high-resolution electrospray ionization mass spectroscopy (HR ESI-MS) in a separate publication. Analytical-grade trifluoroacetic acid (TFA) and methanol (MeOH) were bought from Chengdu Kelon Chemical Reagent Factory (Chengdu, China). HPLC-grade acetonitrile (ACN) was purchased from Sigma (St. Louis, MO).
Table 1. Macamides and Fatty Acid Derivatives Identified in the Air-Dried Maca.
| peak no. | RT (min) | [M – H]− (m/z) | [M + H]+ (m/z) | formula | mode | fragment ions MS/MS (m/z) | identified compounds | UV max (nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.1 | 307.1909 | C18H28O4 | – | 307, 289, 235, 211, 209, 185, 121 | 16-hydroxy-9-oxo-10E,12E,14E-octadecatrienoic acid | 314 | |
| 2 | 4.0 | 305.1754 | C18H26O4 | – | 305, 287, 249, 135, 125 | 9,16-dioxo-10E,12E,14E-octadecatrienoic acid | 317 | |
| 3 | 5.0 | 398.2703 | C25H35NO3 | + | 398, 380, 273, 260, 108, 91 | N-benzyl-16(S)-hydroxy-9-oxo-10E,12E,14E-octadecatrienamide | 314 | |
| 4 | 6.3 | 396.2537 | C25H33NO3 | + | 396, 380, 300, 289, 271, 260, 108, 91 | N-benzyl-9,16-dioxo-10E,12E,14E-octadecatrienamide | 317 | |
| 5 | 6.7 | 234.1863 | C15H23NO | + | 234, 108, 91 | N-benzyl-octanamide | ||
| 6 | 7.5 | 293.2114 | C18H30O3 | – | 293, 275, 235, 171, 121 | 9-hydroxy-10E,12Z,15Z-octadecatrienoic acid | 236 | |
| 7 | 8.5 | 291.1956 | C18H28O3 | – | 291, 273, 235, 195, 111, 97 | 13-oxo-9E,11E,15E-octadecatrienoic acid | 276 | |
| 8 | 8.9 | 291.1956 | C18H28O3 | – | 291, 273, 197, 185, 125, 121 | 9-oxo-10E,12Z,15Z-octadecatrienoic acid | 279 | |
| 9 | 9.2 | 291.1963 | C18H28O3 | – | 291, 273, 197, 185, 125, 121 | 9-oxo-10E,12E,14E-octadecatrienoic acid | 276 | |
| 10 | 9.4 | 295.2280 | C18H32O3 | – | 295, 277, 195, 133 | 13-hydroxy-9Z,11E-octadecadienoic acid | 234 | |
| 11 | 9.5 | 295.2271 | C18H32O3 | – | 295, 277, 195, 171, 125 | 9-hydroxy-10E,12Z-octadecadienoic acid | 234 | |
| 12 | 10.3 | 293.2112 | C18H30O3 | – | 293, 275, 249, 195, 185, 113 | 13-oxo-9Z,11E-octadecadienoic acid | 279 | |
| 13 | 10.5 | 293.2117 | C18H30O3 | – | 293, 275, 249, 195, 185, 113 | 13-oxo-9E,11E-octadecadienoic acid | 276 | |
| 14 | 10.9 | 293.2117 | C18H30O3 | – | 293, 275, 249, 197, 185, 125 | 9-oxo-10E,12Z-octadecadienoic acid | 279 | |
| 15 | 11.3 | 293.2117 | C18H30O3 | – | 293, 275, 249, 197, 185, 125 | 9-oxo-10E,12E-octadecadienoic acid | 276 | |
| 16a | 11.6 | 382.2726 | C25H35NO2 | + | 382, 364, 286, 275, 260, 257, 108, 91 | N-benzyl-13-oxo-9E,11E,15E-octadecatrienamide | 276 | |
| 17a | 11.9 | 382.2742 | C25H35NO2 | + | 382, 364, 288, 275, 260, 108, 91 | N-benzyl-9-oxo-10E,12Z,15Z-octadecatrienamide | 279 | |
| 18a | 12.2 | 382.2745 | C25H35NO2 | + | 382, 364, 288, 275, 260, 108, 91 | N-benzyl-9-oxo-10E,12E,14E-octadecatrienamide | 276 | |
| 19a | 13.2 | 384.2901 | C25H37NO2 | + | 384, 366, 286, 277, 260, 108, 91 | N-benzyl-13-oxo-9Z,11E-octadecadienamide | 279 | |
| 20 | 13.4 | 384.2896 | C25H37NO2 | + | 384, 366, 286, 277, 260, 108, 91 | N-benzyl-13-oxo-9E,11E-octadecadienamideN-benzyl-13-oxo-9E,11E-octadecadienamideN-benzyl-13-oxo-9E,11E-octadecadienamide | 276 | |
| 21 | 14.0 | 384.2908 | C25H37NO2 | + | 384, 366, 277, 260, 108, 91 | N-benzyl-9-oxo-10E,12Z-octadecadienamide | 279 | |
| 22 | 14.4 | 384.2913 | C25H37NO2 | + | 384, 366, 277, 260, 108, 91 | N-benzyl-9-oxo-10E,12E-octadecadienamide | 276 | |
| 23 | 15.9 | 386.3047 | C25H39NO2 | + | 386, 368, 279, 260, 232, 108, 91 | N-benzyl-9-oxo-12E-octadecadienamide | ||
| 24 | 16.4 | 277.2172 | C18H30O2 | – | 277 | (Z,Z,Z)-9,12,15-octadecatrienoic acid | ||
| 25 | 18.5 | 398.3046 | + | 398, 138, 121 | N-(3-methoxybenzyl)-9Z,12Z,15Z-octadecatrienamide | |||
| 26 | 18.9 | 368.2949 | + | 368, 108, 91 | N-benzyl-9Z,12Z,15Z-octadecatrienamide | |||
| 27 | 19.0 | 279.2332 | C18H32O2 | – | 279 | (Z,Z)-9,12-octadecatrienoic acid | ||
| 28 | 20.8 | 400.3208 | + | 400, 138, 121 | N-(3-methoxybenzyl)-9Z,12Z-octadecadienamide | |||
| 29 | 21.1 | 370.3109 | + | 370, 108, 91 | N-benzyl-9Z,12Z-octadecadienamide | |||
| 30 | 21.2 | 255.2332 | C16H32O2 | – | 255 | 1-Pentadecanecarboxylic acid | ||
| 31 | 21.7 | 281.2494 | C18H34O2 | – | 281 | (Z)-9-octadecatrienoic acid | ||
| 32 | 22.6 | 376.3211 | + | 376, 138, 121 | N-(3-methoxybenzyl)-hexadecanamide | |||
| 33 | 22.9 | 346.3102 | + | 346, 108, 91 | N-benzyl-hexadecanamide | |||
| 34 | 23.1 | 402.3367 | + | 402, 138, 121 | N-(3-methoxybenzyl)-9Z-octadecadienamide | |||
| 35 | 23.4 | 372.3261 | + | 372, 108, 91 | N-benzyl-9Z-octadecadienamide |
Novel macamides identified in this study.
2.2. Materials and Postharvest Processing
Fresh maca tubers were harvested in a farm at an altitude of 3700 m in Lasa, Tibet Autonomous Region, China, in November 2016, and authenticated by Prof. Wanyi Li. Half of the fresh maca tubers were dried naturally in an open plate at room temperature until they were crisp. The other half of the fresh maca were cut into slices and dried at −60 °C at a vacuum of 0.1 kPa using an FD-1D-50 freeze dryer (Beijing Bilang Experimental Equipment Co., Ltd., Beijing, China). All of the dried maca tubers and slices were milled into fine powder and stored at −18 °C for preparation of tests.
2.3. Sample Preparation
Test sample solutions were prepared according to the method described in a previous study,19 with some modification. Briefly, 1 g of dry maca powder was weighed and homogenized with methanol at a ratio of 1:10 (w/v) and extracted at 40 °C for 1 h with ultrasound assistance. All testing solutions were filtered through 0.22 μm nylon membrane filters before injection into the UPLC-QTOF-MS and HPLC system.
2.4. Identification and Quantification of Macamides and Fatty Acid Derivatives
Identification of UPLC-QTOF-MS analyses was carried out using an Agilent 1290 HPLC system interfaced with Agilent 6545 QTOF MS (Agilent Technologies). The 1290 HPLC system was equipped with a quaternary pump, online vacuum degasser, autosampler, and a diode-array detector. The separation of the compounds was carried out on a Poroshell 120 EC-C18 column (3 × 100 mm2, 2.7 μm) operated at 40 °C. The mobile phase that consists of 0.1% acetic acid (5 mM/L ammonium acetate) in water (A) and acetonitrile (B) was delivered at a flow rate of 0.3 mL/min under a gradient program, which was set at 0–5 min (50–40% B), 5–22 min (40–5% B), and 22–25 min (5% B). The sample injection volume was 2 μL. The diode-array detector was set to detect at 280 nm and the online UV spectra were recorded in the scanning range of 200–400 nm.
A mass spectrometer was operated in the negative and positive electrospray ionization mode. Mass spectra were recorded by scanning the mass range of m/z 50–1000 in both MS and MS/MS modes. Nitrogen was used as a drying, nebulizing, and collision gas. The drying gas flow rate was 12 L/min. The heated capillary temperature was set at 350 °C and the nebulizer pressure at 45 psi. The source parameters capillary voltage (Vcap) and fragmentor, skimmer, and octupole voltages were set to 3500, 175, 65, and 750 V, respectively. For the MS/MS analysis, collision energy was set at 10 and 20 eV. Accurate mass data of the molecular ions were processed through the Mass Hunter version B 04.00 (Agilent Technology Santa Clara, CA).
2.5. Quantification via HPLC
Quantification analyses were performed according to our previous publication with minor revision,12 using an Agilent 1260 HPLC system (Agilent Technologies). A Zorbax XDB-C18 column (250 mm × 4.6 mm, 5 μm particle size, Agilent Technologies) was used for separation. The mobile phase consisted of 0.005% trifluoroacetic acid in water (solvent A) and 0.005% trifluoroacetic acid in acetonitrile (solvent B). The gradient program was set at 0–35 min (55–95% B), 35–40 min (95–100% B), and 40–45 min (100% B), with a flow rate of 1 mL/min. The column temperature was 40 °C. The injection volume was 10 μL and detection wavelengths were both 210 and 280 nm.
All tests were performed in triplicate.
3. Results and Discussion
3.1. Characterization of Macamides and Fatty Acid Derivatives
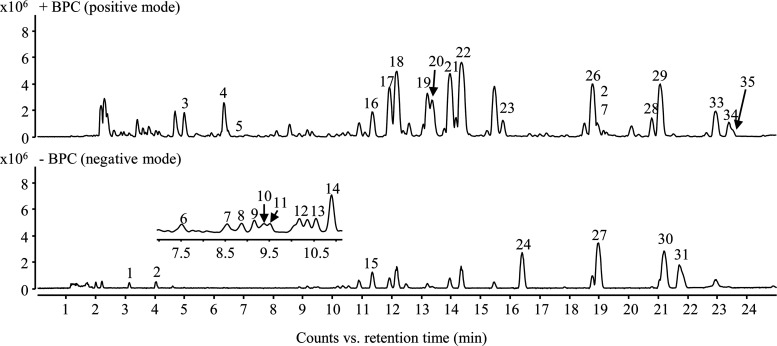
There are more than 100 compounds that have been identified and characterized in maca and 30% of them are fatty acid derivatives and macamides.3,20 A private database of fatty acid derivatives and macamides was established according to the reported structure. A total of 35 compounds including 16 fatty acid derivatives and 19 macamides in the air-dried maca were tentatively identified by UPLC-QTOF-MS (Table 1). The chemical profiling of air-dried maca was evaluated under optimized chromatographic conditions (Figure 1).
Figure 1.
Macamide and fatty acid derivative profiles of air-dried maca.
Our previous study indicated that the diagnosed ions of N-benzyl-type macamides and N-(3-methoxybenzyl)-type macamides were m/z 91 [C7H7]+ and 108 [C7H10N]+ as well as m/z 121 [C8H9O]+and 138 [C8H12ON]+, respectively.18 This allows us to rapidly distinguish macamides and non-macamides in chemical profiling. The target peaks based on their product ions were divided into three groups: N-benzyl-type macamides, N-(3-methoxybenzyl)-type macamides, and non-macamides.
For N-benzyl-type macamides (peaks 3–5, 16–23, 26, 29, 33, and 35), except for peaks 16–19, most of them were known compounds.21,22 Peaks 25, 28, 32, and 34 were the N-(3-methoxybenzyl)-type macamides.23 Characterization was processed using the “Find by formula” algorithm in Agilent Mass Hunter Workstation Software with mass accuracies of <20 ppm between the theoretical mass and calculated mass. The high-resolution mass data, MS/MS fragment ions, and UV maximal absorption wavelengths of identified compounds are listed in Table 1.
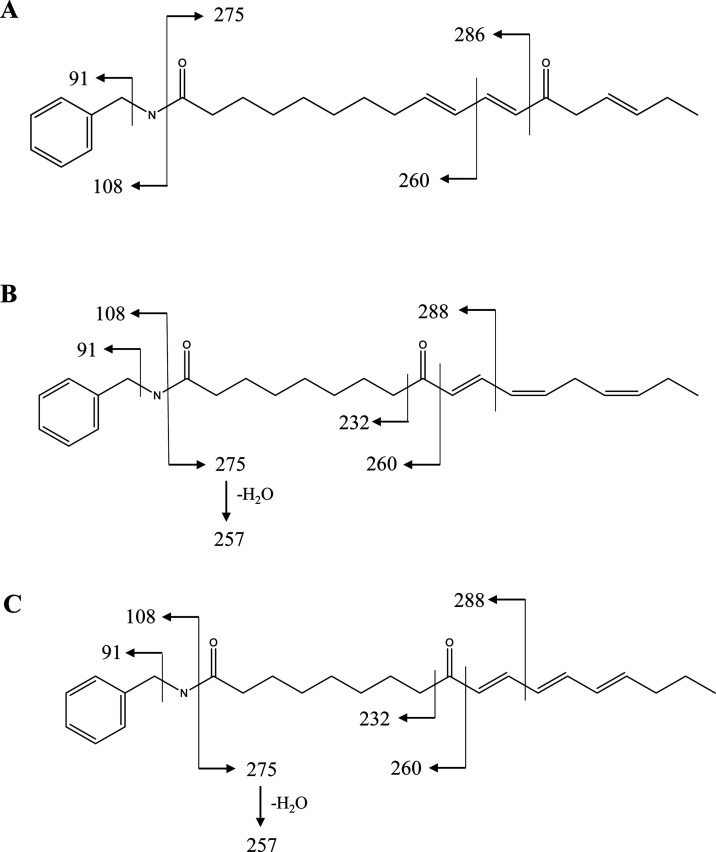
According to the UPLC-QTOF-MS data, peaks 16–18 are a group of isomers with the N-benzyl nucleus. Their common fragmentation ions are m/z 364, m/z 275, m/z 260, and m/z 257, which are assigned to [M – H2O + H]+, [M – C7H9N + H]+, [M – C9H14 + H]+, and [M – C7H9N – H2O + H]+, respectively. Peaks 17 and 18 share highly similar fragmentation ions (Table 1). In the same manner, m/z 288 is assigned as [M – C7H10 + H]+ through losing neutral moieties (CH3CH2CH=CHCH2C≡CH). The only difference in them is the UV maximal absorption wavelengths, implying peaks 17 and 18 are cis–trans-isomers of N-benzyl-9-oxo-10,12,15-octadecatrienamide. Their fragmentation patterns are shown in Figure 2. In comparison with the UV maximal absorption wavelengths of peak 14 (λmax 279 nm) and peak 15 (λmax 276 nm), peaks 17 and 18 are identified as N-benzyl-9-oxo-10E,12Z,15Z-octadecatrienamide and N-benzyl-9-oxo-10E,12E,14E-octadecatrienamide by the reference standards, respectively. For peak 16, m/z 286 was assigned as [M – C6H8O + H]+, which was derived from cleavage of C12–C13. Compared with the UV maximal absorption wavelengths of peak 18 (λmax 276 nm), peak 16 was tentatively identified as N-benzyl-13-oxo-9E,11E,15E-octadecatrienamide.
Figure 2.
Fragmentation patterns of peak 16 (A), peak 17 (B), and peak 18 (C).
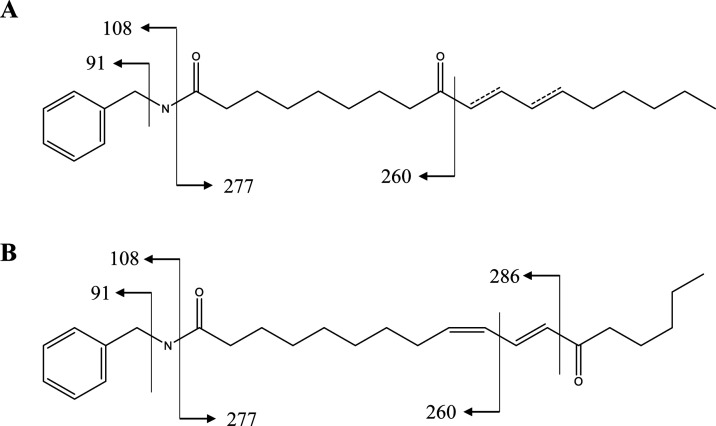
Additionally, peaks 19–21 were another group of the N-benzyl nucleus sharing the same parent ion of m/z 384.2901. Among them, peaks 21 and 22 were identified as N-benzyl-9-oxo-10E,12Z-octadecadienamide and N-benzyl-9-oxo-10E,12E-octadecadienamide based on published data22 (Figure 3A). The characteristic fragmentation ion (m/z 286) [M – C6H8O + H]+ was observed in MS2 data of peaks 19 and 20 (Figure 3B), but it was absent in that of peaks 21 and 22. By further confirmation using synthetic reference standard, peak 19 was tentatively identified as N-benzyl-13-oxo-9Z,11E-octadecadienamide. Notably, this is the first report of four novel macamides being observed in maca.
Figure 3.
Fragmentation patterns of peaks 21 and 22 (A) and peak 19 (B).
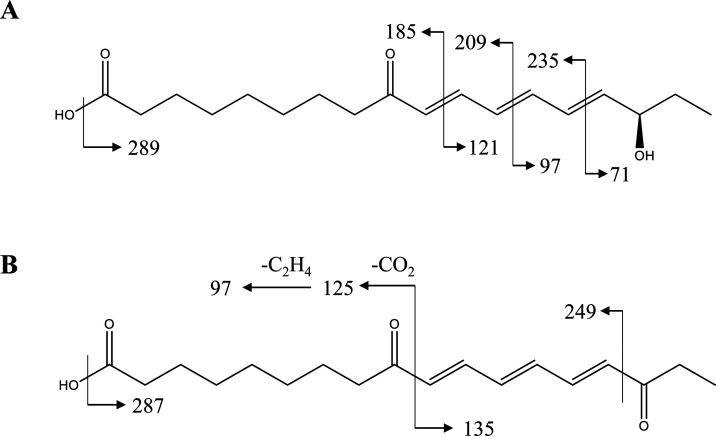
Other peaks are fatty acid derivatives displaying the [M – H]− ions in the negative ion mode. In this case, peak 1 (m/z 307.1909) and peak 2 (m/z 305.1754) were selected as examples for the characterization of fatty acid derivatives. According to its fragmentation pattern and literature data,24 peak 1 was tentatively identified as 16-hydroxy-9-oxo-10E,12E,14E-octadecatrienoic acid. A series of cleavages were observed at the conjugated triene structure (C10–C11, C12–C13, and C14–C15), which corresponded to m/z 235 ([M – C4H7O – H]−), m/z 209 ([M – C6H9O – H]−), m/z 185 ([M – C4H7O – H]−), m/z 121 ([M – C10H17O – H]−), m/z 97 ([M – C12H17O3 – H]−), and m/z 71 [M – C14H19O3 – H]− (Figure 4A). Compared with the fragmentation pattern of peak 1, peak 2 (Figure 4B) was the dehydrogenation product of 16-hydroxy-9-oxo-10E,12E,14E-octadecatrienoic acid, namely, 9,16-dioxo-10E,12E,14E-octadecatrienoic acid.25 Notably, peaks 1 and 2 were also detected in Maca for the first time.
Figure 4.
Fragmentation patterns of peak 1 (A) and peak 2 (B).
3.2. Quantification of Macamides and Fatty Acid Derivatives
For comparative studies, 11 macamides and fatty acid derivatives in both air-dried and freeze-dried Maca hypocotyls were examined quantifiably using HPLC. HPLC method calibrations of the 11 compounds are presented in Table 2. The linearity was satisfactory with determination coefficients (R2) greater than 0.9998 in the concentration range of 0.25–220 μg/mL. The limit of detection (LOD) and limit of quantitation (LOQ) were calculated based on signal-to-noise (S/N) ratios of 3 and 10, respectively.
Table 2. Calibrations of the 11 Macamides and Fatty Acid Derivatives.
| compound | RT (min) | equation | linear range (μg/mL) | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| 13-oxo-9Z,11E-octadecadienoic acid | 15.52 | Y = 106.6X | 0.25–63.18 | 0.9998 | 0.146 | 0.045 |
| 9-oxo-10E,12Z-octadecadienoic acid | 16.40 | Y = 107.52X | 0.49–62.5 | 0.9999 | 0.145 | 0.044 |
| 9-oxo-10E,12E-octadecadienoic acid | 17.09 | Y = 106.99X | 0.28–144.55 | 0.9999 | 0.147 | 0.044 |
| N-benzyl-9-oxo-10E,12Z,15Z-octadecadienamide | 17.95 | Y = 82.349X | 0.38–97.80 | 0.9998 | 0.159 | 0.047 |
| N-benzyl-9-oxo-10E,12E,14E-octadecadienamide | 18.29 | Y = 81.146X | 0.53–69.24 | 0.9999 | 0.164 | 0.049 |
| N-benzyl-13-oxo-9Z,11E-octadecadienamide | 20.54 | Y = 1.301X | 0.34–86.98 | 0.9998 | 0.169 | 0.051 |
| N-benzyl-9-oxo-10E,12Z-octadecadienamide | 21.56 | Y = 81.487X | 0.143–73.1 | 0.9999 | 0.236 | 0.071 |
| N-benzyl-9-oxo-10E,12E-octadecadienamide | 22.16 | Y = 83.293X | 0.25–127.06 | 0.9999 | 0.161 | 0.048 |
| N-benzyl-9Z,12Z,15Z-octadecatrienamide | 29.7 | Y = 56.774X | 0.58–118.5 | 0.9999 | 0.275 | 0.082 |
| N-benzyl-9Z,12Z-octadecadienamide | 34.0 | Y = 39.552X | 0.4–118.5 | 0.9998 | 0.276 | 0.083 |
| N-benzyl-hexadecanamide | 37.6 | Y = 32.199X | 0.6–220 | 0.9999 | 0.272 | 0.082 |
It is apparent from Table 3 that there were only three macamides and one fatty acid detected with very low contents (3.97–34.36 μg/g, on dry tuber, same below) in the freeze-dried maca. On the contrary, the level of macamides and fatty acid derivatives in air-dried maca is far more than that in freeze-dried maca, showing the eight macamides with contents ranging from 31.39 to 1163.19 μg/g and fatty acids from 18.71 to 181.99 μg/g. These results are consistent with our previous study.18N-Benzyl-hexadecanamide (1163.19 ± 0.49 μg/g) exhibited the highest macamide content and 9-oxo-10E,12E-octadecadienoic acid (181.99 ± 0.77 μg/g) was concluded to have the highest contribution to the content of fatty acid derivatives. Although freeze drying could efficiently decrease the drying time, it concurrently interrupted the biosynthetic pathway of macamides. Previous study showed that a negative correlation was observed between postharvest time of maca and glucosinolate content, which is the metabolic precursor of macamides.13 The content of macamides was increased with the increase in storage time. In a combination of our results, presumably, the drying process may have a huge impact on the key enzyme catalyzing the formation of macamides. This suggests that an optimized postharvest promotes the activation of the biosynthetic pathway of macamides.
Table 3. Contents of Macamides and Fatty Acid Derivatives in Maca Using HPLC Quantification.
| contenta (mean ± SD μg/g) |
||
|---|---|---|
| compounds | freeze drying | air drying |
| 13-oxo-9Z,11E-octadecadienoic acid | nd | 18.71 ± 0.14 |
| 9-oxo-10E,12Z-octadecadienoic acid | 3.97 ± 0.05 | 77.15 ± 0.41 |
| 9-oxo-10E,12E-octadecadienoic acid | nd | 181.99 ± 0.77 |
| N-benzyl-9-oxo-10E,12Z,15Z-octadecadienamide | nd | 58.28 ± 0.44 |
| N-benzyl-9-oxo-10E,12E,14E-octadecadienamide | nd | 199.98 ± 0.14 |
| N-benzyl-13-oxo-9Z,11E-octadecadienamide | nd | 31.39 ± 0.06 |
| N-benzyl-9-oxo-10E,12Z-octadecadienamide | nd | 165.97 ± 0.73 |
| N-benzyl-9-oxo-10E,12E-octadecadienamide | nd | 432.05 ± 1.45 |
| N-benzyl-9Z,12Z,15Z-octadecatrienamide | 34.36 ± 0.43 | 354.33 ± 4.82 |
| N-benzyl-9Z,12Z-octadecadienamide | 28.07 ± 0.75 | 595.494 ± 1.04 |
| N-benzyl-hexadecanamide | 6.72 ± 0.38 | 1163.19 ± 0.49 |
nd: not detected.
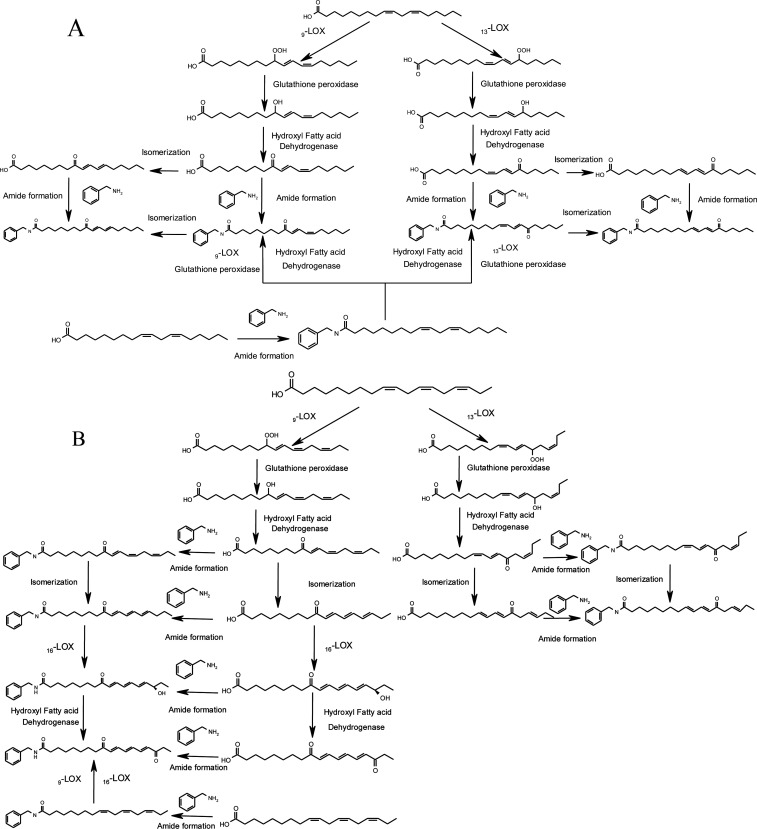
3.3. Biosynthetic Pathway of N-Benzyl-oxo-octadecadienamides and N-Benzyl-oxo-octadecatrienamides
According to the quantification analysis, N-benzyl-oxo-octadecadienamides and N-benzyl-oxo-octadecatrienamides are the most abundant macamides in the air-dried sample. Briefly, macamide formation is based on the amidation reaction of macaenes and benzylamine.17 In this case, we propose biosynthetic pathways of these macamides as following.
9-Hydroperoxy-10E,12Z-octadecadienoic acid and 13-hydroperoxy-9Z,12E-octadecadienoic acid were synthesized by linoleic acid through catalyzing by lipoxygenase enzymes.26 Then, 9 or 13-hydroperoxy-10E,12Z-octadecadienoic acid was transferred into the basic skeleton of macaenes 9 or 13-hydroxy-10E,12Z-octadecadienoic acid and 9 or 13-oxo-10E,12Z-octadecadienoic acid via catalyzing by glutathione peroxidase and hydroxy fatty acid dehydrogenase. More octadecadienoic acid isomers were generated by isomerization, resulting in the chemical diversity of macamides. In the same manner, linolenic acid was transferred into a variety of octadecatrienoic acid isomers. The putative biosynthetic pathways of N-benzyl-oxo-octadecadienamides and N-benzyl-oxo-octadecatrienamides are shown in Figure 5A,B.
Figure 5.
Putative biosynthetic pathways of N-benzyl-oxo-octadecadienamides (A) and N-benzyl-oxo-octadecatrienamides (B).
Additionally, the structures of 13-oxo-9Z,11E,15Z-octadecatrienoic acid and N-benzyl-13-oxo-9Z,11E,15Z-octadecatrienoamide were unstable and easily converted to 13-oxo-9E,11E,15E-octadecatrienoic acid and N-benzyl-13-oxo-9E,11E,15E-octadecatrienamide, respectively, through the cis–trans isomerization of C12. Because of the reactivity of allylic hydrogen in 9-oxo-10E,12E,14E-octadecatrienoic acid and N-benzyl-9-oxo-10E,12E,14E-octadecadienamide, the hydrogen atom at the C16 position was actively and easily oxidized, leading to the formation of 9-oxo,16-hydroxy-10E,12E,14E-octadecatrienoic acid, 9,16-dioxo-10E,12E,14E-octadecatrienoic acid, N-benzyl-16-hydroxy-9-oxo-10E,12E,14E-octadecatrienamide, and N-benzyl-9,16-dioxo-10E,12E,14E-octadecatrienamide.
To sum up, the final oxidized products including 9-oxo-10E,12E-octadecadienoic acid, 13-oxo-9E,11E-octadecadienoic acid, 13-oxo-9E,11E,15E-octadecatrienoic acid, and 9,16-dioxo-10E,12E,14E-octadecatrienoic acid were the most stable derivatives of fatty acids. By the same token, N-benzyl-9-oxo-10E,12E-octadecadienamide, N-benzyl-13-oxo-9E,11E-octadecadienamide, and N-benzyl-9,16-dioxo-10E,12E,14E-octadecatrienamide were the most stable macamides in maca. Although freeze drying has higher dehydration efficiency than air drying, the enzymatic activity related to macamide formation, such as lipoxygenase enzymes, could be reduced due to low temperature and low water content.27 The dramatic decrease of temperature and water content may inhibit the enzymatic activity and interfere with the reaction of enzymes and substrates, which may provide a rational explanation for low content of macamides in freeze-dried samples. On the contrary, the air-drying process may provide a mild condition for the reaction on macamide formation. Furthermore, other factors, such as pH value, should also be taken into consideration in the future works.
4. Conclusions
A total of 35 constituents comprised of 19 macamides (including four novel ones) and 16 fatty acid derivatives (two found in maca for the first time) were simultaneously identified in air-dried maca via UPLC-QTOF-MS. Among them, eight macamides (including four novel ones) and three fatty acids were quantified using HPLC-DAD analysis and revealed the differences in constituents between air-dried and freeze-dried maca tubers. Air-dried maca showed higher content levels of macamides and fatty acid derivatives. The possible biosynthetic pathways of N-benzyl-oxo-octadecadienamides and N-benzyl-oxo-octadecatrienamides in maca tubers during the air-drying process were rationally described. These results provide more valuable insights of the phytochemical metabolism of maca in postharvest processing. The results are also helpful to explain the health benefits of maca.
Acknowledgments
This work was supported by the Innovation Team Project of Sichuan Province Authentic Chinese Medicine (SCCXTD-2020-19) and the Fund for Talented Scholars of Sichuan Academy of Agricultural Sciences and Sichuan Province Industrial Technology Research and Development Project (Grant No. 2013XM054), China. The authors appreciate Xia Ke (Chengdu Bencao Tianyun Biological Technology Co., Ltd., Chengdu, China) for his assistance on mass analysis.
Author Contributions
§ C.X., J.D., and Y.P contributed equally.
The authors declare no competing financial interest.
References
- da Silva Leitão Peres N.; Cabrera Parra Bortoluzzi L.; Medeiros Marques L. L.; Formigoni M.; Fuchs R. H. B.; Droval A. A.; Reitz Cardoso F. A. Medicinal effects of Peruvian maca (Lepidium meyenii): a review. Food Funct. 2020, 11, 83–92. 10.1039/C9FO02732G. [DOI] [PubMed] [Google Scholar]
- Dini A.; Migliuolo G.; Rastrelli L.; Saturnino P.; Schettino O. Chemical composition of Lepidium meyenii. Food Chem. 1994, 49, 347–349. 10.1016/0308-8146(94)90003-5. [DOI] [Google Scholar]
- Wang S.; Zhu F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. 10.1016/j.foodchem.2019.02.071. [DOI] [PubMed] [Google Scholar]
- Meissner H. O.; Mscisz A.; Kedzia B.; Pisulewski P.; Piatkowska E. Peruvian maca: Two Scientific Names Lepidium meyenii Walpers and Lepidium peruvianum Chacon–Are They Phytochemically-Synonymous?. Int. J. Biomed. Sci. 2015, 11, 1–15. [Google Scholar]
- Gonzales C.; Rubio J.; Gasco M.; Nieto J.; Yucra S.; Gonzales G. F. Effect of short-term and long-term treatments with three ecotypes of Lepidium meyenii (Maca) on spermatogenesis in rats. J. Ethnopharmacol. 2006, 103, 448–454. 10.1016/j.jep.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Zhang J.; Li H.; Wang Y.; Li W. Characteristic fingerprinting based on macamides for discrimination of Maca (Lepidium meyenii) by LC-MS/MS and multivariate statistical analysis. J. Sci. Food Agric. 2016, 96, 4475–4483. 10.1002/jsfa.7660. [DOI] [PubMed] [Google Scholar]
- Rubio J.; Caldas M.; Davila S.; Gasco M.; Gonzales G. F. Effect of three different cultivars of Lepidium meyenii (Maca) on learning and depression in ovariectomized mice. BMC Complement. Altern. Med. 2006, 6, 23 10.1186/1472-6882-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.; Sun Q.; Zhang X.; Bao X.; Wang Y.; Rasheed M.; Guo B. Authentication of the geographical origin of Maca (Lepidium meyenii Walp.) at different regional scales using the stable isotope ratio and mineral elemental fingerprints. Food Chem. 2020, 311, 126058 10.1016/j.foodchem.2019.126058. [DOI] [PubMed] [Google Scholar]
- Wu X.; Chen W.; Li L.; Xu B.; Guo Y. Qualitative Identification and Semi-Quantitative Comparison of Sucrose in Maca (Lepidium meyenii) by Infrared Spectrum Analysis. Am. J. Anal. Chem. 2018, 09, 322–329. 10.4236/ajac.2018.96025. [DOI] [Google Scholar]
- Yang S.; Sun X.; Gao Y.; Chen R. Differentiation of Lepidium meyenii (Maca) from Different Origins by Electrospray Ionization Mass Spectrometry with Principal Component Analysis. ACS Omega 2019, 4, 16493–16500. 10.1021/acsomega.9b02128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Li P.; Brantner A.; Wang H.; Shu X.; Yang J.; Bian B. Chemical profiling analysis of Maca using UPLC-ESI-Orbitrap MS coupled with UPLC-ESI-QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 7, 1 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.; Deng J.; Chen J.; Zhu Y.; Song Y.; Zhang Y.; Lin C.; et al. Simultaneous determination of macaenes and macamides in maca using an HPLC method and analysis using a chemometric method (HCA) to distinguish maca origin. Rev. Bras. Farmacogn. 2019, 29, 702–709. 10.1016/j.bjp.2019.05.009. [DOI] [Google Scholar]
- Yábar E.; Pedreschi R.; Chirinos R.; Campos D. Glucosinolate content and myrosinase activity evolution in three maca (Lepidium meyenii Walp.) ecotypes during preharvest, harvest and postharvest drying. Food Chem. 2011, 127, 1576–1583. 10.1016/j.foodchem.2011.02.021. [DOI] [Google Scholar]
- Melnikovova I.; Fait T.; Kolarova M.; Fernandez E.; Milella L. Effect of Lepidium meyenii Walp. on semen parameters and serum hormone levels in healthy adult men: a double-blind, randomized, placebo-controlled pilot study. Evid. Based Complement. Altern. Med. 2015, 2015, 324369 10.1155/2015/324369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Zhao Q.; Liu Y.; Gong P.; Cao L.; Wang X.; Zhao B. Macamides present in the commercial maca (Lepidium meyenii) products and the macamide biosynthesis affected by postharvest conditions. Int. J. Food Prop. 2017, 20, 3112–3123. 10.1080/10942912.2016.1274905. [DOI] [Google Scholar]
- Chen J. J.; Gong P. F.; Liu Y. L.; Liu B. Y.; Eggert D.; Guo Y. H.; Zhao B.; et al. Postharvest Ultrasound-Assisted Freeze-Thaw Pretreatment Improves the Drying Efficiency, Physicochemical Properties, and Macamide Biosynthesis of Maca (Lepidium meyenii). J. Food Sci. 2018, 83, 966–974. 10.1111/1750-3841.14083. [DOI] [PubMed] [Google Scholar]
- Esparza E.; Hadzich A.; Kofer W.; Mithofer A.; Cosio E. G. Bioactive maca (Lepidium meyenii) alkamides are a result of traditional Andean postharvest drying practices. Phytochemistry 2015, 116, 138–148. 10.1016/j.phytochem.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Zhang J.; Li H.; Wang Y.; Li W. Simultaneous Analysis of Macamides in Maca (Lepidium meyenii) with different drying process by liquid chromatography tandem mass spectrometry. Food Anal. Methods 2016, 9, 1686–1695. 10.1007/s12161-015-0346-9. [DOI] [Google Scholar]
- Ganzera M.; Zhao J.; Ilias M.; Khan I. Chemical Profiling and Standardization of Lepidium meyenii (Maca) by Reversed Phase High Performance Liquid Chromatography. Chem. Pharm. Bull. 2002, 50, 988–991. 10.1248/cpb.50.988. [DOI] [PubMed] [Google Scholar]
- Carvalho F. V.; Ribeiro P. R. Structural diversity, biosynthetic aspects, and LC-HRMS data compilation for the identification of bioactive compounds of Lepidium meyenii. Food Res. Int. 2019, 125, 108615 10.1016/j.foodres.2019.108615. [DOI] [PubMed] [Google Scholar]
- Mccollom M. M.; Villinski J. R.; Mcphail K. L.; Craker L. E.; Gafner S. Analysis of macamides in samples of Maca (Lepidium meyenii) by HPLC-UV-MS/MS. Phytochem. Anal. 2005, 16, 463–469. 10.1002/pca.871. [DOI] [PubMed] [Google Scholar]
- Xia C.; Chen J.; Deng J.-L.; Zhu Y.-Q.; Li W.-Y.; Jie B.; Chen T.-Y. Novel macamides from maca (Lepidium meyenii Walpers) root and their cytotoxicity. Phytochem. Lett. 2018, 25, 65–69. 10.1016/j.phytol.2018.03.001. [DOI] [Google Scholar]
- E Chain F.; Grau A.; Martins J. C.; Catalán C. A. N. Macamides from wild ‘Maca’, Lepidium meyenii Walpers (Brassicaceae). Phytochem. Lett. 2014, 8, 145–148. 10.1016/j.phytol.2014.03.005. [DOI] [Google Scholar]
- Yoshikawa M.; Murakami T.; Shimada H.; Yoshizumi S.; Saka M.; Yamahara J.; Matsuda H. Medicinal foodstuffs. XIV. On the bioactive constituents of moroheiya.(2): New fatty acids, corchorifatty acids A, B, C, D, E, and F, from the leaves of Corchorus olitorius L.(Tiliaceae): Structures and inhibitory effect on NO production in mouse peritoneal macrophages. Chem. Pharm. Bull. 1998, 46, 1008–1014. 10.1248/cpb.46.1008. [DOI] [PubMed] [Google Scholar]
- Bréant L.; Vonthron-Sénécheau C.; Brelot L.; Lobstein A. (10E, 12E, 14E)-9, 16-Dioxooctadeca-10, 12, 14-trienoic acid. Acta Crystallogr., Sect. E 2012, 68, o2624. 10.1107/S1600536812027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosblech A.; Feussner I.; Heilmann I. Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. 10.1016/j.plaphy.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bhushan B.; Thomas P. Effects of. gamma. irradiation and storage temperature on lipoxygenase activity and carotenoid disappearance in potato tubers (Solanum tuberosum L.). J. Agric. Food Chem. 1990, 38, 1586–1590. 10.1021/jf00097a033. [DOI] [Google Scholar]