Abstract
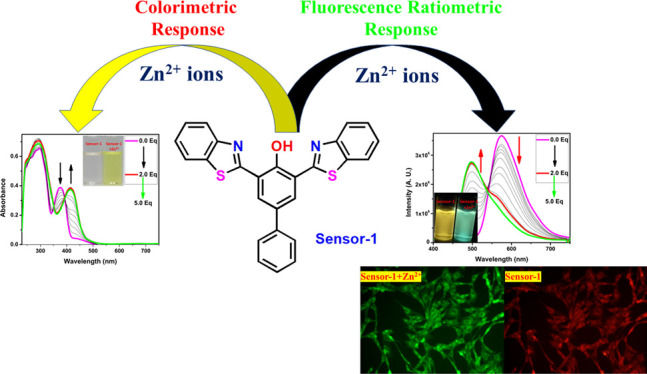
Metal ions play a very important role in environmental as well as biological fields. The detection of specific metal ions at a minute level caught much attention, and hence, several probes are available in the literature. Even though benzothiazole-based molecules have a special place in the medicinal field, only very few chemosensors are reported based on this moiety. The current work describes the design and synthesis of the benzothiazole-based chemosensor for a highly selective and sensitive detection of biologically important metal ions such as Zn2+, Cu2+, and Ni2+. The sensing studies of compound-1 showed a ratiometric as well as colorimetric response toward Zn2+, Cu2+, and Ni2+ ions and color changes from colorless to yellow and is found to be insensitive toward various metal ions (Cd2+, Cr3+, Mn2+, Pb2+, Ba2+, Al3+, Ca2+, Fe2+, Fe3+, Mg2+, K+, and Na+). Further, compound-1 exhibited ratiometric as well as turn-on-enhanced fluorescence response toward Zn2+ ions and turn off response for Cu2+ and Ni2+ ions. The Job plots revealed that the binding stoichiometry of compound-1 and metal ions is 2:1. The detection limits were found to be 0.25 ppm for Zn2+, while it was 0.30 ppm and 0.34 ppm for Ni2+ and Cu2+, respectively. In addition, density functional theory results strongly support the colorimetric response of metals, and the reversibility studies suggested that compound-1 can be used as a powerful chemosensor for the detection of Zn2+, Cu2+, and Ni2+ ions. The bioimaging data illustrated that compound-1 is a very effective ratiometric sensor for Zn2+ ions in live cells.
Introduction
It is not surprising that in the advanced scientific era humans in an effort to ease their lives are unaware that their activities are harming nature and organisms. Importantly, the toxic metal ions, which harm the environment, have made us look back and think about the environmental concerns. Efforts are underway to find such toxic metal ions in the environment. Even though researchers have shown progress in this regard, more work is required to gain an upper hand in finding the toxic metal ions in the environment. The role of chemosensors in detecting the toxic metal ions in water bodies is outstanding.
Novel chemosensors continue to be an exciting area of the present research world. In the past, some traditional highly expensive instrumental methods such as atomic absorption spectroscopy,1 inductively coupled plasma atomic emission,2 anodic stripping voltammetry,3a potentiometry,3b spectrophotometry, and so on have been used for the detection of ions. Practically, these methods were considered to be unpropitious in terms of time consumption, need experts for the handling and cost.4 Compared with the methods mentioned above, fluorescent chemosensors have drawn remarkable attention from the biological, chemical, and environmental research groups due to their ease of synthesis, high efficiency, selectivity, very fast response, low cost, as well as low detection limits toward metal ions particularly for transition-metal ions.5 In addition to fluorescence enhancement or quenching, in some cases, the probe would also act as a colorimetric chemosensor for sensing the metal ions.6 The colorimetric method of sensing is quick, equally selective and sensitive and associated with naked eye detection without the aid of any instrument. Colorimetric receptors have gained much attention, and researchers have focused on the synthesis of several artificial dyes for the selective recognition of specific metal ions.6b,7 On the other hand, the development of highly sensitive ratiometric and “turn-on” fluorescent probes have superior significance due to their capability of detection of metal ions even in biological systems,5b,6d,8 which was found to display different emission behavior upon metal binding. Due to the great impact on fluorescence turn-on receptors, lately, researchers have been devoted to the synthesis and structural modifications on BODIPY,9 anthracene,10 coumarin,11 rhodamine,12 and so on. However, several synthetic modifications on fluorescent dyes are found to have difficulties in purification and many of them lack the selectivity toward particular metal ions.
Zinc is the second most abundant metal in the human body and plays an important role in the biological reactions such as regulatory functions in enzyme catalysis, neurophysiology, gene expression, and DNA binding.13 However, the imbalance of these metal ions can ultimately lead to neuron dysfunctions causing Alzheimer’s, Parkinson’s disease, amyotrophic lateral sclerosis, Creutzfeldt–Jakob disease, multisystem atrophy, diabetes, and prostate cancer.14 Moreover, notably an excess of Zn leads to environmental pollution via the reduction of soil microbial activity causing phytotoxic effects.15 Accordingly, due to the great relevance of Zn, it is very important to monitor its level in the biological system. Owing to the advantages and disadvantages of Zn, the design and synthesis of novel receptors which can detect and monitor its concentration have been a concern for chemists and are necessary. Moreover, recently, there have been several fluorescent receptors developed for the selective recognition of Zn2+ ions such as chelating peptides, proteins, and macrocyclic compounds.16
Copper is the third most abundant transition-metal ion found in all body tissues after Fe3+ and Zn2+ owing to several essential biological processes. Moreover, copper is present in everything including soil, water, and air and is also an essential element for the all living beings such as human, animals, and plants.17a Several copper-containing pesticides and herbicides are extensively used for the treatment of plant diseases.17b,17c Additionally, it plays an important role in the human body, for example, iron absorption, maintaining nerves, blood vessels, and active catalytical cofactor in several metalloenzymes, for instance, superoxide dismutase, tyrosinase, and cytochrome c oxidase.18 However, the high amount of copper intake also becomes toxic to the body; destructive consequences causing irritation of the nose and throat, nausea, vomiting, and diarrhea. Further, a very high concentration of copper leads to the annihilation of organs in infants, whereas the deficiency of copper leads to abnormal growth of bones. The abnormal levels of copper lead to the disorder of the cellular homeostasis, which causes oxidative stress accompanied by several neurodegenerative syndromes, including Menkes, Wilson, familial amyotrophic lateral sclerosis, and Alzheimer’s.19
Nickel is also an important trace element for a living system in respiration, biosynthesis, and metabolism.20a The deficiency of the nickel(II) ion leads to harmfulness to prokaryotic and eukaryotic organisms.20 However, the accumulation of excessive nickel can cause lung cancer, asthma, sinus, pneumonitis, acute pneumonitis, syndromes of the central nervous system, and abnormal increment of blood cells.21 In addition, Ni2+ ions are also an environmental pollutant along with other heavy metal ions. Consequently, there is a very high demand for novel receptors of nickel in various chemical and biological samples.22
Even though numerous different types of chemosensors are available for the recognition of these biologically important metal ions (Zn2+, Cu2+, and Ni2+), ratiometric, fluorescence turn-on, as well as colorimetric “naked eye” chemosensors have gained great attention due to their easy sample preparation, naked eye detection, effortlessness, high sensitivity, easy way of functioning, and usefulness of identifying metal ions in biological media via cellular imaging.23 Additionally, the development of the single receptor, which could target multiple analytes, has been anomalously increasing in the past decade due to several advantages, such as potential cost and analytical time reduction. Recently, various research groups around the world focused on receptors which respond to multiple metal ions simultaneously, including Cr3+/Al3+,24 Cu2+/Hg2+,25 Cu2+/Zn2+,26 Zn2+/Cd2+,27 Ag+/Mn2+,28 Al3+/Fe3+,29 and Cr3+/Fe3+.30
In this context, we have proposed functionalized thiazole derivative compound 1 as a superior chemosensor for the recognition of Zn2+, Cu2+, and Ni2+ with multiple binding sites such as N–O–N or S–O–S chelation. Heterocyclic compounds containing nitrogen and sulfur such as quinoline31 and benzothiazole32 correspondents were extensively studied in the field of the metal ion sensing chemistry as well as biological aspects. However, benzothiazole-based ratiometric and fluorescent turn-on chemosensors for Zn2+ ions are scarce in the literature.36 Herein, we have designed and synthesized biphenyl-based benzothiazole (1) via a condensation reaction between the 4-phenyl 2,6-diformylphenol and 2-aminobenzenethiol. The receptor was found to show a colorimetric response toward Zn2+, Cu2+, and Ni2+ with a significant color change from colorless to yellow in DMSO/CHCl3 (50:50, v/v) and no change upon the addition of other metal ions such as Al3+, Ca2+, Cd2+, Co2+, Fe2+, Fe3+, Hg2+, K+, Mg2+, Mn2+, Na+, Cd2+, and Pb2+. Further, fluorescence titration of 1 was found to be ratiometric and fluorescence turn-on for Zn2+, whereas turn off for Cu2+ and Ni2+. Moreover, the receptor showed the detection limit for Zn2+ as low as 0.25 ppm and was very low as compared to Ni2+ and Cu2+ ions, which were 0.30 and 0.34 ppm, respectively.
Results and Discussion
The synthetic procedure for the new sensor is described as follows. First, 4-phenylphenol was treated with (hexamethylenetetramine) HMTA/(trifluoroacetic acid) TFA mixture and was refluxed for 48 h. The crude product was purified through column chromatography and the pure compound was obtained, 4-phenyl 2,6-diformylphenol as a yellow solid with 78% yield.33 Further, the condensation reaction was carried out between 4-phenyl 2,6-diformylphenol and aminothiophenol in EtOH under reflux conditions for 24 h and a white compound was obtained as a target molecule with good yields, which was purified via column chromatography (Scheme 1).32e The target compound was structurally characterized by 1H, 13C NMR, as well as high-resolution mass spectrometry (HRMS) (see in the Supporting Information).
Scheme 1. Synthetic Scheme for the Target Molecule.
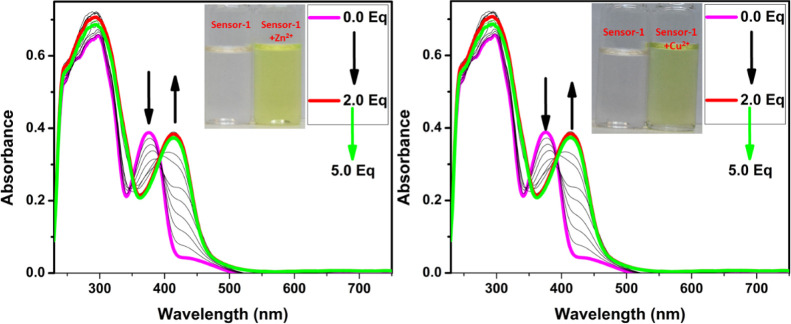
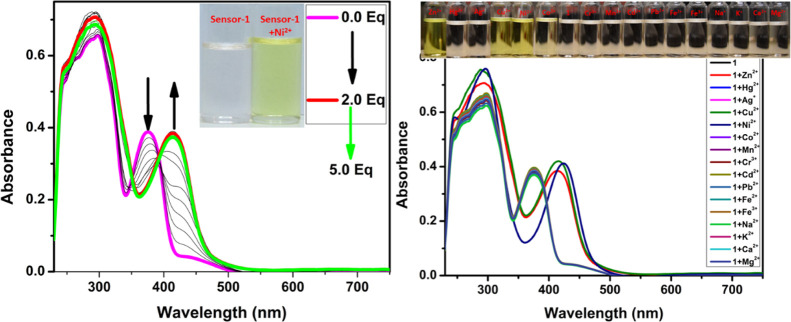
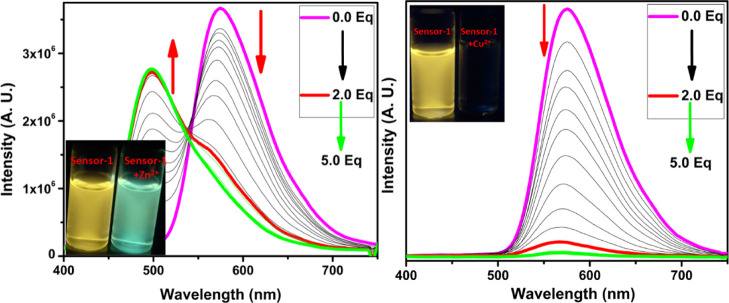
After successfully obtaining the pure form of compound-1, we have evaluated the sensing ability toward diverse cations in a 1:1 ratio of DMSO and CHCl3 solutions. The absorption spectrum of compound-1 in DMSO displayed two strong bands around 300 and 370 nm, which can be attributed to π–π* and n−π* transitions, respectively. The metal ion sensing studies were carried out using UV–visible and fluorescence spectroscopic studies. 1 μM of compound-1 was titrated with Zn2+ ions and found that upon incremental addition, the absorption band at 370 nm was gradually decreased and simultaneously a new absorption band was generated at 440 nm. As a result, the color of the solution changed from colorless to dark yellow. Under similar conditions, Ni2+ and Cu2+ also showed similar changes in absorption bands, but a drastic change was seen in the case of Ni2+ as compared with Zn2+ and Cu2+. Further, compound-1 exhibited a ratiometric response toward Ni2+, Cu2+, and Zn2+ (see the Supporting Information). The isosbestic point was found to be at 393 nm for Zn2+, while for Cu2+ and Ni2+ were found to be at 392 and 405 nm, respectively, which clearly indicated the smooth molecular conversion of free ligand to metal complex. Therefore, the new bathochromic peaks can be accredited to the metal to ligand charge transfer, which is accountable for the naked eye detection of Zn2+, Cu2+, and Ni2+ ions (Figures 1 and 2). Furthermore, we have checked the colorimetric response toward the other competitive metal ions such as Cd2+, Cr3+, Mn2+, Pb2+, Ba2+, Al3+, Ca2+, Fe2+, Fe3+, Mg2+, K+, and Na+, which caused no change or very slight change to the absorption spectra even in the presence of excess amounts of these metal ions.
Figure 1.
Change in absorption spectra of compound-1 (1 μM) upon the addition of Zn2+ (left) and Cu2+ (right) in a 1:1 ratio of DMSO and CHCl3 (inset photographs were taken in the presence of normal light).
Figure 2.
Change in absorption spectra of compound-1 (1 μM) upon the addition of Ni2+ (left) and different cations (right) in a 1:1 ratio of DMSO and CHCl3 (inset photographs were taken in the presence of normal light).
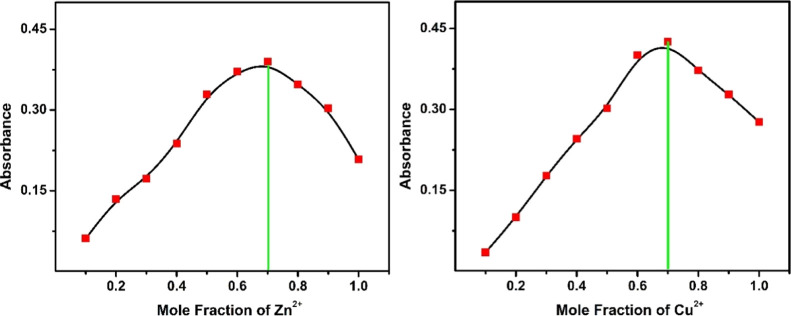
The complete saturation of compound-1 was obtained after the addition of 2.5 equiv of metal ions (Ni2+, Cu2+, and Zn2+). Moreover, the binding stoichiometry of the receptor with metals (Ni2+, Cu2+, and Zn2+) was determined using a Job's plot. This is an unceasing process as well as well known for the determination of the stoichiometric ratio of complexes. Compound-1 displayed an absorption maximum around 440 nm upon the addition of metal ions, which was monitored for Job’s plot to determine the ratios of compound-1 and metal ions. Various mole fractions of metal ions and compound-1 were mixed in different proportions from 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9. The maximum absorbance for the complex was observed at 0.3 mole fraction corresponding to a 2:1 stoichiometry of the metal–compound-1 complex (Figures 3 and 4). Additionally, we have determined the association constants (ka) of compound-1 with Zn2+, Cu2+, and Ni2+ ions which were found to be 4.9 × 1011, 6.0 × 1010, and 1.6 × 1011M-1, respectively. These results strongly suggested that the binding nature of Zn2+ ions to the compound-1 is much stronger as compared to Cu2+ and Ni2+ ions.
Figure 3.
Job’s plot according to the method of continuous variations, indicating the 1:2 stoichiometry for compound-1 + Zn2+ (left) and compound-1 + Cu2+ (right).
Figure 4.
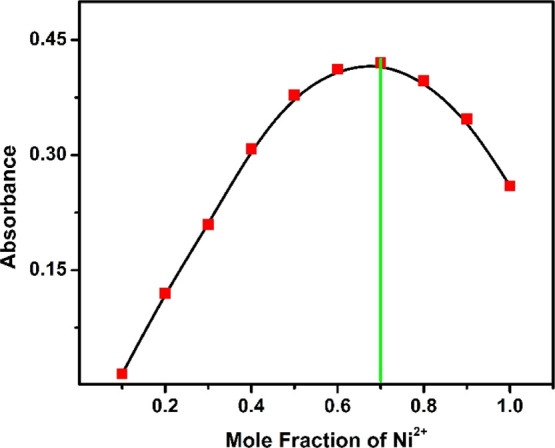
Job’ plot according to the method of continuous variations, indicating the 1:2 stoichiometry for compound-1 + Ni2+ ions.
The fluorescence emission of compound-1 exhibited a very bright yellow color (575 nm) with 0.55 quantum yields, upon excitation at 375 nm in a 1:1 ratio of DMSO and CHCl3 solutions. To better understand the sensing ability of compound-1, we have carried out metal binding studies using fluorescence spectroscopy. During UV–visible titrations, we observed a color change in the solution as well as different fluorescence upon the addition of Zn2+ ions. For better investigation, we have carried out the fluorescence titration of compound-1 with the subsequent addition of Zn2+ and found that the emission intensity at 575 nm was gradually decreased and consequently, a new emission band emerged at 520 nm. The ratiometric and fluorescence enhancement of compound-1 witnessed the visual detection of Zn2+ ions by the naked eye, when the samples were exposed to a UV lamp. The quantum yields of compound-1 increased from 0.55 to 0.69 upon the binding of Zn2+. Accordingly, compound-1 could be utilized as an excellent turn-on fluorescence chemo receptor for Zn2+ ions (Figure 5). On the other hand, the titration of Cu2+ and Ni2+ ions completely quenched the fluorescence intensity (Figures 5 and 6). The fluorescence quenching effect by Cu2+ and Ni2+ might be due to the presence of unpaired electrons and the energy-transfer process from the probe to the open shell d–orbitals of metal ions, which allows the non-radiation attenuation of the excited states to be more rapid and effective. The detection limit of compound-1 toward Zn2+, Cu2+, and Ni2+ was obtained using the calibration curve of emission versus composition, which is an important factor for a better sensor and should be less than the limit set by the U.S. EPA (∼20 μM or less) regulations.34 The detection limits of compound-1 toward metal ions, for example, Zn2+, Cu2+, and Ni2+ were found to be as low as 0.25, 0.30, and 0.34 ppm, respectively. The quenching constant was calculated from the Stern–Volmer equation and found to be 2.0 × 105, 2.4 × 105, and 5.6 × 105 for Zn2+, Cu2+, and Ni2+, respectively (Table S1). Further, the quenching efficiency of compound-1 with Zn2+, Cu2+, and Ni2+ was obtained to be 69, 96, and 98%, respectively, and further, the quantum yields of Zn2+, Cu2+, and Ni2+ strongly supported the quenching efficiency (see the Supporting Information). From these results, one can conclude that the quenching efficiency and quenching constant for Ni2+ is high as compared with that of Zn2+ and Cu2+ ions.
Figure 5.
Change in emission spectra of compound-1 (1 μM) upon the addition of Zn2+ (left) and Cu2+ (right) in a 1:1 ratio of DMSO and CHCl3 (inset photographs were taken in the presence of UV light).
Figure 6.
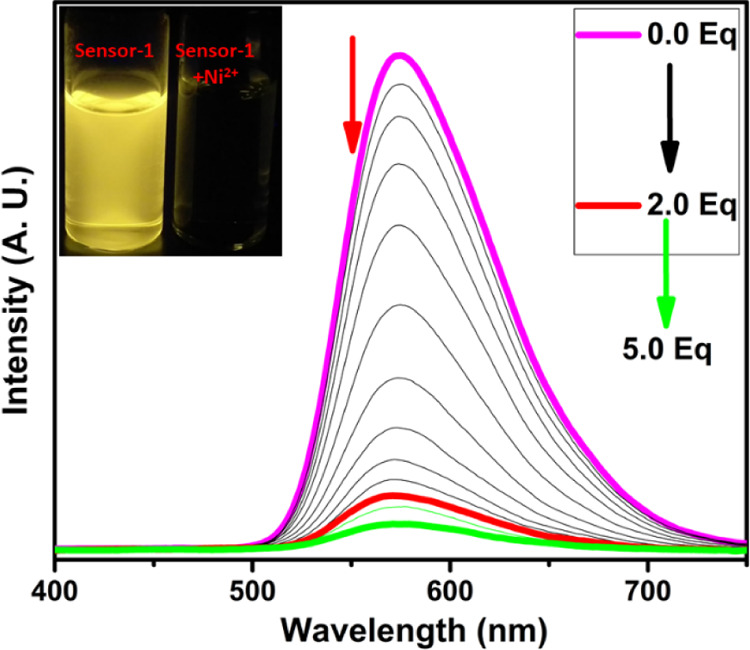
Change in emission spectra of compound-1 (1 μM) upon the addition of Ni2+ ions in a 1:1 ratio of DMSO and CHCl3 (inset photographs were taken in the presence of UV light).
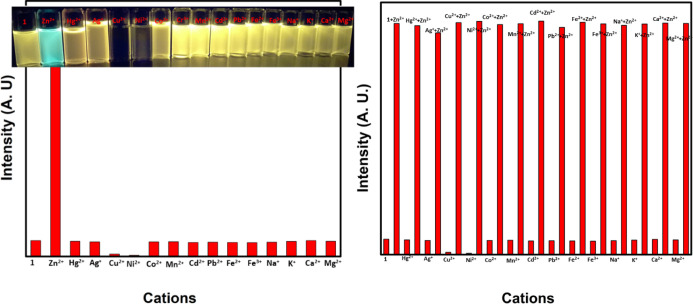
Furthermore, we explored the sensitivity and selectivity of compound-1 toward several metal ions and was compared with Zn2+. A 1 μM stock solution of compound-1 in a 1:1 ratio of DMSO and CHCl3 solutions was treated with excess amounts of diverse metal ions such as Cd2+, Cr3+, Mn2+, Pb2+, Ba2+, Al3+, Ca2+, Fe2+, Fe3+, Mg2+, K+, and Na+ for the investigation of selectivity. Compound-1 exhibited high selectivity and ratiometric as well as fluorescence turn-on response toward Zn2+, while Cu2+ and Ni2+ metal ions displayed fluorescence turn-off, other metal ions do not influence the emission spectra (Figure 7).
Figure 7.
Examination of selectivity (left) and competitive (right) studies of compound-1 (1 μM) toward various metal ions. The competitive binding ability of compound-1 (1 μM) were observed toward Zn2+ in the presence of other interfering metal ions and the emission band was monitored at 520 nm. Inset photographs were taken in UV light.
In addition, competitive selectivity of compound-1 with respect to Zn2+ in the presence of other interfering candidates such as Cu2+, Ni2+, Cd2+, Cr3+, Mn2+, Pb2+, Ba2+, Al3+, Ca2+, Fe2+, Fe3+, Mg2+, K+, and Na+ was studied. The initial emission band of compound-1 with excess amounts of various other metal ions exhibited very low and constant intensity which upon the addition of 5 equiv of Zn2+ led to efficient enhancement of green emission with good quantum yields (Figure 7). These results strongly suggest the excellent selectivity of compound-1 for Zn2+ ions in the presence of other mentioned interfering metal ions.
To get an insight into the photophysical responses of the compound-1 with metal ions, we carried out the quantum mechanical calculations of the free probe and its metal coordinated complexes with the help of density functional theory (DFT) method using a Gaussian 09 program35 at the level of B3LYP/631G/LANL2DZ (d,p) for C, H, N, S, O, and metal ions (Zn2+, Cu2+, and Ni2+). The energy minimized structure of compound-1 and compound-1–Zn2+ complexes is shown in Figure 8, whereas for complexes of Cu2+ and Ni2+ are given in the Supporting Information.
Figure 8.
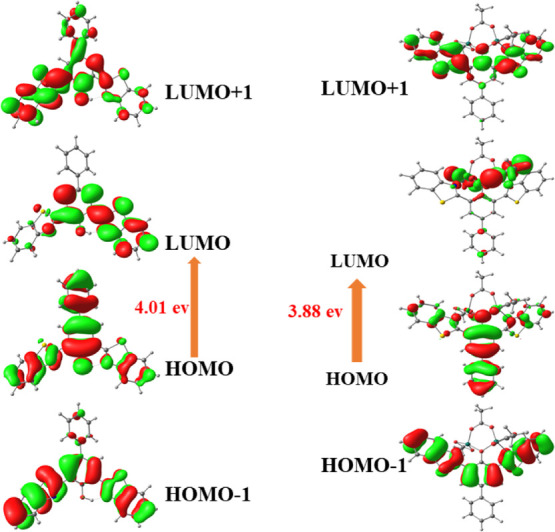
Selected MOs of compound-1 and compound-1 + Zn2+ (not to scale; isovalue = 0.02).
In addition, the frontier molecular orbitals reveal that compound-1 as well as metal complexes of Zn2+, Cu2+, and Ni2+ will provide the valuable information about electronic transitions. Using the optimized structures, time-dependent DFT (TDDFT) calculations were carried out and created a checkpoint file which was used to generate frontier molecular orbital cubes. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of compound-1 were mostly concentrated on the whole π-moiety of benzothiazole as well as biphenyl units with a HOMO–LUMO gap of 4.02 eV, which indicated the transition of π to π* of the benzothiazole unit. As evident from UV–visible and fluorescence titration spectra, the stoichiometry of compound-1 and Zn2+, Cu2+, and Ni2+ was confirmed strongly as 1:2 and hence, the optimization of the metal complex was carried out with 1:2 coordinated complexes taking acetate as an auxiliary ligand and optimized using LAN2DZ basis sets. In the compound-1–Zn2+ complex, the HOMO is situated completely on the whole biphenyl–benzothiazole moiety, whereas LUMO is spread over Zn2+ ions with a band gap of 3.88 eV (Figure 6). These results clearly revealed the interruption of internal charge transfer after the appendage of Zn2+ ions to compound-1. Similarly, the calculated HOMO–LUMO gap of other metal complexes (Cu2+ and Ni2+) were small compared to that of compound-1. These findings intensely supported the reason for the colorimetric response upon the addition of metal ions (Zn2+, Cu2+, and Ni2+) to compound-1.
Furthermore, the TDDFT of compound-1 and metal complexes (Zn2+, Cu2+, and Ni2+) were carried out in the LAN2DZ basis set to investigate the electronic transition in absorption spectra in vacuum. Further, the compared oscillator strength of compound-1 and its metal complexes provided the valuable evidence for the colorimetric response. It was noticed that the absorption spectrum of compound-1 consists of two major peaks at 295 and 375 nm with energies 3.8894904 and 4.480113 eV for the key electron transitions, respectively. However, TDDFT data showed two major strong bands at 389.37 and 279.24 nm with energies of 3.1842 and 4.4401 eV correspondingly. As evident from TDDFT data, the absorption bands of the Zn2+ complex displayed at 411.32 and 309.69 nm and the strong absorption band in the visible region are attributed to intraligand charge-transfer bands, which also matched with the experimental data. These outcomes are strongly supported by the oscillator strength of compound-1 before and after metal complexes with a change from 0.2110 to 1.8996. The formation of the metal ion complex with compound-1 had gradually enhanced the absorption band in the visible region, which might be due to the change in electronic transition and marginal sensitivity of the metal ions. For example, compound-1 and its complexation with Zn2+ was found to give a bathochromic shift with 40 nm as noted in UV–visible spectra. On the other hand, the resultant Ni2+ complex led to a huge bathochromic shift in the absorption of (Δλ) 52 nm and Cu2+ prompts instead similar red shifts (Δλ ≈ 42 nm) as compared with Zn2+. Further, these arguments are completely supported by the change in energy gap values, which are observed from DFT studies. In addition, we carried out DFT/TDDFT calculations of compound-1–Cu2+ and compound-1–Ni2+ complexes under similar conditions and found that the oscillator strength values were relatively unchanged compared with compound-1–Zn2+. Also, these data provided further evidence for the observed selectivity of the compound-1 with Zn2+.
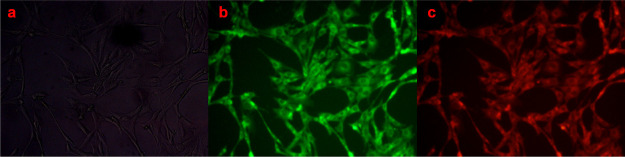
The strong affinity of compound-1 toward Zn2+ and the ratiometric response of compound-1 encouraged us to explore the applicability of compound-1 for the ratiometric fluorescence imaging of Zn2+ ions in live cells. Initially, the cytotoxicity of compound-1 toward HeLa cells was studied by the standard MTT assay, explaining the least cytotoxic nature to the cells. Further, HeLa cells were cultured in Dulbecco’s modified Eagle medium (DMEM) and the cells were incubated with compound-1 for 30 min at 25 °C. Later, the cells were imaged through a fluorescence microscope, a high red fluorescence was observed only inside the cellular regions and was clearly supported by the ICT mechanism in biological samples also. Furthermore, 10 μM of Zn2+ solution (DMEM) was added prior to the incubation and was kept for 10 min. Later, the cells were washed with PBS buffer solutions to remove an excess amount of Zn2+ ions and were subjected to fluorescence microscopy imaging and the bright green emission was observed inside the cells (Figure 9). From these results, one can conclude that compound-1 could be a promising candidate for the practical protocol in ratiometric imaging of living cells in biology.
Figure 9.
Cellular images of Zn2+ ions in HeLa cell lines at 37 °C (a) bright field image of compound-1 + Zn2+ ion-treated HeLa cells; (b) fluorescence imaging of HeLa cells with compound-1 after 10 min of treatment of 10 μM Zn2+ ions; and (c) HeLa cells incubated with compound-1 for 30 min.
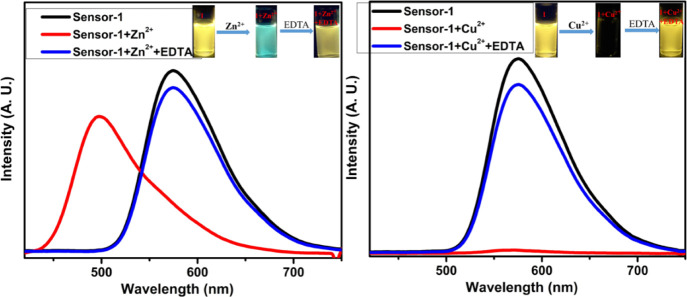
A good sensor should exhibit better selectivity as well as reversibility nature; thus, we have examined the reversibility of compound-1 with Zn2+ in a 1:1 ratio of DMSO and CHCl3 solutions. Upon the addition of 5 equiv of the EDTA solution to the Zn2+ + compound-1 solution; the original emission of compound-1 was regenerated (Figure 10), which indicated that the EDTA was involved in captivating the Zn2+ ion. Cu2+ and Ni2+ complexes with compound-1 also furnished the same results (Figures 10 and 11). These experiments strongly confirm that compound-1 has very good reversibility and thus, it can be employed as a useful and better chemosensor probe selectively for Zn2+ ions.
Figure 10.
Reversibility studies of compound-1 upon the addition of EDTA to compound-1 + Zn2+ (left) and compound-1 + Cu2+ (right).
Figure 11.
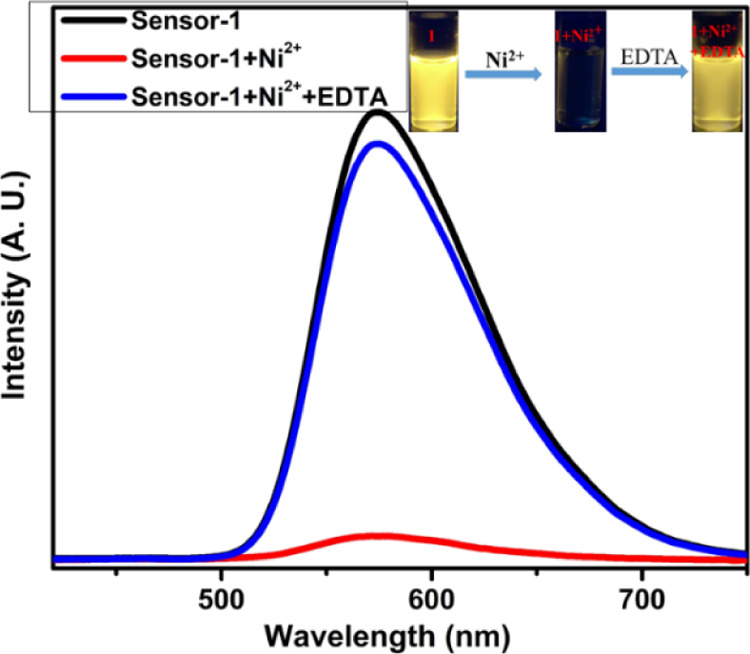
Reversibility studies of compound-1 upon the addition of EDTA to compound-1 + Ni2+.
Conclusions
In summary, we have designed and synthesized a biphenyl–benzothiazole-based chemosensor and was characterized by 1H, 13C NMR, as well as HRMS. The novel sensor exhibited ratiometric and colorimetric responses toward biologically important metal ions such as Zn2+, Cu2+, and Ni2+ with significant color changes from colorless to yellow in DMSO/CHCl3 (50:50, v/v) over other metal cations (Cd2+, Cr3+, Mn2+, Pb2+, Ba2+, Al3+, Ca2+, Fe2+, Fe3+, Mg2+, K+, and Na+). Further, fluorescence studies revealed that there was an enhanced fluorescence in the emission spectra, upon the addition of Zn2+ ions and that make compound-1 as a potential ratiometric as well as fluorescence turn-on chemosensor, which was strongly supported by the elevating quantum yields, whereas a turn-off chemosensor for Cu2+ and Ni2+ ions. The selectivity and competitive studies also strongly suggested that compound-1 was excellent for Zn2+ ions in the presence of other mentioned interfering metal ions. Moreover, compound-1 showed the detection limit for Zn2+ ions as 0.25 ppm which was lower compared to that of Ni2+ and Cu2+ ions which were 0.30 and 0.34 ppm, respectively. The binding stoichiometry of the metal complex was determined using a Job's plot and was found to be 1:2 for compound-1 with all the three metal ions. The reversibility of compound-1 was studied upon the addition of EDTA and found that the green color emission of compound-1 was changed to yellow, restoring the emission of compound-1, proving the reversibility of the chemosensor. Similarly, metal complexes of compound-1 with Ni2+ and Cu2+ ions follow similar trends of Zn2+ ions. The ratiometric response of compound-1 toward Zn2+ ions extend its applicability in the fluorescence cellular imaging of Zn2+ ions in live cells and found a color change from red to green fluorescence in cells. Due to the presence of these novel properties, compound-1 can act as an excellent chemosensor for the detection of biologically important metal ions such as Zn2+, Cu2+, and Ni2+ in the environment as well as bioimaging (Zn2+ ions) of live cells.
Experimental Section
All reagents were of analytical grade and used without further purification, otherwise noted the purification process. Glassware was oven dried at 140 °C for at least 2 h prior to use and allowed to cool under vacuum. 4-Phenylphenol, aminothiophenol, (Alfa Aesar), TFA, EtOH, MeOH, diethylether, and DMSO (Spectrochem). HMTA and all the metal salts were used as such without purification (Merck India). Flash chromatography was carried out on Merck Kieselgel 60 (230–400 mesh) as the stationary phase under a positive pressure using AR grade solvents; the procedure includes the removal of solvents under reduced pressure. Thin-layer chromatography was performed on precoated Merck Kieselgel silica gel plates (60 F254, Merck, Germany) monitored by UV light and iodine. Electrospray ionization time-of-flight mass spectrometry (TOF MS ES+) and HRMS were recorded on a Waters QTOFMS Xevo G2 spectrometer in the positive ion mode. The 1H, 13C{1H} NMR were recorded on a Bruker AVANCE III 400 MHz NMR spectrometer. All solution 1H and 13C spectra were referenced internally to the solvent signal (TMS). In rare instances where other NMR solvents have been used, an appropriate mention has been made in the text. Chemical shifts are expressed in parts per million (ppm) and coupling constants (J) are in Hz. The 1H and 13C{1H} NMR spectra were referenced using residual H impurities in the deuterated solvents. Deuterated solvents (CDCl3) were purchased from Cambridge Isotope Laboratories, dried over calcium hydride, degassed by three freeze–pump–thaw cycles, and vacuum-transferred prior to use. Electronic absorption spectra were recorded on a PerkinElmer LAMBDA 750 UV/visible spectrophotometer. The solutions were prepared using a microbalance (±0.1 mg) and volumetric glassware and then charged in quartz cuvettes with sealing screw caps. Fluorescence emission studies were carried out on a Horiba JOBIN YVON Fluoromax-4 spectrometer. All aqueous solutions were prepared with double distilled deionized water. The standard stock solution of Ag+, Co2+, Pb2+, Zn2+, Cd2+, Cr3+, Mn2+, Pb2+, Ba2+, Al3+, Ca2+, Fe2+, Fe3+, Mg2+, K+, Cu2+, Na+, Cd2+, and Hg2+ was prepared by dissolving their nitrate salts.
Synthesis of 4-Phenyl 2,6-Diformylphenol (A)
4-Phenylphenol (5 g, 29 mmol) was dissolved in TFA (100 mL). Hexamethylene tetramine (32 g, 232 mmol) was added to the solution and refluxed at 160 °C for 48 h (Scheme 1). The reaction mixture was stirred in 6 N HCl (100 mL) for 30 min. The crude reaction mixture was extraction with DCM and purified through silica gel column chromatography (2:8 ratios of ethyl acetate and hexanes). A yellow colored powder was obtained with yield 78%. 1H NMR (400 MHz, CDCl3) δ 11.63 (s, 1H), 10.32 (s, 2H), 8.19 (s, 2H), 7.59 (d, J = 7.3 Hz, 2H), 7.48 (t, J = 7.5 Hz, 2H), 7.41 (d, J = 7.3 Hz, 1H) (the 1H NMR data was matching with literature reports). HRMS (EI): calcd for C14H10NaO3 [M + Na]+m/z, 249.0528; found m/z, 249.0532.
Synthesis of Compound-1
A stirred mixture of 4-phenyl 2,6-diformylphenol (0.5 g, 2.2 mmol) and aminothiophenol (0.7 g, 5.5 mmol) in 60 mL of EtOH was refluxed for 24 h. The crude product was purified by column chromatography using silica gel and solvent was dried under vacuum. A dirty white product was obtained with 71% yield. 1H NMR (400 MHz, CDCl3): δ 14.24 (s, 1H), 8.48 (s, 2H), 8.10 (d, J = 8.0 Hz, 2H), 7.98 (d, J = 7.5 Hz, 2H), 7.80–7.71 (m, 2H), 7.61–7.48 (m, 4H), 7.49–7.39 (m, 3H). 13C NMR (101 MHz, CDCl3): δ 155.6, 151.8, 139.6, 133.2, 128.9, 127.5, 127.1, 126.6, 125.5, 122.6, 121.6, 116.7. HRMS (EI): calcd for C26H17N2OS2 [M + H]+m/z, 437.0782; found m/z, 437.0780.
Acknowledgments
C.A.S.P thanks the Department of Chemistry at National Institute of Technology, Calicut, for the financial support. A.J. thanks National Institute of Technology, Calicut, for Institute-JRF fellowship. C.A.S.P thanks to Dr. Parameswaran P. for the use of his computational work station.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02855.
1H NMR, 13C NMR, HRMS, and characterization data for all the compounds, photophysical data, association constants, detection limits, and DFT results (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mirzaei M.; Behzadi M.; Abadi N. M.; Beizaei A. Simultaneous separation/preconcentration of ultra-trace heavy metals in industrial wastewaters by dispersive liquid–liquid microextraction based on solidification of floating organic drop prior to determination by graphite furnace atomic absorption spectrometry. J. Hazard. Mater. 2011, 186, 1739–1743. 10.1016/j.jhazmat.2010.12.080. [DOI] [PubMed] [Google Scholar]
- Sitko R.; Janik P.; Zawisza B.; Talik E.; Margui E.; Queralt I. Green Approach for Ultratrace Determination of Divalent Metal Ions and Arsenic Species Using Total-Reflection X-ray Fluorescence Spectrometry and Mercapto-Modified Graphene Oxide Nanosheets as a Novel Adsorbent. Anal. Chem. 2015, 87, 3535–3542. 10.1021/acs.analchem.5b00283. [DOI] [PubMed] [Google Scholar]
- a Curdová E.; Vavrusková L.; Suchánek M.; Baldrian P.; Gabriel J. ICP-MS determination of heavy metals in submerged cultures of wood-rotting fungi. Talanta 2004, 62, 483–487. 10.1016/j.talanta.2003.08.030. [DOI] [PubMed] [Google Scholar]; b Petrović S.; Guzsvany V.; Ranković N.; Beljin J.; Rončević S.; Dalmacija B.; Ashrafi A. M.; Konya Z.; Švancara I.; Vytřas K. Trace level voltammetric determination of Zn(II) in selected nutrition related samples by bismuth-oxychloride-multiwalled carbon nanotube composite based electrode. Microchem. J. 2018, 146, 178–186. 10.1016/j.microc.2018.12.053. [DOI] [Google Scholar]
- a Li S.; Zhang C.; Wang S.; Liu Q.; Feng H.; Ma X.; Guo J. Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 2018, 143, 4230–4246. 10.1039/c8an01067f. [DOI] [PubMed] [Google Scholar]; b Li Y.; Chen Y.; Yu H.; Tian L.; Wang Z. Portable and smart devices for monitoring heavy metal ions integrated with nanomaterials. TrAC, Trends Anal. Chem. 2018, 98, 190–200. 10.1016/j.trac.2017.11.011. [DOI] [Google Scholar]
- a Sahoo S. K.; Sharma D.; Bera R. K.; Crisponi G.; Callan J. F. Iron(iii) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195–7227. 10.1039/c2cs35152h. [DOI] [PubMed] [Google Scholar]; b Carter K. P.; Young A. M.; Palmer A. E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. 10.1021/cr400546e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jiang P.; Guo Z. Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord. Chem. Rev. 2004, 248, 205–229. 10.1016/j.cct.2003.10.013. [DOI] [Google Scholar]
- a Baljeet K.; Navneet K.; Subodh K. Colorimetric metal ion sensors – A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. 10.1016/j.ccr.2017.12.002. [DOI] [Google Scholar]; b Ali Q. A.; Mohamed A.; Sami A. Z. Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene. Chemosensors 2020, 8, 1–10. 10.3390/chemosensors8010001. [DOI] [Google Scholar]; c Hao Z.; Jiangli F.; Benhua W.; Xiaojun P. Fluorescent, MRI, and colorimetric chemical sensors for the first-row d-block metal ions. Chem. Soc. Rev. 2015, 44, 4337–4366. 10.1039/C4CS00285G. [DOI] [PubMed] [Google Scholar]; d Sanchari P.; Nabanita C.; Parimal K. B. Selectively sensing first-row transition metal ions through fluorescence enhancement. RSC Adv. 2014, 4, 26585–26620. 10.1039/C4RA02054E. [DOI] [Google Scholar]; e Erdemir S.; Malkondu S. Calix[4]arene based a NIR-fluorescent sensor with an enhanced stokes shift for the real-time visualization of Zn(II) in living cells. Sens. Actuators, B 2020, 306, 127574. 10.1016/j.snb.2019.127574. [DOI] [Google Scholar]
- Shalini U.; Ajay S.; Riya S.; Shivangi O.; Kiran N. Colorimetric chemosensors for d-metal ions: A review in the past, present and future prospect. J. Mol. Struct. 2019, 1193, 89–102. 10.1016/j.molstruc.2019.05.007. [DOI] [Google Scholar]; b Jung J. M.; Lee S. Y.; Kim C. A novel colorimetric chemosensor for multiple target metal ions Fe2+, Co2+, and Cu2+ in a near-perfect aqueous solution: experimental and theoretical studies. Sens. Actuators, B 2017, 251, 291–301. 10.1016/j.snb.2017.05.055. [DOI] [Google Scholar]
- a Luo A.; Wang H.; Wang Y.; Huang Q.; Zhang Q. A novel colorimetric and turnon fluorescent chemosensor for iron(III) ion detection and its application to cellular imaging. Spectrochim. Acta, Part A 2016, 168, 37–44. 10.1016/j.saa.2016.05.048. [DOI] [PubMed] [Google Scholar]; b Yuan L.; Lin W.; Zheng K.; Zhu S. FRET-based small-molecule fluorescent probes: rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. 10.1021/ar300273v. [DOI] [PubMed] [Google Scholar]; c Guo Z.; Hu T.; Sun T.; Li T.; Chi H.; Niu Q. A colorimetric and fluorimetric oligothiophene-indenedione-based sensor for rapid and highly sensitive detection of cyanide in real samples and bioimaging in living cells. Dyes Pigm. 2019, 163, 667–674. 10.1016/j.dyepig.2018.12.057. [DOI] [Google Scholar]
- a Ahmed N. K.; Elif B.; Ersin G.. Importance of BODIPY-based Chemosensors for Cations and Anions in Bio-imaging Applications, Curr. Anal. Chem. 2020, 17, 10.2174/1573411017666201215105055; [DOI] [Google Scholar]; b Apurba M.; Utsav G.; Dipanjan G.; Devdeep M.; Tapas K. M.; Sanjib K. P. A water-soluble BODIPY based ‘OFF/ON’ fluorescent probe for the detection of Cd2+ ions with high selectivity and sensitivity. Dalton Trans. 2019, 48, 2108–2117. 10.1039/C8DT04016H. [DOI] [PubMed] [Google Scholar]
- a Chinna Ayya Swamy P.; Jeyabalan S.; Subramanian S.; Sivaraman G.; Duraisamy C. Anthracene-Based Highly Selective and Sensitive Fluorescent “Turn-on” Chemodosimeter for Hg2+. ACS Omega 2018, 3, 12341–12348. 10.1021/acsomega.8b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kaur N.; Kaur B. Recent development in anthracene possessing chemosensors for cations and anions. Microchemical Journal 2020, 158, 105131. 10.1016/j.microc.2020.105131. [DOI] [Google Scholar]
- Duxia C.; Zhiqiang L.; Peter V.; Seyoung K.; Paramesh J.; Jong S. K.; Weiying L. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. 10.1021/acs.chemrev.9b00145. [DOI] [PubMed] [Google Scholar]
- a Ha N. K.; Min H. L.; Hyun J. K.; Jong S. K.; Juyoung Y. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. 10.1039/B802497A. [DOI] [PubMed] [Google Scholar]; b Ruiqi Z.; Fanyong Y.; Yicun H.; Depeng K.; Qianghua Y.; Jinxia X.; Li C. Rhodamine-based ratiometric fluorescent probes based on excitation energy transfer mechanisms: construction and applications in ratiometric sensing. RSC Adv. 2016, 6, 50732–50760. 10.1039/C6RA06956H. [DOI] [Google Scholar]
- a Berg J. M.; Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]; d Frederickson C. J.; Koh J.-Y.; Bush A. I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]; e Fraker P. J.; King L. E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004, 24, 277–298. 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]; f Que E. L.; Domaille D. W.; Chang C. J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517. 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]; g Suhy D. A.; Simon K. D.; Linzer D. I. H.; O’Halloran T. V. Metallothionein is part of a zincscavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem. 1999, 274, 9183–9192. 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- a Bush A. I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003, 26, 207–214. 10.1016/s0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]; b Strausak D.; Mercer J. F. B.; Dieter H. H.; Stremmel W.; Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res. Bull. 2001, 55, 175–185. 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]; c Rolfs A.; Hediger M. A. Metal ion transporters in mammals: structure, function and pathological implications. J. Physiol. 1999, 518, 1–12. 10.1111/j.1469-7793.1999.0001r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Finney L. A.; Halloran T. V. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 2003, 300, 931–936. 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]; e Nelson N. Metal ion transporters and homeostasis. EMBO J. 1999, 18, 4361–4371. 10.1093/emboj/18.16.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Kozlowski H.; Janicka-Klos A.; Brasun J.; Gaggelli E.; Valensin D.; Valensin G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coord. Chem. Rev. 2009, 253, 2665–2685. 10.1016/j.ccr.2009.05.011. [DOI] [Google Scholar]; g Berg J. M.; Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]; h Xie X.; Smart T. G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature 1991, 349, 521–524. 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]; i Maret W. Zinc biochemistry, physiology, and homeostasis–recent insights and current trends. BioMetals 2001, 14, 187–190. 10.1023/a:1012945110820. [DOI] [Google Scholar]; j Vallee B. L.; Falchuk K. H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- a Dong W.-K.; Akogun S. F.; Zhang Y.; Sun Y.-X.; Dong X.-Y. A reversible “turn-on” fluorescent sensor for selective detection of Zn2+. Sens. Actuators, B 2017, 238, 723–734. 10.1016/j.snb.2016.07.047. [DOI] [Google Scholar]; b Shayegan H.; Farahani Y. D.; Safarifard V. A pillar-layer metal-organic framework as a turn-on luminescent sensor for highly selective and sensitive detection of Zn(II) ion. J. Solid State Chem. 2019, 279, 120968. 10.1016/j.jssc.2019.120968. [DOI] [Google Scholar]
- a Chen D.; Liu Z.; Huang W.; Zhao Y.; Dong S.; Zeng M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J. Funct. Foods 2013, 5, 689–697. 10.1016/j.jff.2013.01.012. [DOI] [Google Scholar]; b Kristin K.; Mark A. S.; Per K. Novel Zn2-Chelating Peptides Selected from a Fimbria-Displayed Random Peptide Library. Appl. Environ. Microbiol. 2001, 67, 5467–5473. 10.1128/AEM.67.12.5467-5473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Joseph K.-H.; Wong M.; Todd H.; Peter J. R. Recent Advances in Macrocyclic Fluorescent Probes for Ion Sensing. Molecules 2017, 22, 200–218. 10.3390/molecules22020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Donald G. B.; Donald B. Copper. Clin. Toxicol. 1999, 37, 217–230. 10.1081/CLT-100102421. [DOI] [Google Scholar]; b Lamichhane J. R.; Osdaghi E.; Behlau F.; Köhl J.; Jones J. B.; Aubertot J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustainable Dev. 2018, 38, 28. 10.1007/s13593-018-0503-9. [DOI] [Google Scholar]; c Schoffer J. T.; Sauvé S.; Neaman A.; Ginocchio R. Role of Leaf Litter on the Incorporation of Copper-Containing Pesticides into Soils under Fruit Production: a Review. J. Soil Sci. Plant Nutr. 2020, 20, 990–1000. 10.1007/s42729-020-00186-1. [DOI] [Google Scholar]
- Amie K. B.; Amy C. R. Structural Biology of Copper Trafficking. Chem. Rev. 2009, 109, 4760–4779. 10.1021/cr900104z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kaler S. G. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 2011, 7, 15–29. 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lutsenko S. Atp7b–/– mice as a model for studies of Wilson’s disease. Biochem. Soc. Trans. 2008, 36, 1233–1238. 10.1042/bst0361233. [DOI] [PubMed] [Google Scholar]; c Que E. L.; Domaille D. W.; Chang C. J. Metals in Neurobiology: Probing Their Chemistry and Biology with Molecular Imaging. Chem. Rev. 2008, 108, 1517–1549. 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]; d Savelieff M. G.; Lee S.; Liu Y.; Lim M. H. Untangling Amyloid-β, Tau, and Metals in Alzheimer’s Disease. ACS Chem. Biol. 2013, 8, 856–865. 10.1021/cb400080f. [DOI] [PubMed] [Google Scholar]; e Davies P.; McHugh P. C.; Hammond V. J.; Marken F.; Brown D. R. Contribution of Individual Histidines to Prion Protein Copper Binding. Biochemistry 2011, 50, 10781–10791. 10.1021/bi2012349. [DOI] [PubMed] [Google Scholar]
- a Satish K.; Trivedi A. V. A Review on Role of Nickel in the Biological System. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 719–727. 10.20546/ijcmas.2016.503.084. [DOI] [Google Scholar]; b Nickel and Its Surprising Impact in Nature; Sigel A., Sigel H., Sigel R. K. O., Eds.; John Wiley & Sons Ltd.: U.K., 2007; Vol. 2. [Google Scholar]; c Kusal K. D.; Chandramouli R. R.; Ishwar B. B.; Swastika D.; Shrilaxmi B.; Lata M.; Jyoti P. K.; Biradar M. S. Primary concept of nickel toxicity – an overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [DOI] [PubMed] [Google Scholar]; d Cempel M.; Nikel G. Nickel: A Review of Its Sources and Environmental Toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- a Giuseppe G.; Alessia C.; Graziantonio L.; Maria S. S.; Alessia C. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. 10.3390/ijerph17030679. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chivers P. T.; Sauer R. T. NikR is a ribbon-helix-helix DNA-binding protein. Protein Sci. 1999, 8, 2494–2500. 10.1110/ps.8.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Dosanjh N. S.; Michel S. L. Microbial nickel metalloregulation: NikRs for nickel ions. Curr. Opin. Chem. Biol. 2006, 10, 123–130. 10.1016/j.cbpa.2006.02.011. [DOI] [PubMed] [Google Scholar]; d Hausinger R. P.; Zamble D. B. In Molecular Microbiology of HeaVy Metals; Nies D. H., Silver S., Eds.; Springer: Heidelberg, Germany, 2007; pp 287–320; [Google Scholar]; e Carrington P. E.; Chivers P. T.; Al-Mjeni F.; Sauer R. T.; Maroney M. J. Nickel coordination is regulated by the DNA-bound state of NikR. Nat. Struct. Biol. 2003, 10, 126–130. 10.1038/nsb890. [DOI] [PubMed] [Google Scholar]; f Schreiter E. R.; Sintchak M. D.; Guo Y.; Chivers P. T.; Sauer R. T.; Drennan C. L. Crystal structure of the nickel-responsive transcription factor NikR. Nat. Struct. Biol. 2003, 10, 794–799. 10.1038/nsb985. [DOI] [PubMed] [Google Scholar]
- Chakraborty S.; Rayalu S. Detection of nickel by chemo and fluoro sensing technologies. Spectrochim. Acta, Part A 2021, 245, 118915. 10.1016/j.saa.2020.118915. [DOI] [PubMed] [Google Scholar]
- a Xu Z.; Chen X.; Kim H. N.; Yoon J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. 10.1039/b907368j. [DOI] [PubMed] [Google Scholar]; b Lodeiro C.; Pina F. Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coord. Chem. Rev. 2009, 253, 1353–1383. 10.1016/j.ccr.2008.09.008. [DOI] [Google Scholar]; c Basabe-Desmonts L.; Reinhoudt D. N.; Crego-Calama M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. 10.1039/b609548h. [DOI] [PubMed] [Google Scholar]; e de Silva A. P.; Gunaratne H. Q. N.; Gunnlaugsson T.; Huxley A. J. M.; McCoy C. P.; Rademacher J. T.; Rice T. E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]; g Kim H.; Kim K. B.; Song E. J.; Hwang I. H.; Noh J. Y.; Kim P.-G.; Jeong K.-D.; Kim C. Turn-on selective fluorescent probe for trivalent cations. Inorg. Chem. Commun. 2013, 36, 72–76. 10.1016/j.inoche.2013.08.025. [DOI] [Google Scholar]
- Jung J. Y.; Han S. J.; Chun J.; Lee C.; Yoon J. New thiazolothiazole derivatives as fluorescent chemosensors for Cr3+ and Al3+. Dyes Pigm. 2012, 94, 423–426. 10.1016/j.dyepig.2012.02.005. [DOI] [Google Scholar]
- Tang L.; Li F.; Liu M.; Nandhakumar R. Single sensor for two metal ions: Colorimetric recognition of Cu2+ and fluorescent recognition of Hg2+. Spectrochim. Acta, Part A 2011, 78, 1168–1172. 10.1016/j.saa.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Wang S.; Men G.; Zhao L.; Hou Q.; Jiang S. Binaphthyl-derived salicylidene Schiff base for dual-channel sensing of Cu, Zn cations and integrated molecular logic gates. Sens. Actuators, B 2010, 145, 826–831. 10.1016/j.snb.2010.01.060. [DOI] [Google Scholar]
- Swamy K. M. K.; Kim M.-J.; Jeon H.-R.; Jung J.-Y.; Yoon J.-Y. New 7-hydroxycoumarinbased fluorescent chemosensors for Zn(II) and Cd(II). Bull. Korean Chem. Soc. 2010, 31, 3611–3616. 10.5012/bkcs.2010.31.12.3611. [DOI] [Google Scholar]
- Singh N.; Kaur N.; Ni Choitir C.; Callan J. F. A dual detecting polymeric sensor: chromogenic naked eye detection of silver and ratiometric fluorescent detection of manganese. Tetrahedron Lett. 2009, 50, 4201–4204. 10.1016/j.tetlet.2009.04.108. [DOI] [Google Scholar]
- Wang L.; Li H.; Cao D. A new photoresponsive coumarin-derived Schiff base: Chemosensor selectively for Al3+ and Fe3+ and fluorescence turn-on under room light. Sens. Actuators, B 2013, 181, 749–755. 10.1016/j.snb.2013.01.090. [DOI] [Google Scholar]
- Wang M.; Wang J.; Xue W.; Wu A. A benzimidazole-based ratiometric fluorescent sensor for Cr3+ and Fe3+. Dyes Pigm. 2013, 97, 475–480. 10.1016/j.dyepig.2013.02.005. [DOI] [Google Scholar]
- a Wu C.; Wang J.; Shen J.; Zhang C.; Wu Z.; Zhou H. A colorimetric quinoline-based chemosensor for sequential detection of copper ion and cyanide anions. Tetrahedron 2017, 73, 5715–5719. 10.1016/j.tet.2017.08.010. [DOI] [Google Scholar]; b Wang W.; Wei J.; Liu H.; Liu Q.; Gao Y. A novel colorimetric chemosensor based on quinoline for the sequential detection of Fe3+ and PPi in aqueous solution. Tetrahedron Lett. 2017, 58, 1025–1029. 10.1016/j.tetlet.2017.01.010. [DOI] [Google Scholar]; c Mahalingam M.; Irulappan M.; Kasirajan G.; Palathurai Subramaniam M.; Ramasamy S.; Unnisa N. Synthesis of bisbenzimidazo quinoline fluorescent receptor for Fe2+ ion in the aqueous medium - An experimental and theoretical approach. J. Mol. Struct. 2015, 1099, 257–265. 10.1016/j.molstruc.2015.06.070. [DOI] [Google Scholar]; d Kia Y.; Osman H.; Suresh Kumar R.; Basiri A.; Murugaiyah V. Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1815–1819. 10.1016/j.bmcl.2014.02.019. [DOI] [PubMed] [Google Scholar]; e Karmakar M.; Bhatta S. R.; Giri S.; Thakur A. Oxidation induced differentially selective turn-on fluorescence via photoinduced electron transfer based on a ferrocene-appended coumarin-quinoline platform: Application in cascaded molecular logic. Inorg. Chem. 2020, 59, 4493–4507. 10.1021/acs.inorgchem.9b03650. [DOI] [PubMed] [Google Scholar]
- a Minyan H.; Jintao Z.; Xudong Z.; Yuzhe Z.; Song X.; Zhongyu L. Novel spiropyran derivative based reversible photo-driven colorimetric and fluorescent probes for recognizing Fe3+, Cr3+ and Al3+ metal ions. Inorg. Chem. Commun. 2020, 117, 107968. 10.1016/j.inoche.2020.107968. [DOI] [Google Scholar]; b Kiani M.; Bagherzadeh M.; Meghdadi S.; Rabiee N.; Abbasi A.; Schenk-Joß K.; Tahriri M.; Tayebi L.; Webster T. J. Development of a Novel Carboxamide-Based Off-On Switch Fluorescence Sensor: Hg2+, Zn2+ and Cd2+. New J. Chem. 2020, 44, 11841. 10.1039/D0NJ02595J. [DOI] [Google Scholar]; c Uahengo V.; Hamukwaya E. N.; Endjala P. T.; Naimhwaka J. H. A potential naphthyl-thiazole-based organic dye and a ditopic chromogenic probe for CN- and Fe3+ with molecular logic functions. New J. Chem. 2020, 44, 18588. 10.1039/D0NJ03806G. [DOI] [Google Scholar]; d Salman A. K. Multi-step synthesis, photophysical and physicochemical investigation of novel pyrazoline a heterocyclic D- p -A chromophore as a fluorescent chemosensor for the detection of Fe3p metal ion. J. Mol. Struct. 2020, 1211, 128084. 10.1016/j.molstruc.2020.128084. [DOI] [Google Scholar]; e Zixuan Y.; Yaqiang T.; Sheng C.; Leiyu Z.; Liang L. The synthesis of a series of fluorescent emitters and their application for dye lasing and cation sensing. Spectrochim. Acta, Part A 2021, 246, 118978. 10.1016/j.saa.2020.118978. [DOI] [PubMed] [Google Scholar]; f Ammar R. A.; Alturiqi A. S.; Alaghaz A.-N. M. A.; Zayed M. E. Synthesis, spectral characterization, quantum chemical calculations, in-vitro antimicrobial and DNA activity studies of 2-(2 ’-mercaptophenyl) benzothiazole complexes. J. Mol. Struct. 2018, 1168, 250–263. 10.1016/j.molstruc.2018.05.043. [DOI] [Google Scholar]; g Daravath S.; Kumar M. P.; Rambabu A.; Vamsikrishna N.; Ganji N.; Shivaraj Design, synthesis, spectral characterization, DNA interaction and biological activity studies of copper(II), cobalt(II) and nickel(II) complexes of 6-amino benzothiazole derivatives. J. Mol. Struct. 2017, 1144, 147–158. 10.1016/j.molstruc.2017.05.022. [DOI] [Google Scholar]; h Tian X.; Zhao Y.; Li Y.; Yang C.; Zhou Z. Sensitive and selective ratiometric nanosensors for visual detection of Cu2+ based on ions promoted oxidation reaction. Sens. Actuators, B 2017, 247, 139–145. 10.1016/j.snb.2017.03.011. [DOI] [Google Scholar]; i Jadhao M.; Das C.; Rawat A.; Kumar H.; Joshi R.; Maiti S.; Ghosh S. K. Development of multifunctional heterocyclic Schiff base as a potential metal chelator: a comprehensive spectroscopic approach towards drug discovery. JBIC, J. Biol. Inorg. Chem. 2017, 22, 47–59. 10.1007/s00775-016-1407-2. [DOI] [PubMed] [Google Scholar]; j Sharma A. K.; Kim J.; Prior J. T.; Hawco N. J.; Rath N. P.; Kim J.; Mirica L. M. Small Bifunctional Chelators That Do Not Disaggregate Amyloid beta Fibrils Exhibit Reduced Cellular Toxicity. Inorg. Chem. 2014, 53, 11367–11376. 10.1021/ic500926c. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Gulcan M.; Karataş Y.; Işık S.; Öztürk G.; Akbaş E.; Şahin E. Transition Metal(II) Complexes of a Novel Symmetrical Benzothiazole-Based Ligand: Synthesis, Spectral/Structural Characterization and Fluorescence Properties. J. Fluoresc. 2014, 24, 1679–1686. 10.1007/s10895-014-1455-3. [DOI] [PubMed] [Google Scholar]; l Barreto J.; Venkatachalam T. K.; Joshi T.; Kreher U.; Forsyth C. M.; Reutens D.; Spiccia L. Synthesis, characterization and coordination chemistry of aminophenylbenzothiazole substituted 1,4,7-triazacyclononane macrocycles. Polyhedron 2013, 52, 128–138. 10.1016/j.poly.2012.10.029. [DOI] [Google Scholar]; m Banci L.; Bertini I.; Durazo A.; Girotto S.; Gralla E. B.; Martinelli M.; Valentine J. S.; Vieru M.; Whitelegge J. P. Metal-free superoxide dismutase forms soluble oligomers under physiological conditions: A possible general mechanism for familial ALS. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 11263–11267. 10.1073/pnas.0704307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis R.; Ludger S.; Victor B.; Frank R.; Michael M. Synthesis of para-Aryl-Substituted Salicyldialdehydes. Eur. J. Org. Chem. 2015, 3274–3285. 10.1002/ejoc.201500228. [DOI] [Google Scholar]
- Chaitali V. M.; Jayashree D. Review of heavy metals in drinking water and their effect on human health. Int. J. Innov. Res. Sci., Eng. Technol. 2013, 7, 2992–2996. [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Peralta J. E. Jr.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Keith T.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford CT, 2010.
- a Qi W.; Li H. F.; Jian B. C.; Yu W.; Shaomin S. Ratiometric sensing of Zn2+ with a new benzothiazole-based fluorescent sensor and living cell imaging. Analyst 2021, 146, 4348–4356. 10.1039/D1AN00749A. [DOI] [PubMed] [Google Scholar]; b Suman G. R.; Bubbly S. G.; Gudennavar S. B.; Gayathri V. Benzimidazole and benzothiazole conjugated Schiff base as fluorescent sensors for Al3+ and Zn2+. J. Photochem. Photobiol., A 2019, 382, 1119472. 10.1016/j.jphotochem.2019.111947. [DOI] [Google Scholar]; c Mayank G.; Sunanda S.; Vivekanand S.; Parimal K. B. Benzothiazole integrated into a cryptand for ESIPT-based selective chemosensor for Zn2+ ions. Dalton Trans. 2019, 48, 7801–7808. 10.1039/C9DT00548J. [DOI] [PubMed] [Google Scholar]; d Suparna P.; Priyabrata B. An ESIPT based turn on fluorochromogenic sensor for low level discrimination of chemically analogous Zn2+ and Cd2+ & aqueous phase recognition of bio-hazardous CN-: From solution state analysis to prototype fabrication. Sens. Actuators, B 2021, 329, 129172. 10.1016/j.snb.2020.129172. [DOI] [Google Scholar]; e Zheng L.; Jing W.; Liwei X.; Jiayu W.; Hanlei Y. A dual-response fluorescent probe for Al3+ and Zn2+ in aqueous medium based on benzothiazole and its application in living cells. Inorg. Chim. Acta 2021, 516, 120147. 10.1016/j.ica.2020.120147. [DOI] [Google Scholar]; f Begum T.; Hayder M. A. A.; Serkan E. Fast and Reversible “Turn on” Fluorescent Sensors Based on Bisphenol-a for Zn2+ in Aqueous Solution. J. Fluoresc. 2019, 29, 1079–1087. 10.1007/s10895-019-02419-8. [DOI] [PubMed] [Google Scholar]; g Barbara P.; Rosita D.; Simona C.; Lucia S.; Angela T.; Stefano P.; Ugo C. Fluorescence pH-dependent sensing of Zn(II) by a tripodal ligand. A comparative X-ray and DFT study. J. Lumin. 2019, 212, 200–206. 10.1016/j.jlumin.2019.04.052. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.