Abstract
Medicinal plants have been a major resource for drug discovery. Emerging evidence shows that in addition to pharmacologically active components, medicinal plants also contain phytochemical nanomaterials, or phytonanomaterials, which form nanoparticles for drug delivery. In this review, we examine the evidence supporting the existence of phytonanomaterials. Next, we review identification, isolation, and classification of phytonanomaterials, characteristics of phytonanomaterial-derived nanoparticles, and molecular mechanisms of phytonanomaterial assembly. We will then summarize the current progress in exploring phytonanomaterial-derived NPs as therapeutic agents and drug delivery carriers for disease treatment. Last, we will provide perspectives on future discovery and applications of phytonanomaterials.
Keywords: Phytonanomaterials, nanoparticles, drug delivery, medicinal plants, nanostructural assembly
Graphical Abstract
1. Introduction
Medicinal plants are widely used in traditional medicine worldwide. It was estimated that approximately 65% of the world’s population relied on traditional medicine for their primary health care in 1985 and traditional medicine continues to play an important role in many countries as of today [1]. Following the paradigm of western medicine, recent efforts to modernize traditional medicine have focused on the isolation of pharmacologically active compounds from medicinal plants. These efforts have led to the successful discovery of various small molecule drugs. Analysis of 1,073 new small molecule drugs developed over the period 1981–2010 found that 51% of them were of phytochemical origin [2].
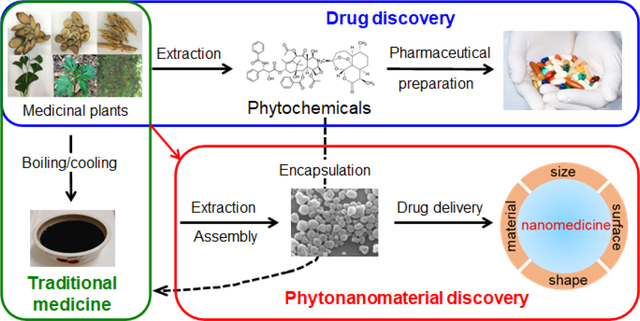
In addition to pharmacologically active compounds, medicinal plants have recently been found to contain phytochemicals capable of self-assembly into nanostructures, such as nanoscale particles, fibers, and gels [3–9]. Some of them have been characterized and demonstrate the capacity for efficient drug encapsulation and targeted delivery. These observations suggest that the value of medical plants is not limited to drug discovery, as they can also be a valuable resource for identifying phytochemical nanomaterials, or phytonanomaterials, for drug delivery. In this article, we will review recent progress in the discovery and applications of phytonanomaterials, with a focus on those forming nanoparticles (NPs), and provide our perspectives on future development in this area.
2. Theory and evidence for the existence of phytonanomaterials
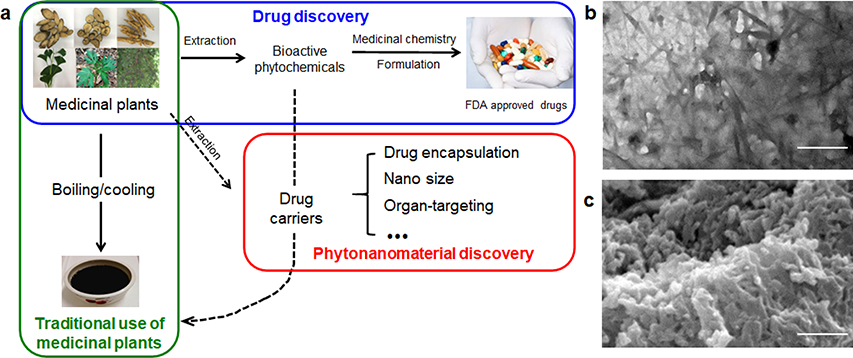
In traditional Chinese medicine (TCM), there are over 100,000 different herbal formulas, which are prescribed empirically based on a synergism principle called “Jun–Chen–Zuo–Shi” [10–13]. The “Jun (emperor)” and “Chen (minister)” represent the primary and secondary medicinal herbs that provide therapeutic activities. The “Zuo (adjuvant)” and “Shi (courier)” represent the herbs that assist “Jun” and “Chen” by reducing systemic toxicity, facilitating synergism, and providing targeted delivery. During the recent process of TCM modernization, medicinal herbs in the “Jun” and “Chen” categories are often selected for the identification of pharmacologically active phytochemicals [10–13]. Due to the lack of pharmacologically active components, the adjuvant “Zuo” and “Shi” herbs are often considered redundant and their roles in TCM appear unjustified. It has been shown that the majority of pharmacologically active compounds isolated from herbal materials cannot be directly used as drugs because of their poor stability, solubility, or pharmacokinetics. Among those phytochemical-originated drugs developed over the period 1981–2010, only 12% of them are used in their natural form without chemical modification [2], while others require chemical modification or formulation. Interestingly, those pharmacologically active phytochemicals with limited bioavailability can exert their pharmacological activities in TCM after processing, such as boiling/cooling or emulsification (Fig. 1a) [14]. It appears that following such processing, those phytochemicals with low bioavailability are converted to a bioavailable form. This enigma led to a hypothesis that certain medicinal plants contain carrier materials that can enhance the bioavailability of those pharmacologically active phytochemicals, which otherwise cannot be directly utilized for therapeutic applications [3]. If such carriers exist, they are likely in nano size, as larger carriers are unfavorable for blood circulation and tissue penetration [15].
Figure 1.
Theory and evidence for the existence of phytonanomaterials. (a) Schematic diagram of utilization of medicinal plants for traditional medicine, drug discovery and nanomaterial discovery. Representative TEM image (b) and SEM image (c) of Poria cocos soup. Scale bar: 500 nm. Images b and c were adapted from Ref.[3], with permission from Springer.
To test the hypothesis, our group recently studied Poria cocos, one of the most commonly used “Zuo (adjuvant)” or “Shi (courier)” herbs in TCM formulas [3]. Following the traditional boiling-cooling processing procedures, supernatant of Poria cocos soup was obtained through filtration. Analysis of the supernatant by transmission electron microscopy (TEM) revealed the existence of rod-shaped NPs (Fig. 1b), whose structure was maintained after lyophilization, as determined by scanning electron microscopy (SEM) (Fig. 1c). These observations suggest the existence of phytonanomaterials in medicinal herbs.
3. Identification and characterization of phytonanomaterials
3.1. Isolation of phytonanomaterials
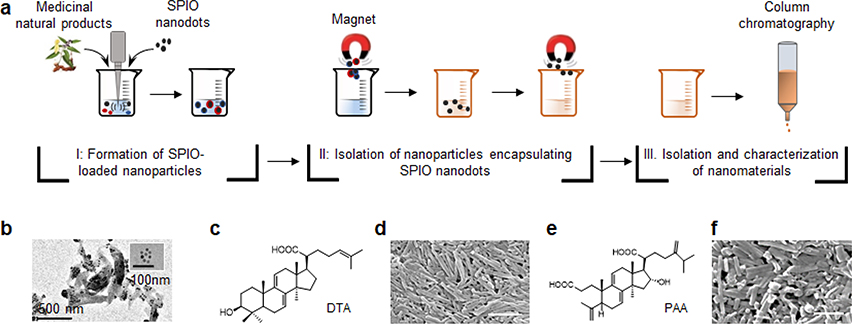
Previous studies suggest that certain phytochemicals can form NPs [6]. However, systemic discovery and isolation of phytonanomaterials from medicinal plants have not been possible until the recent development of a chemical extraction approach, in which we employed hydrophilic, oleic acid-coated superparamagnetic iron oxide (SPIO) nanodots, as a payload to isolate those nanomaterials capable of drug encapsulation (Fig. 2a) [3, 16]. By using Poria cocos as an example, we showed that, following the boiling-cooling procedures, SPIO-loaded NPs could be pulled down using a magnet (Fig. 2b). To improve the efficiency of nanomaterial isolation, we refined the extraction approach by replacing boiling/cooling with standard emulsion procedures [17]. After co-dissolving SPIO and Poria cocos extract in organic solvents, such as dichloromethane, the mixture was emulsified in an aqueous solution containing surfactant poly(vinyl alcohol). SPIO-containing NPs were collected using magnetic force. Nanomaterials were then extracted by removing SPIO, purified using conventional chromatography, and characterized by 1H NMR, 13C NMR and mass spectrometry (MS). Through these procedures, dehydrotrametenolic acid (DTA) and poricoic acid A (PAA), both of which form rod-shaped NPs (Fig. 2c–f), were successfully isolated [18].
Figure 2.
Chemical approach for isolation of phytonanomaterials. (a) Schematic diagram of a chemical approach for the isolation of phytonanomaterials. (b) Representative images of SPIO (insert) and SPIO-encapsulated NPs as captured by TEM. Molecular structures of DTA (c) and PAA (e) and representative SEM images of NPs consisting of DTA NPs (d) and PAA NPs (f). Scale bar: 500 nm. Adapted from Ref. [3], with permission from Springer.
3.2. Classification of phytonanomaterials
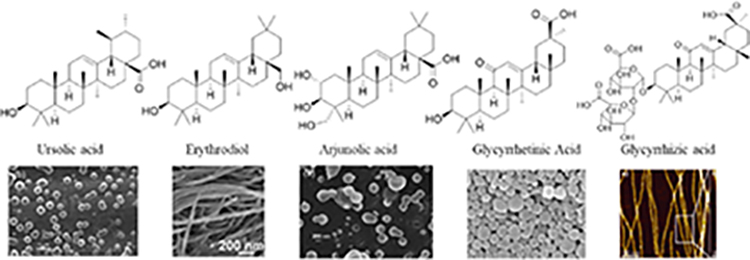
Since the development of the chemical extraction approach, we have screened ~100 medicinal plants [3, 5, 8, 9, 16, 19–25]. Together with those reported by other groups [26–50], over 30 phytonanomaterials, which can be categorized into at least 5 classes (Table 1), have been identified, including 1) tricyclic diterpene resin acids, which are 20-carbon tricyclic carboxylic acids with diversity introduced by double-bond isomers, diastereoisomers, and additional moieties, such as abietic acid (AA) from Pinus kesiya and isopimaric acid (IPA) from Pinus elliottii; 2) phytosterols, which are 3-hydroxy cyclopentane steroids with various numbers of double bonds in the main skeleton, such as stigmasterol (ST) from Taxus chinensis (Pilger) Rehd and β-sitosterol from Artemisia carvifolia; 3) lupane type pentacyclic triterpenes, which have a typical 6–6-6–6-5 main skeleton with α-isopropyl group in the position 19 of the five-membered ring, such as lupeol from Pinus elliottii, and betulinic acid (BA) from Eucommia ulmoides [61]; and 4) oleanane type pentacyclic triterpenes, which have a typical 6–6-6–6-6 main skeleton with hydroxy in position 3 and double bond in position 12, such as glycyrrhetic acid (GA) from Glycyrrhiza uralensis Fisch and sumaresinolic acid (SA) from Melastoma candidum D.Don; and 5) others, mainly including terpenes and alkaloids, such as PAA from P. cocos. It is important to point out that those phytonanomaterials not only can form single-component NPs through self-assembly but also may interact with each other and co-assemble into multiple-component NPs.
Table 1.
Classification of phytonanomaterials.
| Classification | Representative structure | Phytonanomaterial molecular structure and derived NPs | References |
|---|---|---|---|
|
| |||
| Diterpene resin acids |
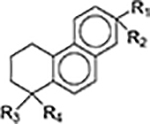
|
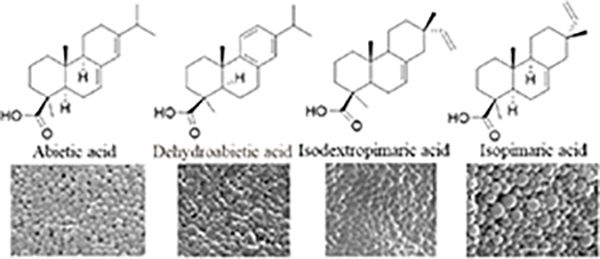
|
[3] |
| Phytosterols |
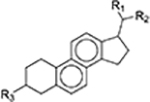
|
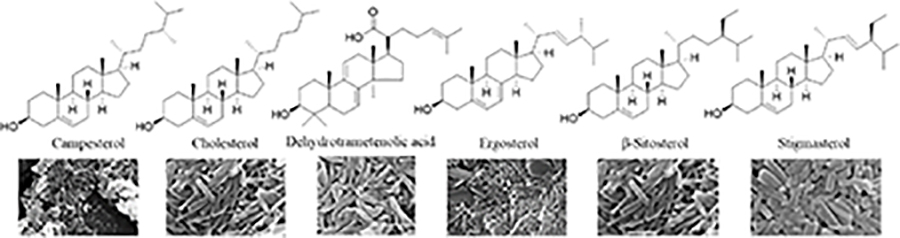
|
[3, 45, 51] |
| Lupane type pentacyclic triterpenes |
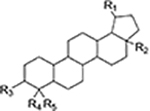
|
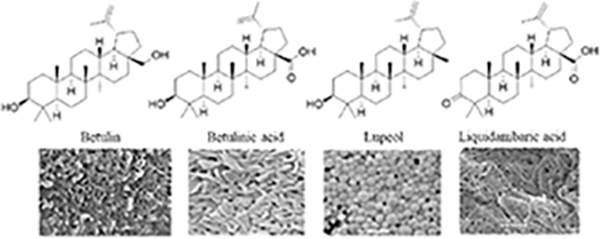
|
[3, 7, 8, 20, 32] |
| Oleanane type pentacyclic triterpenes |
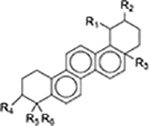
|
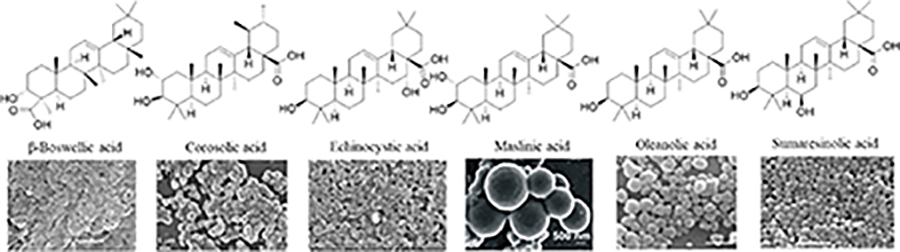
|
[3, 8, 21, 26, 28, 29, 31, 33–35, 37, 39, 40, 48, 52–54] |
|
|||
| Others |
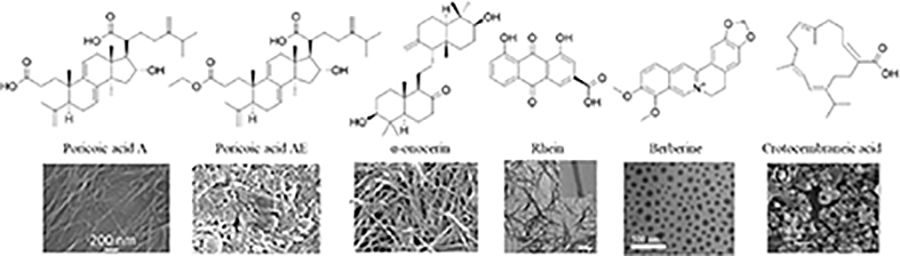
|
[22, 30, 38, 42, 44, 49, 55, 56] | |
3.3. Physical characteristics of phytonanomaterial-derived NPs
Physical characteristics of NPs, including their size, shape, and surface charge, greatly impact their interaction with target cells and tissues in vitro and in vivo [57–59]. Compared to spherical NPs, rod-shaped NPs in a comparable length are easier to be internalized by cells and short rod-shaped NPs have greater internalization efficiency than long rod-shaped NPs [60–63]. Size is particularly important for blood circulation. After intravenous administration, NPs with a diameter of less than 8 nm are subjected to rapid renal clearance [64], while NPs with a size greater than 500 nm are more likely to be phagocytized and eliminated by the reticuloendothelial system (RES) [59]. Surface charge plays a key role in determining the interaction of NPs with tissue, with anionic surface charge advantageous over neutral or positive surface charge in avoiding non-specific organ uptake [65].
Recent studies show that most phytonanomaterial-derived NPs are negatively charged with zeta potential ranging from −18.2 to −25.6 mV [3, 7]. Unlike surface charge, size and shape of phytonanomaterial-derived NPs can vary significantly (Table 1). For example, BA, oleanolic acid (OA), ursolic acid (UA), betulin, arjunolic acid (AJA), maslinic acid (MA) and crotocembraneic acid (CA), can self-assemble into spherical particles of nano- to micrometer in diameter or rod-/fiber-shaped nanostructures [7, 31, 32, 40]. It is particularly interesting that, depending on the synthesis conditions, the same phytonanomaterial can form NPs in different sizes and shapes, which are determined by a combination of various parameters, including solvent, drug feeding concentration, flow rate, temperature, and surfactant [7, 35].
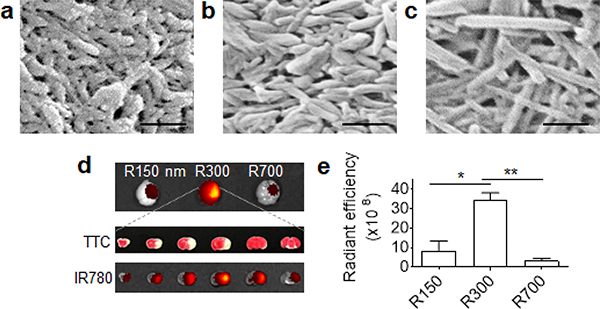
The great flexibility in the synthesis of NPs in various sizes and shapes makes it possible to test and compare multiple NP formulations consisting of the same material and select the one that is optimal for specific biological applications. For example, synthesis through combination of various solvents and temperatures allowed for production of rod-shaped BA NPs in three different sizes and shapes, including length of ~156 nm and diameter of ~45 nm (156(l) x 45(d)) (Fig. 3a), 315(l) x 60(d) (Fig. 3b), or 730(l) x 35(d) (Fig. 3c), designating as R150, R300, and R700, respectively. After intravenous administration, R300 demonstrated an efficiency significantly greater than R150 and R700 in penetration into the ischemic brain (Fig. 3d,e) [7].
Figure 3.
Impact of size and shape of BA NPs on penetration into the ischemic brain. (a-c) Representative SEM images of R150 (a), R300 (b), and R700 (c) BA NPs. Scale bar: 500 nm. (d) Representative images of infarction and NPs in the brain. (e) Quantification of the accumulation of NPs in the brains of mice treated with the indicated NPs. RE: Radiance efficiency. Adapted from Ref. [7], with permission from Ivyspring.
4. Supramolecular assembly of phytonanomaterials
Self-assembly of nanomaterials is a spontaneous process driven by thermodynamics and kinetics and is determined by non-covalent interactions of adjacent molecules. While the precise mechanism of self-assembly is yet to be determined, it is suggested that the formation of phytonanomaterial NPs results from synergistic interactions of multiple intermolecular forces, such as hydrogen bonding, π-π interaction, hydrophobic interaction, and electrostatic interaction. Among them, hydrogen bonding and π-π interactions often play the most important roles.
4.1. Hydrogen bonding
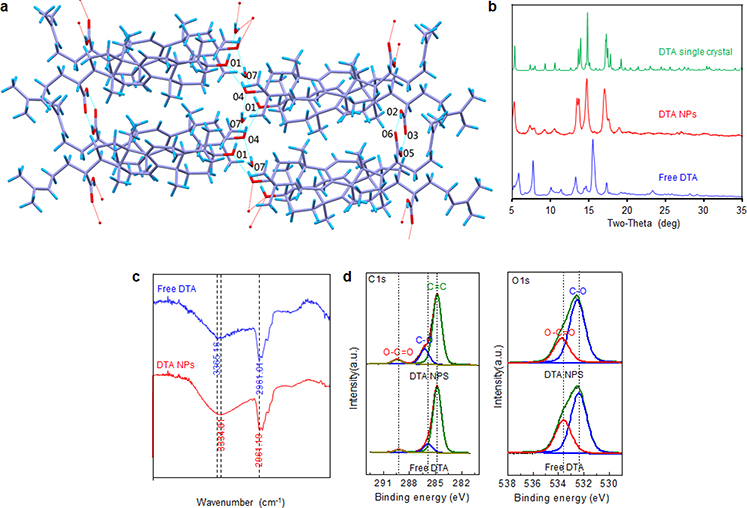
The strength and stability of hydrogen bonding are weak in aqueous solution due to the presence of water molecules. Most phytonanomaterials are hydrophobic and have limited solubility in water. Without competition of water molecules, saturated and directional hydrogen bonding within nanomaterials are capable of providing sufficient force to drive the formation of nanostructures [67]. Our group recently investigated the molecular mechanism of DTA NP self-assembly by solving the crystal structure of DTA using single-crystal X-ray diffraction (SXRD) and comparing the X-ray diffraction (XRD) patterns of DTA crystals with those of DTA NPs. Through these approaches, we revealed the existence of two types of classic hydrogen bonds in the molecular conformations, including a R(22 8) hydrogen bond formed among the rings via carboxyl O2-H2…O6 and O5-H5…O3, and a second hydrogen bond formed between hydroxyl group O (O4, O1) and the water molecule (O7) in DTA NPs (Fig. 4a, b). The presence of hydrogen bonds within DTA NPs was further confirmed by Fourier-transform infrared spectroscopy (FTIR) (Fig. 4c) and X-ray photoelectron spectroscopy (XPS), which identified significant upshifts in both C-O and O-C=O spectra (Fig. 4d) [3]. Consistent with this finding, Bag and He showed that hydrogen bonding plays a key role in the formation of nanostructures derived from α-onocerin 1 [38], and β-sitosterol [51].
Figure 4.
Molecular mechanism of DTA NP self-assembly. (a) Capped sticks representation of DTA packing and intramolecular interactions. (b) XRD patterns of NPs, free DTA, and simulated XRD patterns of DTA crystals. FTIR spectra (c) and C1s (left) and O1s (right) XPS spectra (d) of NPs and free DTA. Adapted from Ref. [3], with permission from Springer.
4.2. π-π stacking interaction
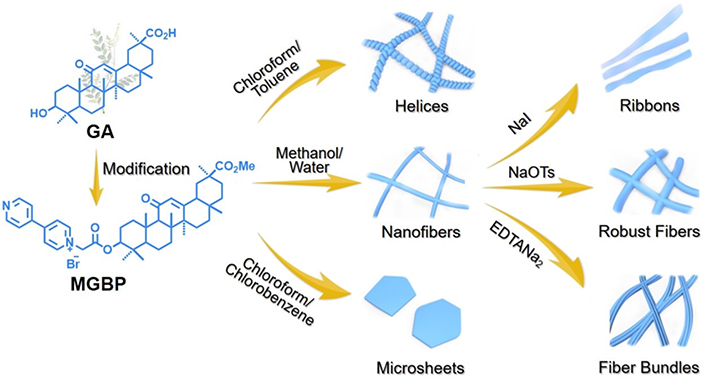
π-π interaction represents the attractive force between adjacent aromatic rings and has been shown to be sufficient to drive formation of nanostructures. One key feature of phytonanomaterials is that all of them bear aromatic rings on the backbone (Table 1). This feature makes it possible to have individual phytonanomaterial molecules stack on each other through π-π interaction, leading to the growth of a 3D nanostructure in a specific direction. Hu and colleagues designed and synthesized a π-chromophore-containing glycyrrhetinic acid amphiphile, 1-[2-(methyl glycyrrhetate)-2-oxoethyl]-[4,4’]bipyridinium bromide (MGBP) (Fig. 5). In this amphiphile, the hydrophobic glycyrrhetinic acid is connected to a hydrophilic bipyridinium cation through a short alkyl chain. Bipyridinium not only improves the hydrophilicity of the molecule, but also endows it with additional π-π stacking forces. As a result, MGBP can self-assemble into various nanostructures in the presence of different solvents largely through π-π stacking [50]. In another study, Yang studied the self-assembly process of PAA, which contains a carbon-carbon double bond (C=C) functional groups related to π-π interaction. It was shown in the UV–vis spectrum that the maximum absorption peak of PAA shifted during the formation of nanostructures (moved from 242.0 nm to 250.5 nm), suggesting that the electron transition energy of C=C is reduced and the C=C drives the self-assembly through enhancement of π-π interaction [22].
Figure 5.
Schematic representation of hierarchical assembly of MGBP. Adapted from ref. [50], with permission from Elsevier.
4.3. Synergistic interactions of multiple weak intermolecular forces
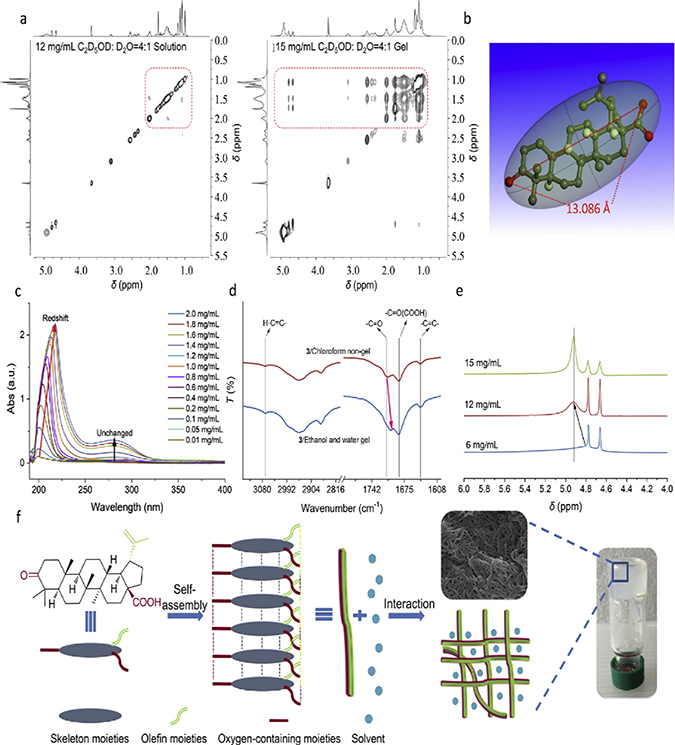
Besides hydrogen bonding and π-π interaction, several other weak intermolecular forces are involved in phytonanomaterial self-assembly. In fact, the formation of complex ordered structures in self-assembly systems is usually driven by multiple non-covalent interactions. These intermolecular forces include: (1) hydrophobic interaction. Hydrophobic interaction is known to be a major driving force for the assembly of various hydrophobic nanomaterials [68, 69]. While composing of hydrophobic parent nucleus and hydrophilic groups, each phytonanomaterial as a whole is hydrophobic. It is likely that, during the emulsion process in aqueous solution, hydrophobic interaction promotes nonpolar parts of phytonanomaterials to stack each other to eliminate water molecules and thus reduce the total energy, which is favorable for the stability of nanostructures [70]; (2) electrostatic interactions. Li and colleagues showed that berberine, a natural hydrophobic cation, was able to form NPs with flavonoid glycoside baicalin, which bear negative charge. They demonstrated that electrostatic attraction between the two molecules contributes significantly to formation of the nanostructures [42]; and (3) interaction between nanomaterials and solvents. Yang and colleagues studied formation of natural product gels by a group of triterpenoids through a combination of two-dimensional nuclear magnetic resonance (NMR) - nuclear overhauser effect spectroscopy (NOSEY), Ultraviolet-visible (UV-vis) spectroscopy, and infrared (IR)/1D 1H NMR, which measure van der Waals force, π-π interaction and hydrogen bonding, respectively. They found that formation of the specific nanostructure is driven not only by weak non-covalent forces among triterpenoid molecules, but also by the interaction of phytonanomaterials with solvents (Fig. 6) [20].
Figure 6.
Formation of injectable natural product gel scaffold. (a) 2D NOESY spectra of compound 3 in mixed solvent of deuterated ethanol and deuterated water (4:1) at different concentrations. (b) Molecular length of compound 3. (c) UV spectra of compound 3 in ethanol/water mixed solvent (1:1) at different concentrations. (d) IR spectra of compound 3 obtained from non-gel and gel. (e) 1H NMR spectra of compound 3 in mixed solvent of deuterated ethanol and deuterated water (4:1) at different concentrations. (f) A possible self-assembly formation process of natural product gels. Adapted from ref. [20], with permission from Elsevier.
5. Application of phytonanomaterials as therapeutics and drug delivery carriers
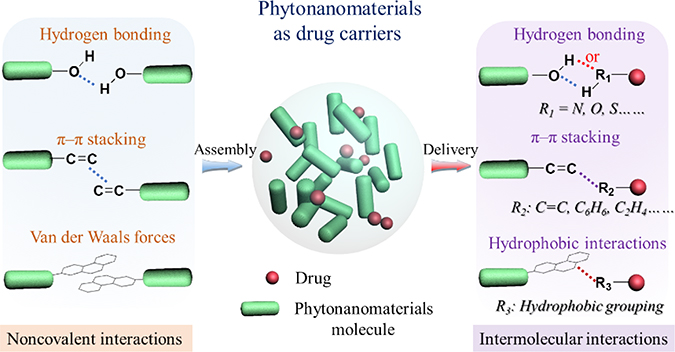
Most phytonanomaterials identified thus far are known to have pharmacological activities. Formulating phytonanomaterials into NPs has several advantages for their biological applications. First, as the majority of phytonanomaterials are hydrophobic, formulating them into NPs enhances their solubility, making it possible to be administered intravenously in large quantitates for disease treatment. Second, compared to sub-nanometer parent small molecules, phytonanomaterial-derived NPs are nanometers in size, which is optimal for blood circulation and disease targeting [71]. Consequently, the NP formulation improves the therapeutic potential of parent phytonanomaterial molecules by improving their pharmacokinetic properties, facilitating their interaction with targeted cells, and reducing their non-specific toxicity. Besides pharmacological activities, phytonanomaterial-derived NPs bear nanostructure, which allows for encapsulation of therapeutic molecules through intermolecular noncovalent interactions, such as hydrogen bonding, π-π stacking interaction, and hydrophobic interactions (Fig. 7). This feature makes it possible to employ phytonanomaterial-derived NPs as carriers for drug delivery. By far, phytonanomaterial-derived NPs have been evaluated as therapeutics and/or drug delivery carriers for the treatment of various diseases, such as neurological disorders, cancer, infectious diseases, and diabetes (Table 2).
Figure 7.
Schematic diagram of phytonanomaterial-derived NPs for drug encapsulation. Phytonanomaterials-assembled NPs allow for encapsulation of therapeutic molecules through intermolecular hydrogen bonding, π-π stacking interaction, and hydrophobic interactions.
Table 2.
Phytonanomaterial-derived NPs as therapeutic agents and drug delivery carriers.
| Phytonanomaterial | Molecular force | Size/Morphology | Payload | Target | Application | Reference | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Single molecule assembly | Dehydrotrametenolic acid | Hydrogen bond | 400(l) × 70(d) nm/Rod | Paclitaxel/GLP-1 | ASBT | Cancer/diabetes | [3] |
| Betulinic acid | NA | 156(l) × 45(d) nm/Rod | Glyburide | CB 1 | Ischemic stroke | [7] | |
| Betulonic acid | π−π/hydrophobic | ~231.4 nm/Sphere | Ce6 | EPR | Cancer | [5] | |
| Arjunolic acid | - | 135 nm/Sphere | Doxorubicin | - | Cancer | [31] | |
| Corosolic acid | - | 90.32 nm/Sphere | Doxorubicin | - | Cancer | [39] | |
| Crotocembraneic acid | - | 200 nm/Sphere | Doxorubicin | - | Cancer | [36] | |
| Ergosterol | π−π/hydrophobic | ~207 nm/Sphere | Ce6 | EPR | Cancer | [19] | |
| Lithocholic acid | - | 90–140 nm/Sphere | Paclitaxel/Doxorubicin | - | Cancer | [30] | |
| Maslinic acid | - | 187.6 nm/Sphere | Doxorubicin | - | Cancer | [40] | |
| Oleanolic acid | Hydrogen bond | ~259.7 nm/Sphere | Paclitaxel | EPR | Cancer | [4] | |
| Oleanolic acid | Hydrogen bond/hydrophobic | ~266 nm/Sphere | Paclitaxel | EPR | Cancer | [21] | |
| Oleanolic acid | - | 284.9 nm/Sphere | - | - | Liver injury | [26] | |
| Oleanolic Acid | - | <100 nm/Sphere | - | - | Cancer | [53] | |
| Ursolic acid | Hydrogen bond/hydrophobic | ~190.1 nm/Sphere | Paclitaxel | EPR | Cancer | [8] | |
| Ursolic acid | - | 100 nm, 300 nm/Sphere | - | - | Cancer | [35] | |
| Ursolic acid | Hydrogen bond | 120–360 nm/Sphere | Doxorubicin | - | Cancer | [37] | |
| Multiple molecule assembly | Berberine + Baicalin/Wogonoside | Electrostatic/hydrophobic | ~100 nm/Sphere or fiber | - | - | Antibacteria | [42, 47] |
| Berberine + Cinnamic acid | Hydrogen bond/π−π | ~66 nm/Sphere | - | - | Antibacteria | [41] | |
| Berberine + Rhein | π−π/electrostatic | ~174 nm/Sphere | - | - | Antibacteria | [49] | |
| Berberine + 3,4,5-TCA | Hydrogen bond/π−π | - | - | - | Antibacteria | [46] | |
| Betulinic acid + Paclitaxel | - | ~282 nm/Rod | - | - | Cancer | [43] | |
| Betulonic acid + Paclitaxel | Hydrogen bond/hydrophobic | ~240 nm/Sphere | - | EPR | Cancer | [23] | |
| Betulinic acid (BA)-s-s + Ce6 | Hydrogen bond/π−π | 207.8 nm/Sphere | - | EPR | Cancer | [24] | |
| Glycyrrhetinic acid + Liquidambaric acid | Hydrogen bond/hydrophobic | ~228 nm/Sphere | - | EPR | Cancer | [25] | |
| Oleanolic acid + Betulinic acid | Hydrogen bond/hydrophobic | ~341 nm/Rod | - | EPR | Cancer | [25] | |
| Oleanolic acid + Glycyrrhetinic acid | Hydrogen bond/hydrophobic | 140–350 nm/Sphere | Paclitaxel | EPR | Cancer | [9] | |
5.1. Neurological disorders
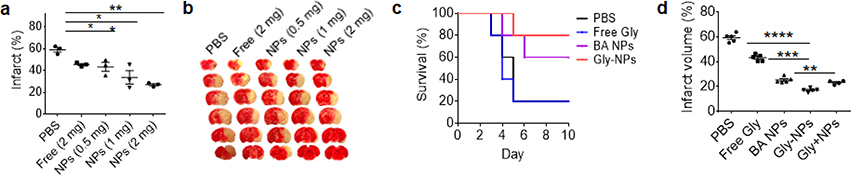
Treatment of neurological disorders is a major challenge, because of the existence of the blood-brain barrier (BBB), which significantly reduces penetration of most therapeutic agents into the brain [18, 72]. This barrier can potentially be overcome through utilization of phytonanomaterial-derived NPs, some of which have the physical characteristics optimal for brain penetration and/or interaction with receptor molecules expressed in brain tissue. We recently characterized BA NPs for their penetrability into the ischemic brain by comparing BA NPs of various sizes and shapes [7]. We found that rod-shaped R300 NPs, but not those with a smaller or greater size, have the greatest brain targeting efficiency (Fig. 3), suggesting size and shape of NPs as key parameters for brain penetration. We further showed that BA NPs interact with cannabinoid receptor 1 (CB1), which is highly expressed in brain tissue after ischemic insult. We found that the interaction between the NPs and CB1 facilitates brain penetration, as blocking CB1 using SR141716A, a CB1 specific inhibitor, significantly reduced the accumulation of NPs in the brain. BA is known to have excellent antioxidant properties [73, 74]. We showed that, as a result of the high efficiency in brain penetration, BA NPs after intravenous administration effectively improved the recovery of mice after stroke. In comparison, administration of free BA at the same dose showed limited therapeutic effects (Fig. 8a, b). We further demonstrated that BA NPs were capable of efficiently encapsulating and delivering glyburide, a sulfonylurea used as an anti-edema therapy, to the brain and the combination of anti-edema therapy provided by glyburide and antioxidant therapy provided by BA NPs significantly prolonged the survival of stroke-bearing mice and reduced cerebral infarction (Fig. 8c, d).
Figure 8.
BA NPs as therapeutics and drug carriers for stroke treatment. (a, b) Quantification (a) and representative images (b) of brain infarction in MCAO mice received treatment of BA NPs at the indicated dose. Kaplan-Meier survival analysis (c), and infarct volume (d) of MCAO mice receiving the indicated treatments. Adapted from Ref. [7], with permission from Ivyspring.
5.2. Cancer
Phytonanomaterial-derived NPs have been tested as therapeutics and/or drug carriers for the treatment of various cancers, including lung cancer, breast cancer, and breast cancer brain metastasis [3–5, 8, 19, 21, 24, 35, 43]. It was found that NPs consisting of DTA, OA, BA, UA, betulonic acid (BC), or ergosterol were able to efficiently accumulate in tumors after intravenous administration, likely due to passive targeting as a result of enhanced permeability and retention (EPR) effect [3–5, 8, 19, 21, 24, 43]. It was also shown that BA NPs could be further modified to respond to glutathione, which is enriched in the tumor microenvironment [75], and the modification significantly reduced systemic toxicity of BA NP treatment [24]. As drug carriers, all of those NPs demonstrated a great capacity for encapsulation and delivery of therapeutic agents, such as paclitaxel and doxorubicin for chemotherapy [3, 4, 30], and photosensitizer Ce6 for photodynamic therapy [5, 19]. In all cases, drug-loaded NPs effectively inhibited the development of tumors. It is particularly interesting that DTA NPs can efficiently penetrate the gastrointestinal tract through interaction with the apical sodium-dependent bile transporter (ASBT) and thus enable oral delivery of chemotherapy for cancer treatment [3].
5.3. Infectious diseases
Many phytochemicals are known to have antibacterial, antifungal, and antiviral properties [76–78]. However, many of them in their natural form have limited antimicrobial activities. Recent studies showed that, for those capable of self-assembly, this limitation could be potentially overcome through formulation into NPs. Lei and colleagues developed a series of phytonanomaterial-derived NPs with high antibacterial activity through dual molecules assembly of berberine with other phytochemicals, such as baicalin, cinnamic acid, and rhein [41, 42, 46, 49]. Berberine is a natural hydrophobic cation that exists in herbal plants, such as Coptidis rhizome, and is known to have weak antibacterial activity [79]. It was shown that berberine and flavonoid glycoside baicalin are able to co-assemble into NPs through electrostatic and hydrophobic interactions [42]. As a result, the hydrophilic glucuronic acid in berberine was displayed toward the outside of NPs, which enhances the interaction of berberine with bacteria, leading to killing bacteria and reducing biofilm formation with significantly greater efficiency than free berberine. They applied the same approach to formulate berberine into NPs through co-assembly with cinnamic acid or antibacterial phytochemicals, such as rhein, and found that, in all cases, the resulting NPs exhibit antibacterial activities greater than selected antibiotics [49].
5.4. Others
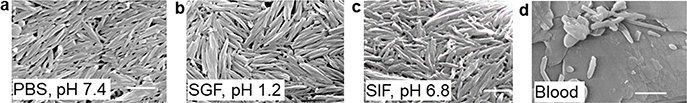
Phytonanomaterial-derived NPs have been explored for the treatment of diabetes [3] and liver injury [26]. It was shown that DTA NPs were capable of efficient encapsulation of glucagon-like peptide-1 (GLP-1), a 30-aa peptide, and oral administration of GLP-1-loaded NPs significantly decreased blood glucose through stimulation of insulin production. It was demonstrated that DTA NPs are stable in artificial gastric fluid (SGF, pH 1.2) and intestinal fluid (SIF, pH 6.8) (Fig. 9a–c), and the NPs in an integrated form could be collected from the circulatory system after oral administration (Fig. 9d). These findings suggest that certain phytonanomaterial-derived NPs, such as DTA NPs, have the stability to survive the gastrointestinal environment, and, thus, can be employed for oral drug delivery [3]. In another study, self-assembled OA NPs were shown to protect the liver from carbon tetrachloride-induced injury through lowering serum alanine aminotransferase activity and liver malondialdehyde content [26].
Figure 9.
Characterization of the stability of DTA NPs for oral drug delivery. (a-c) SEM images of the DTA NPs after incubation in the indicated medium for 2 h. Simulated gastric fluid, SGF; simulated intestinal fluid, SIF. (d) A representative SEM image of DTA NPs recovered from the blood. Scale bar: 500 nm. Adapted from Ref. [3], with permission from Springer.
In summary, existing evidence suggests that phytonanomaterials in NP form are promising for treatment of various diseases, including neurological disorders, cancer, infectious diseases, diabetes, and liver injury, in preclinical studies. In light of the fact that phytonanomaterials have diverse pharmacological activities, further exploration of phytonanomaterial-derived NPs for other disease indications, such as inflammatory diseases, is warranted. It is also worthwhile mentioning it is likely that, in real situations when medicinal plants are used, more than one or two phytonanomaterials are co-assembled together to form NPs as drug carriers. Therefore, further characterization of NPs co-assembled from multiple phytonanomaterials may represent a new important research direction.
6. Conclusions and Perspectives
Growing evidence shows the existence of phytonanomaterials that are capable of forming NPs for drug delivery. This finding is significant in that it may provide valuable insight into the philosophy of traditional medicine, through which an effective formula requires prescribing a combination of those medicinal plants with therapeutic activities of others to facilitate synergism. It is possible that the later ones contain phytonanomaterials that can enhance the bioavailability of bioactive components. This finding may also help resolve the enigma that many pharmacologically active phytochemicals without processing with other medicinal plants don’t have the bioavailability required for disease treatment [10–12]. For example, phytochemicals artemisinin [80] and PTX [81] are known to have limited bioavailability and cannot be used as drugs without further modification or formulation. It was shown that within Artemisia annua L and Taxus, where artemisinin and PTX exist, contain nanomaterial ß-sitosterol [3, 19]. One cannot exclude the possibility that during the boiling/cooling or emulsification process, these compounds are converted into a bioavailable form through encapsulation into ß-sitosterol NPs. Conversely, Poria cocos, one of the most commonly used “Zuo” or “Shi” herbal materials, highly enriches phytonanomaterial DTA and PAA [3], justifying its roles in TCM formulas.
Despite the exciting findings to date, identification and application of phytonanomaterials are still in their infancy. Considering the wide molecular diversity of naturally existing molecules, additional phytonanomaterials structurally different from these already identified are expected to be discovered in the future. This progress in turn will significantly impact drug delivery research and development through diversification of nanomaterials suitable for drug encapsulation. Unlike traditional nanomaterials, such as polymers and lipids, phytonanomaterials have a unique feature in that they can form nanostructures in various physical characteristics, such as spherical or rod-shaped NPs, fibers, and gel (Table 1). It is particularly interesting that the same phytonanomaterial is capable of forming NPs in different sizes and shapes (Fig. 3). Considering the importance of physical characteristics of NPs in drug delivery [57–64], phytonanomaterials are uniquely positioned to satisfy the requirements for a variety of biomedical applications. Phytonanomaterials have another major advantage over traditional nanomaterials in that phytonanomaterial-derived NPs may bear intrinsic organ targeting effects and thus allow for targeted drug delivery without additional chemical modifications. For example, BA NPs were shown to interact with the CB1 receptor and the interaction between NPs and the receptor facilitates efficient brain penetration [7]. In comparison, most polymeric or lipid NPs without surface engineering don’t have a targeting ability. Moreover, unlike traditional nanomaterials, which don’t have biological activities, many phytonanomaterials are pharmacologically active [3, 7]. Therefore, NPs consisting of these phytonanomaterials, such as BA [7] or OA [4], can be utilized for disease treatment alone or for synergistically enhancing the efficacy of payload therapeutics.
In conclusion, the value of medical plants is not limited to drug discovery. Modernization of traditional medicine should also focus on isolation and application of phytonanomaterials that can be employed for drug delivery. Due to the simplicity and inherited multifunctionality, phytonanomaterial-derived NPs are expected to significantly impact drug delivery research and development and expedite clinical translation of various therapeutic agents.
Acknowledgements
This work was supported by NIH Grant NS110721 (JZ), AHA grant 18TPA34170180 (JZ), NSFC 31770614 (CM) and NSFC 31972040 (XY).
Abbreviations
- AA
abietic acid
- AJA
arjunolic acid
- ASBT
apical sodium-dependent bile transporter
- BA
betulinic acid
- CA
crotocembraneic acid
- CB1
cannabinoid receptor 1
- DTA
dehydrotrametenolic acid
- EPR
enhanced permeability and retention
- FTIR
Fourier-transform infrared spectroscopy
- GA
glycyrrhetic acid
- GLP-1
glucagon-like peptide-1
- IPA
isopimaric acid
- MA
maslinic acid
- NP
nanoparticle
- OA
oleanolic acid
- PAA
poricoic acid A
- SA
sumaresinolic acid
- SEM
scanning electron microscopy
- SPIO
superparamagnetic iron oxide
- SXRD
single-crystal X-ray diffraction
- TCM
traditional Chinese medicine
- TEM
transmission electron microscopy
- UA
ursolic acid
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray diffraction
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z, Medicinal plants in therapy, Bulletin of the World Health Organization, 63 (1985) 965–981. [PMC free article] [PubMed] [Google Scholar]
- [2].Newman DJ, Cragg GM, Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010, J Nat Prod, 75 (2012) 311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang X, Ma C, Chen Z, Liu J, Liu F, Xie R, Zhao H, Deng G, Chen AT, Gong N, Single small molecule-assembled nanoparticles mediate efficient oral drug delivery, Nano Research, 12 (2019) 2468–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bao Y, Zhang S, Chen Z, Chen AT, Ma J, Deng G, Xu W, Zhou J, Yu Z-Q, Yao G, Synergistic chemotherapy for breast cancer and breast cancer brain metastases via Paclitaxel-loaded Oleanolic Acid nanoparticles, Molecular Pharmaceutics, 17 (2020) 1343–1351. [DOI] [PubMed] [Google Scholar]
- [5].Cheng J, Zhao H, Wang J, Han Y, Yang X, Bioactive Natural Small Molecule-Tuned Coassembly of Photosensitive Drugs for Highly Efficient Synergistic and Enhanced Type I Photochemotherapy, ACS Applied Materials & Interfaces, 12 (2020) 43488–43500. [DOI] [PubMed] [Google Scholar]
- [6].Bag BG, Majumdar R, Self-assembly of Renewable Nano-sized Triterpenoids, Chem Rec, 17 (2017) 841–873. [DOI] [PubMed] [Google Scholar]
- [7].Deng G, Ma C, Zhao H, Zhang S, Liu J, Liu F, Chen Z, Chen AT, Yang X, Avery J, Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles, Theranostics, 9 (2019) 6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Zhao H, Zhi K, Yang X, Exploration of the Natural Active Small-Molecule Drug-Loading Process and Highly Efficient Synergistic Antitumor Efficacy, ACS Applied Materials & Interfaces, 12 (2020) 6827–6839. [DOI] [PubMed] [Google Scholar]
- [9].Wang J, Zhao H, Qiao W, Cheng J, Han Y, Yang X, Nanomedicine-Cum-Carrier by Co-Assembly of Natural Small Products for Synergistic Enhanced Antitumor with Tissues Protective Actions, ACS Applied Materials & Interfaces, 12 (2020) 42537–42550. [DOI] [PubMed] [Google Scholar]
- [10].Zhang E, Shen J, So KF, Chinese traditional medicine and adult neurogenesis in the hippocampus, Journal of traditional and complementary medicine, 4 (2014) 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yao Y, Zhang X, Wang Z, Zheng C, Li P, Huang C, Tao W, Xiao W, Wang Y, Huang L, Yang L, Deciphering the combination principles of Traditional Chinese Medicine from a systems pharmacology perspective based on Ma-huang Decoction, Journal of ethnopharmacology, 150 (2013) 619–638. [DOI] [PubMed] [Google Scholar]
- [12].Su X, Yao Z, Li S, Sun H, Synergism of Chinese Herbal Medicine: Illustrated by Danshen Compound, Evidence-based complementary and alternative medicine : eCAM, 2016 (2016) 7279361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yi YD, Chang IM, An Overview of Traditional Chinese Herbal Formulae and a Proposal of a New Code System for Expressing the Formula Titles, Evidence-based complementary and alternative medicine : eCAM, 1 (2004) 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sucher NJ, Insights from molecular investigations of traditional Chinese herbal stroke medicines: implications for neuroprotective epilepsy therapy, Epilepsy Behav, 8 (2006) 350–362. [DOI] [PubMed] [Google Scholar]
- [15].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R, Engineering precision nanoparticles for drug delivery, Nature reviews. Drug discovery, 20 (2021) 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Strohbehn G, Coman D, Han L, Ragheb RR, Fahmy TM, Huttner AJ, Hyder F, Piepmeier JM, Saltzman WM, Zhou J, Imaging the delivery of brain-penetrating PLGA nanoparticles in the brain using magnetic resonance, Journal of neuro-oncology, 121 (2015) 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng M-Q, Duong N, Schafbauer T, Huttner AJ, Huang Y, Carson RE, Zhang Y, Sullivan DJ Jr., Piepmeier JM, Saltzman WM, Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma, Proc Natl Acad Sci U S A, 110 (2013) 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou J, Atsina KB, Himes BT, Strohbehn GW, Saltzman WM, Novel delivery strategies for glioblastoma, Cancer J, 18 (2012) 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheng J, Zhao H, Yao L, Li Y, Qi B, Wang J, Yang X, Simple and Multifunctional Natural Self-Assembled Sterols with Anticancer Activity-Mediated Supramolecular Photosensitizers for Enhanced Antitumor Photodynamic Therapy, ACS applied materials & interfaces, 11 (2019) 29498–29511. [DOI] [PubMed] [Google Scholar]
- [20].Zhi K, Wang J, Zhao H, Yang X, Self-assembled small molecule natural product gel for drug delivery: a breakthrough in new application of small molecule natural products, Acta Pharmaceutica Sinica B, 10 (2020) 913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang J, Qiao W, Zhao H, Cheng J, Han Y, Yang X, A highly atom-economical bioactive nanocarrier for synergistically enhanced antitumor with reduced liver injury, New Journal of Chemistry, 44 (2020) 16741–16751. [Google Scholar]
- [22].Zhi K, Sun Y, Zhao H, Zhang C, Peng H, Yang X, Self-assembled supramolecular material derived from traditional Chinese medicine: Injectable self-assembled natural product gel for drug delivery with biological activity, Materials Today Communications, 23 (2020) 101149. [Google Scholar]
- [23].Wang J, Qiao W, Zhao H, Yang X, Paclitaxel and betulonic acid synergistically enhance antitumor efficacy by forming co-assembled nanoparticles, Biochemical Pharmacology, 182 (2020) 114232. [DOI] [PubMed] [Google Scholar]
- [24].Cheng J, Li X, Wang S, Han Y, Zhao H, Yang X, Carrier-Free Triterpene Prodrugs with Glutathione Response and Biosafety for Synergistically Enhanced Photochemotherapy, ACS Applied Materials & Interfaces, (2020). [DOI] [PubMed] [Google Scholar]
- [25].Wang J, Qiao W, Li X, Zhao H, Zhang H, Dong A, Yang X, A directed co-assembly of herbal small molecules into carrier-free nanodrugs for enhanced synergistic antitumor efficacy, Journal of Materials Chemistry B, 9 (2021) 1040–1048. [DOI] [PubMed] [Google Scholar]
- [26].Chen Y, Liu J, Yang X, Zhao X, Xu H, Oleanolic acid nanosuspensions: preparation, in-vitro characterization and enhanced hepatoprotective effect, Journal of pharmacy and pharmacology, 57 (2005) 259–264. [DOI] [PubMed] [Google Scholar]
- [27].Bag BG, Dash SS, First self-assembly study of betulinic acid, a renewable nano-sized, 6–6-6–6-5 pentacyclic monohydroxy triterpenic acid, Nanoscale, 3 (2011) 4564–4566. [DOI] [PubMed] [Google Scholar]
- [28].Bag BG, Majumdar R, Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid, RSC advances, 2 (2012) 8623–8626. [Google Scholar]
- [29].Bag BG, Paul K, Vesicular and Fibrillar Gels by Self-Assembly of Nanosized Oleanolic Acid, Asian Journal of Organic Chemistry, 1 (2012) 150–154. [Google Scholar]
- [30].Patil S, Patil S, Gawali S, Shende S, Jadhav S, Basu S, Novel self-assembled lithocholic acid nanoparticles for drug delivery in cancer, RSC advances, 3 (2013) 19760–19764. [Google Scholar]
- [31].Bag BG, Majumdar R, Vesicular self-assembly of a natural triterpenoid arjunolic acid in aqueous medium: study of entrapment properties and in situ generation of gel–gold nanoparticle hybrid material, RSC Advances, 4 (2014) 53327–53334. [Google Scholar]
- [32].Bag BG, Dash SS, Hierarchical self-assembly of a renewable nanosized pentacyclic dihydroxy-triterpenoid betulin yielding flower-like architectures, Langmuir, 31 (2015) 13664–13672. [DOI] [PubMed] [Google Scholar]
- [33].Dash SK, Dash SS, Chattopadhyay S, Ghosh T, Tripathy S, Mahapatra SK, Bag BG, Das D, Roy S, Folate decorated delivery of self assembled betulinic acid nano fibers: a biocompatible anti-leukemic therapy, RSC Advances, 5 (2015) 24144–24157. [Google Scholar]
- [34].Saha A, Adamcik J, Bolisetty S, Handschin S, Mezzenga R, Fibrillar networks of glycyrrhizic acid for hybrid nanomaterials with catalytic features, Angewandte Chemie International Edition, 54 (2015) 5408–5412. [DOI] [PubMed] [Google Scholar]
- [35].Wang Y, Song J, Chow SF, Chow AH, Zheng Y, Particle size tailoring of ursolic acid nanosuspensions for improved anticancer activity by controlled antisolvent precipitation, International journal of pharmaceutics, 494 (2015) 479–489. [DOI] [PubMed] [Google Scholar]
- [36].Bag BG, Barai AC, Wijesekera K, Kittakoop P, First Vesicular Self-Assembly of Crotocembraneic Acid, a Nano-Sized Fourteen Membered Macrocyclic Diterpenic Acid, ChemistrySelect, 2 (2017) 4969–4973. [Google Scholar]
- [37].Bag BG, Das S, Hasan SN, Barai AC, Nanoarchitectures by hierarchical self-assembly of ursolic acid: entrapment and release of fluorophores including anticancer drug doxorubicin, RSC advances, 7 (2017) 18136–18143. [Google Scholar]
- [38].Bag BG, Hasan SN, Pongpamorn P, Thasana N, First Hierarchical Self-Assembly of a Seco-Triterpenoid α-Onocerin Yielding Supramolecular Architectures, ChemistrySelect, 2 (2017) 6650–6657. [Google Scholar]
- [39].Bag BG, Garai C, Ghorai S, Vesicular self-assembly of a natural ursane-type dihydroxy-triterpenoid corosolic acid, RSC advances, 9 (2019) 15190–15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bag BG, Hasan SN, Ghorai S, Panja SK, First Self-Assembly of Dihydroxy Triterpenoid Maslinic Acid Yielding Vesicles, ACS Omega, 4 (2019) 7684–7690. [Google Scholar]
- [41].Huang X, Wang P, Li T, Tian X, Guo W, Xu B, Huang G, Cai D, Zhou F, Zhang H, Self-assemblies based on traditional medicine berberine and cinnamic acid for adhesion-induced inhibition multidrug-resistant staphylococcus aureus, ACS applied materials & interfaces, 12 (2019) 227–237. [DOI] [PubMed] [Google Scholar]
- [42].Li T, Wang P, Guo W, Huang X, Tian X, Wu G, Xu B, Li F, Yan C, Liang X-J, Natural berberine-based chinese herb medicine assembled nanostructures with modified antibacterial application, Acs Nano, 13 (2019) 6770–6781. [DOI] [PubMed] [Google Scholar]
- [43].Wang R, Yang M, Li G, Wang X, Zhang Z, Qiao H, Chen J, Chen Z, Cui X, Li J, Paclitaxel-betulinic acid hybrid nanosuspensions for enhanced anti-breast cancer activity, Colloids and Surfaces B: Biointerfaces, 174 (2019) 270–279. [DOI] [PubMed] [Google Scholar]
- [44].Zheng J, Fan R, Wu H, Yao H, Yan Y, Liu J, Ran L, Sun Z, Yi L, Dang L, Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation, Nature communications, 10 (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bag BG, Barai AC, Self-assembly of naturally occurring stigmasterol in liquids yielding a fibrillar network and gel, RSC Advances, 10 (2020) 4755–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Han N, Huang X, Tian X, Li T, Liu X, Li W, Huo S, Wu Q, Gu Y, Dai Z, Self-assembled Nanoparticles of Natural Phytochemicals (Berberine and 3, 4, 5-methoxycinnamic Acid) Originated from Traditional Chinese Medicine for Inhibiting Multidrug-resistant Staphylococcus Aureus, Current Drug Delivery, (2020). [DOI] [PubMed] [Google Scholar]
- [47].Li L, Cui H, Li T, Qi J, Chen H, Gao F, Tian X, Mu Y, He R, Lv S, Synergistic effect of berberine-based Chinese medicine assembled nanostructures on diarrhea-predominant irritable bowel syndrome in vivo, Frontiers in pharmacology, 11 (2020) 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Panja SK, Bag BG, Flower-and Grass-like Self-Assemblies of an Oleanane-Type Triterpenoid Erythrodiol: Application in the Removal of Toxic Dye from Water, ACS omega, 5 (2020) 30488–30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tian X, Wang P, Li T, Huang X, Guo W, Yang Y, Yan M, Zhang H, Cai D, Jia X, Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination, Acta Pharmaceutica Sinica B, 10 (2020) 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gao Y, Zhao K, Yu X, Li Z, Wu T, Zhang C, Du F, Hu J, Multiple modulations of supramolecular assemblies from a natural triterpenoid-tailored bipyridinium amphiphile, Journal of Colloid and Interface Science, 584 (2021) 92–102. [DOI] [PubMed] [Google Scholar]
- [51].He N, Zhi K, Yang X, Zhao H, Zhang H, Wang J, Wang Z, Self-assembled fibrillar networks induced by two methods: a new unmodified natural product gel, New Journal of Chemistry, 42 (2018) 14170–14178. [Google Scholar]
- [52].Zhao X-L, Chen H-B, Chen Y-J, Yang X-L, Freeze-drying of oleanolic acid-loaded nanosuspensions, Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China journal of Chinese materia medica, 32 (2007) 1874–1876. [PubMed] [Google Scholar]
- [53].Li W, Ng K.-y., Heng PWS, Development and evaluation of optimized sucrose ester stabilized oleanolic acid nanosuspensions prepared by wet ball milling with design of experiments, Biological and Pharmaceutical Bulletin, 37 (2014) 926–937. [DOI] [PubMed] [Google Scholar]
- [54].Li W, Das S, Ng K.-y., Heng PW, Formulation, biological and pharmacokinetic studies of sucrose ester-stabilized nanosuspensions of oleanolic acid, Pharmaceutical research, 28 (2011) 2020–2033. [DOI] [PubMed] [Google Scholar]
- [55].Ma Y-Q, Zhang Z-Z, Li G, Zhang J, Xiao H-Y, Li X-F, Solidification drug nanosuspensions into nanocrystals by freeze-drying: a case study with ursodeoxycholic acid, Pharmaceutical development and technology, 21 (2016) 180–188. [DOI] [PubMed] [Google Scholar]
- [56].Gao Y, Hao J, Yan Q, Du F, Ju Y, Hu J, Natural triterpenoid-tailored phosphate: in situ reduction of heavy metals spontaneously to generate electrochemical hybrid gels, ACS applied materials & interfaces, 10 (2018) 17352–17358. [DOI] [PubMed] [Google Scholar]
- [57].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nature biotechnology, 33 (2015) 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J.r., Rekik A, Knauer SK, Bantz C, Nawroth T, Bier C, Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis, ACS nano, 5 (2011) 7155–7167. [DOI] [PubMed] [Google Scholar]
- [59].He C, Hu Y, Yin L, Tang C, Yin C, Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles, Biomaterials, 31 (2010) 3657–3666. [DOI] [PubMed] [Google Scholar]
- [60].Chithrani BD, Ghazani AA, Chan WCW, Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells, Nano Letters, 6 (2006) 662–668. [DOI] [PubMed] [Google Scholar]
- [61].Meng H, Yang S, Li Z, Xia T, Chen J, Ji Z, Zhang H, Wang X, Lin S, Huang C, Zhou ZH, Zink JI, Nel AE, Aspect Ratio Determines the Quantity of Mesoporous Silica Nanoparticle Uptake by a Small Gtpase-Dependent Macropinocytosis Mechanism, ACS Nano, 5 (2011) 4434–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Qiu Y, Liu Y, Wang L, Xu L, Bai R, Ji Y, Wu X, Zhao Y, Li Y, Chen C, Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods, Biomaterials, 31 (2010) 7606–7619. [DOI] [PubMed] [Google Scholar]
- [63].Liu N, Becton M, Zhang L, Wang X, Mechanism of Coupling Nanoparticle Stiffness with Shape for Endocytosis: From Rodlike Penetration to Wormlike Wriggling, J Phys Chem B, 124 (2020) 11145–11156. [DOI] [PubMed] [Google Scholar]
- [64].Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV, Renal Clearance of Quantum Dots, Nature Biotechnology, 25 (2007) 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K, Long-Circulating Poly(Ethylene Glycol)–Poly(D,L-Lactide) Block Copolymer Micelles with Modulated Surface Charge, Journal of Controlled Release, 77 (2001) 27–38. [DOI] [PubMed] [Google Scholar]
- [66].Bag BG, Das S, Hasan S, C. B., Nanoarchitectures by Hierarchical Self-Assembly of Ursolic Acid: Entrapment and Release of Fluorophores Including Anticancer Drug Doxorubicin, RSC Advances, 7 (2017) 18136–18143. [Google Scholar]
- [67].Kharlampieva E, Kozlovskaya V, Sukhishvili SA, Layer-by-Layer Hydrogen-Bonded Polymer Films: From Fundamentals to Applications, Advanced Materials, 21 (2009) 3053–3065. [Google Scholar]
- [68].Sánchez-Iglesias A, Grzelczak M, Altantzis T, Goris B, Pérez-Juste J, Bals S, Van Tendeloo G, Donaldson SH, Chmelka BF, Israelachvili JN, Liz-Marzán LM, Hydrophobic Interactions Modulate Self-Assembly of Nanoparticles, ACS Nano, 6 (2012) 11059–11065. [DOI] [PubMed] [Google Scholar]
- [69].Chakraborty D, Dinda S, Chowdhury M, Das PK, Morphological transformation of self-assemblies by tuning hydrophobic segment of small amphiphiles, Journal of Colloid and Interface Science, 539 (2019) 414–424. [DOI] [PubMed] [Google Scholar]
- [70].Jung HS, Verwilst P, Sharma A, Shin J, Sessler JL, Kim JS, Organic molecule-based photothermal agents: an expanding photothermal therapy universe, Chem Soc Rev, 47 (2018) 2280–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chen ZG, Small-molecule delivery by nanoparticles for anticancer therapy, Trends in molecular medicine, 16 (2010) 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Patel T, Zhou J, Piepmeier JM, Saltzman WM, Polymeric nanoparticles for drug delivery to the central nervous system, Advanced drug delivery reviews, 64 (2012) 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yogeeswari P, Sriram D, Betulinic acid and its derivatives: a review on their biological properties, Curr Med Chem, 12 (2005) 657–666. [DOI] [PubMed] [Google Scholar]
- [74].Peng J, Lv YC, He PP, Tang YY, Xie W, Liu XY, Li Y, Lan G, Zhang M, Zhang C, Shi JF, Zheng XL, Yin WD, Tang CK, Betulinic acid downregulates expression of oxidative stress-induced lipoprotein lipase via the PKC/ERK/c-Fos pathway in RAW264.7 macrophages, Biochimie, 119 (2015) 192–203. [DOI] [PubMed] [Google Scholar]
- [75].Guo X, Cheng Y, Zhao X, Luo Y, Chen J, Yuan WE, Advances in redox-responsive drug delivery systems of tumor microenvironment, J Nanobiotechnology, 16 (2018) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sánchez E, Nabavi SF, Nabavi SM, Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity, Microbiological research, 196 (2017) 44–68. [DOI] [PubMed] [Google Scholar]
- [77].Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M, Natural product-derived phytochemicals as potential agents against coronaviruses: A review, Virus research, (2020) 197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cheng S-S, Chung M-J, Lin C-Y, Wang Y-N, Chang S-T, Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents, Journal of agricultural and food chemistry, 60 (2012) 124–128. [DOI] [PubMed] [Google Scholar]
- [79].Lv Z, Peng G, Liu W, Xu H, Su J, Berberine blocks the relapse of Clostridium difficile infection in C57BL/6 mice after standard vancomycin treatment, Antimicrobial agents and chemotherapy, 59 (2015) 3726–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Balint GA, Artemisinin and its derivatives: an important new class of antimalarial agents, Pharmacology & therapeutics, 90 (2001) 261–265. [DOI] [PubMed] [Google Scholar]
- [81].Singla AK, Garg A, Aggarwal D, Paclitaxel and its formulations, Int J Pharm, 235 (2002) 179–192. [DOI] [PubMed] [Google Scholar]