Abstract
Ludwigia stolonifera (Guill. & Perr.) P.H.Raven belonging to the family Onagraceae is an important aquatic herbal plant of economic importance in water bioremediation. We explored the compositional heterogeneity in the aroma profile of L. stolonifera aerial parts and roots. Volatile profiling was employed for the first time using volatile solvent extraction (VSE-GC-MS/MS) of both aerial parts and roots. A total of 85 volatiles were identified belonging to eight classes, viz., aliphatic, aromatic, and oxygenated hydrocarbons, monoterpenes, diterpenes, alcohols, acids/esters, and sterols. Aliphatic and aromatic hydrocarbons were found to be the most abundant metabolite groups in both aerial parts and roots. Furthermore, antioxidant and metal chelation activities of aerial parts and roots were investigated, revealing a potent activity as an antioxidant and high metal chelation capacity for heavy metals.
Introduction
Onagraceae, also known as evening primrose or willow herb family, is a family of flowering plants which comprises about 650 species of herbs, shrubs, and trees distributed in 17 genera.1,2 Several phytochemicals which are widely abundant in different species such as phenolic compounds, flavonoids, essential oils, triterpenenoids, and saponins were reported. Essential oils are a strong-smelling secondary metabolite group of complex chemical composition and myriad pharmacological and medicinal value such as antimicrobial, anti-inflammatory, and antioxidant properties.3Ludwigia is a pantropic genus and comprises about 82 species of aquatic plants, well represented in South and North America.4Ludwigia species were reported for their and traditional importance and medicinal uses such as antidiabetic,5 cytotoxic activity,6 and anti-inflammatory activity.7 In Egyptian flora, genus Ludwigia is represented by two species, Ludwigia stolonifera (Guill. & Perr.) P.H.Raven (the dominant aquatic macrophytes) and Ludwigia erecta (L.) Hara, in canals and drains crossing the cultivated lands in the Nile Delta.8L. stolonifera is an important aquatic herbal plant used for eliminating various toxic pollutants associated with hazardous effects on ecosystems. Also, L. stolonifera is used for water bioremediation helping to improve the quality of drinking water.9 Roots and leaves of L. stolonifera were used as heavy metal biofilters for cadmium (Cd) and nickel (Ni) where leaf biomass showed the best metal binding capacity.10 Metabolite profiling of different plants of nutritional and economical value has lately started to implement modern analytical approaches, for example, metabolomics, where samples are examined in a rather untargeted, comprehensive manner.11 Gas chromatography–mass spectrometry (GC–MS) analysis has been extensively adopted for metabolomic profiling in different plant parts, viz., fruits, leaves, seeds, or even the whole plant, to characterize their metabolite components, for example, volatiles and nonvolatiles.12 Essential oil identification in plant materials can be achieved by several methods such as steam distillation, headspace solid-phase microextraction, supercritical fluid extraction, microwave-assisted extraction, and solvent extractions. Unlike common thermal methods for volatile extraction, for example, distillation, volatile solvent extraction (VSE) is a simple analytical technique for the enhancement of volatile recovery from the plant sample.13
To the best of our knowledge, this is the first report of volatile profiling of L. stolonifera aerial parts and roots using GC–MS. Also, in vitro antioxidant and metal chelation potentials of the two parts were investigated using DPPH radical scavenging capacity assay and iron metal chelation assay, respectively.
Results and Discussion
GC–MS Volatile Profiling in L. stolonifera Aerial Parts and Roots
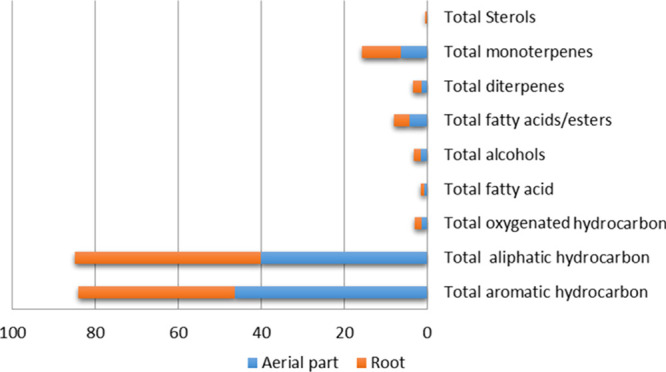
The goal of this study was to explore the volatile metabolite composition in L. stolonifera aerial part and root hexane extract. GC–MS analysis revealed the identification of 85 volatile components (Table 1, Figure 1) belonging to eight classes, viz., aliphatic, aromatic, and oxygenated hydrocarbons, monoterpenes, diterpenes, alcohols, acids/esters, and sterols. Each group includes various numbers of different compounds in which their percentage differs in both hexane extracts of the plant, as shown in Figure 2.
Table 1. Relative Percentile of Volatile Metabolites Detected in Ludwigia stolonifera Aerial Part and Root Hexane Extracts Analyzed Using GC–MS.
| peak | Rt | RI | compound | aerial part | roots |
|---|---|---|---|---|---|
| total alcohol | 1.56 | 1.76 | |||
| 1 | 14.63 | 1548.9 | 2-undecen-1-ol | 0.35 | |
| 2 | 12.8 | 1447 | 1-octanol, 2-butyl | 1.07 | |
| 3 | 14.64 | 1585.7 | 2-dodecen-1-ol | 0.33 | |
| 4 | 15.09 | 1622 | cyclododecanemethanol | 0.41 | 0.36 |
| 5 | 11.43 | 1351.3 | 11-methyldodecanol | 0.8 | |
| total aliphatic hydrocarbons | 41.16 | 46.7 | |||
| 6 | 8.06 | 1141.8 | tritetracontane | 0.23 | |
| 7 | 9.12 | 1201.2 | decane | 3.47 | 4.61 |
| 8 | 9.82 | 1246.7 | 4-methyl decane | 1.59 | 2.25 |
| 9 | 10.33 | 1280 | 3-methyl decane | 1.5 | 0.81 |
| 10 | 10.82 | 1311.6 | decalin | 1.58 | |
| 11 | 10.93 | 1318.8 | 2,5-dimethyl nonane | 0.49 | |
| 12 | 11.14 | 1332.8 | 2-methyl decane | 1.42 | 1.43 |
| 13 | 11.76 | 1372.7 | 1-ethyl-1-methyl cyclohexane | 0.75 | 0.51 |
| 14 | 12.31 | 1410 | undecane | 9.38 | 7.41 |
| 15 | 12.59 | 1430.9 | 2-methyl trans-decalin | 1.01 | |
| 16 | 12.81 | 1447.6 | dodecane | 9.44 | 6.47 |
| 17 | 14.02 | 1538.7 | 5-methyl-undecane | 1.19 | |
| 18 | 14.14 | 1547.8 | 4-methyl-undecane | 0.74 | 0.64 |
| 19 | 14.27 | 1557.6 | 2-methyl-undecane | 1.89 | 1.56 |
| 20 | 14.47 | 1572.9 | 3-methyl-undecane | 1.01 | 0.84 |
| 21 | 15.79 | 1681.4 | 2,5-dimethyl-undecane | 0.37 | |
| 22 | 42.22 | 4830.2 | heneicosane | 2 | 2.65 |
| 23 | 45.44 | 5238.2 | hexacosane | 0.74 | |
| 24 | 46.96 | 5430.9 | tetracontane | 1.23 | 3.76 |
| 25 | 6.64 | 1062.1 | 1,2,3-trimethoxycyclohexane | 0.26 | |
| 26 | 6.95 | 1079.2 | propyl-cyclohexane | 0.37 | |
| 27 | 7.06 | 1085.4 | 3-methyl-nonane | 1.96 | |
| 28 | 7.22 | 1094.7 | 1-ethyl-2,4-dimethyl-cyclohexane | 1.5 | |
| 29 | 7.46 | 1108 | 1-methyl-3-propyl-cyclooctane | 1.95 | |
| 30 | 7.7 | 1121.7 | (E)-3-tetradecene | 0.56 | |
| 31 | 8 | 1138.1 | 2-methyl-nonane | 0.46 | |
| 32 | 9.99 | 1258 | 3,7-dimethyl-decane | 0.33 | |
| 33 | 10.33 | 1280.1 | 3,7-dimethyl-nonane | 1.03 | |
| 34 | 11.5 | 1356 | 1-isopropyl-1-methylcyclohexane | 0.56 | |
| 35 | 11.76 | 1373 | 1-ethyl-1-methyl-cyclohexane | 0.79 | |
| 36 | 12.14 | 1235 | 1-methyl-2-propyl cyclohexane | 0.73 | |
| 37 | 13.98 | 1536 | 2,5-dimethyl-decane | 1.04 | |
| 38 | 15.8 | 1681.9 | 3,6-dimethyl-undecane, | 0.29 | |
| 39 | 49.32 | 5729.8 | 2-methyloctacosane | 0.09 | |
| 40 | 9.25 | 1209.9 | 1,2-dimethoxycyclopentane | 0.53 | |
| 41 | 10.11 | 1265.4 | butylcyclohexane | 1.13 | 1.31 |
| total aromatic hydrocarbons | 46.28 | 37.71 | |||
| 42 | 24.74 | 2616 | 5-phenyl decane | 1.19 | 0.96 |
| 43 | 24.95 | 2643.2 | 4-phenyl eicosane | 1.14 | 0.91 |
| 44 | 25.41 | 2700 | 3-phenyl decane | 1.41 | 1.16 |
| 45 | 26.31 | 2814.5 | 2-phenyl-decane | 2.08 | 1.79 |
| 46 | 27.14 | 2919.5 | 5-phenyl undecane | 4.48 | 3.57 |
| 47 | 27.37 | 2949.6 | 4-phenyl undecane | 2.77 | 2.21 |
| 48 | 27.86 | 3011.3 | 3-phenyl undecane | 3.43 | 2.76 |
| 49 | 28.74 | 3122.2 | 2-phenyl undecane | 4.93 | 3.94 |
| 50 | 29.31 | 3195.2 | 6-phenyl dodecane | 2.17 | 1.76 |
| 51 | 29.42 | 32.9.1 | 5-phenyl dodecane | 2.36 | 1.93 |
| 52 | 29.69 | 3243.7 | 4-phenyl dodecane | 2.09 | 1.69 |
| 53 | 30.18 | 3304.8 | 3-phenyl dodecane | 2.67 | 2.18 |
| 54 | 31.03 | 3413.1 | 2-phenyl dodecane | 3.93 | 3.17 |
| 55 | 31.49 | 3470.8 | 6-phenyl tridecane | 2.61 | 2.11 |
| 56 | 31.63 | 3489.5 | 5-phenyl tridecane | 2.01 | 1.59 |
| 57 | 31.9 | 3522.8 | 4-phenyl tridecane | 1.73 | 1.45 |
| 58 | 32.4 | 3586.3 | 3-phenyl tridecane | 2.1 | 1.77 |
| 59 | 33.23 | 3691.2 | 2-phenyl tridecane | 3.18 | 2.63 |
| 60 | 26.06 | 3759.7 | 3-phenyl tridecane | 0.13 | |
| total diterpenes | 1.36 | 2 | |||
| 61 | 13.12 | 1471.5 | phytol | 1.36 | 1.52 |
| 62 | 9.58 | 1231.3 | tetraprenol | 0.48 | |
| Total fatty acid/ester | 4.33 | 3.71 | |||
| 63 | 14.95 | 1609.9 | oxalic acid, cyclohexylmethyl isohexyl ester | 0.91 | 0.8 |
| 64 | 33.52 | 3728.3 | palmitic acid methyl ester | 0.71 | 0.62 |
| 65 | 44.8 | 5158.1 | bis(2-ethylhexyl)phthalate | 1.61 | 1.53 |
| 66 | 11.63 | 1364.2 | oxalic acid, di(cyclohexylmethyl) ester | 0.38 | |
| 67 | 36.88 | 4154.3 | methyl linoleate | 0.33 | 0.43 |
| 68 | 37.02 | 4171.8 | methyl linolenate | 0.91 | 0.8 |
| total monoterpenes | 5.34 | 7.34 | |||
| 69 | 12.6 | 1431.7 | 2-methyl-trans-decalin | 0.69 | |
| 70 | 6.81 | 1071.5 | 1,1,4,4-tetramethyl-cyclohexane | 0.35 | |
| 71 | 7.55 | 1113.1 | 1-butyl-2-propyl-cyclopentane | 0.15 | |
| 72 | 10.54 | 1293.5 | 1-ethyl-2-propyl-cyclohexane | 0.33 | |
| 73 | 10.63 | 1299.3 | tetrahydrocarvone | 0.2 | |
| 74 | 10.83 | 1312.1 | decalin | 2.15 | |
| 75 | 11.87 | 1380.3 | 1-methyl-2-pentyl-cyclohexane | 1.49 | |
| 76 | 13.36 | 1489.3 | pentyl cyclohexane | 0.98 | 0.92 |
| 77 | 13.5 | 1499.6 | hexylcyclopentane | 0.58 | 0.58 |
| 78 | 7.87 | 1130.9 | 1-ethyl-2,4-dimethyl-cyclohexane | 0.46 | |
| 79 | 8.24 | 313 | p-menthane | 0.83 | 0.48 |
| 80 | 8.56 | 1151.7 | 1-methyl-2-propyl-cyclohexane | 0.86 | |
| 81 | 8.94 | 1191 | 1,2-diethyl-, cis-cyclohexane | 0.2 | |
| 82 | 11.94 | 1384.6 | 1-ethyl-2-propyl-cyclohexane | 0.76 | |
| 83 | 13.77 | 1520 | 1-methyl-2-pentyl-cyclohexane | 0.67 | |
| total oxygenated hydrocarbons | 0.1 | ||||
| 84 | 9.25 | 1209.8 | 1,1-bis(dodecyloxy)-hexadecane | 0.1 | |
| total sterols | 0.22 | 0.92 | |||
| 85 | 56.37 | 6623.6 | γ-sitosterol | 0.22 | 0.92 |
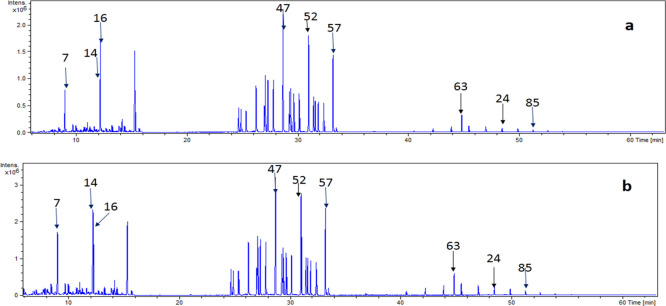
Figure 1.
(a) GC–MS spectrum of the L. stolonifera aerial part n-hexane extract. (b) GC–MS spectrum of the L. stolonifera root n-hexane extract, 7: decane, 14: undecane, 16: dodecane, 47: 4-phenyl undecane, 52: 4-phenyl dodecane, 57: 4-phenyl tridecane, 24: tetratetracontane, and 85: γ-sitosterol.
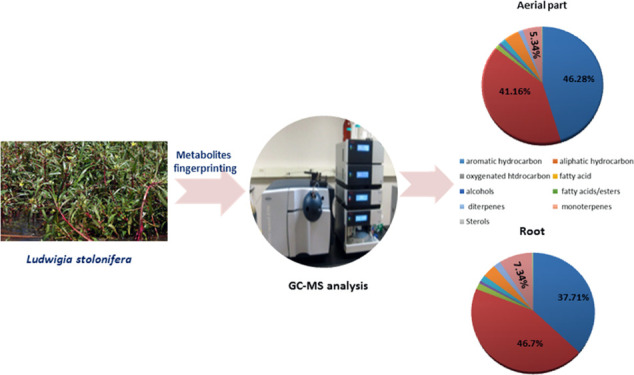
Figure 2.
Chemical composition of L. stolonifera aerial part and root hexane extracts.
Aliphatic Hydrocarbons
Aliphatic hydrocarbons were found to be the major constituents detected in aerial part and root hexane extracts which accounted for 41.16 and 46.70%, respectively, of the volatile composition. Undecane (peak 14), dodecane (peak 16), and decane (peak 7) were prevalent by 9.38, 9.44, and 3.47% in aerial parts, respectively, while in roots were 7.41, 6.47, and 4.61%, respectively, whereas tritetracontane (peak 6), decalin (peak 10), and 2,5-dimethylnonane (peak 11) were represented by 0.23, 1.58, and 0.49%, respectively, and detected only in the aerial part extract. Undecane is a naturally occurring alkane hydrocarbon and has been reported to have anti-inflammatory, antiallergic, and immunosuppressant effects.14
Aromatic Hydrocarbons
Aromatic hydrocarbons constituted the second most abundant volatile class in aerial part and root hexane extracts which represented 46.28 and 37.71% of the total identified compounds, respectively. The highest compounds detected were 5-phenylundecane (peak 46) which constituted 4.48 and 3.57%, 2-phenylundecane (peak 49) which constituted 4.93 and 3.94%, and 2-phenyldodecane which constituted 3.93 and 3.17%, respectively, in both aerial parts and roots. Oxygenated hydrocarbons were detected in trace levels only in the aerial part and represented by 1,1-bis(dodecyloxy) hexadecane (peak 84) and was detected by 0.13%. Aromatic hydrocarbons and their phenylundecane derivatives have been reported for their good antifungal and antibacterial activity.15
Acids/Esters
The relative amounts of fatty acids/esters were detected in the volatile blend of L. stolonifera aerial parts and roots by 4.33 and 3.71%, respectively. Both saturated and unsaturated fatty acid esters were detected in both aerial parts and roots. Palmitic acid methyl ester (peak 64) 0.71 and 0.62%, methyl linoleate 0.33 and 0.43%, and methyl linolenate 0.91 and 0.8%, respectively, were detected as the major components. Oxalic acid derivatives were detected in both aerial parts and roots and represented by oxalic acid, cyclohexylmethyl isohexyl ester (peak 63, 0.91, and 0.8%, respectively), while oxalic acid, di(cyclohexylmethyl)ester (peak 66) was detected only in the aerial parts. In addition, the abundance of phthalic acid derivative (bis(2-ethylhexyl)phthalate, peak 65) was observed in both aerial parts and roots.16 Organic acids such as oxalic acid and phthalic acid were reported to be effective in metal chelation and produced by plants as typical extracellular metal chelators in phytoremediation.17,18 Interestingly, the presence of oxalic acid and phthalic acid derivatives may interfere with the metal chelation capacity of L. stolonifera.
Terpenes/Sterols
Monoterpene compounds were detected by 5.34 and 7.34% in aerial parts and roots, respectively. Dekalin (peak 74), 1-methyl-2-pentyl cyclohexane (peak 75), and 1-methyl-2-pentyl-cyclohexane (peak 75) were detected only in the root extract. Other monoterpenes were detected in both aerial part and root hexane extracts by different percentages such as pentyl cyclohexane (peak 76) 0.98 and 0.92% and p-menthane (peak 79) 0.83 and 0.48%, respectively. Phytol, a diterpene member of the long-chain unsaturated acyclic alcohols, was detected in the aerial part and root extracts and accounted 1.36 and 1.52%, respectively. Phytol is a valuable essential oil of pleasant fragrance and has been focused for its myriad biological activities such as anxiolytic, metabolism-modulating, cytotoxic, antioxidant, apoptosis-inducing, antinociceptive, anti-inflammatory, immune-modulating, and antimicrobial effects.19
Sterols were detected in trace levels in both aerial part and root extracts represented by γ-sitosterol which accounted for 0.22 and 0.92%, respectively. Plant sterols are found in the highest amounts in vegetable oils, nuts, and seeds and considered as potent hypolipidemic agents.20
Alcohols
Alcohols were detected by comparable levels in both aerial part and root hexane extracts of 1.56 and 1.76%, respectively. Cyclododecanemethanol (peak 4) was detected in aerial and root extracts at levels ca. 0.41 and 0.36%, respectively, whereas 11-methyldodecanol (peak 5) and trans-2-undecen-1-ol (peak 3) were predominant only in the aerial part extract by 0.8 and 0.35%, respectively. On the other hand, 1-octanol, 2-butyl (peak 2) and trans-2-dodecen-1-ol (peak 3) were detected only in the root extract by 1.07 and 0.33%, respectively.
Biological Activity
Antioxidant Assay
Free radicals could be produced as a result of different metabolic reactions or initiated by other environmental factors. Therefore, there are great interests in antioxidants, especially those from the natural origin as being safer and desirable than synthetic drugs, which can reduce these free radicals in the human body.21 Medicinal plants are rich in phenolic compounds that possess antioxidant activity. 2,2-Diphenyl-1-picrylhydrazyl assay (DPPH) is the most common assay for antioxidant activity evaluation via testing radical scavenging capacity of the plant extract.
DPPH inhibition results of the different concentrations used compared with ascorbic acid as reference antioxidants are summarized in Table 2 and Figure 3. Calculated SC50 for ascorbic acid and different fractions of L. stolonifera are shown in Figure 4.
Table 2. Calculated SC50 for Ascorbic Acid and Different Fractions of L. stolonifera and Metal Chelation Activity Represented by % of Inhibition and μM EDTA eq/mg Extract.
| radical
scavenging capacity |
metal
chelation assay |
||
|---|---|---|---|
| SC50 (μg/mL) | % of inhibition | μM EDTA eq/mg extract | |
| aerial total ex. | 19.2 ± 1.6 | 52.8 | 36.36 ± 3.58 |
| ethyl acetate Fr. | 7.6 ± 0.4 | ||
| n-butanol Fr. | 16.0 ± 1.2 | ||
| roots total ex. | 61.6 ± 3.4 | 43.6 | 29.67 ± 1.52 |
| ascorbic acid R. S. | 10.6 ± 0.8 | ||
Figure 3.
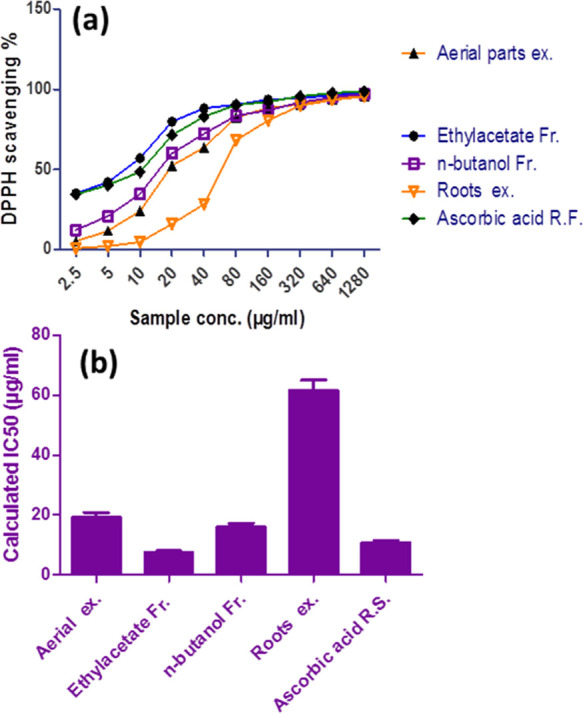
(a) Antioxidant activity of Ludwigia stolonifera different fractions represented as % inhibition. (b) Calculated SC50L. stolonifera fractions compared to ascorbic acid expressed as average ± standard deviation (scanning electron microscopy), and P < 0.05 was used.
Figure 4.
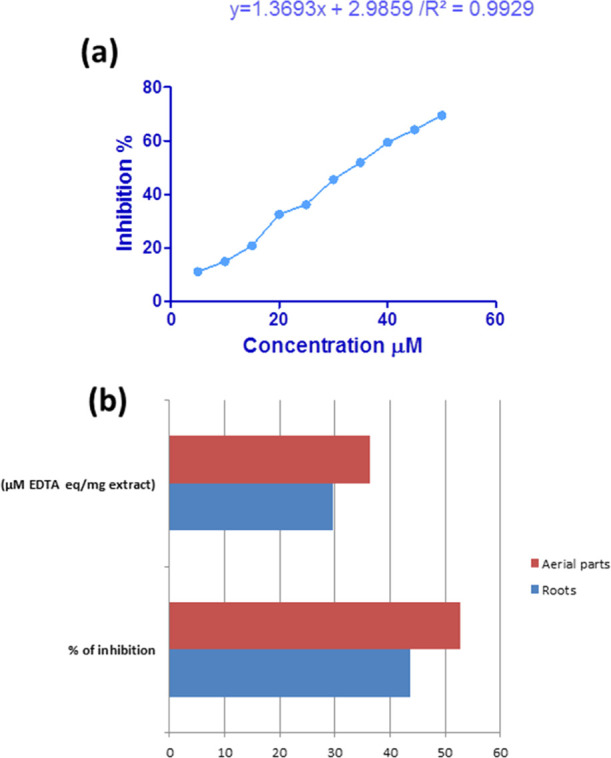
(a) Linear dose–inhibition curve of EDTA. (b) L. stolonifera aerial part and root metal chelation activity represented by % inhibition and μM EDTA eq/mg extract expressed as average ± standard deviation (SD).
The samples showed variable antioxidant scavenging activity against the DPPH radical concluded from the SC50 value of the aerial part total methanolic extract, ethyl acetate, n-butanol fractions, and root total methanolic extract recorded as 19.2, 7.6, 16, and 61.6 μg/mL, respectively, compared to ascorbic acid SC50 10.6 μg/mL (Figure 3). The aerial part ethyl acetate fraction showed to be the most effective sample (SC50: 7.6 μg/mL), and the root total methanolic extract showed the modest effect with SC50: 61.6 μg/mL. The results are confirmed by the reported biological review for the family Onagraceae which has common potent antioxidant activity as in the Circaea lutetiana L. aqueous methanolic extract and aerial part which possess strong scavenging activity toward DPPH (SC50 33.1 μg/mL).22
Metal Chelation Assay
Reactive oxygen species are formed in vivo by the presence of the iron metal (Fe3+) such as superoxide radicals (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (−OH), which are very strong oxidant agents, so they need strong antioxidants to overcome the oxidant agents.23 Antioxidants may be classified according to the mechanism of action as chelation of transition metals, electron transfer, or hydrogen atom transfer.24 In this study, we assessed the ability of samples to chelate transition metals such as Fe2+ which may induce degenerative diseases such as cardiovascular, Parkinson’s, and Alzheimer’s diseases.
The results of the tested samples (aerial and root total methanolic extracts) are presented as μM EDTA equivalent/mg sample using the linear regression equation extracted from the calibration curve (Figure 4), and compounds of samples bind a certain amount of Fe2+, but the remaining Fe2+ reacts with ferrozine, forming a blue-colored complex that can be monitored spectrophotometrically, and the data were labeled in Table 2 and Figure 4.
Samples were prepared in a concentration of 1 mg/mL in methanol using the linear regression equation. The results revealed that 1 mg of the aerial part total extract is equal to 36.36 μM EDTA, and from the calibration curve, the percentage of inhibition was 52.8%. On the other hand, 1 mg of the root total extract equal to 29.67 μM EDTA revealed 43.6% of inhibition. Data showed that the same concentration for two samples was equivalent to different concentrations of EDTA and so was recorded different % of inhibition. The extract of the aerial parts was more effective than the root extract of L. stolonifera as the metal chelator.
Experimental Section
Plant Material
L. stolonifera (Guill. & Perr.) P.H.Raven was collected from the Nile River at Al-Qanatir Al-Khayriyah, September 2019, and kindly identified by Dr. Rim Hamdy, Professor of Plant Taxonomy, Botany Department, Faculty of Science, Cairo University, Egypt. A voucher specimen (01Lst/2019) was deposited at the Herbarium of Pharmacognosy department, Faculty of Pharmacy, Helwan University. The plant materials (roots and aerial parts), 500 g each, were dried in shade and then coarsely grinded separately. Part of the coarsely divided powdered roots and aerial parts were extracted with n-hexane for GC–MS analysis. Another part of both aerial parts and roots was macerated and extracted with the methanol solvent and then further fractionated with ethylacetate and n-butanol for biological study.
GC–MS Analysis
GC–MS analysis was performed at Pharmacognosy Department, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. Mass spectra were recorded using Shimadzu GCMS-QP2010 (Koyoto, Japan) equipped with an Rtx-5MS fused bonded column (30 m × 0.25 mm i.d. X 0.25 μm film thickness) (Restek, USA) equipped with a split–splitless injector. The initial column temperature was kept at 50 °C for 3 min (isothermal) and programmed to 300 °C at a rate of 5 °C/min and kept constant at 300 °C for 10 min (isothermal). The injector temperature was 280 °C. The helium carrier gas flow rate was 1.37 mL/min. All the mass spectra were recorded by applying the following condition: (equipment current) filament emission current, 60 mA; ionization voltage, 70 eV; and ion source, 220 °C. Diluted samples (1% v/v) were injected with the split mode (split ratio 1: 15).
Identification of essential oil composition was performed by comparing their retention indices in relation to n-alkanes (C6–C20), mass matching to NIST, Wiley library database, and standards if available. Peaks were first deconvoluted using AMDIS software (www.amdis.net (accessed on 28 November 2019)) before mass spectral matching.12,25
Biological Investigation
DPPH Radical Scavenging Activity
Radical scavenging activity of L. stolonifera aerial part and root total methanolic extracts, n-butanol, and ethyl acetate fractions against the stable free radical DPPH (2,2-diphenyl-2-picrylhydrazyl hydrate) was determined spectrophotometrically. The 3 mL DPPH (0.2 mM) solution was mixed with 77 μL extract solution in 1 cm path length cuvettes, and the samples were kept in the dark for 30 min at room temperature, and then, the decrease in absorption was measured at 517 nm. Ascorbic acid was used as a positive control, and the results were expressed as % inhibition for DPPH.26 All measurements were performed in three replicates, and results are calculated as mean and standard deviation. The percentage inhibition of the DPPH radical was calculated according to the formula
where AB = absorbance of the control at t = 0 min and AA = absorbance of the sample at t = 16 min.
Metal Chelation Activity
Iron metal chelation assay was used to determine the chelating activity of L. stolonifera aerial part and root methanol extracts.27 Trolox stock solution of 0.1 mM in methanol was prepared, and 10 serial dilutions were prepared in different concentrations and preparation of samples at a concentration of 1 mg/mL in methanol. The freshly prepared ferrous sulfate (20 μL, 0.3 mM) was mixed with 50 μL of the sample/compound in 96-well plates (n = 6); then, 30 μL of ferrozine (0.8 mM) was added to each well. The reaction mixture was incubated at room temperature for 10 min. At the end of the incubation time, the decrease in the produced color intensity was measured at 562 nm and EDTA was used as the standard chelating agent. Finally, the data are represented as means ± SD according to the following equation, and the results were recorded using microplate reader FluoStar Omega.
Conclusions
L. stolonifera (Guill. & Perr.) P.H.Raven is an important aquatic macrophyte in the Nile Delta. This plant is common with its economic importance and high potential in eliminating various toxic pollutants from the aquatic environment. In this study, we investigated the difference between aerial part and root essential oil contents by GC–MS analysis. A total of 85 metabolites were identified in both aerial parts and roots with the abundance of aliphatic and aromatic hydrocarbons. Biological investigation on the plant parts revealed its potential activities as antioxidants and metal chelation capacity. Further studies on the nonvolatile metabolites and more deep biological evaluation are recommended.
Acknowledgments
M.H.B. acknowledges Prof. Dr. Rim Hamdy, Professor of Plant Taxonomy, Faculty of Science, Cairo University, for her efforts in identifying the plant of study in Egypt.
The authors did not receive support from any organization for the submitted work.
The authors declare no competing financial interest.
References
- Shawky E. M.; Elgindi M. R.; Ibrahim H. A.; Baky M. H. The potential and outgoing trends in traditional, phytochemical, economical, and ethnopharmacological importance of family Onagraceae: A comprehensive review. J. Ethnopharmacol. 2021, 281, 114450. 10.1016/j.jep.2021.114450. [DOI] [PubMed] [Google Scholar]; , In press
- Xu Z.; Deng M.. Identification and Control of Common Weeds; Springer: Switzerland AG, 2017; Vol. 2. [Google Scholar]
- Kaškonienė V.; Maruška A.; Akuņeca I.; Stankevičius M.; Ragažinskienė O.; Bartkuvienė V.; Kornyšova O.; Briedis V.; Ugenskienė R. Screening of antioxidant activity and volatile compounds composition of Chamerion angustifolium (L.) Holub ecotypes grown in Lithuania. Nat. Prod. Res. 2016, 30, 1373–1381. 10.1080/14786419.2015.1058792. [DOI] [PubMed] [Google Scholar]
- Zardini E.; Raven P. H. A new section of Ludwigia (Onagraceae) with a key to the sections of the genus. Syst. Bot. 1992, 17, 481–485. 10.2307/2419486. [DOI] [Google Scholar]
- Lin W.-S.; Lo J.-H.; Yang J.-H.; Wang H.-W.; Fan S.-Z.; Yen J.-H.; Wang P.-Y. Ludwigia octovalvis extract improves glycemic control and memory performance in diabetic mice. J. Ethnopharmacol. 2017, 207, 211–219. 10.1016/j.jep.2017.06.044. [DOI] [PubMed] [Google Scholar]
- Smida I.; Sweidan A.; Souissi Y.; Rouaud I.; Sauvager A.; Torre F.; Calvert V.; Le Petit J.; Tomasi S. Anti-Acne, Antioxidant and Cytotoxic Properties of Ludwigia peploides Leaf Extract. Int. J. Pharmacogn. Phytochem. Res. 2018, 10, 271–278. [Google Scholar]
- Praneetha P.; Reddy Y.; Kumar B. In vitro and In vivo hepatoprotective studies on methanolic extract of aerial parts of Ludwigia hyssopifolia G. Don Exell. Pharmacogn. Mag. 2018, 14, 546. 10.4103/pm.pm_85_18. [DOI] [Google Scholar]
- Amer W. M.; Hamdy R. S.; Hamed A. B.. In Macro-and micromorphological variations of Ludwigia stolonifera (Guill. & Perr.) PH Raven in Egypt and its taxonomic assessment, Egypt. Journal of Botany, 6 th International Conference, 2016; pp 11–12.
- Saleh H. M.; Aglan R. F.; Mahmoud H. H. Ludwigia stolonifera for remediation of toxic metals from simulated wastewater. Chem. Ecol. 2019, 35, 164–178. 10.1080/02757540.2018.1546296. [DOI] [Google Scholar]
- Elifantz H.; Tel-Or E. Heavy metal biosorption by plant biomass of the macrophyte Ludwigia stolonifera. Water, Air, Soil Pollut. 2002, 141, 207–218. 10.1023/A:1021343804220. [DOI] [Google Scholar]
- Baky M. H.; Farag M. A.; Rasheed D. M. Metabolome-Based Analysis of Herbal Cough Preparations Via Headspace Solid-Phase Microextraction GC/MS and Multivariate Data Analyses: A Prospect for Its Essential Oil Equivalency. ACS Omega 2020, 5, 31370–31380. 10.1021/acsomega.0c04923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M. A.; Khattab A. R.; Shamma S.; Afifi S. M. Profiling of Primary Metabolites and Volatile Determinants in Mahlab Cherry (Prunus mahaleb L.) Seeds in the Context of Its Different Varieties and Roasting as Analyzed Using Chemometric Tools. Foods 2021, 10, 728. 10.3390/foods10040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Glory L. F.; Pino J. A.; Santiago L. S.; Sauri-Duch E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007, 103, 1032–1043. 10.1016/j.foodchem.2006.07.068. [DOI] [Google Scholar]
- Choi D.; Kang W.; Park T. Anti-allergic and anti-inflammatory effects of Undecane on mast cells and keratinocytes. Molecules 2020, 25, 1554. 10.3390/molecules25071554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-Hefny M.; Mohamed A. A.; Salem M. Z. M.; Abd El-Kareem M. S. M.; Ali H. M. Chemical composition, antioxidant capacity and antibacterial activity against some potato bacterial pathogens of fruit extracts from Phytolacca dioica and Ziziphus spina-christi grown in Egypt. Sci. Hortic. 2018, 233, 225–232. 10.1016/j.scienta.2018.01.046. [DOI] [Google Scholar]
- Zorníková G.; Jarošová A.; Hřivna L. Distribution of phthalic acid esters in agricultural plants and soil. Acta Univ. Agric. Silvic. Mendelianae Brun. 2014, 59, 233–238. 10.11118/ACTAUN201159030233. [DOI] [Google Scholar]
- Prasad R.; Shivay Y. S. Oxalic Acid/Oxalates in Plants:From Self-Defence to Phytoremediation. Curr. Sci. 2017, 112, 1665–1667. 10.18520/cs/v112/i08/1665-1667. [DOI] [Google Scholar]
- Xu P.; Leng Y.; Zeng G.; Huang D.; Lai C.; Zhao M.; Wei Z.; Li N.; Huang C.; Zhang C.; Li F.; Cheng M. Cadmium induced oxalic acid secretion and its role in metal uptake and detoxification mechanisms in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2015, 99, 435–443. 10.1007/s00253-014-5986-y. [DOI] [PubMed] [Google Scholar]
- Islam M. T.; Ali E. S.; Uddin S. J.; Shaw S.; Islam M. A.; Ahmed M. I.; Chandra Shill M.; Karmakar U. K.; Yarla N. S.; Khan I. N.; Billah M. M.; Pieczynska M. D.; Zengin G.; Malainer C.; Nicoletti F.; Gulei D.; Berindan-Neagoe I.; Apostolov A.; Banach M.; Yeung A. W. K.; El-Demerdash A.; Xiao J.; Dey P.; Yele S.; Jóźwik A.; Strzałkowska N.; Marchewka J.; Rengasamy K. R. R.; Horbańczuk J.; Kamal M. A.; Mubarak M. S.; Mishra S. K.; Shilpi J. A.; Atanasov A. G. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. 10.1016/j.fct.2018.08.032. [DOI] [PubMed] [Google Scholar]
- Balamurugan R.; Stalin A.; Aravinthan A.; Kim J.-H. γ-sitosterol a potent hypolipidemic agent: In silico docking analysis. Med. Chem. Res. 2015, 24, 124–130. 10.1007/s00044-014-1075-0. [DOI] [Google Scholar]
- Santos S. A. O.; Villaverde J. J.; Sousa A. F.; Coelho J. F. J.; Neto C. P.; Silvestre A. J. D. Phenolic composition and antioxidant activity of industrial cork by-products. Ind. Crops Prod. 2013, 47, 262–269. 10.1016/j.indcrop.2013.03.015. [DOI] [Google Scholar]
- Granica S.; Piwowarski J. P.; Kiss A. K. Polyphenol composition of extract from aerial parts of Circaea lutetiana L. and its antioxidant and anti-inflammatory activity in vitro. Acta Biol. Cracov., Ser. Bot. 2013, 55, 1. 10.2478/abcsb-2013-0005. [DOI] [Google Scholar]
- Kehrer J. P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Prior R. L.; Wu X.; Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Ribeiro V. P.; Arruda C.; Mejía J. A. A.; Candido A. C. B. B.; Santos R. A.; Magalhães L. G.; Bastos J. K. Brazilian southeast brown propolis: gas chromatography method development for its volatile oil analysis, its antimicrobial and leishmanicidal activities evaluation. Phytochem. Anal. 2021, 32, 404–411. 10.1002/pca.2988. [DOI] [PubMed] [Google Scholar]
- Serag A.; Baky M. H.; Döll S.; Farag M. A. UHPLC-MS metabolome based classification of umbelliferous fruit taxa: a prospect for phyto-equivalency of its different accessions and in response to roasting. RSC Adv. 2020, 10, 76–85. 10.1039/C9RA07841J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. S.; Alvarenga Brizola V. R.; Granato D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. 10.1016/j.foodchem.2016.07.091. [DOI] [PubMed] [Google Scholar]