Abstract

This article introduces an environmentally friendly and more economical method for preparing red selenium nanoparticles (Se-NPs) with high stability, good biocompatibility, and narrow size using yeast as a bio-reducing agent with high antioxidant, immune regulation, and low toxicity than inorganic and organic Se. The yeast-derived Se-NPs were characterized by scanning electron microscopy, transmission electron microscopy, energy dispersive spectroscopy, Fourier transform infrared spectroscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. The results revealed spherical-shaped particles of Se-NPs with an average diameter of 71.14 ± 18.17 nm, an amorphous structure, and surface enhancement with an organic shell layer, that provide precise geometry and stability in the formation of bio-inert gray or black Se-NPs instead of red Se-NPs. Furthermore, the addition of 0.3–0.8 mg/kg Se-NPs in the feed significantly improved the health of mice. As Se-NPs stimulated the oxidative state of mice, it significantly increased the level of GSH-Px, SOD, and AOC, and decreased the level of MDA. The yeast-derived Se-NPs alleviated the immunosuppression induced by cyclophosphamide, whereas protected the liver, spleen, and kidney of mice, stimulated the humoral immune potential of the mice, and significantly increased the levels of I g M, IgA, and I g G. These results indicated that the yeast-derived Se-NPs, as a trace element feed additive, increased the defense of the animal against oxidative stress and infectious diseases and therefore Se-NPs can be used as a potential antibiotic substitute for animal husbandry.
Introduction
With the improvement of people’s living standards, the demand for meat products is higher and higher and the expansion of breeding industry has become an inevitable choice.1 However, outbreaks of endemic diseases, leading to high mortality of animals and significant economic losses are considered to be the most important factor restricting the development of animal husbandry. Antibiotics are often used to solve this problem, but long-term and large-scale use of antibiotics causes intestinal flora imbalance,2 increases drug resistance by pathogenic bacteria, and destructs the micro-ecological environment. In addition, excessive intake of meat with antibiotics in the body leads to autoimmune decline and organ disease and thus causes harm to human health.3 Many countries in the world have clearly stipulated that the use of antibiotic drugs in animal husbandry is strictly prohibited and it is an inevitable trend to completely eliminate antibiotic residues in animal products.4 Functional feed additives are considered as a potential substitute for antibiotics in feed because of their functions of improving organism sub-health, improving immunity and anti-stress ability, and reducing disease incidences.5
Selenium (Se) is an essential micronutrient, often added to animal feed to promote animal growth.6 Se can affect the antioxidation and immune function of animal organism through glutathione peroxidase (GSH-Px) and selenoproteins with various biological activities, so as to increase the defense against oxidative stress and infectious diseases in animals. At present, Se in feeds on the market is mainly added in the form of inorganic and organic Se.7 However, inorganic Se has been criticized for its high toxicity and negative impact on the environment due to its high excretion in feces.8 Organic Se is less toxic than inorganic Se, but organic diselenides (such as selenocysteine and selenocystamine) are converted to selenols (RSeH) in the presence of thiols, which also cause production of reactive oxygen species and subsequent oxidative stress.9 Recently, due to the higher bioavailability and lower toxicity of Se nanoparticle (Se-NP) trace elements than inorganic salt, people’s interest in them as animal feed supplements has increased.10 Studies have shown that Se-NPs are less toxic to animals than other Se compounds (e.g., selenite, selenomethionine, and Se-methylselenocysteine).11,12 Adding Se-NPs to animal feed can modulate gut microbiota and improve the growth performance, feed conversion rate, immunity, and oxidative stress ability of animals.13,14
The unique properties, functions, and applications of NPs such as silver,15 gold, iron,16 and Se-NPs have a lasting connection with their size and shape. Chemical and physical methods to synthesize Se-NPs, apart from being expensive, easy to aggregate,17 and of unstable shape and size, there are other problems such as complex synthesis process,18 not being environmentally friendly, and the requirement of strict conditions19 limit the application of Se-NPs. By contrast, the biotemplate method (green synthesis method) for preparing Se-NPs from microorganisms, proteins, polysaccharides, and plant extracts20 is considered as a potential substitute for chemical and physical methods because of its eco-friendliness,21 less toxicity, high stability,22 and narrow size and shape distribution. Among them, microorganisms are considered to be suitable templates for the preparation of Se-NPs when considering biological activity and health care function. Due to the diversity of reducing enzymes in microorganisms, Se-NPs synthesized by microorganisms have a unique and complex nanostructure arrangement of Se atoms. Yeast is rich in proteins, B vitamins, amino acids, and other substances, widely used as animal feed protein supplement.23 Additionally, yeast has a strong tolerance to inorganic Se salts and can convert toxic selenite into nontoxic element Se through aerobic or anaerobic conditions.24 However, there are relatively few studies on yeast-derived Se-NPs as feed additives, so the effect of yeast-derived Se-NPs in improving animal health needs further verification.
In this study, yeast was used as a biological template to synthesize Se-NPs. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersion spectroscopy (EDS), Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) were used to investigate the size, shape, stability, material composition, and structure of Se-NPs. The antioxidant activity of yeast-derived Se-NPs at different levels and its ameliorative effect on immune injury induced by cyclophosphamide (CTX) were evaluated in specific pathogen-free (SPF) male rats. The results obtained from the above studies will be helpful for the development of yeast-derived Se-NPs as a functional feed additive and further incorporated into the formulation of Se-containing feed in the market.
Results and Discussion
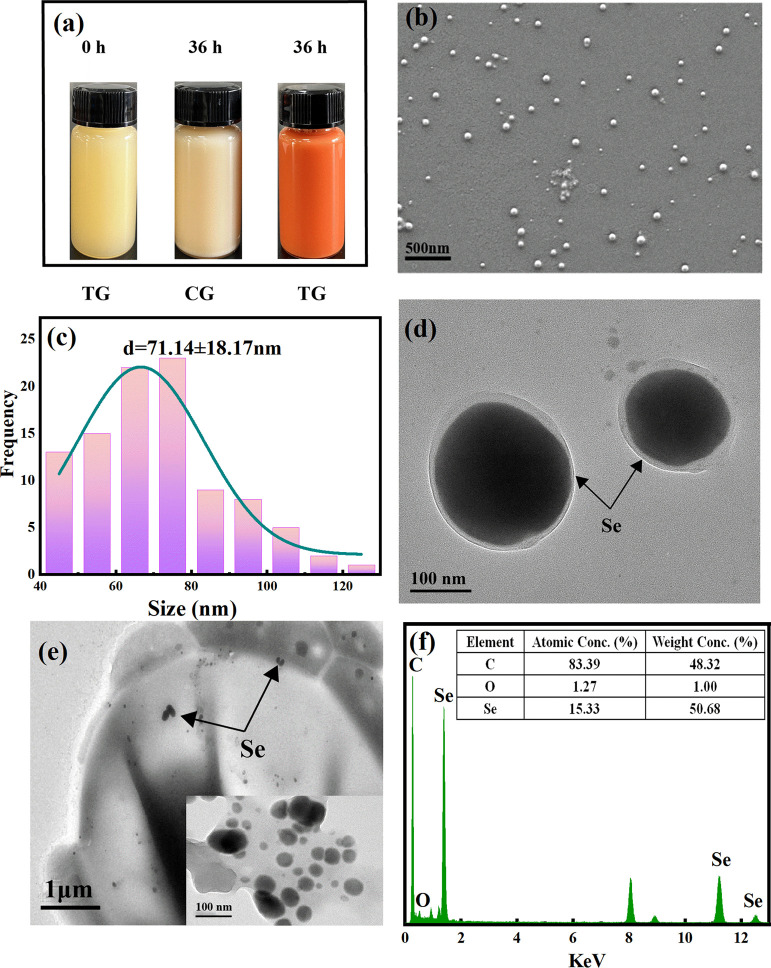
Immune regulation and antioxidative response of environmentally friendly and economical yeast-derived Se-NPs with high stability and good biocompatibility was assessed using CTX-induced mice and their biomedical application illustration is shown in schematic Figure 1. The color change during the synthesis of Se-NPs is shown in Figure 2a. The mixed solution with sodium selenite was the test group (TG), while the control group (CG) was not. After 36 h of incubation, the CG had no obvious change, whereas the TG changed from light yellow to brick red. Color change was the main visual indicator of successful synthesis of Se-NPs, which was related to the excitation of surface plasma vibration of Se-NPs.25,26
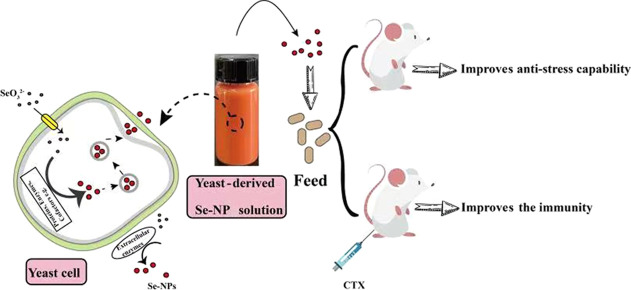
Figure 1.
Schematic illustration of yeast-mediated synthesis of Se-NPs with application.
Figure 2.
Morphology and microstructure of Se-NPs: (a) color changes during the synthesis of Se-NPs; (b) SEM image of Se-NPs in medium solution; (c) diameter distribution histogram of Se-NPs; (d) TEM image of Se-NPs in medium solution; (e) TEM image of Se-NPs in yeast cells and partially enlarged view (inset); and (f) EDS spectra and elemental composition of Se-NPs.
Morphological Analysis of Se-NPs
The microstructure of Se-NPs in the medium was further observed by SEM, as shown in Figure 2b. The NPs are clearly spherical in shape, but the size of the particles is not uniform. The nanospheres are composed of interconnected three-dimensional Se networks in which the stable chain and ring structure results in the spherical shape of the nanospheres.27,28 Besides, the lack of uniformity of Se-NPs may be due to the short-term thermodynamic interaction, as the resulting particles tend to be adhere to each other.29 After that, by measuring the diameter of 100 nm size of particles in different areas of the SEM image, the histogram of the diameter distribution was plotted as shown in Figure 2c. The average diameter is 71 ± 18.17 nm. Among them, 91.84% of the particles are smaller than 100 nm in diameter, which is an acceptable particle size.30 The size of Se-NPs determines their potential biological activity, and it seems that Se-NPs with small particle size (particle size smaller than 100 nm) and good stability have higher biological activity because the increase of Se-NP diameter can reduce their antioxidant activity, Se retention ability, and upregulation of GSH S-transferase activity.31 Furthermore, the diameter of yeast-derived Se-NPs was smaller than the Se-NPs synthesized with Pseudomonas putida KT2440 (266 nm) reported by Avendaño et al.32 and Se-NPs synthesized with Bacillus cereus (150–200 nm) reported by Dhanjal and Cameotra,33 and others.34−36 Where the size ranges 85–709 nm37−39 and the range between 100 and 300 nm is found significantly abundant,40,41 it indicated that yeast can better control the size of the Se-NPs.
TEM images were used to further observe the deposition of Se-NPs in the medium and yeast cells, as shown in Figure 2d,e. A large number of Se-NPs are distributed in the culture medium and yeast cells, confirming the deposition of Se-NPs inside and outside the cell. Interestingly, the larger Se-NPs in yeast cells were concentrated around the cell membrane or protruded from the cell surface, and the Se-NPs in the culture medium had a clear organic shell around the cells. The presence of Se particles in medium solution and cells is an indication of a vesicular mechanism to repel bio-transformed Se.
Zhang et al. believe that the yeast cells transport the overloaded Se out of the cell through a vesicle-like structure.24 In order to determine the elemental composition and composition change of the product, the Se-NPs were analyzed by EDS. In Figure 2f, the EDS spectra of Se-NPs identified elements C (0.29 keV), O (0.51 keV), and Se (1.39, 11.23, and 12.53 keV), with mass percentages of 48.32, 1.0, and 50.68%, respectively. These results strongly support the presence of Se, and the high carbon ratio suggests that the organic shell layer on the surface of Se-NPs may be a protein from yeast cells, acting as a capping agent during particle formation, to prevent the red Se-NPs into a biologically inert gray or black.42
Structural Spectrogram Analysis
In this study, except for Se, C, and O, other elements were also found in the obtained Se-NPs. In order to further determine the molecular structure and bonding principle of NPs, FTIR analysis was conducted on yeast cells and Se-NPs, the results of which are shown in Figure 3a. The broad absorption peak of Se-NPs at 3287 cm–1 corresponded to the stretching vibration of hydroxyl (−OH), while the absorption peak at 2930 cm–1 was generated by the stretching vibration of the C–H bond on the alkyl chain.43 Furthermore, the absorption bands at 1700–2000 cm–1 were attributed to the specific functional groups of the protein. The characteristic absorption bands of the amide bond (−CO–NH) appeared in the protein at 1642 cm–1 (the stretching vibration of C=O in amide) and 1544 cm–1 (the bending vibration of N–H in amide II).44 The absorption peaks of the curve at 1398 and 1239 cm–1 corresponded to the symmetrical deformation of −CH3 and the C–O deformation vibration of the carboxyl group, respectively. The strong absorbance at 1083 cm–1 was generated by the C–O–C group in the polysaccharide.45 The main characteristic peak of infrared spectrum of Se-NPs was similar to that of yeast cells, which indicated that Se-NPs contained some active substances of yeast cells. Moreover, compared with yeast cells, Se-NPs had a stronger absorption band peak at 1700–2000 cm–1, and characteristic peaks of the amide bond and C–O appeared at 1544 and 1239 cm–1, indicating that the active substance in Se-NPs was protein. Its function was to transport Se-NPs outside the cell, control the size of NPs and stabilize Se-NPs.
Figure 3.
Structure characterization: (a) FTIR analysis of (i) yeast cells and (ii) Se-NPs; (b) XRD analysis of Se-NPs; and (c) zeta potentials of Se-NPs.
In order to further confirm the crystal structure and phase composition of the NPs, XRD analysis of Se-NPs was performed. In the XRD pattern, the sharp and strong bands point to the crystalline structure, whereas the low θ-degree broadband represents the amorphous structure.46 The XRD pattern of Se-NPs is shown in Figure 3b, showing wide and overlapping curves with wide diffraction fringes at a 2θ angle of 20–25, indicating that it is an amorphous phase structure. Similar XRD patterns had been found by Akcay et al. when preparing Se-NPs with Bacillus sp. EKT1,41 whereas different XRD patterns of Se-NP-loaded chitosan microspheres had been reported by Bai et al.32 This indicated that the crystal structure of Se-NPs can be affected by different manufacturing conditions.
Zeta potential is often used to evaluate the stability of colloidal solutions. Se-NPs were dispersed in deionized water to measure the zeta potential, and the surface charge and stability of Se-NPs solution were also studied. As shown in Figure 3c, the zeta potential of Se-NP solution was −34.00 mV. It is noteworthy that the strong surface charge (positive and negative, above 30 mV) is beneficial to inhibit the agglomeration of NPs.40 Considering the relationship between the stability of NPs and their zeta potential, Se-NPs can be stabilized in solution for a long time.47
XPS analysis of Se-NPs was performed to reveal the bonding mechanism and element valence of NPs. In Figure 4a, the C 1s, O 1s, and Se 3d signals are detected, which is consistent with the EDS analysis. Whereas, N was detected in the XPS spectrum, but not in the EDS spectrum, which could be due to the overlap of C and O signals on the N peak in the EDS spectrum.48
Figure 4.
XPS spectra: (a) Se-NPs (inset); (b) Se-in-Se-NPs; (c) C-in-Se-NPs; and (d) O-in-Se-NPs.
The 3d peak Se (0) of typical Se was found at 55.38, which confirmed that the valence state of Se in Se-NPs in elementary status. However, the signal of Se 3d is much weaker than that of C 1s and O 1s. This is because XPS is a typical surface analysis method. Although X-ray can penetrate Se-NPs, only the photoelectrons emitted by C and O in the shell layer on the surface of the sample can escape, while the photoelectrons emitted by Se inside the NP are difficult to escape, which makes the Se 3d signal weak.49 Besides, the Se 3d (Figure 4b) spectrum of Se-NPs consists of two sub-peaks located at 56.04 and 55.28 eV corresponding to Se 3d3/2 and Se 3d5/2, respectively. The sub-peaks at 288.04, 286.12, 284.78, and 284.15 eV in the C 1s (Figure 4c) spectrum of Se-NPs correspond to the C=O or O–C–O bonds in carbonyl and amide, the C–C or C–N bond in amide, and the C–O and C–H bonds in amino acid side chains, respectively.50 The O 1s (Figure 4d) spectrum of Se-NPs consists of three sub-peaks located at 532.83, 532.79, and 532.46 eV, which are generated by C–O or C=O bonds. These functional groups were consistent with FTIR results, which indicated that proteins, polysaccharides, and Se in Se-NPs were formed autonomously through the interaction of various chemical bonds.45
Effect of Yeast-Derived Se-NPs on the Antioxidant Status in Rats
The activities of major antioxidant enzymes (GSH-Px, SOD, and AOC) and the level of MDA in serum of rats are shown in Table 1. Compared with the CG, the contents of GSH-Px and T-SOD in serum of 0.8 mg/kg Se-NP group were significantly increased by 38.39% (p < 0.01) and 13.52% (p < 0.01), respectively. The T-AOC content in 0.3 mg/kg Se-NP group was significantly increased by 12.70% (p < 0.01). Besides, compared with the CG, the content of MDA in the serum of the yeast-derived Se-NP group was decreased to some extent, and the content of MDA in the 0.8 mg/kg Se-NP group was significantly decreased by 31.52% (p < 0.01). The antioxidant capacity is an important index reflecting the growth performance and health of animals. MDA is the main product of lipid peroxidation and its accumulation can reflect the degree of lipid peroxidation in animal organism, however, SOD, GSH-Px, and catalase activity are its good scavengers and therefore these indicators are often used to reflect the animal’s antioxidant status.51,52 Se is a co-factor of the antioxidant enzyme GSH-Px, which is involved in scavenging free radicals and blocking the lipid peroxidation of cells, membranes, and organelles.53 However, the existence of Se ester bonds (Se–O–C) in the yeast-derived Se-NPs makes the physical and chemical properties of the compound change greatly, and it has a higher biological activity. Studies show that organic Se-NPs are more beneficial to improve the antioxidant function of animal organism. Li et al. found that, compared with the control, the activities of T-SOD and GSH-Px in the hippocampus of mice in the Se-NP group were significantly increased, while the MDA level was significantly decreased.54 Dawood et al. reported that dietary supplementation of 0.5, 1, and 2 mg/kg Se-NPs significantly increased the antioxidant potential of red sea bream compared with the CG.55 It has been reported that the GSH-Px activity and antioxidant status were improved when Se-NPs were added to the diets in crucian carp.56 This is basically consistent with the results of this study. Despite the effective earlier reports for anti-biofilm, wound healing, cytotoxic and anti-viral activities57 in cancer treatment58 and in HT-29 cell line59 in vitro, the prepared Se had shown remarkable potential in vivo. In conclusion, Se-NPs can protect mice from the damage of reactive oxygen species and free radicals by delaying lipid oxidation and improving the activity of antioxidant enzymes in the organism.
Table 1. Effect of Yeast-Derived Se-NPs on GSH-Px, SOD, AOC, and MDA Activities in Ratsa.
| dietary Se (mg/kg) | GSH-Px (μmol/L) | T-SOD (U/mL) | T-AOC (U/mL) | MDA (nmol/L) |
|---|---|---|---|---|
| control | 2680.29 ± 207.42B | 91.40 ± 6.59bB | 0.63 ± 0.053bB | 4.95 ± 0.76aA |
| 0.3 mg/kg | 2878.47 ± 275.70B | 93.79 ± 8.05b | 0.71 ± 0.025aA | 4.91 ± 0.72aA |
| 0.5 mg/kg | 2848.40 ± 389.25B | 91.61 ± 15.72bB | 0.62 ± 0.045bB | 4.47 ± 0.54a |
| 0.8 mg/kg | 3709.05 ± 569.13A | 103.76 ± 70.5aA | 0.68 ± 0.047a | 3.39 ± 0.62bB |
In the same column, values with different small letter superscripts mean significant difference (P <0.05), and with different capital letter superscripts mean obvious significant difference (p < 0.01), while with the same or no letter superscripts mean no significant difference (p > 0.05). The same as below.
Effects of Yeast-Derived Se-NPs on the Immune Organ Index in CTX-Treated Rats
The organ indexes of rats are shown in Table 2. Compared with the CG, 0.3 mg/kg yeast-derived Se-NPs can significantly increase the spleen index (p < 0.01) and significantly decrease the kidney index and thymus index (p < 0.05). Simultaneously, 0.5 and 0.8 mg/kg yeast-derived Se-NPs could significantly increase the liver index and spleen index, and significantly decrease the thymus index (p < 0.01). Moreover, compared with the model group, 0.3 mg/kg yeast-derived Se-NPs significantly increased the renal index (p < 0.05); 0.5 and 0.8 mg/kg yeast-derived Se-NPs significantly increased the liver index, spleen index, and kidney index (p < 0.01); the thymus index of each group had no significant difference. The immune system is a defensive structure for the organism to protect itself. It is mainly composed of immune organs, immune cells, and immunologically active substances. Among them, the immune organs such as the liver, spleen, and thymus are one of the important indicators to measure the organism’s immunity. The liver is a front-line immune organ that contains the largest collection of phagocytes in the organism, specifically designed to detect and remove potential pathogens from the blood.60 Immune dysfunction or abnormal immune function is the main cause of renal disease.61 Furthermore, the thymus and spleen contain a large number of lymphocytes, which are directly related to the organism’s immunity.62 A series of evidences show that Se-NPs can enhance the immune response of the organism. Kohshahi et al. demonstrated that dietary addition of curcumin and Se-NPs (Se-NPs + CUR) significantly increased the activities of lysozyme and surrogate hemolytic complement and promoted the innate immune response of rainbow trout.63 It has been reported that Se-NPs metabolizes into selenocysteine to promote functional T lymphocyte development and macrophage migration, in addition to improving the structure of animal immune organs and restoring the morphological and functional state of these organs.64 Additionally, Bai et al. reported that Se-NPs can improve the liver injury induced by Con A and provide a protective effect on the liver of rats.65 In contrast, Burk et al. demonstrated that a decrease in glutathione caused by Se deficiency could lead to liver and kidney diseases.66 Moreover, Li et al. treated CTX-treated mice with Se-polysaccharide (SE-GFP-22) and found that SE-GFP-22 significantly increased the thymus and spleen indexes of mice and improved CTX-induced immune organ atrophy.67 These results are basically consistent with the present study. Suitable Se-NPs increased the liver index, spleen index and kidney index of rats. Therefore, we conclude that Se-NPs can alleviate the immunosuppression induced by CTX in rats. However, the thymus index of each group was significantly lower than that of the CG, which might be caused by the over-effect of CTX.
Table 2. Effects of Yeast-Derived Se-NPs on Immune Organ Index in CTX-Treated Rats.
| viscera
index (%) |
||||
|---|---|---|---|---|
| dietary treatment | liver | spleen | kidney | thymus |
| control | 3.73 ± 0.3bB | 0.27 ± 0.04cC | 0.92 ± 0.10aA | 0.24 ± 0.02a |
| model control | 3.75 ± 0.3bB | 0.54 ± 0.23B | 0.77 ± 0.05cC | 0.17 ± 0.03b |
| 0.3 mg/kg Se-NPs | 3.61 ± 0.26bB | 0.51 ± 0.16bB | 0.85 ± 0.08b | 0.18 ± 0.04b |
| 0.5 mg/kg Se-NPs | 4.39 ± 0.53aA | 0.86 ± 0.19aA | 0.90 ± 0.11aA | 0.16 ± 0.05b |
| 0.8 mg/kg Se-NPs | 4.45 ± 0.65aA | 0.91 ± 0.32aA | 0.89 ± 0.07aA | 0.18 ± 0.04b |
Effect of Yeast-Derived Se-NPs on Serum I g Contents in CTX-Treated Rats
The activities of immunoglobulin (I g M, I g A, and I g G) in serum of rats fed with four diets are shown in Table 3. In the model group, almost all I g M, I g A, and I g G activities in serum were decreased in comparison with the CG (p > 0.05). The dose of 0.3 mg/kg yeast-derived Se-NPs significantly increased I g M, I g A, and I g G levels (p < 0.01). Moreover, 0.5 mg/kg yeast-derived Se-NPs significantly increased I g M level (p < 0.01); 0.8 mg/kg yeast-derived Se-NPs could significantly increase I g A and I g G levels (p < 0.01). Humoral immunity is an important mechanism for organisms to resist tumors and infections. Immunoglobulin (I g M, I g A, and I g G) is the main index to measure humoral immunity, with functions of sterilization, activation of complement, and stimulation of phagocytic immunity. Immunosuppressive substances can lead to a decrease in immunoglobulin levels, but a series of studies have shown that Se-NPs indirectly affect the humoral immune regulation through cellular immune responses. Al-Deriny et al. reported that Se-NPs significantly increased Ig M levels while improving the health status of Nile tilapia.68 Similarly, as reported by Raahati et al. application of the Se-NP diet resulted in increased Ig A and Ig G levels in serum and saliva of rats, demonstrating that Se-NPs were effective in inducing immune cell effects at both humoral and mucosal levels.69 Shubhangi et al. found that the levels of Ig M and Ig G in enrofloxacin group were significantly lower than those in the CG in broiler chicken, but the dose of 0.3 and 0.6 mg/kg yeast-derived Se-NPs can significantly increase I g M and I g G levels.70 These results are basically consistent with the present study, indicating that Se-NPs from yeast can increase the content of immunoglobulin in serum and improve the humoral immunosuppression induced by CTX.
Table 3. Effect of Yeast-Derived Se-NPs on I g G, I g A, and I g M Levels in CTX-Treated Rats.
| dietary treatment | Ig M (mg/mL) | I g A (mg/mL) | I g G (mg/mL) |
|---|---|---|---|
| control | 0.172 ± 0.020bcB | 1.64 ± 0.13bB | 2.76 ± 0.29cC |
| model | 0.169 ± 0.016cC | 1.54 ± 0.13bB | 2.75 ± 0.52cC |
| 0.3 mg/kg Se-NPs | 0.200 ± 0.015aA | 1.87 ± 0.14aA | 2.84 ± 0.456aA |
| 0.5 mg/kg Se-NPs | 0.209 ± 0.0096aA | 1.80 ± 0.14ab | 2.80 ± 0.55ab |
| 0.8 mg/kg Se-NPs | 0.178 ± 0.018bB | 1.86 ± 0.11aA | 2.83 ± 0.34aA |
Conclusions
In the present study, Se-NPs were synthesized using yeast as the biotemplate, and its structure was characterized. SEM images showed spherical particles of Se-NPs with an average diameter of 71.14 ± 18.17 nm. EDS and XPS spectra confirmed the presence of element Se. XRD, FTIR, and XPS analysis showed that the Se-NPs had an amorphous structure and were covered with bioactive substances, which may act as a linker and stabilizer during the formation of Se-NPs and prevent the red Se-NPs from turning into a biologically inert gray or black. Besides, the addition of 0.3–0.8 mg/kg Se-NPs to the diet could improve the health of mice. Se-NPs can stimulate the oxidative state of mice, significantly increase the levels of GSH-Px, SOD, and AOC, and decrease the level of MDA. Se-NPs can alleviate CTX-induced immunosuppression in rats, improve the liver index, spleen index and kidney index of rats, stimulate the humoral immunity potential of the body, and significantly increase Ig M, Ig A, and Ig G levels. The results showed that Se-NPs from yeast were safe and had high bioavailability, and the supplement of Se-NPs in feed can increase the defense of animal body against oxidative stress and infectious diseases.
Materials and Methods
Sodium selenite was supplied by Aladdin Co., Ltd. Baker’s yeast was purchased from Angel Yeast Co., Ltd. Malt powder was provided by Hongyang Chinese medicine shop and the SPF SD male rats were purchased from the animal center of Guangdong Medical College of China. All diagnostic kits for detecting antioxidation and immunity were purchased from Nanjing Jiancheng Hongda Biotechnology Co., Ltd.
Synthesis of Se-NPs from Yeast
Malt powder and deionized water were mixed evenly at the mass ratio of 1:4, heated at 60 °C in a water bath for 4 h and then the mash was filtered and centrifuged to obtain the malt juice culture. Later, 100 mL of malt juice culture with 5% baker’s yeast and 100 mg of sodium selenite were mixed. The mixed solution was adjusted to a pH of 4 and the culture was carried out for 36 h in a constant temperature shaker at 30 °C. After fermentation, the sample solution was centrifuged for 15 min at 4500 r/min to obtain the brick-red yeast cells rich in Se-NPs. In addition, the centrifuged medium solution was centrifuged at 8000 r/min for 20 min to collect Se-NPs in the medium. The NPs were washed with deionized water three times and then freeze-dried to obtain the brick-red Se-NPs.
Morphological Observation
Se-NPs were freeze-dried using a vacuum freeze dryer (α 1-2 LD Plus, Christ, Germany). 5 mg of Se-NPs was dispersed in 10 mL of deionized water and then the sample solution was dripped on the silicon wafer and dried. The morphology and particle size distribution of the samples were observed by SEM (Sigma300, Zeiss, Germany). The dispersed sample was dropped on a copper grid and dried and then the Se-NPs in the culture medium and the Se-NPs in the yeast cells were observed using a TEM FEI Talos F200S, Thermo Fisher Scientific, USA. Meanwhile, EDS was applied during TEM experiments.
Structural Characterization
A Fourier infrared spectrometer (Spectrum100, PerkinElmer, USA) was used to analyze Se-NPs in the range of 4000–400 cm–1 according to the KBr pellet pressing method. Cu Kα was used as the radiation source (λ = 0.15406 nm), powder X-ray diffraction (Ultima IV, Rigaku, Japan) was used for XRD analysis of Se-NPs with a tube voltage of 40 kV and a tube current of 40 mA. The zeta potential of Se-NPs was measured using a Zetasizer Nano (90Plus PALS, Brookhaven Instruments Corporation, USA) after dispersing Se-NPs in deionized water. Using Al Kα as the excitation source (HV = 1486.6 eV), the XPS analysis of Se-NPs was performed by XPS (K-Alpha, Thermo Fisher Scientific, USA) with an operating voltage of 12 kV and a current of 6 mA.
Experimental Animals and Treatments
The SPF male rats (5 weeks old) in this study were purchased from the animal center of Guangdong Medical College of China. Rats were housed in individual stainless-steel cage with controlled temperature (25 °C), relative humidity (60%), and light/dark cycle (12 h). Before the experiment, the rats were acclimated for 3 days and fed with basal diet to adapt to the experimental environment. After acclimation, 40 rats were randomly divided into four groups with 10 rats in each group for the antioxidant index test. The CG was fed with basal diet and the other groups were fed with 0.3, 0.5, and 0.6 mg/kg yeast-derived Se-NP feed, respectively. Herein, mice were given dried yeast-derived Se-NPs at three doses well below the LD50.71 In addition, 50 rats were randomly divided into five groups (control, model, 0.3, 0.5, and 0.6 mg/kg group), 10 rats in each group, for the immunosuppression experiment. From day 17 to day 20, the CG was administered with 1 mL normal saline and the other groups were administered with 1 mL CTX (50 mg/kg/d) for immunosuppression. The experiment lasted for 28 days. Rats were fed at 9:00 a.m. and 5:00 p.m. each day and a special equipment was used to avoid contamination of urine and feces.
Sample Collection
At the end of the feeding period, all mice were fasted for 24 h before sampling. The blood was sampled by eyeball extirpation, stored at 4 °C for 4 h until the blood was significantly stratified and then centrifugation occurred at 3500 rpm for 15 min. The serum was collected to evaluate the antioxidant and immunological parameters in rats including glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOD), malondialdehyde (MDA), immunoglobulin M (Ig M), immunoglobulin A (Ig A), and immunoglobulin G (Ig G). Enzyme activity and immune parameters were analyzed using an automated biochemical analyzer (Infinite 200, Tecan Austria GmbH), following the methods and instructions for the specific kit. Moreover, the abdomens of the rats were slit open, and the liver, spleen, kidney, and thymus were collected, washed with normal saline, dried with absorbent paper, and weighed. The relative organ index was expressed as
Animal handling and sampling procedures referred to the Jiangsu Academy of Agricultural Sciences guidelines (permit no. SCXK Su 2002-0029).
Statistical Analysis
All experimental data were expressed as mean ± standard deviation (SD). The data in this study were processed by SPSS 13.0 (SPSS, USA) statistical software and analyzed through the one-way analysis of variance. Values with differences P < 0.05 and P < 0.01 were regarded as statistically significant..
Acknowledgments
The authors acknowledge the financial support provided by Guangdong science and technology planning project (2016A020210066 and Guangzhou science and technology planning project (201707010461)) Guangdong Provincial Department of Agriculture and Rural Affairs Project, Grant/Award Number: 2020KJ115, 2021KJ115.
The authors declare no competing financial interest.
References
- Luo F.; Wang M.; Huang L.; Wu Z.; Wang W.; Zafar A.; Tian Y.; Hasan M.; Shu X. Synthesis of Zinc Oxide Eudragit FS30D Nanohybrids: Structure, Characterization, and Their Application as an Intestinal Drug Delivery System. ACS Omega 2020, 5, 11799–11808. 10.1021/acsomega.0c01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Hao C.; Yao W.; Zhu D.; Lu H.; Li L.; Ma B.; Sun B.; Xue D.; Zhang W. Intestinal Flora Imbalance Affects Bile Acid Metabolism and Is Associated with Gallstone Formation. BMC Gastroenterol. 2020, 20, 59. 10.1186/s12876-020-01195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. X.; Tan L. T.-H.; Ab Mutalib N.-S.; Pusparajah P.; Goh B.-H.; Chan K.-G.; Letchumanan V.; Lee L.-H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. 10.3390/antibiotics10050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin-Laprade D.; Brouard J.-S.; Gagnon N.; Turcotte A.; Langlois A.; Matte J. J.; Carrillo C. D.; Zaheer R.; McAllister T. A.; Topp E.; Talbot G. Resistance Determinants and Their Genetic Context in Enterobacteria from a Longitudinal Study of Pigs Reared under Various Husbandry Conditions. Appl. Environ. Microbiol. 2021, 87, e02612–e02620. 10.1128/AEM.02612-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.; Yuan Q.; Mathieu J.; Stadler L.; Senehi N.; Sun R.; Alvarez P. J. J. Antibiotic Resistance Genes from Livestock Waste: Occurrence, Dissemination, and Treatment. npj Clean Water 2020, 3, 4. 10.1038/s41545-020-0051-0. [DOI] [Google Scholar]
- Ahmad W.; Shams S.; Ahmad A.; Wei Y.; Yuan Q.; Khan A. U.; Khan M. S.; Ur Rahman A.; Iqbal M. Synthesis of Selenium–Silver Nanostructures with Enhanced Antibacterial, Photocatalytic and Antioxidant Activities. Appl. Nanosci. 2020, 10, 1191–1204. 10.1007/s13204-019-01213-z. [DOI] [Google Scholar]
- Dawood M. A. O.; Zommara M.; Eweedah N. M.; Helal A. I.; Aboel-Darag M. A. The Potential Role of Nano-Selenium and Vitamin C on the Performances of Nile Tilapia (Oreochromis Niloticus). Environ. Sci. Pollut. Res. 2020, 27, 9843–9852. 10.1007/s11356-020-07651-5. [DOI] [PubMed] [Google Scholar]
- Gangadoo S.; Dinev I.; Willson N.-L.; Moore R. J.; Chapman J.; Stanley D. Nanoparticles of Selenium as High Bioavailable and Non-Toxic Supplement Alternatives for Broiler Chickens. Environ. Sci. Pollut. Res. 2020, 27, 16159–16166. 10.1007/s11356-020-07962-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A.; Basu A.; Bhattacharya S. Selenium Nanoparticles Are Less Toxic than Inorganic and Organic Selenium to Mice in Vivo. Nucleus 2019, 62, 259–268. 10.1007/s13237-019-00303-1. [DOI] [Google Scholar]
- Hasan M.; Rafique S.; Zafar A.; Loomba S.; Khan R.; Hassan S. G.; Khan M. W.; Zahra S.; Zia M.; Mustafa G.; Shu X.; Ihsan Z.; Mahmood N. Physiological and Anti-Oxidative Response of Biologically and Chemically Synthesized Iron Oxide: Zea Mays a Case Study. Heliyon 2020, 6, e04595 10.1016/j.heliyon.2020.e04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Zhang J.; Yu H. Elemental Selenium at Nano Size Possesses Lower Toxicity without Compromising the Fundamental Effect on Selenoenzymes: Comparison with Selenomethionine in Mice. Free Radical Biol. Med. 2007, 42, 1524–1533. 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Shoeibi S.; Mozdziak P.; Golkar-Narenji A. Biogenesis of Selenium Nanoparticles Using Green Chemistry. Top. Curr. Chem.. 2017, 375, 88. 10.1007/s41061-017-0176-x. [DOI] [PubMed] [Google Scholar]
- Alian H. A.; Samy H. M.; Ibrahim M. T.; Mahmoud M. M. A. Nanoselenium Effect on Growth Performance, Carcass Traits, Antioxidant Activity, and Immune Status of Broilers. Environ. Sci. Pollut. Res. 2020, 27, 38607–38616. 10.1007/s11356-020-09952-1. [DOI] [PubMed] [Google Scholar]
- Qasim S.; Zafar A.; Saif M. S.; Ali Z.; Nazar M.; Waqas M.; Haq A. U.; Tariq T.; Hassan S. G.; Iqbal F.; Shu X.-G.; Hasan M. Green Synthesis of Iron Oxide Nanorods Using Withania Coagulans Extract Improved Photocatalytic Degradation and Antimicrobial Activity. J. Photochem. Photobiol., B 2020, 204, 111784. 10.1016/j.jphotobiol.2020.111784. [DOI] [PubMed] [Google Scholar]
- Zulfiqar H.; Zafar A.; Rasheed M. N.; Ali Z.; Mehmood K.; Mazher A.; Hasan M.; Mahmood N. Synthesis of Silver Nanoparticles Using: Fagonia Cretica and Their Antimicrobial Activities. Nanoscale Adv. 2019, 1, 1707–1713. 10.1039/c8na00343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M.; Gulzar H.; Zafar A.; ul Haq A.; Mustafa G.; Tariq T.; Khalid A.; Mahmmod A.; Shu X.; Mahmood N. Multiplexing Surface Anchored Functionalized Iron Carbide Nanoparticle: A Low Molecular Weight Proteome Responsive Nano-Tracer. Colloids Surf., B 2021, 203, 111746. 10.1016/j.colsurfb.2021.111746. [DOI] [PubMed] [Google Scholar]
- Athar M.; Fiaz M.; Farid M. A.; Tahir M.; Asghar M. A.; Ul Hassan S.; Hasan M. Iron and Manganese Codoped Cobalt Tungstates Co1-(X+ y)Fe XMn YWO4as Efficient Photoelectrocatalysts for Oxygen Evolution Reaction. ACS Omega 2021, 6, 7334–7341. 10.1021/acsomega.0c05412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munawar T.; Nadeem M. S.; Mukhtar F.; Hasan M.; Mahmood K.; Arshad M. I.; Hussain A.; Ali A.; Saif M. S.; Iqbal F. Rare Earth Metal Co-Doped Zn0·9La0.05M0.05O (M = Yb, Sm, Nd) Nanocrystals; Energy Gap Tailoring, Structural, Photocatalytic and Antibacterial Studies. Mater. Sci. Semicond. Process. 2021, 122, 105485. 10.1016/j.mssp.2020.105485. [DOI] [Google Scholar]
- Munawar T.; Mukhtar F.; Nadeem M. S.; Riaz M.; Naveed ur Rahman M.; Mahmood K.; Hasan M.; Arshad M. I.; Hussain F.; Hussain A.; Iqbal F. Novel Photocatalyst and Antibacterial Agent; Direct Dual Z-Scheme ZnO–CeO2-Yb2O3 Heterostructured Nanocomposite. Solid State Sci 2020, 109, 106446. 10.1016/j.solidstatesciences.2020.106446. [DOI] [Google Scholar]
- Hasan M.; Altaf M.; Zafar A.; Hassan S. G.; Ali Z.; Mustafa G.; Munawar T.; Saif M. S.; Tariq T.; Iqbal F.; Khan M. W.; Mahmood A.; Mahmood N.; Shu X. Bioinspired Synthesis of Zinc Oxide Nano-Flowers: A Surface Enhanced Antibacterial and Harvesting Efficiency. Mater. Sci. Eng., C 2021, 119, 111280. 10.1016/j.msec.2020.111280. [DOI] [PubMed] [Google Scholar]
- Akbar S.; Haleem K. S.; Tauseef I.; Rehman W.; Ali N.; Hasan M. Raphanus Sativus Mediated Synthesis, Characterization and Biological Evaluation of Zinc Oxide Nanoparticles. Nanosci. Nanotechnol. Lett. 2017, 9, 2005–2012. 10.1166/nnl.2017.2550. [DOI] [Google Scholar]
- Hasan M.; Ullah I.; Zulfiqar H.; Naeem K.; Iqbal A.; Gul H.; Ashfaq M.; Mahmood N. Biological Entities as Chemical Reactors for Synthesis of Nanomaterials: Progress, Challenges and Future Perspective. Mater. Today Chem. 2018, 8, 13–28. 10.1016/j.mtchem.2018.02.003. [DOI] [Google Scholar]
- Castillo-Castillo Y.; Ruiz-Barrera O.; Burrola-Barraza M. E.; Marrero-Rodriguez Y.; Salinas-Chavira J.; Angulo-Montoya C.; Corral-Luna A.; Arzola-Alvarez C.; Itza-Ortiz M.; Camarillo J. Isolation and Characterization of Yeasts from Fermented Apple Bagasse as Additives for Ruminant Feeding. Braz. J. Microbiol. 2016, 47, 889–895. 10.1016/j.bjm.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Li D.; Gao P. Expulsion of Selenium/Protein Nanoparticles through Vesicle-like Structures by Saccharomyces Cerevisiae under Microaerophilic Environment. World J. Microbiol. Biotechnol. 2012, 28, 3381–3386. 10.1007/s11274-012-1150-y. [DOI] [PubMed] [Google Scholar]
- Arif A.; Bhatti A.; John P. Therapeutic Potential of Foeniculum Vulgare Mill. Derived Selenium Nanoparticles in Arthritic Balb/c Mice. Int. J. Nanomed. 2019, 14, 8561–8572. 10.2147/IJN.S226674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N.; Mukhopadhyay M. Biosynthesis and Structural Characterization of Selenium Nanoparticles Mediated by Zooglea Ramigera. Powder Technol 2013, 244, 26–29. 10.1016/j.powtec.2013.03.050. [DOI] [Google Scholar]
- Oremland R. S.; Herbel M. J.; Blum J. S.; Langley S.; Beveridge T. J.; Ajayan P. M.; Sutto T.; Ellis A. V.; Curran S. Structural and Spectral Features of Selenium Nanospheres Produced by Se-Respiring Bacteria. Appl. Microbiol. 2004, 70, 52–60. 10.1128/AEM.70.1.52-60.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M.; Mehmood K.; Mustafa G.; Zafar A.; Tariq T.; Hassan S. G.; Loomba S.; Zia M.; Mazher A.; Mahmood N.; Shu X. Phytotoxic Evaluation of Phytosynthesized Silver Nanoparticles on Lettuce. Coating 2021, 11, 225. 10.3390/coatings11020225. [DOI] [Google Scholar]
- Zhang W. Nanoparticle Aggregation: Principles and Modeling. Adv. Exp. Med. Biol. 2014, 811, 19–43. 10.1007/978-94-017-8739-0_2. [DOI] [PubMed] [Google Scholar]
- Salunke B. K.; Sawant S. S.; Lee S.-I.; Kim B. S. Microorganisms as Efficient Biosystem for the Synthesis of Metal Nanoparticles: Current Scenario and Future Possibilities. World J. Microbiol. Biotechnol. 2016, 32, 88. 10.1007/s11274-016-2044-1. [DOI] [PubMed] [Google Scholar]
- Bai K.; Hong B.; He J.; Hong Z.; Tan R. Preparation and Antioxidant Properties of Selenium Nanoparticles-Loaded Chitosan Microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. 10.2147/IJN.S129958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendaño R.; Chaves N.; Fuentes P.; Sánchez E.; Jiménez J. I.; Chavarría M. Production of Selenium Nanoparticles in Pseudomonas Putida KT2440. Sci. Rep. 2016, 6, 37155. 10.1038/srep37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanjal S.; Cameotra S. Aerobic Biogenesis of Selenium Nanospheres by Bacillus Cereus Isolated from Coalmine Soil. Microb. Cell Fact. 2010, 9, 52. 10.1186/1475-2859-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaie M.; Salari Mohazab N.; Ayatollahi Mousavi S. A. Antifungal Activity of Selenium Nanoparticles Synthesized by Bacillus Species Msh-1 against Aspergillus Fumigatus and Candida Albicans. Jundishapur J. Microbiol. 2015, 8, e26381 10.5812/jjm.26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Shu X.; Hou J.; Lu W.; Zhao W.; Huang S.; Wu L. Selenium Nanoparticle Synthesized by Proteus Mirabilis YC801: An Efficacious Pathway for Selenite Biotransformation and Detoxification. Int. J. Mol. Sci. 2018, 19, 3809. 10.3390/ijms19123809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara H. H.; Guisbiers G.; Mendoza J.; Mimun L. C.; Vincent B.; Lopez-Ribot J. L.; Nash K. L. Synergistic Antifungal Effect of Chitosan-Stabilized Selenium Nanoparticles Synthesized by Pulsed Laser Ablation in Liquids against Candida Albicans Biofilms. Int. J. Nanomed. 2018, 13, 2697–2708. 10.2147/IJN.S151285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdati M.; Tohidi Moghadam T.. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10. 10.1038/s41598-019-57333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov S. V.; Shafeev G. A.; Glinushkin A. P.; Shkirin A. V.; Barmina E. V.; Rakov I. I.; Simakin A. V.; Kislov A. V.; Astashev M. E.; Vodeneev V. A.; Kalinitchenko V. P. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 5, 510. 10.1021/acsomega.0c02448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faramarzi S.; Anzabi Y.; Jafarizadeh-Malmiri H. Nanobiotechnology Approach in Intracellular Selenium Nanoparticle Synthesis Using Saccharomyces Cerevisiae—Fabrication and Characterization. Arch. Microbiol. 2020, 202, 1203–1209. 10.1007/s00203-020-01831-0. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Li Z.; Dai C.; Wang P.; Fan S.; Yu B.; Qu Y. Antibacterial Properties and Mechanism of Selenium Nanoparticles Synthesized by Providencia Sp. DCX. Environ. Res. 2021, 194, 110630. 10.1016/j.envres.2020.110630. [DOI] [PubMed] [Google Scholar]
- Akçay F. A.; Avcı A. Effects of Process Conditions and Yeast Extract on the Synthesis of Selenium Nanoparticles by a Novel Indigenous Isolate Bacillus Sp. EKT1 and Characterization of Nanoparticles. Arch. Microbiol. 2020, 202, 2233–2243. 10.1007/s00203-020-01942-8. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Zhai X.; Zhao G.; Ren F.; Leng X. Synthesis, Characterization, and Controlled Release of Selenium Nanoparticles Stabilized by Chitosan of Different Molecular Weights. Carbohydr. Polym. 2015, 134, 158–166. 10.1016/j.carbpol.2015.07.065. [DOI] [PubMed] [Google Scholar]
- Anu K.; Devanesan S.; Prasanth R.; AlSalhi M. S.; Ajithkumar S.; Singaravelu G. Biogenesis of Selenium Nanoparticles and Their Anti-Leukemia Activity. J. King Saud Univ., Sci. 2020, 32, 2520–2526. 10.1016/j.jksus.2020.04.018. [DOI] [Google Scholar]
- Hasan M.; Iqbal J.; Awan U.; Saeed Y.; Ranran Y.; Liang Y.; Dai R.; Deng Y. Mechanistic Study of Silver Nanoparticle’s Synthesis by Dragon’s Blood Resin Ethanol Extract and Antiradiation Activity. J. Nanosci. Nanotechnol. 2015, 15, 1320–1326. 10.1166/jnn.2015.9090. [DOI] [PubMed] [Google Scholar]
- Song C.; Sun X.-F.; Xing S.-F.; Xia P.-F.; Shi Y.-J.; Wang S.-G. Characterization of the Interactions between Tetracycline Antibiotics and Microbial Extracellular Polymeric Substances with Spectroscopic Approaches. Environ. Sci. Pollut. Res. 2014, 21, 1786–1795. 10.1007/s11356-013-2070-6. [DOI] [PubMed] [Google Scholar]
- Munawar T.; Yasmeen S.; Mukhtar F.; Nadeem M. S.; Mahmood K.; Saqib Saif M.; Hasan M.; Ali A.; Hussain F.; Iqbal F. Zn0.9Ce0.05M0.05O (M = Er, Y, V) Nanocrystals: Structural and Energy Bandgap Engineering of ZnO for Enhancing Photocatalytic and Antibacterial Activity. Ceram. Int. 2020, 46, 14369–14383. 10.1016/j.ceramint.2020.02.232. [DOI] [Google Scholar]
- Dang H.; Meng M. H. W.; Zhao H.; Iqbal J.; Dai R.; Deng Y.; Lv F. Luteolin-Loaded Solid Lipid Nanoparticles Synthesis, Characterization, & Improvement of Bioavailability, Pharmacokinetics in Vitro and Vivo Studies. J. Nanopart. Res. 2014, 16, 2347. 10.1007/s11051-014-2347-9. [DOI] [Google Scholar]
- Song C.; Li X.; Wang S.; Meng Q. Enhanced Conversion and Stability of Biosynthetic Selenium Nanoparticles Using Fetal Bovine Serum. RSC Adv 2016, 6, 103948–03954. 10.1039/c6ra22747c. [DOI] [Google Scholar]
- Korin E.; Froumin N.; Cohen S. Surface Analysis of Nanocomplexes by X-Ray Photoelectron Spectroscopy (XPS). ACS Biomater. Sci. Eng. 2017, 3, 882–889. 10.1021/acsbiomaterials.7b00040. [DOI] [PubMed] [Google Scholar]
- Karmakar A.; Karthick K.; Sankar S. S.; Kumaravel S.; Kundu S. Self-Assembling of Metallic Rh over DNA as Nano-Chains: An Effective Organosol for Catalysis and SERS Studies. Appl. Surf. Sci. 2020, 527, 2411–2502. 10.1016/j.apsusc.2020.146777. [DOI] [Google Scholar]
- Luo F.; Fu Z.; Wang M.; Ke Z.; Wang M.; Wang W.; Hasan M.; Shu X. Growth Performance, Tissue Mineralization, Antioxidant Activity and Immune Response of Oreochromis Niloticus Fed with Conventional and Gluconic Acid Zinc Dietary Supplements. Aquacult. Nutr. 2021, 27, 897–907. 10.1111/anu.13234. [DOI] [Google Scholar]
- Hasan M.; Iqbal J.; Awan U.; Xin N.; Dang H.; Waryani B.; Saeed Y.; Ullah K.; Rongji D.; Deng Y. LX Loaded Nanoliposomes Synthesis, Characterization and Cellular Uptake Studies in H2O2 Stressed SH-SY5Y Cells. J. Nanosci. Nanotechnol. 2014, 14, 4066–4071. 10.1166/jnn.2014.8201. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Guo Y.; Zhang W.; Chai H.; Xing H.; Xing M. Neurotoxicity Induced by Arsenic in Gallus Gallus: Regulation of Oxidative Stress and Heat Shock Protein Response. Chemosphere 2017, 166, 238–245. 10.1016/j.chemosphere.2016.09.060. [DOI] [PubMed] [Google Scholar]
- Li Q.; Chen G.; Chen H.; Zhang W.; Ding Y.; Yu P.; Zhao T.; Mao G.; Feng W.; Yang L.; Wu X. Se-enriched G. frondosa polysaccharide protects against immunosuppression in cyclophosphamide-induced mice via MAPKs signal transduction pathway. Carbohydr. Polym. 2018, 196, 445–456. 10.1016/j.carbpol.2018.05.046. [DOI] [PubMed] [Google Scholar]
- Dawood M. A. O.; Koshio S.; Zaineldin A. I.; Van Doan H.; Ahmed H. A.; Elsabagh M.; Abdel-Daim M. M. An Evaluation of Dietary Selenium Nanoparticles for Red Sea Bream (Pagrus Major) Aquaculture: Growth, Tissue Bioaccumulation, and Antioxidative Responses. Environ. Sci. Pollut. Res. 2019, 26, 30876–30884. 10.1007/s11356-019-06223-6. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Wang Y.; Gu Q.; Li W. Effects of Different Dietary Selenium Sources (Selenium Nanoparticle and Selenomethionine) on Growth Performance, Muscle Composition and Glutathione Peroxidase Enzyme Activity of Crucian Carp (Carassius Auratus Gibelio). Aquaculture 2009, 291, 78–81. 10.1016/j.aquaculture.2009.03.007. [DOI] [Google Scholar]
- Ramya S.; Shanmugasundaram T.; Balagurunathan R. Biomedical Potential of Actinobacterially Synthesized Selenium Nanoparticles with Special Reference to Anti-Biofilm, Anti-Oxidant, Wound Healing, Cytotoxic and Anti-Viral Activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. 10.1016/j.jtemb.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Li H.; Liu D.; Li S.; Xue C. Synthesis and Cytotoxicity of Selenium Nanoparticles Stabilized by α-D-Glucan from Castanea Mollissima Blume. Int. J. Biol. Macromol. 2019, 129, 818–826. 10.1016/j.ijbiomac.2019.02.085. [DOI] [PubMed] [Google Scholar]
- Ranjitha V. R.; Ravishankar V. R.. Extracellular Synthesis of Selenium Nanoparticles from an Actinomycetes Streptomyces Griseoruber and Evaluation of Its Cytotoxicity on HT-29 Cell Line. Pharm. Nanotechnol. 2018, 6. 10.2174/2211738505666171113141010. [DOI] [PubMed] [Google Scholar]
- Kubes P.; Jenne C.. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36. 10.1146/annurev-immunol-051116-052415. [DOI] [PubMed] [Google Scholar]
- Basso P. J.; Andrade-Oliveira V.; Câmara N. O. S.. Targeting Immune Cell Metabolism in Kidney Diseases. Nat. Rev. Nephrol. 2021, 17. 10.1038/s41581-021-00413-7. [DOI] [PubMed] [Google Scholar]
- Yang F.; Liao J.; Yu W.; Pei R.; Qiao N.; Han Q.; Hu L.; Li Y.; Guo J.; Pan J.; Tang Z.. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol. Environ. Saf. 2020, 200. 10.1016/j.ecoenv.2020.110715. [DOI] [PubMed] [Google Scholar]
- Kohshahi A. J.; Sourinejad I.; Sarkheil M.; Johari S. A.. Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2019, 45. 10.1007/s10695-018-0585-y. [DOI] [PubMed] [Google Scholar]
- Staroverov S. A.; Volkov A. A.; Mezhenny P. V.; Domnitsky I. Y.; Fomin A. S.; Kozlov S. V.; Dykman L. A.; Guliy O. I.. Prospects for the Use of Spherical Gold Nanoparticles in Immunization. Appl. Microbiol. Biotechnol. 2019, 103. 10.1007/s00253-018-9476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K.; Hong B.; He J.; Huang W.. Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin a-Induced Liver Injury in Mice. Nutrients 2020, 12. 10.3390/nu12030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk R. F.; Hill K. E.; Awad J. A.; Morrow J. D.; Lyons P. R.. Liver and Kidney Necrosis in Selenium-Deficient Rats Depleted of Glutathione. Lab. Invest. 1995. [PubMed] [Google Scholar]
- Wang H.; Wang M.; Chen J.; Tang Y.; Dou J.; Yu J.; Xi T.; Zhou C.. A Polysaccharide from Strongylocentrotus Nudus Eggs Protects against Myelosuppression and Immunosuppression in Cyclophosphamide-Treated Mice. Int. Immunopharmacol. 2011, 11. 10.1016/j.intimp.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Al-Deriny S. H.; Dawood M. A. O.; Elbialy Z. I.; El-Tras W. F.; Mohamed R. A.. Selenium Nanoparticles and Spirulina Alleviate Growth Performance, Hemato-Biochemical, Immune-Related Genes, and Heat Shock Protein in Nile Tilapia (Oreochromis Niloticus). Biol. Trace Elem. Res. 2020, 198. 10.1007/s12011-020-02096-w. [DOI] [PubMed] [Google Scholar]
- Raahati Z.; Bakhshi B.; Najar-Peerayeh S.. Selenium Nanoparticles Induce Potent Protective Immune Responses against Vibrio Cholerae WC Vaccine in a Mouse Model. J. Immunol. Res. 2020, 2020. 10.1155/2020/8874288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirsat S.; Kadam A.; Mane R. S.; Jadhav V. V.; Zate M. K.; Naushad M.; Kim K. H.. Protective Role of Biogenic Selenium Nanoparticles in Immunological and Oxidative Stress Generated by Enrofloxacin in Broiler Chicken. Dalton Trans 2016, 45. 10.1039/c6dt00120c. [DOI] [PubMed] [Google Scholar]
- Bai K.; Hong B.; Hong Z.; Sun J.; Wang C.. Selenium Nanoparticles-Loaded Chitosan/Citrate Complex and Its Protection against Oxidative Stress in d-Galactose-Induced Aging Mice. J. Nanobiotechnol. 2017, 15. 10.1186/s12951-017-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]