Abstract
Durian peels are an agricultural waste in Asian countries, including Thailand, Indonesia, and Malaysia, which can be used as a precursor for the production of activated carbon. The objective of this work is to produce activated carbon from durian peels by chemical activation using sodium sulfite (Na2SO3) as an activating and sulfur-doping agent. The process parameter investigated in this study was the activation temperature (500–900 °C) at a fixed impregnation ratio (durian to activating agent of 1:1, by weight). Specific surface areas and pore structures were determined by nitrogen adsorption and desorption measurements, and elemental compositions were characterized by CHNSO analysis. The chemical structure and surface functionality were examined by X-ray photoelectron spectroscopy. The electrochemical behavior of the obtained activated carbon was characterized in 6 M KOH using a three-electrode configuration. It was found that the sulfur content decreases with activation temperature. In contrast, the specific surface area of the activated carbon increases with activation temperature. However, the sample activated at 900 °C with the highest specific surface area (1499 m2 g–1) has a lower specific capacitance (166 F g–1) than the one activated at 700 °C (183 F g–1). This could be due to the presence of a pseudocapacitance caused by the organic sulfur functional groups such as thiophene, sulfone, and sulfoxide, which can trigger a surface redox reaction, leading to a higher capacitance.
1. Introduction
There are many materials that can be used as adsorbents in gas adsorption and wastewater treatment, and the most commonly used adsorbents are activated carbons (ACs). ACs are carbonaceous materials that have high porosity, surface area, and surface functional groups. ACs can be widely used not only in gas adsorption and wastewater treatment but also in numerous other applications such as catalyst supports, gas separation and storage, solvent recovery and decolorization, and an electrode for supercapacitors.
ACs can be produced from various precursors, which are generally low-cost biomass. The precursors for the preparation of AC usually contain carbon, such as coals1,2 and coconut shells.3 Their adsorption capacity, wettability, and ion storage capacity can be enhanced by introducing heteroatoms such as nitrogen, oxygen, and sulfur into the carbon skeleton.4−7 Heteroatoms are usually introduced into the carbon framework of ACs by the following approaches: (i) post-treatment of ACs by chemical reactions with reagents containing the desired heteroatoms,8 (ii) molecular grafting of heteroatoms onto the carbon framework,9 and (iii) carbonization and activation of heteroatom-rich carbonaceous materials.10 Chemical impregnation with a chemical agent such as ZnCl2, H3PO4, and KOH can prevent tar formation and enhance carbon conversion. In addition, certain chemical agents can increase certain functional groups in ACs (heteroatom-doped carbon).11−13
There are numerous reports on AC preparation from biomass waste, but the uses of durian peels have rarely been investigated. It is desirable to develop the effective utilization of such biomass as it is cost-effective. Durian peels are one of the most abundant biomasses from agriculture in Thailand, Indonesia, and Malaysia. They naturally contain about 60% carbon but low sulfur content (0.1%).14 Sulfur-containing compounds such as Na2O3S2, Na2SO4, and H2SO4 have been used as activating agents to incorporate sulfur into the carbon frameworks.15−17 However, chemical activation with sodium sulfite (Na2SO3) has never been reported to our knowledge.
In this work, S-doped activated carbon is prepared from durian peels using sodium sulfite (Na2SO3) as an activating agent. The aim of this study is to investigate the effects of activation temperature on the sulfur content of ACs as well as their surface characteristics. Moreover, the electrochemical behavior of the obtained ACs will be investigated. It was found that the sulfur content can decrease the specific surface area of the ACs. Interestingly, the specific capacitance does not depend on the specific surface area of the ACs. Therefore, it is suggested that sulfur would play an important role in the electrochemical capacitance of electrodes made of S-doped activated carbon. Although there are many methods to synthesize sulfur-doped carbon from wasted biomass such one-pot synthesis and post-treatment synthesis and H2SO4 have been used to incorporate sulfur with the carbon framework, it unfortunately requires post-treatment to create the porosity.17,18 In addition, H2SO4 is a strong acid, and the process involved inevitably suffers from corrosion. Instead, salt-containing sulfur, such as Na2SO4, Na2SO3, and Na2O3S2, can be used as a sulfur source where the etching effect would play a role in creating the porosity.16 Beneficially, sulfur-doped carbon prepared with Na2SO3 is easy to handle without any pre- or post-treatment as using Na2O3S215 and K2SO4.19 Although the price of Na2SO3 is somewhat higher than that of H2SO4 (as its usage is still limited to food preservative and antioxidant), taking the aforementioned advantages as well as the abundant amount of raw materials used into consideration, this work could realistically be applied commercially.
2. Results and Discussion
2.1. Elemental Analysis
The elemental compositions of the raw materials (dried durian peels, DP) and the obtained ACs, including carbon, hydrogen, nitrogen, and sulfur, are listed in Table 1. Compared with the sulfur content of the raw material (DP, 0.19% w/w), the sulfur content of all ACs is drastically increased. By increasing the activation temperature, the carbon content of the sample is increased up to the optimum at 800 °C and then slightly decreased. However, the sulfur content decreases exponentially with increasing activation temperature. This can be clearly seen in Figure 1 when considering S/C as a function of activation temperature. These trends are common as a result of many reactions at high temperatures such as dehydrogenation, desulfurization, and condensation.20 This relationship shows that sulfur content could be decomposed at high temperatures as COS, SO2, H2S, and CS2.21,22
Table 1. Carbon, Hydrogen, and Nitrogen Contents of the Raw Materials and the As-Prepared ACs.
| elemental
composition |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| sample | C (%w w–1) | H (%w w–1) | N (%w w–1) | S (%w w–1) | S/C | SBET (m2 g–1) | Vtotal (cm3 g–1) | Vmicro (cm3 g–1) | Vmeso (cm3 g–1) |
| DP | 43.64 | 6.32 | 0.78 | 0.19 | 0.0043 | ||||
| DP900 | 74.67 | 1.62 | 1.44 | 0.04 | 0.0005 | 4.36 | 0.062 | 0.002 | 0.060 |
| AC500 | 51.23 | 2.84 | 0.82 | 17.55 | 0.3426 | 24 | 0.23 | 0.01 | 0.22 |
| AC600 | 59.59 | 2.80 | 1.07 | 11.19 | 0.1878 | 133 | 0.30 | 0.06 | 0.24 |
| AC700 | 71.08 | 2.77 | 0.93 | 2.20 | 0.0309 | 852 | 0.75 | 0.33 | 0.42 |
| AC800 | 79.12 | 2.62 | 0.87 | 1.86 | 0.0235 | 1279 | 1.09 | 0.47 | 0.62 |
| AC900 | 73.56 | 5.78 | 1.35 | 0.65 | 0.0088 | 1499 | 1.19 | 0.56 | 0.63 |
Figure 1.
Relation between the sulfur content (S/C) of the ACs and the activation temperature.
2.2. Porosity Analysis
N2-sorption measurement was employed to characterize the porosity of ACs. Figure 2a reveals that DP900 and AC500 adsorb deficient nitrogen gas, indicating the nonporosity of the material. This result is also confirmed in Table 1, which shows that these two samples only consist of less than 25 m2 g–1SBET. The pore size distribution calculated by the NLDFT method in Figure 2b shows a plateau line for these samples (DP900 and AC500). When the activation temperature is increased, the sorption isotherm transitions to the IV type, indicating the combination of mesopore and micropore structures.23 The hysteresis loops in the range of relative pressure of 0.4–1.0 become more evident by increasing the activation temperature, indicating the abundance of mesopore structures.24 These results are also confirmed by the pore size distribution shown in Figure 2b. As listed in Table 1, AC900 has the highest porosity in terms of SBET, Vtotal, Vmicro, and Vmeso. Decreasing the activation temperature leads to a decrease in the surface area and pore volume of the samples.
Figure 2.
(a) Nitrogen-sorption isotherms at −196 °C and (b) DFT pore size distributions of the obtained ACs.
The relationship between the activation temperature and the pore characteristic of ACs is shown in Figure 3a,b. As the activation temperature increases, the surface area and total pore volume increase linearly. As shown in Figure 3b, the total pore volume is dominated by mesopores at low activation temperature. At higher activation temperature, more micropores are formed so that the micropore volume and mesopore volume are almost equal. In contrast, the surface area decreases exponentially with the S/C value (Figure 3c), suggesting that sulfur in the carbon framework could collapse the structure or enlarge the pores, leading to a decrease in the specific surface area.17,25,26 This explanation is also supported by the fact that the sample activated at 500 °C (the highest S/C value) is dominated by mesopores, as shown in Figure 3d. Moreover, volatile sulfur could be evaporated at high activation temperatures, resulting in a more ordered and condensed carbon structure, leading to the formation of micropores.27
Figure 3.
(a) Dependence of the specific surface area on the activation temperature, (b) effect of activation temperature on the formation of pore size, and (c, d) the relations of S/C to the specific surface area and pore volume, respectively.
2.3. Surface Characteristics
The surface functionality of the obtained ACs was investigated by FTIR spectroscopy (Figure S1). The spectra show several vibrations including C=C (aromatic, 1580–1615 cm–1), SO2 symmetric stretching (1120–1190 cm–1), S=O stretching (1020–1060 cm–1), C–S stretching (600–700 cm–1), and S–S stretching (450–550 cm–1).28,29 Further investigation on the surface functionality has been done by XPS, and their S 2p spectra are shown in Figure 4. Based on the deconvolution using the Gaussian equation, each AC consists of sulfur functional groups in the form of mercaptan (C–SH3, 161.2–163.6 eV), thiophene (C–S–C, 164.0–164.4 eV), sulfoxide (C–SO–C, 165.0–166.0 eV), sulfone (C–SO2–C, 167.0–168.3 eV), and inorganic sulfur (168.4–175.0 eV).30−32 The atomic percentage estimated from the peak area after the deconvolution of each sulfur functional group is listed in Table 2. With the exception of DP900, all samples contain a small amount of mercaptan (less than 3.5%), while the content of thiophene, sulfoxide, and sulfone varies from 10 to 35%.
Figure 4.
S 2p spectra of (a) DP900, (b) AC500, (c) AC600, (d) AC700, (e) AC800, and (f) AC900 samples.
Table 2. Atomic Percentages of Each Sulfur Functionality Estimated from XPS Analysis.
| atomic
percentage (%) |
|||||
|---|---|---|---|---|---|
| sample | mercaptan | thiophene | sulfoxide | sulfone | inorganic sulfur/sulfate |
| DP900 | 10.49 | 15.29 | 15.25 | 31.50 | 27.47 |
| AC500 | 1.71 | 31.82 | 14.23 | 34.65 | 17.59 |
| AC600 | 2.11 | 24.91 | 17.02 | 27.54 | 28.42 |
| AC700 | 2.87 | 19.63 | 19.06 | 10.32 | 48.12 |
| AC800 | 2.34 | 19.12 | 17.54 | 19.94 | 41.06 |
| AC900 | 3.50 | 21.37 | 11.33 | 32.75 | 31.05 |
To understand the change of the surface functional group during activation, the relationship between the atomic percentage of each sulfur functional group and the activation temperature is established, as shown in Figure 5. However, the chemical transformations of the sulfur surface functional group during pyrolysis are still a complex issue. Mercaptan, which combines aliphatic C with S, has a lower content at each activation temperature. This is the cleavage of S–C at low temperatures to form S radicals (·S·), which further react with carbon sources to form other sulfur functional groups.21 If the activation temperature is increased up to 700 °C, the thiophene content decreases to a certain extent and then remains constant. This could be due to the presence of unstable thiol groups, which transform into a stable thiophene structure at high temperatures.33,34 The sulfone content decreases to the minimum at an activation temperature of 700 °C, which is due to the thermal reduction of the sulfone to a sulfoxide group, so that the sulfoxide content increases up to 700 °C. Another possible explanation for the unpredictable thermal decomposition of the sulfone is due to the chemical neighborhood of the sulfone. Sulfone with aliphatic carbon can decompose at low temperatures, while sulfone with cyclic carbon can decompose at the temperatures up to 500 °C.35 In addition, thiophene and oxygen-containing sulfur functional groups (sulfone and sulfoxide) can decompose as H2S, SO2, or COS, which could further reduce the sulfur content.21,36
Figure 5.

Correlation of each sulfur functional group to the activation temperature.
2.4. Electrochemical Capacitive Behaviors
The electrochemical behavior of the obtained ACs was investigated using cyclic voltammetry (CV, a sweep rate of 1 mV s–1) and galvanostatic charge–discharge measurement (GCD, a current density of 1 A g–1). As shown in Figure 6a,b, the voltammograms of the ACs (except AC500) have a quasi-rectangular shape and GCD straight discharge lines typical of the EDLC mechanism. However, AC, which was activated at 500 °C, shows low CV and GCD performances due to its low electrical conductivity, consistent with a large IR drop in the initial discharge curve. Cyclic voltammograms at various scan rates, galvanostatic charge/discharge curves at various current densities, and specific capacitance as a function of current density of the obtained ACs can be found in the Supporting Information (Figures S2–S4).
Figure 6.
(a) Cyclic voltammograms and (b) discharge curves of the obtained ACs. The scan rate and current density were 5 mV s–1 and 1 A g–1, respectively, using a three-electrode configuration in 6 M KOH.
According to the results of CV and GCD, the specific capacitance of ACs was estimated as listed in Table 3. AC700 exhibits the highest specific capacitance of 183 F g–1, which is comparable to those in the literature using the same electrolyte (Table S1),15,17,33,37 and decreases slightly at higher activation temperature. Considering in terms of specific energy and power (Figure S5), the obtained S-doped ACs still show compatible performances to those reported in the literature.15,17,37 Moreover, the obtained ACs exhibit excellent capacitance retention after 1000 cycles of the cyclability test (Figure S6). To understand the effects of surface functional groups on the charge storage capacity, the normalized capacitance is considered by dividing the specific capacitance by the specific surface area. As can be seen in Table 3, AC500 drives an excellent normalized capacitance (1.65 F m–2), which is higher than the others. This could be due to the highest sulfur content of this sample with the lowest specific surface area. However, as mentioned earlier, this sample has low conductivity, which could hinder the ion transport of the electrode. Moreover, the normalized capacitance decreases at high activation temperature when the sulfur content decreases as aforementioned (Figure 7a). This clearly indicates the role of sulfur in altering the surface properties and charge storage capability of ACs.
Table 3. Specific Capacitance and Normalized Capacitance of the Obtained ACs.
| sample | specific capacitance (F g–1) | normalized capacitance (F m–2) |
|---|---|---|
| AC500 | 41 | 1.65 |
| AC600 | 118 | 0.89 |
| AC700 | 183 | 0.21 |
| AC800 | 170 | 0.13 |
| AC900 | 166 | 0.11 |
Figure 7.
Relations of (a) normalized capacitance versus S/C and (b–f) normalized capacitance versus atomic percentage of each sulfur functional group.
The relationship between normalized capacitance and the atomic percentage of each sulfur functional group is shown in Figure 7b–f. Although it can be seen in Figure 7b that mercaptan-sulfur has a negative effect on the normalized capacitance, its content is 10 times lower than those of the other functional groups (see Figure 5). Therefore, these effects could be reasonably negligible in this work.
It can be seen that thiophene (Figure 7c) and inorganic sulfur (or sulfate, Figure 7f) could play a significant role in normalized capacitance, while sulfoxide (Figure 7d) and sulfone (Figure 7e) do not seem to have a clear tendency. As shown in Figure 7c, thiophene sulfur has a positive impact on normalized capacitance. Although sulfone and sulfoxide species are expected to play a positive role in the overall capacitance by undergoing faradic redox reactions,38 it has been reported that thiophenic-like sulfur can also contribute to the pseudocapacitance.33,39 Moreover, sulfur can induce structural defects in the carbon framework due to its relatively large covalent radius, resulting in more active sites for charge localization.40 In addition, sulfur doping could alter the electronic density of the carbonaceous material, and sulfur possesses a lone pair, leading to an increase in the reactivity of ACs.15,41 This further supports and highlights the use of S-doped AC as an electrode in electrochemical energy storage devices.
3. Conclusions
In conclusion, S-doped activated carbon was successfully synthesized from durian peels by chemical activation with a mild activating agent, Na2SO3. The results indicate that the sulfur content plays an important role in the specific surface area and the formation of the microporous structure of ACs. AC700 exhibits the highest capacitance of 183 F g–1 at 5 mV s–1. Nevertheless, AC500 with the highest sulfur content can store a large amount of charge even at a low SBET. It is suggested that the presence of thiophene species could contribute to increase the charge storage capacity of S-doped AC.
4. Materials and Methods
4.1. Preparation of Activated Carbons
Durian peels (DP) were obtained from a local market (Talaad Thai, Pathum Thani province, Thailand). It was washed and dried at 105 °C for 24 h. It was then ground into powder form and impregnated with the activating agent, Na2SO3 (weight ratio 1:1). After that, the impregnated sample was placed in an alumina boat and inserted into a horizontal tube furnace. The furnace was heated to 500–900 °C (5 °C min–1) under a nitrogen atmosphere and held for 1 h. After the carbonization/activation, it was cooled to room temperature under a N2 flow. The AC products were washed with RO water and dried at 110 °C for 24 h. The samples were labeled as AC500, AC600, AC700, AC800, and AC900, depending on the activation temperature (500, 600, 700, 800, and 900 °C, respectively). For comparison, the sample that was simply carbonized at 900 °C for 1 h (without Na2SO3) was labeled as DP900.
4.2. Characterization
The chemical composition of the dried durian peels and the obtained ACs was analyzed by elemental analysis (CHNSO analyzer, model: 628 series, Leco Corporation, USA). The specific surface area (SBET) was calculated from the N2 adsorption isotherms at 77 K (−196 °C) using the Brunauer–Emmett–Teller (BET) equation. The total pore volume (Vtotal) was estimated from the N2 adsorption amount at a relative pressure of 0.95. The micropore volume (Vmicro) was calculated using the Dubinin–Radushkevich (DR) method.42 The mesopore volume (Vmeso) was determined by subtracting the micropore volume from the total pore volume. The pore size distribution was characterized by density functional theory (DFT). The chemical structure and surface functionality of the obtained ACs were examined by X-ray photoelectron spectroscopy (XPS) using Mg Kα radiation (12 kV and 25 mA). The obtained ACs were also characterized by Fourier transform infrared spectroscopy (FTIR, Nicole iS50, Thermo Fisher Scientific, 2.5 wt % in KBr). The number of scans and resolution were 16 and 4, respectively.
Electrochemical characterizations were performed using a three-electrode configuration. To prepare the electrodes, the obtained ACs were mixed with carbon black and polytetrafluoroethylene (PTFE) in a weight ratio of 9:0.5:0.5 to form a uniform solid sheet. The sheet was then cut into a square shape and pressed into a stainless-steel mesh. After that, it was impregnated (overnight) with the electrolyte before the measurements. Cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) were performed to observe the electrochemical behavior of the AC electrodes. The electrolyte, reference, and counter electrodes are 6 M KOH, Ag/AgCl in 3 M KCl, and a platinum rod, respectively. The specific capacitances (F g–1) were calculated from the integrated voltammogram using the following equation:
| 1 |
where ∫E1E1i(E)dE is the total charge resulting from the integration of the positive and negative sweep of the cyclic voltammogram (C), i(E) is the instantaneous current (A), E1 and E2 are the cut-off potentials (V), m is the mass of active material in the samples (g), and v is the scan rate (V s–1). In addition, charge/discharge measurements were performed on the obtained ACs, and the resulting specific capacitance was calculated using the following equation:
| 2 |
where C is
the specific capacitance (F g–1), I is the current density, and 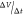 is the
slope of the charge/discharge plot
(V s–1). The energy density (E,
Wh kg–1) and power density (P,
W kg–1) (on an active mass) were calculated according
to the following equations:
is the
slope of the charge/discharge plot
(V s–1). The energy density (E,
Wh kg–1) and power density (P,
W kg–1) (on an active mass) were calculated according
to the following equations:
| 3 |
| 4 |
where V is the cell voltage after ohmic drop (V) and t is the discharge time (h).
Acknowledgments
This work was supported by Thammasat University Research Fund, Contact No. TUFT 053/2563 and the thesis support from the Scholarship for Excellent Foreign Student (EFS), Sirindhorn International Institute of Technology (SIIT), Thammasat University (TU). This work was also funded by National Research Council of Thailand (NRCT): N42A640324 and has been partially supported by the National Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand, through its program of Research Network of NANOTEC (RNN).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03760.
FTIR spectra of the obtained ACs, cyclic voltammograms of the obtained ACs at various scan rates in 6 M KOH, galvanostatic charge/discharge curves of the obtained ACs at various current densities in 6 M KOH, specific capacitance of the obtained ACs as a function of current density in 6 M KOH, comparison of several S-doped activated carbon in 6 M KOH electrolyte, Ragone plots of the obtained ACs, and cyclability test over 1000 cycles at 5 A g–1 in 6 M KOH (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Teng H.; Yeh T.-S. Preparation of Activated Carbons from Bituminous Coals with Zinc Chloride Activation. Ind. Eng. Chem. Res. 1998, 37, 58–65. 10.1021/ie970534h. [DOI] [Google Scholar]
- Lozano-Castelló D.; Lillo-Ródenas M. A.; Cazorla-Amorós D.; Linares-Solano A. Preparation of activated carbons from Spanish anthracite I Activation by KOH. Carbon 2001, 39, 741–749. 10.1016/S0008-6223(00)00185-8. [DOI] [Google Scholar]
- Daud W. M.; Ali W. S. Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresour. Technol. 2004, 93, 63–69. 10.1016/j.biortech.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Mondal A. K.; Kretschmer K.; Zhao Y.; Liu H.; Fan H.; Wang G. Naturally nitrogen doped porous carbon derived from waste shrimp shells for high-performance lithium ion batteries and supercapacitors. Microporous Mesoporous Mater. 2017, 246, 72–80. 10.1016/j.micromeso.2017.03.019. [DOI] [Google Scholar]
- Zhang D.; Han M.; Wang B.; Li Y.; Lei L.; Wang K.; Wang Y.; Zhang L.; Feng H. Superior supercapacitors based on nitrogen and sulfur co-doped hierarchical porous carbon: Excellent rate capability and cycle stability. J. Power Sources 2017, 358, 112–120. 10.1016/j.jpowsour.2017.05.031. [DOI] [Google Scholar]
- Chen F.; Yang J.; Bai T.; Long B.; Zhou X. Biomass waste-derived honeycomb-like nitrogen and oxygen dual-doped porous carbon for high performance lithium-sulfur batteries. Electrochim. Acta 2016, 192, 99–109. 10.1016/j.electacta.2016.01.192. [DOI] [Google Scholar]
- Treeweranuwat P.; Boonyoung P.; Chareonpanich M.; Nueangnoraj K. Role of Nitrogen on the Porosity, Surface, and Electrochemical Characteristics of Activated Carbon. ACS Omega 2020, 5, 1911–1918. 10.1021/acsomega.9b03586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce Y.; Aktas Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. 10.1016/j.apsusc.2014.05.214. [DOI] [Google Scholar]
- Menanteau T.; Benoît C.; Breton T.; Cougnon C. Enhancing the performance of a diazonium-modified carbon supercapacitor by controlling the grafting process. Electrochem. Commun. 2016, 63, 70–73. 10.1016/j.elecom.2015.12.014. [DOI] [Google Scholar]
- Gao F.; Qu J.; Zhao Z.; Wang Z.; Qiu J. Nitrogen-doped activated carbon derived from prawn shells for high-performance supercapacitors. Electrochim. Acta 2016, 190, 1134–1141. 10.1016/j.electacta.2016.01.005. [DOI] [Google Scholar]
- Sriprom P.; Krusong W.; Assawasaengrat P. Preparation of Activated Carbon from Durian Rind with Difference Activations and Its Optimization. J. Renewable Mater. 2021, 9, 311–324. 10.32604/jrm.2021.012560. [DOI] [Google Scholar]
- Sevilla M.; Carro-Rodríguez J.; Díez N.; Fuertes A. B. Straightforward synthesis of Sulfur/N,S-codoped carbon cathodes for Lithium-Sulfur batteries. Sci. Rep. 2020, 10, 4866. 10.1038/s41598-020-61583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrellas S. Á.; García Lovera R.; Escalona N.; Sepúlveda C.; Sotelo J. L.; García J. Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem. Eng. J. 2015, 279, 788–798. 10.1016/j.cej.2015.05.104. [DOI] [Google Scholar]
- Chandra T. C.; Mirna M. M.; Sunarso J.; Sudaryanto Y.; Ismadji S. Activated carbon from durian shell: Preparation and characterization. J. Taiwan Inst. Chem. Eng. 2009, 40, 457–462. 10.1016/j.jtice.2008.10.002. [DOI] [Google Scholar]
- Yaglikci S.; Gokce Y.; Yagmur E.; Aktas Z. The performance of sulphur doped activated carbon supercapacitors prepared from waste tea. Environ. Technol. 2020, 41, 36–48. 10.1080/09593330.2019.1575480. [DOI] [PubMed] [Google Scholar]
- Roberts A. D.; Li X.; Zhang H. Hierarchically porous sulfur-containing activated carbon monoliths via ice-templating and one-step pyrolysis. Carbon 2015, 95, 268–278. 10.1016/j.carbon.2015.08.004. [DOI] [Google Scholar]
- Hao E.; Liu W.; Liu S.; Zhang Y.; Wang H.; Chen S.; Cheng F.; Zhao S.; Yang H. Rich sulfur doped porous carbon materials derived from ginkgo leaves for multiple electrochemical energy storage devices. J. Mater. Chem. A 2017, 5, 2204–2214. 10.1039/C6TA08169J. [DOI] [Google Scholar]
- Chen H.; Yu F.; Wang G.; Chen L.; Dai B.; Peng S. Nitrogen and Sulfur Self-Doped Activated Carbon Directly Derived from Elm Flower for High-Performance Supercapacitors. ACS Omega 2018, 3, 4724–4732. 10.1021/acsomega.8b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Antonietti M. Moderating black powder chemistry for the synthesis of doped and highly porous graphene nanoplatelets and their use in electrocatalysis. Adv. Mater. 2013, 25, 6284–6290. 10.1002/adma.201302034. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Xu H.; Zhang T.; Nan X.; Ma F. Effect of pyrolysis temperature on sulfur content, extractable fraction and release of sulfate in corn straw biochar. RSC Adv. 2018, 8, 35611–35617. 10.1039/C8RA06382F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Yang J.; Li Y.; Liu Z. Behavior of organic sulfur model compounds in pyrolysis under coal-like environment. Fuel Process. Technol. 2004, 85, 1013–1024. 10.1016/j.fuproc.2003.11.036. [DOI] [Google Scholar]
- Song Z.; Wang M.; Batts B. D.; Xiao X. Hydrous pyrolysis transformation of organic sulfur compounds: Part 1. Reactivity and chemical changes. Org. Geochem. 2005, 36, 1523–1532. 10.1016/j.orggeochem.2005.07.002. [DOI] [Google Scholar]
- Feng W.; He P.; Ding S.; Zhang G.; He M.; Dong F.; Wen J.; Du L.; Liu M. Oxygen-doped activated carbons derived from three kinds of biomass: preparation, characterization and performance as electrode materials for supercapacitors. RSC Adv. 2016, 6, 5949–5956. 10.1039/C5RA24613J. [DOI] [Google Scholar]
- Ariyanto T.; Kurniasari M.; Laksmana W. T.; Prasetyo I. Pore size control of polymer-derived carbon adsorbent and its application for dye removal. Int. J. Environ. Sci. Technol. 2019, 16, 4631–4636. 10.1007/s13762-018-2166-0. [DOI] [Google Scholar]
- Zhao J.; Guan B.; Ma C.; Hu B.; Zhang H. Effect of elemental sulfur in precursors on the pore structure and surface chemical characteristics of high-surface area activated carbon. J. Saudi Chem. Soc. 2017, 21, 691–697. 10.1016/j.jscs.2017.03.001. [DOI] [Google Scholar]
- Fang K.; Sheng J.; Yang R. Synthesis of Highly Microporous Sulfur-Containing Activated Carbons by a Multistep Modification Process. J. Wuhan Univ. Technol., Mater. Sci. Ed. 2020, 35, 856–862. 10.1007/s11595-020-2330-5. [DOI] [Google Scholar]
- Liu S.; Cai Y.; Zhao X.; Liang Y.; Zheng M.; Hu H.; Dong H.; Jiang S.; Liu Y.; Xiao Y. Sulfur-doped nanoporous carbon spheres with ultrahigh specific surface area and high electrochemical activity for supercapacitor. J. Power Sources 2017, 360, 373–382. 10.1016/j.jpowsour.2017.06.029. [DOI] [Google Scholar]
- White J. L. Interpretation of Infrared Spectra of Soil Minerals. Soil Sci. 1971, 112, 22–31. 10.1097/00010694-197107000-00005. [DOI] [Google Scholar]
- Guo Y.; Zeng Z.; Liu Y.; Huang Z.; Cui Y.; Yang J. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics. J. Mater. Chem. A 2018, 6, 4055–4067. 10.1039/C7TA09814F. [DOI] [Google Scholar]
- Shan J.; Huang J.-J.; Li J.-Z.; Li G.; Zhao J.-T.; Fang Y.-T. Insight into transformation of sulfur species during KOH activation of high sulfur petroleum coke. Fuel 2018, 215, 258–265. 10.1016/j.fuel.2017.09.117. [DOI] [Google Scholar]
- Li W.; Zhou M.; Li H.; Wang K.; Cheng S.; Jiang K. A high performance sulfur-doped disordered carbon anode for sodium ion batteries. Energy Environ. Sci. 2015, 8, 2916–2921. 10.1039/C5EE01985K. [DOI] [Google Scholar]
- Qie L.; Chen W.; Xiong X.; Hu C.; Zou F.; Hu P.; Huang Y. Sulfur-Doped Carbon with Enlarged Interlayer Distance as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2015, 2, 1500195. 10.1002/advs.201500195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Zhang Q.; Chen C.-M.; Zhang B.; Reiche S.; Wang A.; Zhang T.; Schlögl R.; Sheng Su D. Aromatic sulfide, sulfoxide, and sulfone mediated mesoporous carbon monolith for use in supercapacitor. Nano Energy 2012, 1, 624–630. 10.1016/j.nanoen.2012.04.003. [DOI] [Google Scholar]
- Telfer M.; Zhang D.-K. The influence of water-soluble and acid-soluble inorganic matter on sulphur transformations during pyrolysis of low-rank coals. Fuel 2001, 80, 2085–2098. 10.1016/S0016-2361(01)00078-3. [DOI] [Google Scholar]
- Weh R.; de Klerk A. Thermochemistry of Sulfones Relevant to Oxidative Desulfurization. Energy Fuels 2017, 31, 6607–6614. 10.1021/acs.energyfuels.7b00585. [DOI] [Google Scholar]
- Guo Z.; Fu Z.; Wang S. Sulfur distribution in coke and sulfur removal during pyrolysis. Fuel Process. Technol. 2007, 88, 935–941. 10.1016/j.fuproc.2007.05.003. [DOI] [Google Scholar]
- Elmouwahidi A.; Castelo-Quibén J.; Vivo-Vilches J. F.; Pérez-Cadenas A. F.; Maldonado-Hódar F. J.; Carrasco-Marín F. Activated carbons from agricultural waste solvothermally doped with sulphur as electrodes for supercapacitors. Chem. Eng. J. 2018, 334, 1835–1841. 10.1016/j.cej.2017.11.141. [DOI] [Google Scholar]
- Ji H.; Wang T.; Liu Y.; Lu C.; Yang G.; Ding W.; Hou W. A novel approach for sulfur-doped hierarchically porous carbon with excellent capacitance for electrochemical energy storage. Chem. Commun. 2016, 52, 12725–12728. 10.1039/C6CC05921J. [DOI] [PubMed] [Google Scholar]
- Wang T.; Wang L. X.; Wu D. L.; Xia W.; Jia D. Z. Interaction between nitrogen and sulfur in co-doped graphene and synergetic effect in supercapacitor. Sci. Rep. 2015, 5, 9591. 10.1038/srep09591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.; Zeng Z.; Li Y.; Huang Z.; Yang J. Catalytic oxidation of 4-chlorophenol on in-situ sulfur-doped activated carbon with sulfate radicals. Sep. Purif. Technol. 2017, 179, 257–264. 10.1016/j.seppur.2017.02.006. [DOI] [Google Scholar]
- Zhang J.; Yang Z.; Wang X.; Ren T.; Qiao Q. Homogeneous sulphur-doped composites: porous carbon materials with unique hierarchical porous nanostructure for super-capacitor application. RSC Adv. 2016, 6, 84847–84853. 10.1039/C6RA17231H. [DOI] [Google Scholar]
- Dubinin M. M.Physical Adsorption of Gases and Vapors in Micropores. In Progress in Surface and Membrane Science ;Cadenhead D.A.; Danielli J. F.; Rosenberg M. D., Eds. Academic Press, Inc.: New York, 1975; Vol. 9, pp. 1–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









