Introduction
One of the complications of severe pneumonia due to novel coronavirus disease-2019 (COVID-19) and ARDS is pulmonary fibrosis;1 , 2 it is estimated that it affects around a third of hospitalized patients with Severe Acute Respiratory Syndrome Secondary to Coronavirus -2 (SARS-COV-2).3 A potential role for anti-fibrotic therapies is suggested based on anecdotal evidence and a proposed similarity in the mechanism of post-COVID-19 interstitial lung disease (ILD) with idiopathic pulmonary fibrosis, hence clinical trials are underway, and we await their results.4 , 5 The second complication of COVID-19 infection is usually pulmonary hypertension (PH); the incidence of PH is reported as 15%,6 although a real prevalence is very difficult to assess; Furthermore, the pathophysiology of the development of PH could be multifactorial due to sequelae of ILD (group 3), associated to chronic thromboembolic pulmonary hypertension (group 4), myocardial involvement (group 2) and more recently to direct damage of the vessel wall (group 1).7 , 8
Case Presentation
A 58-year-old woman with significant relevant medical history of controlled systemic hypertension and history of exposure to wood smoke in childhood, with no other important antecedents, attended our ILD-PH clinic at the General Hospital of Mexico with the chief complain of progressively worse dyspnea on exertion 3 months post severe COVID-19 pneumonia.
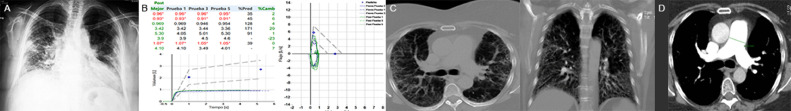
On her initial evaluation, he was on supplemental oxygen use at 4 liters/min, BMI 26.9, central and peripheral cyanosis, generalized decreased breath sounds, diffuse bilateral crackles, voice transmission, and vocal vibrations without alterations. Cardiac auscultation was remarkable for regular rhythm and rate, and fixed split of S2 on the pulmonary foci. Upper extremities with cyanosis, without clubbing. Lower extremities with evidence of chronic venous insufficiency and edema. Arterial blood gases at room air were performed: pH: 7.45, PaCO2: 26 mm Hg, PaO2: 47 mm Hg, SO2 82%, HCO3: 21 mmol/L, Lactate: 2.2 mmol/L. Laboratories with glucose: 149 mg/dL, hemoglobin: 14.9 g/dL, hematocrit: 45.6%, platelets: 315-103/µL, D-Dimer: 2407 µg/L, normal kidney function, hepatic thyroid hormonal profile, immunological profile with anti- Sm, anti-ro, anti-la, anti-Scl-70, anti jo-1, RNP and anti-DNA negative. ANA with a cytoplasmic immunofluorescent pattern with a titer of 1:160. BNP 160 pg/ml. Electrocardiogram in sinus rhythm, rate 92 bpm, heart axis to the right, and data suggestive of RV pressure overload. Comprehensive pulmonary function tests (PFTs)with bronchodilator challenge reports a suggestive pattern of restriction (Fig 1 A-1B).
FIG 1.
(A): Chest radiograph with peripheral confluent ground glass infiltrate, cardiomegaly. (B): Post-bronchodilator spirometry with a severe restrictive functional pattern. (C): CT showing peripheral septal thickening and traction bronchiectasis. (D): CTA of the chest showing dilation >29 mm of the pulmonary artery trunk.
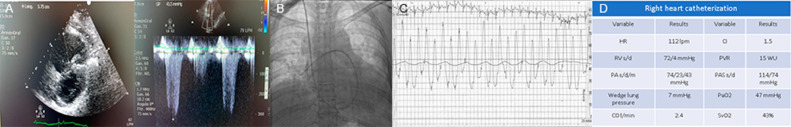
Six-minute walk test resulted in 192 meters walked, interrupted at minute 4 due to dyspnea and fatigue (Borg scale) of 10 out of 10, respectively. Computed tomographic angiogram (CTA) of the chest showed diffuse bilateral reticular infiltrates and traction bronchiectasis with significant pulmonary trunk dilatation (Fig 1C and D). Transthoracic echocardiogram (TTE) showed dilated right ventricle, TAPSE: 17 mm and AF 20%, left ventricle with concentric remodeling, LVEF 55%, tricuspid valve with mild insufficiency and maximum velocity of 3.17 m/s. Dilated vein cava with a diameter of 2.1 cm with an inspiratory collapse of 40% (Fig 2 A).
FIG 2.
(A): Transthoracic echocardiogram (TTE) showed dilated right ventricle, TAPSE: 17 mm and AF 20%, left ventricle with concentric remodeling, LVEF 55%, tricuspid valve with mild insufficiency and maximum velocity of 3.17 m/s. Dilated vein cava with a diameter of 2.1 cm with an inspiratory collapse of 40%. (B): 7-Fr Swan-Ganz flotation catheter within the main right pulmonary artery. (C) and (D): Right heart catheterization performed at rest with detailed cardiopulmonary hemodynamics, demonstrating remarkable severe pulmonary arterial (precapillary) hypertension, decreased cardiac index and a significant increase in pulmonary vascular resistance.
Given her initial remarkable non-invasive work up for PH, it was decided to perform a right heart catheterization (RHC) at rest with a 7 Fr flotation catheter through the right internal jugular vein demonstrated, to have objective diagnosis and documentation of her possible pulmonary arterial hypertension (PAH). Detailed accurate cardiopulmonary hemodynamics, which were remarkable for severe PAH (precapillary), decreased cardiac index (CI) and a significant increase in pulmonary vascular resistance. Acute Vasodilator challenge test was not performed (Fig 2B and D).
Discussion
According to data referring to previous epidemics, 70-80% of patients who survived coronavirus infections such as SARS and MERS, presented respiratory complications such as chronic cough, bronchiectasis, ILD, pulmonary vascular disease, as well as mild alterations in the diffusion of carbon monoxide.9 Likewise, the association with PH has been demonstrated in several studies of critically ill patients. Associated derangements in pulmonary vascular hemodynamics during ARDS caused by COVID-19 are multifactorial and depend on hypoxia, pulmonary vascular arteriolar remodeling, or compression due to edema or fibrosis, increased alveolar pressure, vasoconstriction, pulmonary vascular in situ thrombosis or acute pulmonary embolism (PE), reduction of lung compliance and use of PEEP when the subject is undergoing mechanical ventilation.10 The observed prevalence of PH and RHF among non-ICU and ICU hospitalized patients with COVID-19 was 12.0% and 14.5%, respectively.11
Unlike MERS and SARS, moderate and severe SARS-COV-2 pneumonia presents a high frequency of venous thromboembolism events, thrombosis in situ, and a state of hypercoagulability for a prolonged time that, added to immobilization and the severity of lung damage, are additional risk factors for venous thromboembolism, for which reason during follow-up there should be a low-threshold to further investigate and to perform work up for long-term thromboembolic complications like chronic thromboembolic pulmonary hypertension, especially patients in which they had objective evidence of pulmonary vascular in situ thrombosis in the acute setting of COVID-19.
The hypothesis that SARS-CoV-2 has mechanisms that promote the pathogenesis of PAH was recently published and that some individuals infected with this virus become susceptible to developing clinically significant PAH in the future.12 The hypothesis is based on histological findings of thickened pulmonary vascular walls in cadavers of patients who died of COVID-19; therefore, it is logical to assume that at least a subset of patients infected with SARS-CoV-2 may be predisposed to developing severe PAH in the future.13
Likewise, ILD will soon be an emerging complication; the mechanism of post-viral pulmonary fibrosis has been extensively studied in other virus-related epidemics such as influenza and SARS, where serum elevations of GFR-B1, a cytokine that is associated with deposition of extracellular matrix proteins, have been observed, stimulation of chemotactic migration of fibroblasts and transition from fibroblasts to myofibroblasts. The prevalence of post-COVID-19 fibrosis will become apparent over time, but early analysis of these patients at hospital discharge suggests that more than a third of recovered patients will develop fibrotic abnormalities.14
To date, the literature on post-COVID-19 PH is scarce. Salcin et al. described a clinical case of COVID-19 with great affection to the lung parenchyma documented PH six weeks post-discharge by RHC. However, in their description of the above case, investigators did not report detailed numbers of cardiopulmonary hemodynamics performed during RHC; having a significant limitation in regards appropriate World Health Organization (WHO) categorization criteria in their patient.15
According to the WHO, PH is classified into five groups.16 In this patient, an extensive evaluation was carried out to establish the etiology of PH, which was finally corroborated during RHC, which is currently considered the ‘gold standard’ for the definitive diagnosis of PH. Immunological origins are reasonably ruled out since the basic profile reports normal values of all the antibodies requested. Group 2 as a cause of PH is ruled out since TTE reported a normal left ventricular ejection fraction, no significant valvular abnormalities and during RHC the mean pulmonary artery wedge pressure was less than 15 mm Hg. Regarding group 4 as a cause of PH, CTA of the chest ruled-out significant proximal and distal thrombotic obstructive lesions; however, the microvascular lesion may have played a role in the development of their PH. The findings of extensive parenchymal lung damage in the form of ILD in the chest CTA-chest plus the restrictive functional pattern observed in the PFTs make it possible that the PH documented in the patient's RHC corresponds to WHO Group 3; however, its severe component (mean PAP >35 mm Hg) is not common, therefore a component of pulmonary arteriolar microvasculature affection may play in important role in the development of severe PAH.
Our patient was discharged with home oxygen, aggressive respiratory physiotherapy, as well with PAH specific vasodilator therapies with sildenafil (a specific inhibitor of type-5 phosphodiesterase), given the significant and disproportionate severe PAH in comparison with the degree of pulmonary restriction shown in PFTs. Some research experts in PAH world have described potential clinical and hemodynamic benefits with the use of specific pulmonary vasodilators, particularly in the setting of ILD-associated PH, patients who received inhaled Treprostinil (a prostacyclin analog) had a significant improvement in the 6-minute walk test and functional capacity.17 It is important to emphasize that large-scale randomized clinical trials, as well as prospective multinational registries, are currently being designed and carried out.18 , 19 On the other hand, pirfenidone was started as a promising anti-fibrotic drug for the management of his diffuse ILD, and the patient will be reassessed in our PH-ILD clinic in the following 8-12 weeks.
Conclusion and Future Perspectives
Pulmonary vascular disorders complicating post-acute COVID-19 syndrome clearly represents a global threat for survivors, which may involve multifactorial physiological components of PH and PAH (eg, a mix of WHO Group 1, 2, 3 or 4 PH). We strongly believe that a multidisciplinary approach with cardiologists, pulmonologists, dedicated pulmonary vascular disease experts, internists with expertise in vascular medicine and thrombosis, and cardiopulmonary physiotherapists may optimize the care of such complex patients. More-over, such integrated care will be of paramount importance for early detection, of long-term complications like multifactorial PH and PAH. A better understanding of implicating physio pathological mechanisms, predisposing risk factors and long-term evolution of severe post-COVID-19 syndrome will trigger the implementation of effective and efficacious therapies, with the aim of improving quality of life and survival for these patients, which they are at significant morbidity and mortality down the road.
Author Contributions
Every single coauthor designed, performed research, helped in writing, crafting, editing, and critically reviewing key elements of the manuscript. All coauthors read and approved the final version of the manuscript.
Footnotes
Disclosures: Written and informed consent was obtained from the patient and available for review if needed. The authors of this case report vouch for accuracy and integrity of the data provided. There is no conflict of interests.
References
- 1.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course, and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single center, study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tale S, Ghosh S, Meitei SP, Kolli M, Garbhapu AK, Pudi S. Post COVID -19 pneumonia pulmonary fibrosis. QJM. 2020;113:837–838. doi: 10.1093/qjmed/hcaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasarmidi E, Tsitoura E, Spandidos DA, Tzanakis N, Antoniou KM. Pulmonary fibrosis in the aftermath of the COVID-19 era (Review) Exp Ther Med. 2020;20:2557–2560. doi: 10.3892/etm.2020.8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhary S, Natt B, Bime C, Kenneth S, Knox KS, Glassberg MK. Antifibrotics in COVID-19 lung disease: Let Us Stay Focused. Front Med (Lausana) 2020;9:539. doi: 10.3389/fmed.2020.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalized patients with COVID-19. MNJ J. Heart. 2020;106:1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tudoran C, Tudoran M, Lazureanu VE, et al. Evidence of Pulmonary hypertension after SARS-CoV-2 infection in subjects without previous significant cardiovascular pathology. J Clin Med. 2021;10:199. doi: 10.3390/jcm10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 10.Alcaianu G, Calcaianu M, Gschwend A, et al. Hemodynamic profile of pulmonary hypertension (PH) in ARDS. Pulm Circ. 2018;8 doi: 10.1177/2045893217753415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.- Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106:1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki Yuichiro J., Nikolaienko Sofia I., Shults Nataliia V., Gychka Sergiy G. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med Hypotheses. 2021;147 doi: 10.1016/j.mehy.2021.110483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki YJ, Nikolaienko SI, Dibrova VA, et al. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vasc Pharmacol. 2021;137 doi: 10.1016/j.vph.2020.106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar-Rai D, Sharma P, Kumar R. Post covid 19 pulmonary fibrosis- Is it reversible? Int J Legal Med. 2020;134:2209–2214. [Google Scholar]
- 15.Salcin S, Fontem F. Recurrent SARS-CoV-2 infection resulting in acute respiratory distress syndrome and development of pulmonary hypertension: A case report. Respir Med Case Rep. 2021;33 doi: 10.1016/j.rmcr.2020.101314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53 doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384:325–334. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 18.Harari S, Elia D, Humbert M. Pulmonary hypertension in parenchymal lung diseases: any future for new therapies? Chest. 2018;153:217–223. doi: 10.1016/j.chest.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Cascino TM, Desai AA, Kanthi Y. At a crossroads: coronavirus disease 2019 recovery and the risk of pulmonary vascular disease. Curr Opin Pulm Med. 2021 doi: 10.1097/MCP.0000000000000792. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]