Abstract
Objective
To assess the seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Oman and longitudinal changes in antibody levels over time within the first 11 months of the coronavirus disease 2019 (COVID-19) pandemic.
Methods
This nationwide cross-sectional study was conducted as a four-cycle serosurvey using a multi-stage stratified sampling method from July to November 2020. A questionnaire was used and included demographics, history of acute respiratory infection and list of symptoms, COVID-19 contact, previous diagnosis or admission, travel history and risk factors.
Results
In total, 17,457 participants were surveyed. Thirty percent were female and 66.3% were Omani. There was a significant increase in seroprevalence throughout the study cycles, from 5.5% (4.8–6.2%) in Cycle 1 to 22% (19.6–24.6%) in Cycle 4. There was no difference in seroprevalence between genders, but significant differences were found between age groups. There was a transition of seroprevalence from being higher in non-Omanis than Omanis in Cycle 1 [9.1% (7.6–10.9%) vs 3.2% (2.6–3.9%)] to being higher in Omanis than non-Omanis in Cycle 4 [24.3% (21.0–27.9%) vs 16.8% (14.9–18.9%)]. There was remarkable variation in the seroprevalence of SARS-CoV-2 according to governorate. Close contacts of people with COVID-19 had a 96% higher risk of having the disease [adjusted odds ratio (AOR) 1.96, 95% confidence intervals (CI) 1.64–2.34]. Labourers had 58% higher risk of infection compared with office workers (AOR 1.58, 95% CI 1.04–2.35).
Conclusion
This study showed a wide variation in the spread of SARS-CoV-2 across governorates in Oman, with higher estimated seroprevalence in migrants in the first two cycles. Prevalence estimates remain low and are insufficient to provide herd immunity.
Keywords: Oman, SARS-CoV-2, COVID-19, Antibody seroprevalence, Herd immunity, Prevalence, Serosurvey, Sero-epidemiological surveys
Introduction
Coronavirus disease 2019 (COVID-19) is a severe, acute respiratory syndrome caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It was first identified in Wuhan, China in December 2019 (Huang et al., 2020; Zhu et al., 2020), and within months had spread to most nations of the world (Hick and Biddinger, 2020; World Health Organization, 2021).
The surveillance of confirmed COVID-19 cases may not represent a particular community, as the manifestation of SARS-CoV-2 infection ranges from asymptomatic to fatal (Stringhini et al., 2020; Xu et al., 2020). Although reverse transcription polymerase chain reaction (RT-PCR) testing is currently recognized as the gold standard for the diagnosis of SARS-CoV-2 infection (Chan et al., 2020), the true number of SARS-CoV-2 infections is much higher than the officially reported number of cases. This is due to several factors, including the occurrence of asymptomatic infections, variable seeking of health care for clinically mild cases, variation in testing strategies between and within countries, and incomplete case reporting (Chen et al., 2021; Rostami et al., 2021). Therefore, the reported number of COVID-19 cases, based on clinical identification with virological confirmation, only represents a small proportion of actual cases, and a large number of asymptomatic and mild infections in the general population might only be identified by sero-epidemiological studies (Munster et al., 2020; Rostami et al., 2021).
Serological studies are an important tool to evaluate the cumulative prevalence of SARS-CoV-2 infection in the general population (Xu et al., 2020; Rostami et al., 2021; Thomas et al., 2021), estimate the proportion of the population previously infected, quantify the magnitude of the transmission of pathogens, estimate the infection fatality rate (Stringhini et al., 2020), assess the effect of interventions (Sughayer et al., 2020), and – when correlates of protection are available – estimate the degree of population immunity (Griffin, 2020; Verity et al., 2020; Chen et al., 2021). It is expected that serological monitoring and surveillance, if undertaken routinely, can be valuable for policy makers and health authorities when making public health decisions (Chen et al., 2021).
Oman has a heterogenous population of 4.6 million people, with migrants accounting for 41% of the population according to the National Centre for Statistical Information, Oman (2021). The first case of COVID-19 in Oman was confirmed on 24 February 2020 when two citizens tested positive for COVID-19 after returning from Iran (Al-Rawahi et al., 2021).
The Government of Oman established a national supreme committee for the COVID-19 response in mid-March 2020 to coordinate the national response to the pandemic using a whole-of-government approach. Oman responded to the COVID-19 pandemic by implementing multiple non-pharmaceutical interventions in phases to control the disease, such as restricting flights from infected countries, closing schools and commercial activities, and restricting mass gatherings. In addition, other public health interventions have been instated, such as increasing testing capacity, addressing migrants' needs under universal health coverage, contact tracing and quarantine, use of face masks, encouraging hand hygiene, and maintaining social distancing (Al Wahaibi et al., 2020).
The overall quality of seroprevalence studies is low; the majority of studies use convenience samples and rely on small or non-random sampling of participants, while only a few studies have used national-scale and multi-stage or stratified random sampling to select study participants (Bellizzi et al., 2021; Chen et al., 2021). This study aimed to assess the seroprevalence of SARS-CoV-2 in the general population in Oman, and to assess the longitudinal changes in antibody levels over time within the first 11 months of the pandemic (prior to the launch of the national vaccination campaign), and how the changes correlate with time, sex, age, district, nationality and disease prevalence in the community.
Methods
Setting
This study was conducted as a series of four-cycle serosurvey nationwide cross-sectional studies. The target population was the entire sultanate of Oman, including all governorates (regions) and all wilayats (districts) in each governorate.
Study duration
The survey was performed in four cycles, and each cycle lasted from 5 to 10 working days. There was a 2–4-week gap between each cycle (not less than one incubation period and not exceeding two incubation periods). The variation between the duration of the cycles and the gaps was due to the presence of public holidays. Hence, Cycle 1 started on 12 July 2020, Cycle 2 started on 16 August 2020, Cycle 3 started on 13 September 2020, and Cycle 4 started on 8 November 2020.
Sampling
Sample size
Sample sizes were calculated based on 95% confidence intervals (CI) and 5% margin of error samples using Epi Info Version 7.2 (Atlanta, GA, USA). To calculate the sample size, Oman was divided administratively into 11 governorates, with each counting as a unique population. Muscat Governorate has a larger population, representing approximately one-third of Oman's population, so this governorate was divided into two areas and considered as two administrative areas; sampling was conducted from each area separately.
Sampling technique
This study was conducted using a multi-stage stratified sampling method. With the help of the telecommunications regulatory authority and the National Centre for Statistical Information, telephone numbers of residents in each governorate were used as the sample frame for the study. This was particularly useful because people who were physically present in governorates were targeted, not only by the registered address but by mobile phones connected to telecom towers in each area. Random samples were selected from each governorate, and telephone numbers were distributed to the focal points in the governorates. The telephone number was used to represent the household. In the household, the family was listed, sorted by age in descending order and numbered. One member from the household was selected at random using a random number generator mobile phone application. The focal points in the governorates were responsible for calling the participants, selecting the household member at random, preparing them for the study, and directing them to the nearest primary healthcare centre to undergo the survey. Table S1 (see online supplementary material) shows the samples for each governorate in each cycle.
Exclusion criteria
In this study, current symptoms consistent with COVID-19 infection were excluded, as the participant would be directed to undergo PCR testing. Other exclusion criteria included age <5 years, contraindication to venepuncture, or lack of willingness to participate.
Data collection
Questionnaire
The questionnaire data included: patient demographics (sex, age, house address, work address, nationality, occupation), history of acute respiratory infection and list of symptoms, COVID contact, previous diagnosis or admission, history of travel, and risk factors (e.g. diabetes mellitus, hypertension) (Table S2, see online supplementary material). The questionnaire was collected electronically by the attending physician in the primary healthcare system through Tarassud Plus, a Ministry of Health electronic record system. After consenting, the participant was directed for collection of a serological sample.
Blood test for serology of COVID-19
Specimens were collected, with appropriate infection control precautions, aseptically by venepuncture, and the serum or plasma was separated from clot, red cells or gel separator after centrifugation of the sample. Samples were then transported to central public health laboratories as soon as possible; if transportation was delayed, samples were stored at 2–8 °C for a maximum of 48–72 h, and if transportation was delayed further, samples were stored at -80 °C.
SARS-CoV-2 immunoglobulin G (IgG) was assessed using the LIAISON IgG chemiluminescence assay (DiaSorin, Saluggia, Italy). The test method was quantitative determination of IgG anti-S1 and IgG anti-S2. The test was performed in accordance with the manufacturer's instructions. The analyser automatically calculated SARS-CoV-2 S1/S2 IgG antibody concentrations expressed as arbitrary units (AU/mL), and graded the results as follows: ≥15.0 was considered positive, 12.0≤x<15.0 was equivocal, and <12.0 was considered negative. The equivocal samples were retested on the same specimen in duplicate with the LIAISON SARS-CoV-2 S1/S2 IgG assay. Samples with at least two of the three results ≥15.0 AU/mL were graded positive. Samples with at least two of the three results <12.0 AU/mL were graded negative. When the results were repeatedly equivocal, a second sample was collected and tested no less than 1–2 weeks later.
Daily SARS-CoV-2 PCR confirmed data
The open access national and governorate daily SARS-CoV-2 PCR-confirmed data were also presented for the same time period as the study to compare with the serological results.
Statistical analysis
Basic sample characteristics were presented as percentages in each cycle and compared using the Chi-squared test. Age was categorized into the following groups: 5–14, 15–30, 31–49 and ≥50 years. The participant was considered positive when the IgG concentration was >15 AU/mL, negative when the concentration was <12.0 AU/mL, and equivocal when the concentration was 12.0–15.0 AU/mL. Only positive serological results were analysed.
Weights were computed for the sample in such a way that they accounted for age group, sex and nationality. Overall weighted seroprevalence rates by cycle, governorate and wilayat were presented. The description was also done by age group, sex and nationality between Omanis and non-Omanis. All the weighted seroprevalence rates were presented with 95% CI, calculated using a design-based likelihood method in the survey package of R software (Lumley, 2004). Mapping the prevalence at wilayat level was conducted using the tmap package of R software (Tennekes, 2018).
Multi-variable binary logistic regression analysis was performed to estimate the odds ratio of positive serology as the dependent variable, with various independent variables, namely: age group, sex, nationality, previous symptoms, previous COVID diagnosis, close contact with a COVID-19 patient, history of travel, comorbidities and occupation.
Simple linear regression analysis was used to estimate national projected near-future (up to January 2021) seroprevalence. All statistical analyses were undertaken using R Version 4.0 (R Core Team, Vienna, Austria).
Results
In total, 17,457 participants took part in these surveys, with 4210, 4403, 4780 and 4064 participants in Cycles 1, 2, 3 and 4, respectively. Thirty percent of the sample were female, 52% were aged 31–49 years, and 66.3% were Omani (Table 1 ). Among all positive symptomatic results (n=279), 100 participants (35.8%) had a fever, 105 (37.6%) participants had loss of smell, and 92 (33.0%) participants had a cough (Table S3, see online supplementary material).
Table 1.
Basic characteristics of the study sample, nationwide serosurvey, Oman, 12 July 2020–8 November 2020.
| Total n (%) | Cycle 1 n (%) | Cycle 2 n (%) | Cycle 3 n (%) | Cycle 4 n (%) | P-value | |
|---|---|---|---|---|---|---|
| Participants | 17,457 | 4210 (24.1) | 4403 (25.2) | 4780 (27.4) | 4064 (23.3) | - |
| Sex | ||||||
| Female | 5463 (31.3) | 1633 (38.8) | 1172 (26.6) | 1414 (29.6) | 1244 (30.6) | <0.001 |
| Age group (years) | ||||||
| 5–14 | 1020 (5.8) | 707 (16.8) | 179 (4.1) | 77 (1.6) | 57 (1.4) | <0.001 |
| 15–30 | 5219 (29.9) | 1243 (29.5) | 1443 (32.8) | 1403 (29.4) | 1130 (27.8) | <0.001 |
| 31–49 | 9155 (52.4) | 1729 (41.1) | 2316 (52.6) | 2726 (57.0) | 2384 (58.7) | <0.001 |
| ≥50 | 2063 (11.8) | 531 (12.6) | 465 (10.6) | 574 (12.0) | 493 (12.1) | <0.001 |
| Nationality | ||||||
| Omani | 11582 (66.3) | 3006 (71.4) | 2851 (64.8) | 3188 (66.7) | 2537 (62.4) | <0.001 |
| Governorate | ||||||
| Buraimi | 1538 (8.8) | 379 (9.0) | 402 (9.1) | 389 (8.1) | 368 (9.1) | <0.001 |
| Dhofar | 1498 (8.6) | 372 (8.8) | 375 (8.5) | 383 (8.0) | 368 (9.1) | <0.001 |
| Dahirah | 1471 (8.4) | 373 (8.9) | 392 (8.9) | 390 (8.2) | 316 (7.8) | <0.001 |
| Dakhiliyah | 1526 (8.7) | 379 (9.0) | 388 (8.8) | 395 (8.3) | 364 (9.0) | <0.001 |
| Muscat | 2649 (15.2) | 664 (15.8) | 674 (15.3) | 679 (14.2) | 632 (15.6) | <0.001 |
| Musandam | 1407 (8.1) | 344 (8.2) | 336 (7.6) | 409 (8.6) | 318 (7.8) | <0.001 |
| North Batinah | 1456 (8.3) | 343 (8.1) | 359 (8.2) | 378 (7.9) | 376 (9.3) | <0.001 |
| North Sharqiyah | 1417 (8.1) | 342 (8.1) | 376 (8.5) | 369 (7.7) | 330 (8.1) | <0.001 |
| South Batinah | 1692 (9.7) | 386 (9.2) | 382 (8.7) | 603 (12.6) | 321 (7.9) | <0.001 |
| South Sharqiyah | 1489 (8.5) | 354 (8.4) | 386 (8.8) | 392 (8.2) | 357 (8.8) | <0.001 |
| Wusta | 1314 (7.5) | 274 (6.5) | 333 (7.6) | 393 (8.2) | 314 (7.7) | <0.001 |
| Comorbidity | 1191 (6.8) | 339 (8.1) | 262 (6.0) | 342 (7.2) | 248 (6.1) | <0.001 |
| Travelled | 1103 (6.3) | 174 (4.1) | 301 (6.8) | 329 (6.9) | 299 (7.4) | <0.001 |
| Respiratory symptoms in the past 2 weeks | 368 (2.1) | 81 (1.9) | 73 (1.7) | 114 (2.4) | 100 (2.5) | 0.026 |
| Respiratory symptoms in the past 3 months | 1316 (7.5) | 208 (4.9) | 306 (7.0) | 431 (9.0) | 371 (9.1) | <0.001 |
| Known contact with COVID-19 | 1230 (7.0) | 158 (3.8) | 266 (6.0) | 400 (8.4) | 406 (10.0) | <0.001 |
| Diagnosed with COVID-19 | 376 (2.2) | 34 (0.8) | 83 (1.9) | 138 (2.9) | 121 (3.0) | <0.001 |
| Admitted with COVID-19 | 8 (1.4) | 1 (1.4) | 2 (1.7) | 2 (1.2) | 3 (1.5) | 0.989 |
| Occupation | ||||||
| Driver | 80 (2.8) | 128 (3.6) | 101 (2.5) | 59 (1.7) | 368 (2.7) | <0.001 |
| Healthcare worker | 67 (2.4) | 199 (5.6) | 374 (9.3) | 370 (10.7) | 1010 (7.3) | |
| Officeworker | 949 (33.6) | 1557 (43.7) | 1639 (40.7) | 1463 (42.4) | 5608 (40.4) | |
| Police | 77 (2.7) | 188 (5.3) | 158 (3.9) | 111 (3.2) | 534 (3.9) | |
| Teacher | 118 (4.2) | 144 (4.0) | 252 (6.3) | 211 (6.1) | 725 (5.2) | |
| Labourer | 50 (1.8) | 37 (1.0) | 39 (1.0) | 45 (1.3) | 171 (1.2) | |
| Housewife | 547 (19.4) | 276 (7.7) | 302 (7.5) | 246 (7.1) | 1371 (9.9) | |
| Shop/restaurant worker | 64 (2.3) | 68 (1.9) | 70 (1.7) | 59 (1.7) | 261 (1.9) | |
| Not provided | 874 (30.9) | 967 (27.1) | 1092 (27.1) | 889 (25.7) | 3822 (27.6) |
COVID-19, coronavirus disease 2019.
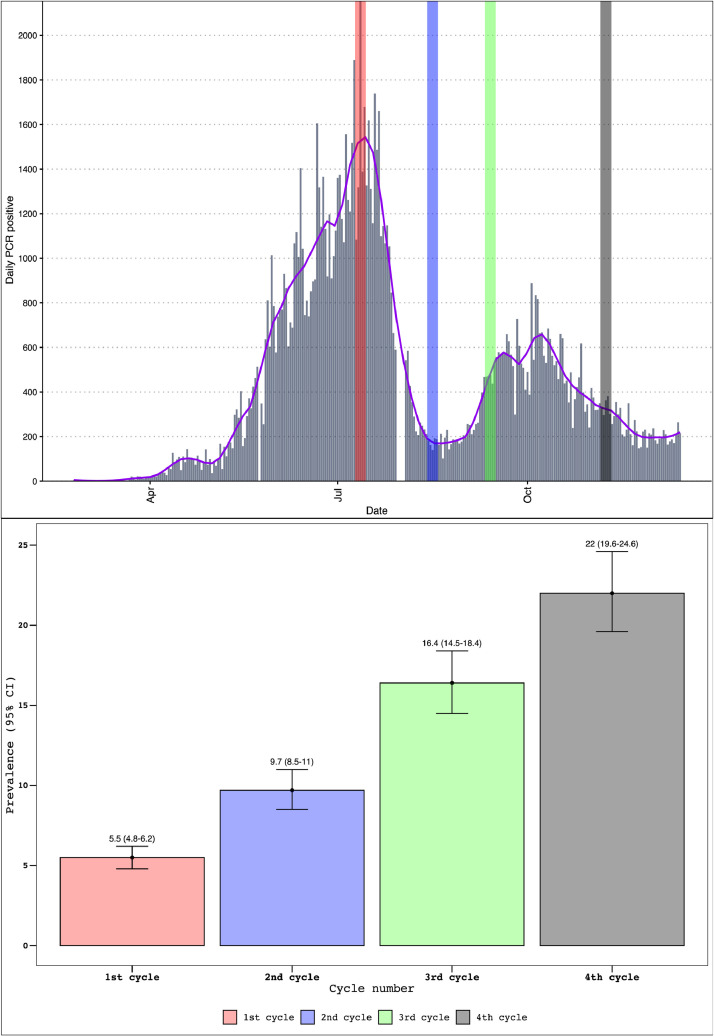
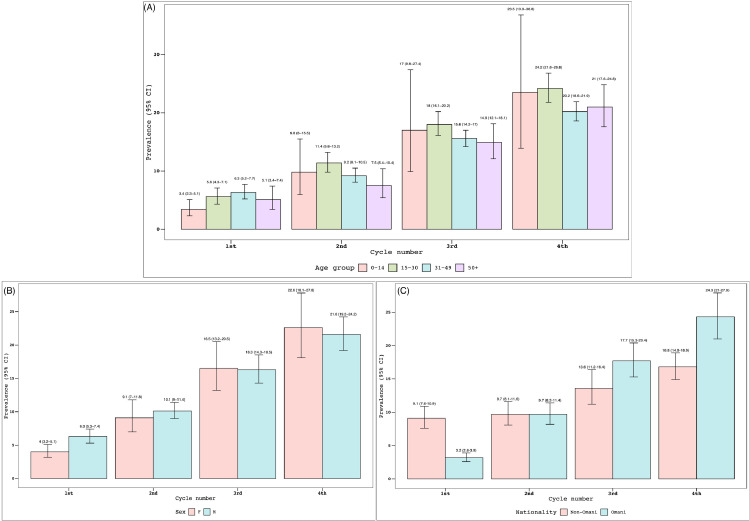
A significant increase in the seroprevalence of COVID-19 was observed throughout the study cycles, from 5.5% (4.8–6.2%) in Cycle 1 to 22% (19.6–24.6%) in Cycle 4 (Figure 1 ). Although no differences in prevalence were noted between age groups in each cycle, the prevalence rates for each age group were notably different between cycles, except for children (5–14 years; Figure 2 A). No difference in seroprevalence was noted between the two sexes throughout the cycles (Figure 2B). Nevertheless, a transition of seroprevalence was observed, from being higher in non-Omanis compared with Omanis in Cycle 1 [9.1% (7.6–10.9%) vs 3.2% (2.6–3.9%)] to being higher in Omanis compared with non-Omanis in Cycle 4 [24.3% (21.0–27.9%) vs 16.8% (14.9–18.9%)] (Figure 2C).
Figure 1.
Serosurvey results by cycle, nationwide serosurvey, Oman, 12 July 2020–8 November 2020. PCR, polymerase chain reaction.
Figure 2.
Serosurvey results by age group (A), sex (B) and nationality (C), nationwide serosurvey, Oman, 12 July 2020–8 November 2020. CI, confidence interval.
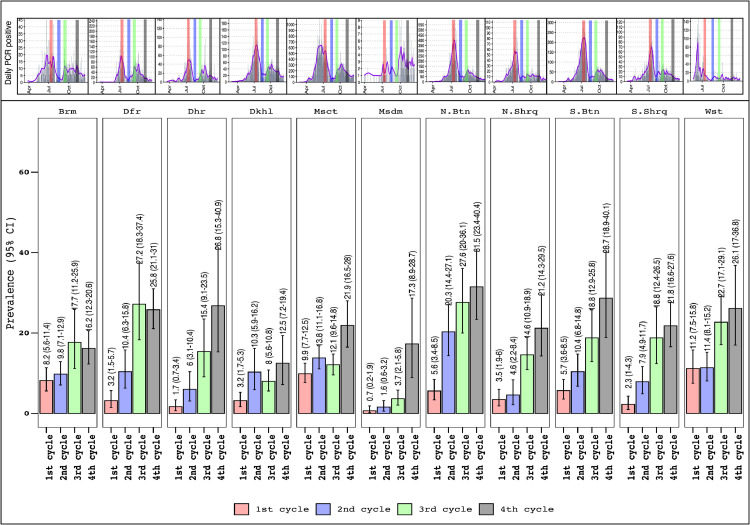
Figure 3 shows the seroprevalence of COVID-19 by governorate. The highest seroprevalence rates in Cycle 4 were found in North Batinah, South Batinah and Al Dahirah Governorates [31.5% (23.4–40.4%), 28.7% (18.9–40.1%) and 26.8% (15.3–40.9%), respectively]. Figure 3 shows that the significant increase in cycle seroprevalence rates differed within each governorate. For example, the change in seroprevalence in Muscat Governorate was only significant when moving from Cycle 3 to Cycle 4 [from 12.1% (9.6–14.8%) to 21.9% (16.5–28%)]. This difference was observed for North Batinah Governorate between Cycle 1 and Cycle 2 [from 5.6% (3.4–8.5%) to 20.3% (14.4–27.1%)], after which seroprevalence did not differ.
Figure 3.
Serosurvey results by governorate and cycle, nationwide serosurvey, Oman, 12 July 2020–8 November 2020. Brm, Alburaimi; Dfr, Dhofar; Dhr, Dhahira; Dkhl, Dakhiliyah; Msct, Muscat; Msdm, Musandam; N. Btn, North Batinah; N. Shrq, North Sharqiyah; S. Btn, South Batinah; S. Shrq, South Sharqiyah; Wst, Alwusta; CI, confidence interval.
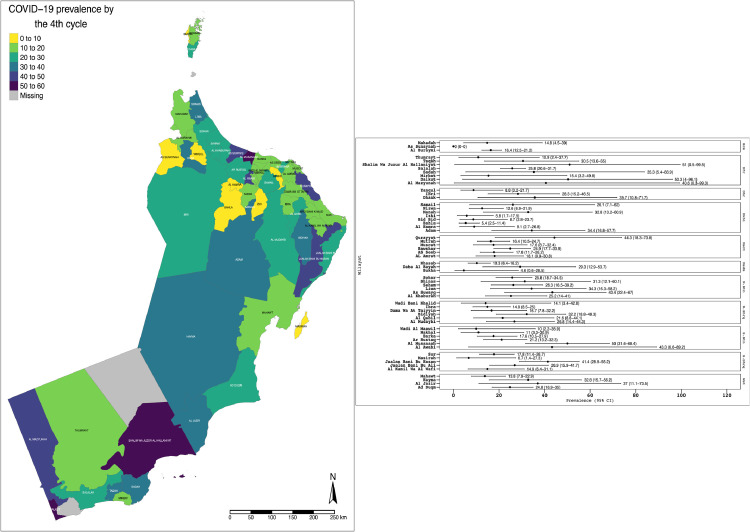
Figure 4 shows the results of seroprevalence in Cycle 4 at wilayat level, both graphically and by geographical distribution. It shows that the seroprevalence rate ranged from 50–60% in Al-Musanah (South Batinah Governorate) to 40–50% in Qurayyat (Muscat Governorate).
Figure 4.
Cycle 4 serosurvey results by wilayat with geographical location, nationwide serosurvey, Oman, 12 July 2020–8 November 2020. COVID-19, coronavirus disease 2019.
Table 2 shows the crude odds ratios (COR) and adjusted odds ratios (AOR) (with 95% CI) of getting COVID-19 infection (positive serology) for certain factors. Older age groups are at higher risk of getting the infection compared with children (5–14 years old), especially the 15–30-year-old category (COR 2.42, 95% CI 1.88–3.17; AOR 2.3, 95% CI 1.31–4.46). The AOR did not capture any difference in seropositivity between sexes and nationalities (AOR 1.1, 95% CI 0.94–1.28; AOR 0.93, 95% CI 0.83–1.04, respectively). Table 2 also shows that close contacts of COVID-19 patients were at 96% higher risk of having the disease than non-contacts (AOR 1.96, 95% CI 1.64–2.34). In addition, travellers and patients with comorbidities were found to be 40% more protected from contracting COVID-19 (AOR 0.58, 95% CI 0.45–0.72; AOR 0.59, 95% CI 0.46–0.75, respectively). For occupations, labourers had a 58% higher risk of infection compared with office workers (AOR 1.58, 95% CI 1.04–2.35), whereas healthcare workers had a 37% lower risk of infection compared with office workers (AOR 0.63, 95% CI 0.49–0.80).
Table 2.
Crude odds ratios (COR) and adjusted odds ratios (AOR) [with 95% confidence intervals (CI)] of getting coronavirus disease 2019 (COVID-19) (serology positive) and studied factors.
| Factors | COR (95% CI) | AOR (95% CI) |
| 15–30 years (ref. 5–14 years) | 2.42 (1.88–3.17) | 2.3 (1.31–4.46) |
| 31–49 years (ref. 5–14 years) | 2.22 (1.73–2.89) | 2.1 (1.2–4.06) |
| ≥50 years (ref. 5–14 years) | 1.94 (1.48–2.59) | 2.16 (1.2–4.23) |
| Male (ref. female) | 1.13 (1.03–1.25) | 1.1 (0.94–1.28) |
| Omani (ref. non-Omani) | 1.06 (0.97–1.17) | 0.93 (0.83–1.04) |
| Known contact with COVID-19 | 3.15 (2.77–3.59) | 1.96 (1.64–2.34) |
| Diagnosed with COVID-19 | 32.3 (25–42.29) | 25.31 (19.1–34.01) |
| Respiratory symptoms in the past 3 months | 3.15 (2.77–3.57) | 2.39 (2–2.84) |
| Respiratory symptoms in the past 2 weeks | 1.67 (1.28–2.15) | 0.74 (0.51–1.06) |
| Travelled | 0.8 (0.65–0.96) | 0.58 (0.45–0.72) |
| Comorbidity | 0.74 (0.61–0.89) | 0.59 (0.46–0.75) |
| Driver (ref. office worker) | 1.16 (0.86–1.54) | 1.29 (0.94–1.73) |
| Healthcare worker (ref. office worker) | 0.81 (0.66–1) | 0.63 (0.49–0.8) |
| Housewife (ref. office worker) | 1 (0.84–1.19) | 1.12 (0.9–1.41) |
| Labourer (ref. office worker) | 1.46 (0.97–2.12) | 1.58 (1.04–2.35) |
| Police (ref. office worker) | 1.37 (1.08–1.72) | 1.08 (0.82–1.4) |
| Shop/restaurant worker (ref. office worker) | 1.14 (0.8–1.6) | 1.31 (0.91–1.85) |
| Teacher (ref. office worker) | 0.74 (0.57–0.94) | 0.84 (0.64–1.09) |
| Not provided (ref. office worker) | 1.05 (0.93–1.18) | 1.16 (1.02–1.31) |
The projected near-future national trend in seroprevalence is shown in Figure S1 (see online supplementary material), showing that the seroprevalence rate in January 2021 is expected to be approximately 30% (21–38%).
Discussion
The low estimated seroprevalence rate among the residents of Oman in Cycles 1 and 2 is comparable to other population-based studies that have been conducted around the same period or before (Pollán et al., 2020; Alsuwaidi et al., 2021; Bellizzi et al., 2021), but was lower than studies conducted in Brazil and India by Murhekar et al. (2021) and Hallal et al. (2020), and studies conducted in Iran (Hallal et al., 2020; Poustchi et al., 2021). The national lockdown and adherence to social distancing in the few months preceding the study (Al Wahaibi et al., 2020) are potential contributors to the observed low seroprevalence rate, especially in Cycles 1 and 2 of the survey.
Over the 19 weeks of this study, seroprevalence increased from approximately 5% to approximately 22%; this was expected considering the time to seroconversion after symptoms, the fact that the peak of the first wave of the epidemic was reached the week before the start of Cycle 1, and Cycle 4 was conducted after the end of the second peak (see Figures 1 and 2). The increase in seroprevalence was similar to findings from other studies (Hallal et al., 2020; Stringhini et al., 2020; Bellizzi et al., 2021; Murhekar et al., 2021).
This study showed the comparatively low risk of healthcare workers being infected compared with other categories in the community, despite the increased risk of exposure in the workplace; this indicates the increased risk awareness and implementation of infection prevention and control measures by healthcare workers. However, another study conducted simultaneously by the present authors showed that the prevalence of COVID-19 in healthcare workers is a reflection of seroprevalence in the community, and that people working in supporting services are at higher risk of COVID-19 than those working in high-risk areas in hospitals, such as COVID-19 wards and intensive care units (Al-Maani et al., 2021).
The seroprevalence of SARS-CoV-2 in the general population also varied across World Health Organization (WHO) regions, with higher seroprevalence in the South-East Asia region (e.g. India, range 10.8–40.8%) and lower seroprevalence in the Western Pacific region (e.g. China, range 0.8–3.3%) (Chen et al., 2021).
Similar to other serosurveys, this study found no difference in SARS-CoV-2 seroprevalence between genders (Pollán et al., 2020; Stringhini et al., 2020; Alsuwaidi et al., 2021; Chen et al., 2021; Rostami et al., 2021). Significant differences were found between age groups, mainly in the 15–30-years age group compared with the 5–14-years age group; this is similar to findings from other studies which showed that children and adolescents (0–19 years) and older people (60 years) had lower seroprevalence rates than other age groups (Hallal et al., 2020; Pollán et al., 2020; Stringhini et al., 2020; Alsuwaidi et al., 2021; Chen et al., 2021). This may be a reflection of school, daycare, nursery and play area closures during the period prior to and during the surveys, as these areas are the main exposure places for children.
Marked regional differences were observed between the different governorates in Oman which generally match the surveillance data (Figure 2); this could be due, in part, to the different introduction times of the epidemic to the governorates, as it started in the capital governorate (Muscat) and then spread to the adjacent governorates. This has also been shown in a study from Washington, USA (Tordoff et al., 2021). There was also earlier spread in the governorates bordering other countries. Regional variations in SARS-CoV-2 seroprevalence have also been reported in many other countries (Pollán et al., 2020; Alsuwaidi et al., 2021; Bellizzi et al., 2021; Núñez-Zapata et al., 2021; Poustchi et al., 2021).
The seropositivity rate was higher in migrants during Cycles 1 and 2, with a subsequent increase in Omanis in Cycles 3 and 4. In addition, the risk of infection was higher in labourers compared with office workers, as supported by the logistic regression model. This is similar to other studies undertaken in three Gulf Cooperation Council countries (Alali et al., 2021; Alsuwaidi et al., 2021; Jeremijenko et al., 2021). This could be explained by the fact that, early in the pandemic, there were many outbreaks in labour camps and dormitories that were densely populated with congregate living facilities, and lack of adherence to social distancing (Al Balushi et al., 2020; Al Fahdi et al., 2021). The Ministry of Health, along with other stakeholders, implemented rigorous structured occupational health measures in workplaces and dormitories with close monitoring to reduce the risk of COVID-19 transmission among this group. Later, most cases were in Omanis because of family and social gatherings (Al-Mahruqi et al., 2021).
Consistent with other studies, fever, loss of smell and taste, and cough were among the symptoms found to have the strongest association with seropositivity (Makaronidis et al., 2020; Alsuwaidi et al., 2021; Chen et al., 2021).
The results of this multi-variable logistic regression model confirm that close contact with people with COVID-19 almost doubles the risk of viral transmission; this is similar to other serosurveys (Pollán et al., 2020; Alsuwaidi et al., 2021; Chen et al., 2021) and highlights the importance of public health measures, including contact tracing, quarantine and self-isolation.
To the authors’ knowledge, this is the first four-cycle study and the largest general-population-based study of the prevalence of SARS-CoV-2 in the WHO Eastern Mediterranean region. A key strength of this study is the random selection of households from the national telecommunication register, which enabled the authors to contact a representative sample of the Omani population with a detailed sampling framework, rigorous sampling methods (i.e. multi-stage, stratified sampling) and adjustments for selection bias. The use of a more sensitive and specific technique to screen for antibodies was an additional strength of this study.
This study has some limitations that should be noted. First, individuals with a history of COVID-19 symptoms or contacts of positive cases may have been more willing to participate in this study. However, because participants were selected at random from the population, the potential effect of this limitation on the findings is expected to be low, especially with the high media coverage that preceded each cycle, which resulted in a high response rate for the study. Second, some participants may not have recalled the answers for all questions in the study questionnaire accurately, resulting in possible recall bias. Nevertheless, this bias is expected to be small, as with growing fears about the pandemic, it was assumed that people would be more likely to remember any respiratory symptoms over the past few months. Third, only children aged ≥5 years were included in this study, and immunological responses and susceptibility to infection may be different in younger children. Fourth, there is a need to consider potential low sensitivity for individuals who were infected long before testing for the study, as antibody titres wane over the months following infection (Accorsi et al., 2021).
This study found a seroprevalence rate of 22% in the general population, and this was projected to be only 30% by January 2021; this suggests that the number of people infected by the end of 2020 was unlikely to satisfy estimates of what would be required to achieve herd immunity (Pollán et al., 2020; Stringhini et al., 2020; Chen et al., 2021; Núñez-Zapata et al., 2021; Rostami et al., 2021). These findings emphasize the importance of prevention strategies (e.g. physical distancing, use of face masks, identifying and isolating new cases and their contacts), which are imperative for controlling the pandemic until community immunity can be achieved through vaccination to further protect the general population from SARS-CoV-2 transmission.
In conclusion, this study provides nationwide and regional estimates of the spread of SARS-CoV-2 in Oman, showing wide variation between the different governorates. The substantially higher seroprevalence estimates in migrants in Cycles 1 and 2 of the study reflects the high efficiency of SARS-CoV-2 spread in congregate living facilities and work settings, and the effectiveness of occupational health measures put into place to reduce transmission in these settings. The prevalence estimates remain low and are insufficient to provide herd immunity, therefore bolstering the need to achieve mass vaccination.
Acknowledgments
Acknowledgements
This study was a collaboration between the Directorate General for Disease Surveillance and Control, the Directorate General of Primary Health Care, and the 11 Directorate Generals for Health Services in all 11 governorates in Oman. The authors wish to thank all the public health and primary healthcare staff, nurses and laboratory technologists who collaborated in this study. The authors also wish to thank the study participants. Finally, the authors wish to thank Lesley Carson for her editorial assistance in finalizing the manuscript.
Conflict of interest statement
None declared.
Ethical approval
The study was approved by the Supreme Committee for COVID-19 and by the Directorate General for Disease Surveillance and Control in Ministry of Health. Written informed consent was obtained from all individuals prior to study participation. Consent for children was obtained from a parent.
Author contributions
SA, AW, AM, AJ, HK, AM, FY, AQ, SK, KH and BR handled the study conception and design, and the analysis and interpretation of data. PJK, AM, ZM, MT, SK, SB, MHS, AYB, IOA, NS and EM were responsible for conducting the study in the governorates and communicating with the study in the Directorate General for Disease Surveillance and Control. AJ, HK, IS and AR were responsible for the study design, processing of samples in Central Public Health Laboratories (CPHL), and logistics management. All of the authors drafted the article or revised it critically for important intellectual content, and provided final approval of the version for submission.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.09.062.
Appendix. Supplementary materials
References
- Accorsi EK, Qiu X, Rumpler E, Kennedy-Shaffer L, Kahn R, Joshi K, et al. How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. Eur J Epidemiol. 2021;36:179–196. doi: 10.1007/s10654-021-00727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Balushi L, Al Fahdi F, Al Ghafri T, Amin M, Singh J, Al Siyabi B, et al. Epidemiological characteristics of COVID-19 confirmed cases in Muscat Governorate, Sultanate of Oman. Open J Epidemiol. 2020;11:56–69. [Google Scholar]
- Al Fahdi F, Kurup PJ, Al Balushi L, Amin M, Al Siyabi B, Al Kalbani M, et al. Work related clusters of COVID-19 in Muscat Governorate in Oman: epidemiology & future implications. Open J Epidemiol. 2021;11:135–151. [Google Scholar]
- Al Wahaibi A, Al Manji A, Al Maani A, Al Rawahi B, Al Harthy K, Alyaquobi F, et al. COVID-19 epidemic monitoring after non-pharmaceutical interventions: the use of time-varying reproduction number in a country with a large migrant population. Int J Infect Dis. 2020;99:466–472. doi: 10.1016/j.ijid.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alali WQ, Bastaki H, Longenecker JC, Aljunid SM, AlSeaidan M, Chehadeh W, et al. Seroprevalence of SARS-CoV-2 in migrant workers in Kuwait. J Travel Med. 2021;28:taaa223. doi: 10.1093/jtm/taaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Maani A, Al Wahaibi A, Al-Sooti J, Al Abri B, Al Shukri I, AlRisi E, et al. The role of supporting services in driving SARS-CoV-2 transmission within healthcare settings: a multicenter seroprevalence study. Int J Infect Dis. 2021;107:257–263. doi: 10.1016/j.ijid.2021.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mahruqi S, Al-Wahaibi A, Khan AL, Al-Jardani A, Asaf S, Alkindi H, et al. Molecular epidemiology of COVID-19 in Oman: a molecular and surveillance study for the early transmission of COVID-19 in the country. Int J Infect Dis. 2021;104:139–149. doi: 10.1016/j.ijid.2020.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rawahi B, Prakash K, Al-Wahaibi A, Al-Jardani A, Al-Harthy K, Kurup PJ, et al. Epidemiological characteristics of pandemic coronavirus disease (COVID-19) in Oman. Sultan Qaboos Univ Med J. 2021;21:e195. doi: 10.18295/squmj.2021.21.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsuwaidi AR, Hosani FIA, Memari SA, Narchi H, Wareth LA, Kamal H, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021 doi: 10.1093/ije/dyab077. https://10.1093/ije/dyab077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellizzi S, Alsawalha L, Ali SS, Sharkas G, Muthu N, Ghazo M, et al. A three-phase population based sero-epidemiological study: assessing the trend in prevalence of SARS-CoV-2 during COVID-19 pandemic in Jordan. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF-W, Yip CC-Y, To KK-W, Tang TH-C, Wong SC-Y, Leung K-H, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58:e00310–e00320. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen Z, Azman AS, Deng X, Sun R, Zhao Z, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e598–e609. doi: 10.1016/S2214-109X(21)00026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin S. Covid-19: Herd immunity is “unethical and unachievable,” say experts after report of 5% seroprevalence in Spain. BMJ. 2020;370:m2728. doi: 10.1136/bmj.m2728. [DOI] [PubMed] [Google Scholar]
- Hallal PC, Hartwig FP, Horta BL, Silveira MF, Struchiner CJ, Vidaletti LP, et al. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hick JL, Biddinger PD. Novel coronavirus and old lessons – preparing the health system for the pandemic. N Engl J Med. 2020;382:e55. doi: 10.1056/NEJMp2005118. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremijenko A, Chemaitelly H, Ayoub HH, Alishaq M, Abou-Samra A-B, Al Ajmi JAAA, et al. Herd immunity against severe acute respiratory syndrome coronavirus 2 infection in 10 communities. Qatar. Emerg Infect Dis. 2021;27:1343–1352. doi: 10.3201/eid2705.204365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19. [Google Scholar]
- Makaronidis J, Mok J, Balogun N, Magee CG, Omar RZ, Carnemolla A, et al. Seroprevalence of SARS-CoV-2 antibodies in people with an acute loss in their sense of smell and/or taste in a community-based population in London, UK: an observational cohort study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- Murhekar MV, Bhatnagar T, Selvaraju S, Saravanakumar V, Thangaraj JWV, Shah N, et al. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:e257–e266. doi: 10.1016/S2214-109X(20)30544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Centre for Statistical Information, Oman. NCSI portal. 2021. Available at: https://www.ncsi.gov.om/Pages/NCSI.aspx (accessed 1 August 2021).
- Núñez-Zapata SF, Benites-Peralta B, Mayta-Tristan P, Rodríguez-Morales AJ. High seroprevalence for SARS-CoV-2 infection in South America, but still not enough for herd immunity! Int J Infect Dis. 2021;109:244–246. doi: 10.1016/j.ijid.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustchi H, Darvishian M, Mohammadi Z, Shayanrad A, Delavari A, Bahadorimonfared A, et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis. 2021;21:473–481. doi: 10.1016/S1473-3099(20)30858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A, Sepidarkish M, Leeflang MMG, Riahi SM, Nourollahpour Shiadeh M, Esfandyari S, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:331–340. doi: 10.1016/j.cmi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sughayer MA, Mansour A, Al Nuirat A, Souan L, Ghanem M, Siag M. Covid-19 seroprevalence rate in healthy blood donors from a community under strict lockdown measures. medRxiv. 2020 doi: 10.1101/2020.06.06.20123919. [DOI] [Google Scholar]
- Tennekes M. tmap: thematic maps in R. J Stat Softw. 2018;84:1–39. [Google Scholar]
- Thomas SN, Altawallbeh G, Zaun CP, Pape KA, Peters JM, Titcombe PJ, et al. Initial determination of COVID-19 seroprevalence among outpatients and healthcare workers in Minnesota using a novel SARS-CoV-2 total antibody ELISA. Clin Biochem. 2021;90:15–22. doi: 10.1016/j.clinbiochem.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff DM, Greninger AL, Roychoudhury P, Shrestha L, Xie H, Jerome KR, et al. Phylogenetic estimates of SARS-CoV-2 introductions into Washington State. medRxiv. 2021 doi: 10.1101/2021.04.05.21254924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2021. Coronavirus disease (COVID-19) pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at. (accessed 1 August 2021) [Google Scholar]
- Xu X, Sun J, Nie S, Li H, Kong Y, Liang M, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.