Abstract
Background
Ames test is used worldwide for detecting the bacterial mutagenicity of chemicals. In silico analyses of bacterial mutagenicity have recently gained acceptance by regulatory agencies; however, current in silico models for prediction remain to be improved. The Japan Pharmaceutical Manufacturers Association (JPMA) organized a task force in 2017 in which eight Japanese pharmaceutical companies had participated. The purpose of this task force was to disclose a piece of pharmaceutical companies’ proprietary Ames test data.
Results
Ames test data for 99 chemicals of various chemical classes were collected for disclosure in this study. These chemicals are related to the manufacturing process of pharmaceutical drugs, including reagents, synthetic intermediates, and drug substances. The structure-activity (mutagenicity) relationships are discussed in relation to structural alerts for each chemical class. In addition, in silico analyses of these chemicals were conducted using a knowledge-based model of Derek Nexus (Derek) and a statistics-based model (GT1_BMUT module) of CASE Ultra. To calculate the effectiveness of these models, 89 chemicals for Derek and 54 chemicals for CASE Ultra were selected; major exclusions were the salt form of four chemicals that were tested both in the salt and free forms for both models, and 35 chemicals called “known” positives or negatives for CASE Ultra. For Derek, the sensitivity, specificity, and accuracy were 65% (15/23), 71% (47/66), and 70% (62/89), respectively. The sensitivity, specificity, and accuracy were 50% (6/12), 60% (25/42), and 57% (31/54) for CASE Ultra, respectively. The ratio of overall disagreement between the CASE Ultra “known” positives/negatives and the actual test results was 11% (4/35). In this study, 19 out of 28 mutagens (68%) were detected with TA100 and/or TA98, and 9 out of 28 mutagens (32%) were detected with either TA1535, TA1537, WP2uvrA, or their combination.
Conclusion
The Ames test data presented here will help avoid duplicated Ames testing in some cases, support duplicate testing in other cases, improve in silico models, and enhance our understanding of the mechanisms of mutagenesis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41021-021-00206-1.
Keywords: Ames test, Mutagenicity, Bacteria, In silico, Structure-activity relationship, Derek Nexus, CASE Ultra
Introduction
The bacterial mutagenicity test, known as Ames test, is used worldwide to detect the mutagenicity of chemicals [1, 2]. Ames test is utilized not only for research purposes but also for submission to regulatory agencies for the approval of chemical substances [3, 4]. Recently, in silico evaluation of bacterial mutagenicity has been accepted by regulatory agencies [e.g., the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) M7 guideline [5] for hazard identification of mutagenic impurities in medicinal drugs]. In recent years, several in silico models for predicting bacterial mutagenicity have been developed. However, the prediction level is not fully satisfactory and remains to be improved [6–8]. One way to improve this is to collect Ames test data, particularly for chemicals in some chemical classes where a limited number of test data are available.
For this reason, the Japan Pharmaceutical Manufacturers Association (JPMA) organized a task force for Ames data sharing. The purpose of this task force was to disclose a piece of pharmaceutical companies’ proprietary Ames test data to make them available to anyone for utilization in research or submission to regulatory agencies, and to improve in silico models by using them as training set examples. Eight Japanese pharmaceutical companies participated in this task force, and Ames test data for 99 chemicals were collected. These chemicals are related to the manufacturing process of pharmaceutical drugs, including reagents, synthetic intermediates, and drug substances. In addition, in silico analyses of these chemicals for bacterial mutagenicity were conducted using a knowledge-based model (Derek Nexus, Lhasa Limited) or a statistics-based model (CASE Ultra, MultiCASE Inc.).
In this report, we present the Ames test data and in silico predictions for 99 chemicals of various chemical classes and discuss their structure-activity relationships in relation to structural alerts for each chemical class.
Materials and methods
Materials
Ninety-nine chemicals were tested and collected by this task force. Table 1 lists the chemical identification (ID), chemical name, CAS registry number (CAS No.), source, purity of the test chemicals used, and test site. Table 2 lists the chemical ID, chemical name (arranged by chemical classes), chemical structure, solvent used to dissolve the test chemicals, summarized Ames test results, and in silico analyses. In this study, free and salt forms were treated as different chemicals.
Table 1.
Chemical ID, test chemical, CAS No. source or supplier of test chemical, purity, and test site
| Chemical ID | Test chemical | CAS No. | Source or supplier of test chemical | Purity (%) | Test site |
|---|---|---|---|---|---|
| 1 | 1-Iodo-4-nitrobenzene | 636–98-6 | Maruzen Chemicals | 99.9 | CERI |
| 2 | 2-Nitro-5-(1-piperazinyl)benzaldehyde HCl | 13236300a | Otsuka Pharmaceutical | 100 | JISHA |
| 3 | Methyl 2-methyl-3-nitrobenzoate | 59382–59-1 | Otsuka Pharmaceutical | 99.57 | JISHA |
| 4 | 2-Nitro-5-(1-piperazinyl)benzaldehyde dimethyl acetal | 101291629a | Otsuka Pharmaceutical | 99.8 | JISHA |
| 5 | 5-Chloro-2-nitrobenzaldehyde dimethyl acetal | 13796–06-0 | Otsuka Pharmaceutical | 99.97 | JISHA |
| 6 | 2-Nitro-5-(1-piperazinyl)cinnamic acid | NR | Otsuka Pharmaceutical | 99.8 | JISHA |
| 7 | 2-Fluoro-4-nitrophenol | 403–19-0 | Tokyo Chemical Industry | 99.6 | BoZo Research Center |
| 8 | 3-Hydroxy-4-nitrobenzoic acid | 619–14-7 | Eisai | 99.6 | UBE |
| 9 | Pranidipine; Methyl (2E)-phenylprop-2-en-1-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | 99522–79-9 | Otsuka Pharmaceutical | 99.97 | Otsuka Pharmaceutical |
| 10 | 4-Amino-2-fluorophenol | 399–96-2 | Tokyo Chemical Industry | 99.4 | BoZo Research Center |
| 11 | Methyl 3-amino-2-methyl benzoate | 18583–89-6 | Otsuka Pharmaceutical | 94.43 | JISHA |
| 12 | Sodium 3-[2-amino-5-(1-piperazinyl)phenyl]propionate | 101328646a | Otsuka Pharmaceutical | 99.5 | JISHA |
| 13 | Methyl 4-amino-2-methoxybenzoate | 27492–84-8 | Tokyo Chemical Industry | 98.9 | BoZo Research Center |
| 14 | Methyl 3-amino-4,6-dibromo-2-methylbenzoate | 119916–05-1 | Otsuka Pharmaceutical | 98.74 | JISHA |
| 15 | 4-(2-Methoxy-phenyl)-thiazol-2-ylamine | 93209–95-1 | Shionogi | 99.99 | CMIC Pharma Science |
| 16 | 4-Hexyl-1,3-thiazol-2-amine | 90770–58-4 | Shionogi | 99.72 | CMIC Pharma Science |
| 17 | 2-Amino-4-hydroxythiazole | 7146–26-1 | Oakwood Products | 98 | LSI Medience |
| 18 | Thiazole-2,4-diamine | 67355–26-4 | Oxchem | 98 | LSI Medience |
| 19 | 6-(2,3-Epoxypropoxy)-2(1H)-quinolinone | 143343–78-6 | Otsuka Pharmaceutical | 94.44 | JISHA |
| 20 | 6-(4-(3,4-Dimethoxybenzoyl)-2,3-dihydroxypiperazin-1-yl)-3,4-dihydriquinolin-2(1H)-one | NR | Otsuka Pharmaceutical | 98.12 | Otsuka Pharmaceutical |
| 21 | 8-Hydroxy-2(1H)-quinolinone | 15450–76-7 | Otsuka Pharmaceutical | 99.55 | JISHA |
| 22 | 3,4-Dimethoxy-N-{2-[(2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)amino]ethyl}benzamide | NR | Otsuka Pharmaceutical | 99.96 | Otsuka Pharmaceutical |
| 23 | 6-(3-Oxopiperazin-1-yl)-3,4-dihydroquinolin-2(1H)-one | NR | Otsuka Pharmaceutical | 99.56 | Otsuka Pharmaceutical |
| 24 | 3,4-Dihydro-5-(1-piperazinyl)-2-(1H) quinolinone | 87154–95-8 | Otsuka Pharmaceutical | > 99.9 | JISHA |
| 25 | 6-(4-(4-Hydroxy-3-methoxybenxoyl)piperazin-1-yl)-3,4-dihydroquinolin-2(1H)-one | NR | Otsuka Pharmaceutical | 99.87 | Otsuka Pharmaceutical |
| 26 | 6-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-2(1H)-quinolinone | 73963–62-9 | Otsuka Pharmaceutical | 100 | Otsuka Pharmaceutical |
| 27 | trans-3,4-Dihydro-6-[4-[1-(4-hydroxycyclohexyl)-1H-tetrazol-5-yl]butoxy]-2(1H)-quinolinone | 87153–04-6 | Otsuka Pharmaceutical | 99.93 | Otsuka Pharmaceutical |
| 28 | Grepafloxacin; (RS)-1-Cyclopropyl-6-fluoro-5-methyl-7-(3-methylpiperazin-1-yl)-4-oxo-quinoline-3-carboxylic acid | 119914–60-2 | Otsuka Pharmaceutical | 99.66 | JISHA |
| 29 | Grepafloxacin HCl; (RS)-1-Cyclopropyl-6-fluoro-5-methyl-7-(3-methylpiperazin-1-yl)-4-oxo-quinoline-3-carboxylic acid monohydrochloride | 161967–81–3 | Otsuka Pharmaceutical | 99.59 | Otsuka Pharmaceutical |
| 30 | Ethyl 1-cyclopropyl-7-brorno-6-fluoro-1,4-dihydro-5-methyl-4-oxo-3-quinolinecarboxylate | 119916–33-5 | Otsuka Pharmaceutical | 99.88 | JISHA |
| 31 | 2,4-Bis(trimethylsiloxy)-5-fluoropyrimidine | 17242–85-2 | Otsuka Pharmaceutical | 99.3 | JISHA |
| 32 | 1,3-Dimethyl-2,4-pyrimidinedione | 874–14-6 | Otsuka Pharmaceutical | 99.6 | JISHA |
| 33 | 1-(Ethoxymethyl)-5-fluoro-pyrimidine-2,4-dione | 57610–22-7 | Otsuka Pharmaceutical | 99.7 | JISHA |
| 34 | 3-(1-Ethoxymethyl-5-fluoro-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-3-yl)carbonylbenzoic acid | 129971–17-1 | Otsuka Pharmaceutical | 99 | JISHA |
| 35 | 3-(3-Benzyloxycarbonylbenzoyl)-1-ethoxymethyl-5-fluoro-2,4-pyrimidinedione | NR | Otsuka Pharmaceutical | 99.8 | JISHA |
| 36 | 1-Hydroxybenzotriazole hydrate | 123333–53-9 | Otsuka Chemical | 99 | BML |
| 37 | 3H-[1,2,3]Triazolo[4,5-b]pyridin-3-ol | 39968–33-7 | Tokyo Chemical Industry | 99 | BML |
| 38 | 1-[Bis (dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate | 148893–10-1 | Sigma-Aldrich | 99 | BML |
| 39 | Methylcarbamoyl-phenyloxadiazole | 1374817–07-8 | Shionogi | 98.77 | CERI |
| 40 | 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride n-hydrate | 3945-69-5 | Tokuyama | 84.8 | BML |
| 41 | N-Phenylbis(trifluoromethanesulfonimide) | 37595–74-7 | Tokyo Chemical Industry | 99.9 | SNBL |
| 42 | 1,1,1-Trifluoro-N-phenylmethanesulfonamide | 456–64-4 | Tokyo Chemical Industry | 99.9 | SNBL |
| 43 | Perfluoro-1-butanesulfonyl fluoride | 375–72-4 | Funakoshi | > 90 | BML |
| 44 | Diisopropyl sulfate | 2973-10-6 | Tokyo Chemical Industry | 97 | BML |
| 45 | Methyl p-toluenesulfonate | 80–48-8 | Kanto Chemical | 98 | BML |
| 46 | Ethyl trifluoromethanesulfonate | 425–75-2 | Tokyo Chemical Industry | 99.8 | SNBL |
| 47 | 2-Nitrobenzenesulfonyl chloride | 1694-92-4 | Mitsubishi Tanabe Pharma | 100.1 | Koei Techno |
| 48 | p-Toluenesulfonyl chloride | 98–59-9 | Tokyo Chemical Industry | 99 | BML |
| 49 | 4,6-Dibromo-3-fluoro-2-methylbenzoyl chloride | 11916–28-8 | Otsuka Pharmaceutical | 99.18 | JISHA |
| 50 | Benzyl 3-chloroformylbenzoate | 67852–96-4 | Otsuka Pharmaceutical | 99.3 | JISHA |
| 51 | 3-(1-Ethoxymethyl-5-fluoro-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-3-yl)carbonylbenzoyl chloride | 1380098–51-0 | Otsuka Pharmaceutical | 91.1 | JISHA |
| 52 | 6-(3-Chloro-2-hydroxypropoxy)-2(1H)-quinolinone | 128669–85-8 | Otsuka Pharmaceutical | 95.18 | JISHA |
| 53 | Chloroacetonitrile | 107–14-2 | Tokyo Chemical Industry | 99.9 | LSI Medience |
| 54 | 1-Bromohexane | 111–25-1 | Tokyo Chemical Industry | 99.8 | BSRC |
| 55 | 2-Chloro-N-methoxy-N-methylacetamide | 67442–07-3 | Tokyo Chemical Industry | 99.9 | CMIC Pharma Science |
| 56 | Ethyl 5-chloro-2-[2-(trifluoromethyl)phenyl]pentanimidate HCl | 1123197–78-3 | Eisai | 97.8 | UBE |
| 57 | Liothyronine sodium | 55–06-1 | Acros Organics | 95 | Taisho |
| 58 | (4-Bromo-3,5-dimethoxyphenyl)methanol | 61367–62-2 | Eisai | 100 | UBE |
| 59 | Ethyl (4,6-dibromo-3-fluoro-2-methylbenzoyl)acetate | 119916–30-2 | Otsuka Pharmaceutical | 98.88 | JISHA |
| 60 | Catena-m-[2-ethoxycarbonyl-3-(4,6-dibromo-3-fluoro-2-methylphenyl)-3-oxidoacrylato(2-)-O,O′,O″,O″′]magnesium (II) | NR | Otsuka Pharmaceutical | 87.2 | JISHA |
| 61 | Methyl 4,6-dibromo-3-fluoro-2-methylbenzoate | 119916–08-4 | Otsuka Pharmaceutical | 99.72 | JISHA |
| 62 | 4,6-Dibromo-3-fluoro-2-methylbenzoic acid | 11916–27-7 | Otsuka Pharmaceutical | 98.79 | JISHA |
| 63 | Sodium 4,6-dibromo-3-fluoro-2-methylbenzoate | NR | Otsuka Pharmaceutical | 91.79 | JISHA |
| 64 | Ethyl 2-(4,6-dibromo-3-fluoro-2-methyl benzoyl)-3-cyclopropylaminopropenoate | NR | Otsuka Pharmaceutical | 99.95 | JISHA |
| 65 | Ethyl 2-(4,6-dibromo-3-fluoro-2-methylbenzoyl)-3-ethoxypropenoate | NR | Otsuka Pharmaceutical | 100 | JISHA |
| 66 | Cinnamyl 3-aminocrotonate | 113898–97-8 | Otsuka Pharmaceutical | 98.4 | JISHA |
| 67 | Cinnamyl acetoacetate | 57582–46-4 | Otsuka Pharmaceutical | 99.4 | JISHA |
| 68 | Benzyl hydrogen isophthalate | 113266–88-9 | Otsuka Pharmaceutical | 100 | JISHA |
| 69 | Sodium benzyl isophthalate | NR | Otsuka Pharmaceutical | 95.1 | JISHA |
| 70 | Dibenzyl isophthalate | 16034–14-3 | Otsuka Pharmaceutical | 99 | JISHA |
| 71 | Diethyl phosphoryl chloride | 814–49-3 | Tokyo Chemical Industry | 99 | BML |
| 72 | Bis(diphenylphosphino)ferrocene | 12150–46-8 | Hokko Chemical | 99.4 | BML |
| 73 | Phosphorus (III) bromide | 7789–60-8 | Tokyo Chemical Industry | 98 | BML |
| 74 | Triethyl phosphonoacetate | 867–13-0 | Tokyo Chemical Industry | 98.4 | BML |
| 75 | Dicyclohexyl(2′,6′-dimethoxybiphenyl-2-yl)phosphine | 657408–07-6 | Johnson Matthey | 100 | BML |
| 76 | 2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (XPhos) | 564483–18-7 | Mitsubishi Tanabe Pharma | 99.87 | Koei Techno |
| 77 | Zinc cyanide | 557–21-1 | Alfa Aesar | 98.9 | BSRC |
| 78 | 3-Cyano-2,6-dihydroxypyidine monosodium salt | 91467–46-8 | Otsuka Pharmaceutical | 98.3 | JISHA |
| 79 | 3-Cyano-2,6-dihydroxypyridine | 35441–10-2 | Otsuka Pharmaceutical | 99.8 | JISHA |
| 80 | 6-Benzoyloxy-3-cyano-2-hydroxypyridine | 103941–70-4 | Otsuka Pharmaceutical | 100 | JISHA |
| 81 | Ethyl oxoacetate | 924–44-7 | Weylchem | 99.7 | FDSC |
| 82 | 2-Fluoro-3-hydroxy-5-methoxybenzaldehyde | 883576–31-6 | Eisai | 99.3 | UBE |
| 83 | 4-Bromobenzaldehyde | 1122-91-4 | Tokyo Chemical Industry | 99.9 | FDSC |
| 84 | 4-Pentyn-1-ol | 5390-04-5 | Avra Synthesis | 97.85 | LSI Medience |
| 85 | (tert-Butoxycarbonyl)hydrazide | 870–46-2 | Shanghai Unibest Biopharma | 84.8 | BML |
| 86 | 4,6-Dibromo-3-methoxycarbonyl-2-methylbenzenediazonium tetrafluoroborate | NR | Otsuka Pharmaceutical | 93.2 | JISHA |
| 87 | 9-Fluorenylmethyl alcohol | 24324–17-2 | Tokyo Chemical Industry | 99.9 | BSRC |
| 88 | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide HCl | 25952–53-8 | Toyobo | 99 | BML |
| 89 | Benzamidoxime | 613–92-3 | Shionogi | > 98 | Koei Techno |
| 90 | Carbethoxymethyl-dimethylsulfonium bromide | 5187-82-6 | Apollo Scientific | 96.5b | FDSC |
| 91 | 3,4-Dihydro-2H-pyran | 110–87-2 | Tokyo Chemical Industry | 99 | BML |
| 92 | (2S)-2-[(tert-Butoxycarbonyl)amino]hexanedioic acid dimethyl ester | 615258–01-0 | Eisai | 96.9 | UBE |
| 93 | tert-Butyl 2-acryloylhydrazine-1-carboxylate | 28689–14-7 | Eisai | 99.9 | UBE |
| 94 | [4-(Hydroxymethyl)-2,6-dimethoxyphenyl]boronic acid | 332394–37-3 | Eisai | 99.9 | UBE |
| 95 | Triethylsilane | 617–86-7 | Tokyo Chemical Industry | > 98 | BML |
| 96 | 1,3-Butanediol | 107–88-0 | Daicel | 99.8 | Nihon Bioresearch |
| 97 | Ammonium acetate | 631–61-8 | Wako Pure Chemical Industries | 100 | CERI |
| 98 | p-Toluenesulfinic acid sodium salt | 7257-26-3 | Tokyo Chemical Industry | 99.7 | BML |
| 99 | 2,2,6,6-Tetramethylpiperidine 1-oxyl (free radical) | 2564-83-2 | Tokyo Chemical Industry | 99.7 | SNBL |
BSRC Biosafety Research Center, Foods, Drug and Pesticides, CERI Chemicals Evaluation and Research Institute, FDSC Hatano Research Institute, Food and Drug Safety Center, JISHA Japan Industrial Safety and Health Association, SNBL Shin Nippon Biomedical Laboratories, UBE UBE Scientific Analysis Laboratory, NR not registered
aPubChem Compound ID
bpurified after purchase
Table 2.
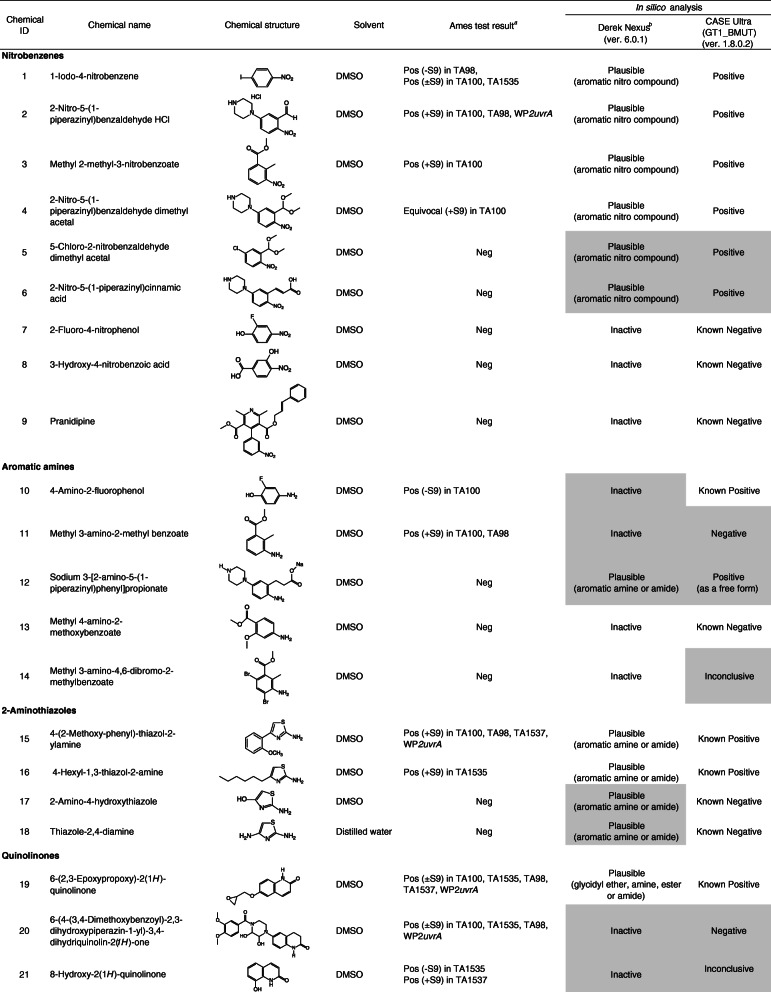
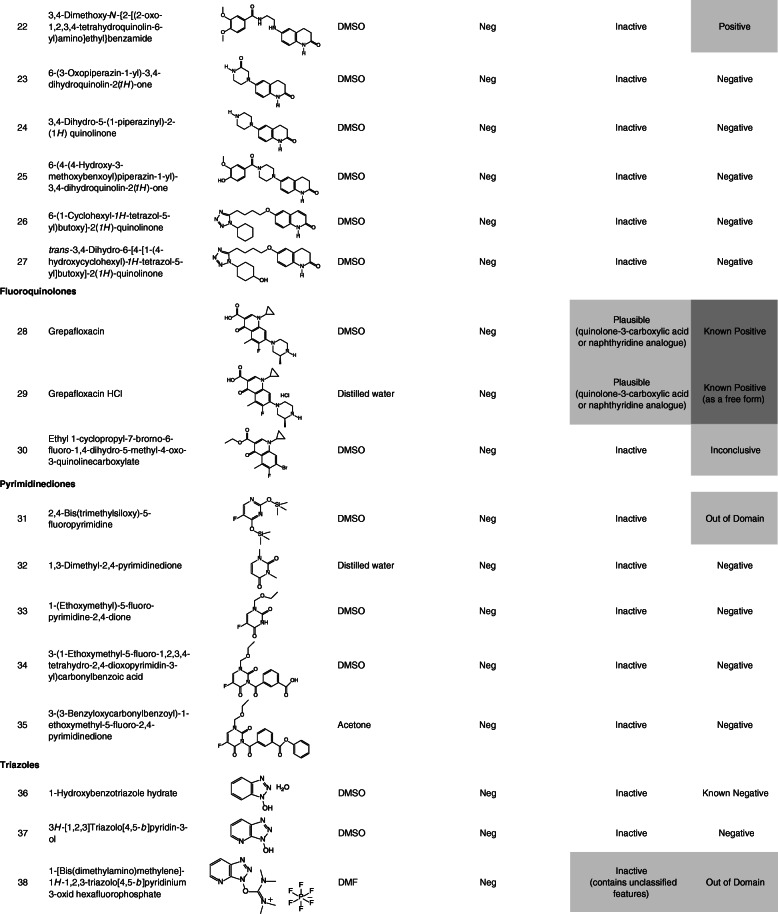
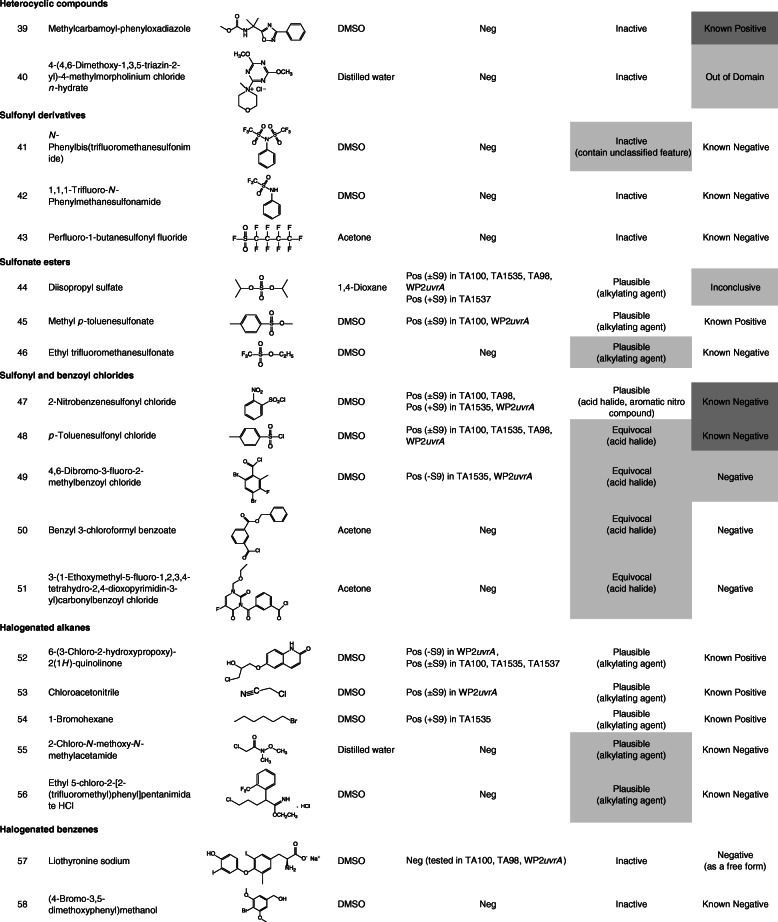
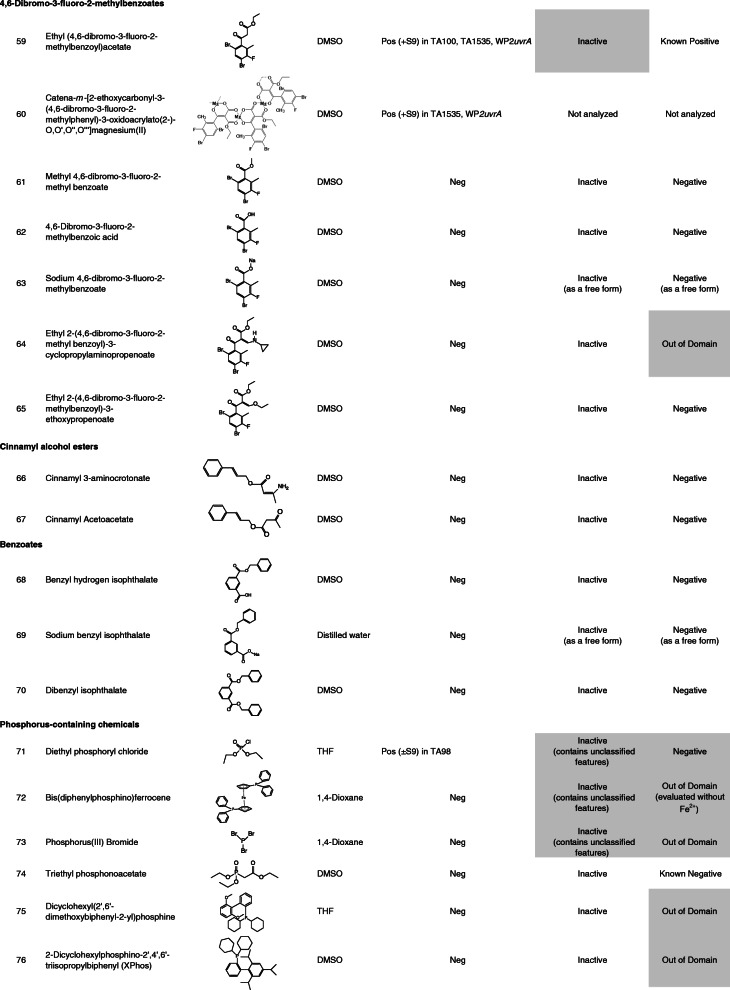
Chemical ID, chemical name, chemical structure, solvent used, Ames test result, and in silico analysis
S9 fraction, prepared from the liver of phenobarbital/5,6-benzoflavone-pretreated male Sprague-Dawley rats, was purchased from Oriental Yeast (Tokyo, Japan) or Kikkoman Biochemifa (Chiba, Japan). The S9 mix consisted of 10% (v/v) S9 fraction (approximately 1.0 mg protein/plate), 8 mM MgCl2, 33 mM KCl, 5 mM glucose-6-phosphate, 4 mM NADPH, 4 mM NADH, and 100 mM sodium phosphate (pH 7.4).
Bacterial strains
Four strains of Salmonella typhimurium, namely TA100, TA1535, TA98, and TA1537, and one strain of Escherichia coli, either WP2uvrA or WP2uvrA/pKM101 (for chemical IDs 21, 56, 58, 82, 93, and 94), were used in each Ames test. Chemical ID 57 was tested using only TA100, TA98, and WP2uvrA. These tester strains are recommended for use in bacterial mutagenicity test by the Organisation for Economic Cooperation and Development (OECD) test guideline 471 [3].
Ames test
All Ames tests were conducted using the preincubation method [9, 10]. Briefly, frozen stock cultures of each strain were inoculated into a conical flask or L-tube containing nutrient broth medium (2.5% w/v; Oxoid Nutrient Broth No.2, Hampshire, UK), and then cultured in a shaking incubator at 37 °C to obtain bacterial cells in the early stationary phase. The cell density of each culture was confirmed to be > 1 × 109 cells/mL. For the tests carried out in the absence of S9 mix, 0.1 mL of the negative (vehicle) control solution, test chemical solution at various concentrations, or positive control solution was added to a test tube, to which 0.5 mL of 100 mM sodium phosphate buffer (pH 7.4) and 0.1 mL of bacterial culture were added. For the tests carried out in the presence of S9 mix, S9 mix was added in place of phosphate buffer. After mixing, the test tubes were preincubated at 37 °C for 20 min in a shaking water bath. After completion of the preincubation, the treatment mixture was immediately added and mixed with 2 mL of 0.05 mM L-histidine/0.05 mM D-biotin molten top agar (for Salmonella strains) or 0.05 mM L-tryptophan (for E. coli strains), and the content was poured onto a plate of minimal-glucose agar medium. The plates were incubated at 37 °C for approximately 48 h, and the revertant colonies that appeared were counted. The sign of bacterial background lawn was examined as an indicator of cytotoxicity. In addition, the presence or absence of a precipitate of the test chemical was checked. When acetone, tetrahydrofuran, N,N-dimethylformamide, or 1,4-dioxane was used as the solvent, 0.05 mL of the vehicle was added to the test tube.
Multiple tests (dose-finding test, main test, or confirmatory test) were conducted for 86 chemicals. For 13 chemicals, a single test was conducted, and a clear conclusion was drawn. All tests were carried out in duplicate (two plates per dose) or triplicate (three plates per dose), except for chemical ID 96 in dose-finding tests (single plate per dose). All solvents used were of high purity and were appropriate for use in Ames test.
Ames test data were generated in-house or in several Japanese contract research organizations in compliance with the Good Laboratory Practice (GLP), except for chemical IDs 47 and 57 (Table 1, Supplementary Tables).
Mutagenicity was evaluated according to the so-called “two-fold” rule [11]. The test chemical was judged to be positive (mutagenic) if the following criteria were satisfied: (1) the maximum number of revertants was two-fold or more relative to the negative (vehicle) control, (2) a dose-dependent increase in the number of revertants was observed, and (3) the results were reproducible between each test (if tests were conducted twice or thrice). Historical negative control counts in each laboratory were also considered for evaluation. Only chemical ID 4 was judged to be equivocal; although there was a clear dose-response relationship with reproducibility, the maximum number of revertants exceeded the upper limit of the historical negative control range, which was less than two-fold higher than the concurrent negative control counts.
In silico analyses
Chemicals were analyzed using a knowledge-based model [Derek Nexus (Derek), ver. 6.0.1; Lhasa Limited, Leeds, UK] and a statistics-based model (CASE Ultra, GT1_BMUT, ver. 1.8.0.2; MultiCASE Inc., OH, USA).
Results and discussion
The data for 99 chemicals, including four chemicals in the free and salt forms (chemical IDs 28 and 29, 62 and 63, 68 and 69, 78, and 79, respectively), were collected by the task force. The four pairs of these chemicals showed the same (negative) result with a similar toxicity between each pair, except for a pair of chemical IDs 28 and 29. Individual data are shown in Supplementary Tables. Table 2 lists the summarized Ames test and in silico analysis data of the test chemicals, which were arranged according to chemical classes. One-third of these chemicals were included in the training set for the latest version of CASE Ultra (where chemicals are presented as “Known positive” or “Known negative” in Table 2). The test chemicals were classified into the following chemical classes: nitrobenzenes, aromatic amines, 2-aminothiazoles, quinolinones, fluoroquinolones, pyrimidinediones, triazoles, heterocyclic compounds, sulfonyl derivatives, sulfonate esters, sulfonyl and benzoyl chlorides, halogenated alkanes, halogenated benzenes, 4,6-dibromo-3-fluoro-2-methylbenzoates, cinnamyl alcohol esters, benzoates, phosphorus-containing compounds, cyanides, aldehydes, and miscellaneous.
Structure-activity relationships
Although some chemical classes have only a few chemicals, we discuss the structure-activity (mutagenicity) relationships in relation to structural alerts.
Nitrobenzenes
The structure of nitroarenes is a representative alert for mutagenicity, although the simplest nitroarene nitrobenzene itself is not mutagenic [12–16]. All Ames-positive nitrobenzene derivatives were predicted to be mutagenic by both in silico models; however, in the present study, approximately half of the nitrobenzenes (5/9 chemicals) were non-mutagenic. The mutagenicity of nitroarenes can be generated through the reduction of the nitro moiety to the corresponding N-hydroxylamines by bacterial nitroreductase, and therefore can be efficiently detected in the absence of S9 mix [12–16]. Interestingly, chemical IDs 2–4 were mutagenic or equivocal only in the presence of S9 mix. One possible reason for nitrobenzene mutagenesis is the nitroreduction inside bacterial cells after oxidative metabolism in the S9 mix [15, 16].
Aromatic amines
The structure of aromatic amines is also a representative indicator of mutagenicity [12–14]. The primary mechanism of mutagenicity by aromatic amines is known to be the production of N-hydroxylations, typically by the CYP 1A2 enzyme, followed by O-esterification with acetate or sulfate [17, 18]. In this study, several aromatic amines (3/5 chemicals) were not mutagenic. Some substituents that generate electronic and/or steric effects probably inhibit mutagenicity through inhibition of drug-metabolizing enzymes involved and/or decreased stability of the nitrenium ion intermediate that was generated through cleavage of the N-O bond of esterified N-hydroxylamines and form adducts with DNA, leading to mutations [18, 19]. The mutagenicity of chemical ID 10 is probably due to reactive para-iminoquinone, which does not require metabolic enzymes.
2-Aminothiazoles
The 2-aminothiazoles tested, which were five-membered aromatic amines containing hetero atoms of sulfur in position 1 and nitrogen in position 3, were half mutagenic (2/4 chemicals) and half non-mutagenic (2/4 chemicals), with a diverse substituent at position 4. 2-Aminothiazoles were all predicted to be mutagenic (as “Plausible” by Derek) through identification of the structural alerts of aromatic amines or amides. 2-Aminothiazole is mutagenic, and the mutagenicity of 2-aminothiazoles is induced via the formation of reactive nitrenium ion intermediates, such as aromatic amines [19–21]. The presence of a substituent at position 4 may enhance or reduce the mutagenicity of 2-aminothiazole.
Quinolinones
The six quinolinone derivatives (chemical IDs 22–27) were non-mutagenic, whereas the other three were mutagenic. The quinolinone structure was not an alert, as shown by both in silico models. Chemical ID 19 was mutagenic, probably because of the presence of epoxide. The mutagenicity of chemical ID 20 may be derived from the dihydroxylated piperazine moiety. Chemical ID 21, an 8-hydroxy derivative of quinolinone, was mutagenic only in TA1535, and TA1537, which shows a small number of negative control counts and is empirically known to be sensitive to some structures.
Fluoroquinolones
The mutagenicity of fluoroquinolones was dependent on WP2uvrA, WP2uvrA/pKM101, or TA102, which have an AT base pair at the primary reversion site [1–3]. Fluoroquinolone antibiotics, including grepafloxacin, were reported to be mutagenic in TA102 [22] and WP2uvrA/pKM101 [23], and the positive result was used as a training set in CASE Ultra. However, in this study, where WP2uvrA was used, the three fluoroquinolone derivatives, including grepafloxacin (chemical ID 28) and grepafloxacin HCl (chemical ID 29), were all non-mutagenic.
The difference of cytotoxicity (reduction in bacterial background lawn) in the two forms (chemical IDs 28 and 29) was much more than would be expected by normal variation. It may be worth looking at the role of the different solvents, including water and DMSO.
Pyrimidinediones
The five pyrimidinedione derivatives were all non-mutagenic. Both in silico models predicted these chemicals to be inactive/negative except for one chemical called the “Out of Domain” owing to the presence of two trimethylsilyl moieties, as shown by CASE Ultra. The structure of pyrimidinedione should not be an alert for mutagenicity.
Triazoles
All three triazole derivatives were non-mutagenic. Both in silico models predicted that these chemicals were inactive/negative except for the “Inactive containing unclassified features” and “Out of Domain” owing to the presence of a tertiary amine moiety, as shown by Derek and CASE Ultra, respectively. The structure of triazole is unlikely to be an indicator of mutagenicity.
Heterocyclic compounds
The two heterocyclic compounds, derivatives of oxadiazole (chemical ID 39) and 1,3,5-triazine (chemical ID 40), were both non-mutagenic. The finding that chemical ID 39 was non-mutagenic was not consistent with the “known positive” from CASE Ultra.
Sulfonyl derivatives
The three sulfonyl derivatives were all non-mutagenic, which was consistent with that in both in silico models, although Derek identified an unclassified feature of sulfonimide in chemical ID 41. The structure of the sulfonyl moiety is not an alert for mutagenicity.
Sulfonate esters
Chemical IDs 44 and 45 were both mutagenic, and this result was consistent with the results of both in silico models. Several sulfonate esters are well-known to be alkylating mutagens, and predicted as “plausible” mutagens by Derek. However, chemical ID 46 was not mutagenic. The mutagenic potency of sulfonates is dependent on both the leaving group and alkylsulfonate moiety, affecting their chemical reaction rate [24, 25] and chemoselectivity [26, 27]. A probable reason for them being non-mutagenic is the rapid hydrolysis (instability) of ethyl trifluoromethanesulfonate [28]. The alertness of some sulfonate esters can be improved by incorporating the chemical properties.
Sulfonyl and benzoyl chlorides
The two sulfonyl chlorides (chemical IDs 47 and 48) and benzoyl chloride (chemical ID 49) were mutagenic in the presence or absence of S9 mix. Dimethyl sulfoxide (DMSO) was used as the solvent. It was reported that when DMSO was used to dissolve sulfonyl chlorides or acyl chlorides (including benzoyl chlorides), these chemicals showed mutagenicity (or false positive results) due to the generation of mutagenic impurity (chlorodimethyl sulfide) in the test chemical formulations, with a few exceptions [29, 30]. Derek predicted sulfonyl and benzoyl chlorides to be equivocal, the definition of which is that there is evidence for and against being mutagenic. These chemicals may not be mutagenic with organic solvents other than DMSO, such as acetone, where sulfonyl and acyl chlorides are stable. Water is probably not appropriate as a solvent, because these chemicals are generally unstable. Further tests on chemical IDs 47–49 are necessary to draw the correct conclusions. Nevertheless, the data presented here may be valuable as data examples when using solvents inappropriate for this chemical class. The other two benzoyl chlorides, chemical IDs 50 and 51, were correctly judged to be non-mutagenic and dissolved in acetone.
Halogenated alkanes
Halogenated alkanes (halogen atoms excluding fluorine) can be alkylating mutagens without requiring metabolic activation. Similar to that of sulfonate esters, their mutagenic activity is dependent on the alkyl moieties and the leaving group of halogen ions. A possible reason why chemical IDs 55 and 56 were non-mutagenic is that the DNA adduct was not formed via inhibition of the SN2 reaction through steric hindrance by the bulky substituent around the carbon center adjacent to the chlorine atom. In this study, chemical ID 54 with a long alkyl chain (hexyl moiety) and a leaving group of bromine ions is marginally positive only in TA1535, which shows a low number of negative control counts in the presence of S9 mix, although n-butyl chloride with a shorter alkyl moiety is reported to be non-mutagenic [31]. Primary alkyl bromides with chains longer than the hexyl moiety are probably non-mutagenic.
Halogenated benzenes
The two halogenated benzenes were non-mutagenic. Chemical ID 57 was tested with three test strains, TA100, TA98, and WP2uvrA; the strains TA100 and TA98 were most sensitive among the five strains that are recommended for use by OECD test guideline 471 [3]. Halogenated benzenes are unlikely to be structural alerts for mutagenicity, as supported by Derek.
4,6-Dibromo-3-fluoro-2-methylbenzoates
Five 4,6-dibromo-3-fluoro-2-methylbenzoate derivatives (chemical IDs 61 to 65) were non-mutagenic, and Derek and CASE Ultra did not show alerts for this structure. Therefore, the structure of 4,6-dibromo-3-fluoro-2-methylbenzoate is not an indicator of mutagenicity. The mutagenicity of chemical ID 59 might involve the enol (tautomerized) form of the 1,3-diketone moiety, followed by epoxidation of the double bond by the drug-metabolizing enzyme in S9 mix. The substitution at position 2 of the 1,3-diketone moiety may inhibit tautomerization, but not lead to the induction of mutagenicity (chemical IDs 64, 65). It remains unclear why chemical ID 60 was mutagenic. Mutagenicity may be associated with the magnesium-oxygen complex.
Cinnamyl alcohol esters
Both cinnamyl esters were non-mutagenic, as predicted by both in silico models. A double bond conjugated with a benzene ring is unlikely to be a structural indicator of mutagenicity.
Benzoates
All benzoates were non-mutagenic, as predicted by both in silico models.
Phosphorus-containing chemicals
Phosphorus-containing chemicals were all non-mutagenic except for chemical ID 71, which is electrophilic and routinely used in organic synthesis for the phosphorylation of amines [32]. For many of the phosphorus-containing chemicals tested, neither of the in silico models were able to make a definite, positive/negative prediction; the reference to negative by Derek contained unclassified features, and CASE Ultra called “Out of Domain”. This indicates that phosphorus-containing chemicals are outside the applicability domain because of the limited number of training set examples for each in silico model.
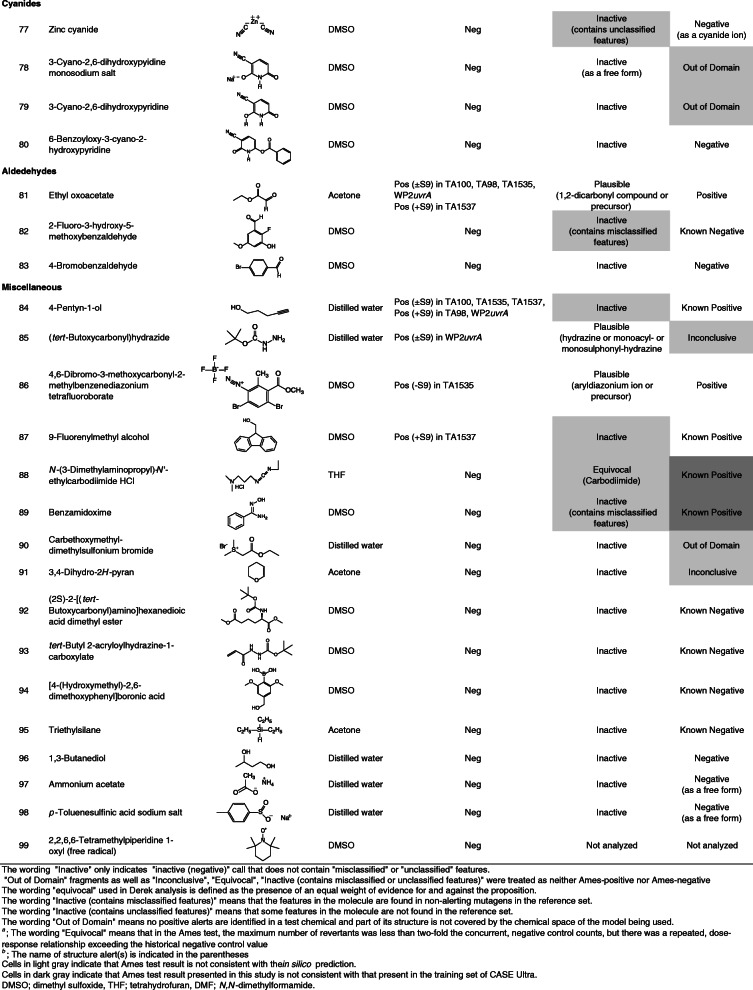
Cyanides
Cyanide ion (Chemical ID 77) and all the cyanide derivatives substituted with an aromatic ring were non-mutagenic. The cyanide moiety is unlikely to be a structural alert for mutagenicity, as supported by Derek.
Aldehydes
Chemical ID 81, an aldehyde conjugated with a single carbonyl moiety, was mutagenic, as predicted by both in silico models. The chemical properties of aldehydes largely differ between aliphatic and aromatic compounds; generally, the former is chemically reactive, whereas the latter is stable. Both aromatic aldehydes (chemical IDs 82 and 83) were non-mutagenic, which can be explained by the extremely low chemical reactivity of aromatic aldehydes.
Miscellaneous
The miscellaneous group consists of chemicals that cannot be simply classified into the above chemical classes. Many of the chemicals tested were non-mutagenic. Chemical ID 84 was mutagenic in the presence and absence of S9 mix, although there were no structural alerts identified by Derek. The cause of the mutagenicity is unclear, but aldehyde might be involved in the induction of mutagenicity, which may be generated from alcohol by the alcohol dehydrogenase present in bacteria [33]. The three chemicals (chemical IDs 85–87) were mutagenic. Chemical IDs 85 and 86 were mutagenic only in WP2uvrA and TA1535, respectively. Both chemicals were predicted to be mutagenic (Derek; Plausible, CASE Ultra; Inconclusive or Positive) by both in silico models. Chemical ID 87 was only mutagenic in TA1537, which would be a tester strain sensitive to some chemical structures, with a small number of negative control counts.
In silico analyses
To calculate the sensitivity, specificity, and accuracy of in silico predictions, ten chemicals (chemical IDs 29, 47–49, 57, 60, 63, 69, 78, and 99) were excluded. Four chemicals tested in both forms were used for calculation in the free form (chemical IDs 28, 62, 68, and 79), but not in the salt form (chemical IDs 29, 63, 69, and 78). Chemical IDs 47–49 were false positive because probable inappropriate solvents were used. Chemical ID 57 was tested in only three strains (TA100, TA98 and WP2uvrA). For chemical IDs 60 and 99, the in silico models could not reach a conclusion because the former is a complex molecule and the latter is a radical. We treated “Out of Domain” fragments as well as “Inconclusive”, “Equivocal”, “Inactive (contains misclassified or unclassified features)”, as neither Ames-positive nor Ames-negative in this study.
In silico analysis using Derek (ver. 6.0.1) revealed the sensitivity, specificity, and accuracy to be 65% (15/23), 71% (47/66), and 70% (62/89), respectively. In contrast, in silico analysis using CASE Ultra (GT1_BMUT, ver. 1.8.0.2) revealed the sensitivity, specificity, and accuracy to be 50% (6/12), 60% (25/42), and 57% (31/54), respectively. Thus, Derek outperformed CASE Ultra (GT1_BMUT) in the predictive level of bacterial mutagenicity for all the parameters in this study, where the limited number of chemicals were compared.
Derek and Case Ultra occasionally called “inactive containing misclassified or unclassified features” (8 chemicals), and “Out of Domain” fragments (10 chemicals), respectively, indicating the need to expand the training or reference set for each in silico model to improve.
It is worth noting that when considering the performance of the in silico models, it is important to account for the ICH M7 approach of combining two complementary systems and an expert review to take a final decision rather than considering them separately [5, 34].
Inconsistency with training set examples
The 35 chemicals (15 “known” positives and 20 “known” negatives) were part of the training set for CASE Ultra. The results for 4 of 35 chemicals (11%) did not agree with the known response for those chemicals in that training set. The four chemicals (chemical IDs 28, 39, 88, and 89) were non-mutagenic but were registered as mutagens in the training set for CASE Ultra. This disagreement ratio (11%) was in the same range as the Ames test non-reproducibility, identified by Piegorsch and Zeiger, who reported a value of approximately 13% [35]. The reasons why the Ames test evaluations did not match were mainly some differences in the test conditions (e.g., plate-incorporation method vs. preincubation method, the type of strains used, source of test strains, preparation of overnight culture), and evaluation criteria (e.g., two-fold rule vs. statistical analysis), and quality of test substances [10, 11, 36].
Two chemicals (chemical IDs 47 and 48) were mutagenic but were registered as non-mutagens in the CASE Ultra training set. This is probably because the solvent used in our study was not appropriate, as previously stated (see the section of “Sulfonyl and benzoyl chlorides” in the Structure-activity relationships section. Our data, together with individual data (Supplementary Tables), provide additional information and will help in reevaluating the Ames test data.
Test strains to detect bacterial mutagens
In this study, 28 chemicals, including three sulfonyl and benzoyl chlorides (chemical IDs 47 to 49) were mutagenic. Among them, three chemicals (chemical IDs 16, 54, and 86), two chemicals (chemical IDs 21 and 87), two chemicals (chemical IDs 53 and 85), and two chemicals (chemical IDs 49 and 60), respectively, were only detected for mutagenicity in either TA1535, TA1537, WP2uvrA, or both TA1535 and WP2uvrA. Williams et al. [36] reported that 93% of bacterial mutagens can be detected with a combination of TA100 and TA98. However, the data of the present study show that only 19 out of 28 chemicals (68%) were detected either by TA100 or TA98. Therefore, the test strains TA1535, TA1537, and WP2uvrA may be useful for the efficient detection of bacterial mutagenicity.
Conclusion
Ames test data were presented for 99 chemicals from eight pharmaceutical companies through the activity of the Ames data sharing task force. The chemicals were related to the manufacturing process of pharmaceutical drugs, including reagents, synthetic intermediates, and drug substances. The Ames test data presented herein will contribute to avoiding duplicated Ames test in some cases, supporting duplicate testing in other cases, improving in silico models, and enhancing our understanding of the mechanisms of mutagenesis.
Supplementary Information
Acknowledgments
We are grateful to Dr. K. Koyama and M. Kurakami of Eisai Co., Ltd. for the in silico analyses and helpful comments on the manuscript, respectively. We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- 9AA
9-aminoacridine
- 2AA
2-aminoanthracene
- B[a]P
Benzo[a]pyrene
- ICR-191
6-chloro-9-[3-(2-chloroethylamino)propylamino]-2-methoxyacridine dihydrochloride
- DMSO
Dimethyl sulfoxide
- AF-2
2-(2-furyl)-3-(5-nitro-2-furyl)acrylamide
- GLP
Good Laboratory Practice
- ICH
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- JPMA
Japan Pharmaceutical Manufacturers Association
- OECD
Organisation for Economic Cooperation and Development
Authors’ contributions
AH analyzed the chemicals using Derek and drafted and edited the manuscript. AH, TA, TS, AO, MY, KK, HO, YD, SO, KS, TK, and EY prepared the Supplementary Tables and reviewed the manuscript. All authors approved the final manuscript.
Funding
All Ames tests were funded or sponsored by each company that participated in this task force. Funding for open access to this publication was provided by the Japan Pharmaceutical Manufacturers Association.
Availability of data and materials
All Ames data are available in the Supplementary Tables. Materials are not applicable.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Atsushi Hakura, Email: a-hakura@hhc.eisai.co.jp.
Takumi Awogi, Email: Awogi.Takumi@otsuka.jp.
Toshiyuki Shiragiku, Email: Shiragiku.Toshiyuki@otsuka.jp.
Atsushi Ohigashi, Email: atsushi.oohigashi@astellas.com.
Mika Yamamoto, Email: mika.yamamoto@astellas.com.
Kayoko Kanasaki, Email: kayoko.kanasaki@shionogi.co.jp.
Hiroaki Oka, Email: hiro-oka@taiho.co.jp.
Yasuaki Dewa, Email: yasuaki.dewa@mb.kyorin-pharm.co.jp.
Shunsuke Ozawa, Email: shunsuke.ozawa@mb.kyorin-pharm.co.jp.
Kouji Sakamoto, Email: ko-sakamoto@taisho.co.jp.
Tatsuya Kato, Email: katou.tatsuya@ma.mt-pharma.co.jp.
Eiji Yamamura, Email: yamamura.eiji@md.mt-pharma.co.jp.
References
- 1.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 2.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455(1-2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 3.OECD . Guideline for the Testing of Chemicals: Bacterial Reverse Mutation Test No. 471 OECD Environment, Health and Safety Publications Series on Testing and Assessment Organization for Economic Cooperation and Development, Paris. 2020. [Google Scholar]
- 4.ICH Harmonized Tripartite Guideline, “Guidance on Genotoxicity testing and data interpretation for pharmaceuticals intended for human use”, S2 (R1), current step 4 version, 2011. https://database.ich.org/sites/default/files/S2_R1_Guideline.pdf.
- 5.Harmonized Tripartite Guideline ICH. “Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk”, M7 (R1), current step 4 version. 2017. [Google Scholar]
- 6.Landry C, Kim MT, Kruhlak NL, Cross KP, Saiakhov R, Chakravarti S, Stavitskaya L. Transitioning to composite bacterial mutagenicity models in ICH M7 (Q) SAR analyses. Regul Toxicol Pharmacol. 2019;109:104488. doi: 10.1016/j.yrtph.2019.104488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benigni R, Bossa C. Data-based review of QSARs for predicting genotoxicity: the state of the art. Mutagenesis. 2019;34(1):17–23. doi: 10.1093/mutage/gey028. [DOI] [PubMed] [Google Scholar]
- 8.Honma M. An assessment of mutagenicity of chemical substances by (quantitative) structure-activity relationship. Genes Environ. 2020;42(1):23. 10.1186/s41021-020-00163-1. [DOI] [PMC free article] [PubMed]
- 9.Kato M, Sugiyama K, Fukushima T, Miura Y, Awogi T, Hikosaka S, Kawakami K, Nakajima M, Nakamura M, Sui H, Watanabe K, Hakura A. Negative and positive control ranges in the bacterial reverse mutation test: JEMS/BMS collaborative study. Genes Environ. 2018;40(1):7. doi: 10.1186/s41021-018-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy DD, Hakura A, Elespuru RK, Escobar PA, Kato M, Lott J, Moore MM, Sugiyama K. Demonstrating laboratory proficiency in bacterial mutagenicity assays for regulatory submission. Mutat Res. 2019;848:403075. doi: 10.1016/j.mrgentox.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Levy DD, Zeiger E, Escobar PA, Hakura A, van der Leede BM, Kato M, Moore MM, Sugiyama K. Recommended criteria for the evaluation of bacterial mutagenicity data (Ames test) Mutat Res. 2019;848:403074. doi: 10.1016/j.mrgentox.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Kazius J, McGuire R, Bursi R. Derivation and validation of toxicophores for mutagenicity prediction. J Med Chem. 2005;48(1):312–320. doi: 10.1021/jm040835a. [DOI] [PubMed] [Google Scholar]
- 13.Benigni R. Structure-activity relationship studies of chemical mutagens and carcinogens: mechanistic investigations and prediction approaches. Chem Rev. 2005;105(5):1767–1800. doi: 10.1021/cr030049y. [DOI] [PubMed] [Google Scholar]
- 14.Benigni R, Bossa C. Mechanisms of chemical carcinogenicity and mutagenicity: a review with implications for predictive toxicology. Chem Rev. 2011;111(4):2507–2536. doi: 10.1021/cr100222q. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu M, Yano E. Mutagenicity of mono-nitrobenzene derivatives in the Ames test and rec assay. Mutat Res. 1986;170(1-2):11–22. doi: 10.1016/0165-1218(86)90077-7. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki J, Takahashi N, Kobayashi Y, Miyamae R, Ohsawa M, Suzuki S. Dependence on Salmonella typhimurium enzymes of mutagenicities of nitrobenzene and its derivatives in the presence of rat-liver S9 and norharman. Mutat Res. 1987;178(2):187–193. doi: 10.1016/0027-5107(87)90268-5. [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Guengerich FP. Cytochrome P450 activation of arylamines and heterocyclic amines. Annu Rev Pharmacol. 2005;45(1):27–49. doi: 10.1146/annurev.pharmtox.45.120403.100010. [DOI] [PubMed] [Google Scholar]
- 18.Gadaleta D, Manganelli S, Manganaro A, Porta N, Benfenati E. A knowledge-based expert rule system for predicting mutagenicity (Ames test) of aromatic amines and azo compounds. Toxicology. 2016;370:20–30. doi: 10.1016/j.tox.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Bentzien J, Hickey ER, Kemper RA, Brewer ML, Jane D, Dyekjær JD, East SP, Whittaker M. An in silico method for predicting Ames activities of primary aromatic amines by calculating the stabilities of nitrenium ions. J Chem Inf Model. 2010;50(2):274–297. doi: 10.1021/ci900378x. [DOI] [PubMed] [Google Scholar]
- 20.McCarren P, Bebernitz GR, Gedeck P, Glowienke S, Grondine MS, Kirman LC, Klickstein J, Schuster HF, Whitehead L. Avoidance of the Ames test liability for aryl-amines via computation. Bioorg Med Chem. 2011;19(10):3173–3182. doi: 10.1016/j.bmc.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 21.Seifried HE, Seifried RM, Clarke JJ, Junghans TB, San RHC. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol. 2006;19(5):627–644. doi: 10.1021/tx0503552CCC. [DOI] [PubMed] [Google Scholar]
- 22.Yim G, McClure J, Surette MG, Davies JE. Modulation of Salmonella gene expression by subinhibitory concentrations of quinolones. J Antibiotics. 2011;64(1):73–78. doi: 10.1038/ja.2010.137. [DOI] [PubMed] [Google Scholar]
- 23.Hayasaki Y, Itoh S, Kato M, Furuhama K. Mutagenesis induced by 12 quinolone antibacterial agents in Escherichia coli WP2uvrA/pKM101. Toxicol in Vitro. 2006;20(3):342–346. doi: 10.1016/j.tiv.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Hakura A, Mizuno Y, Goto M, Kawazoe Y. Studies on chemical carcinogens and mutagens. XXXV. Standardization of mutagenic capacities of several common alkylating agents based on the concentration-time integrated dose. Chem Pharm Bull. 1986;34(2):775–780. doi: 10.1248/cpb.34.775. [DOI] [PubMed] [Google Scholar]
- 25.Hakura A, Kawazoe Y. Studies on chemical carcinogens and mutagens. XXXVI. Apparent activation energy for mutagenic modification induced in E. coli by alkylating agents. Estimation from mutation frequency. Chem Pharm Bull. 1986;34(4):1728–1734. doi: 10.1248/cpb.34.1728. [DOI] [PubMed] [Google Scholar]
- 26.Kawazoe Y, Tamura N, Yoshimura T. Studies on chemical carcinogens. XXIII. A simple method for characterization of the alkylating ability of compounds by using 4-(p-nitrobenzyl)pyridine. Chem Pharm Bull. 1982;30(6):2077–2086. doi: 10.1248/cpb.30.2077. [DOI] [PubMed] [Google Scholar]
- 27.Snodin DJ. Residues of genotoxic alkyl mesylates in mesylate salt drug substances: real or imaginary problems? Regul Toxicol Pharmacol. 2006;45(1):79–90. doi: 10.1016/j.yrtph.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Streitwieser A, Jr, Wilkins C, Kiehlmann E. Kinetics and isotope effects in solvolyses of ethyl trifluoromethanesulfonate. J Am Chem Soc. 1968;90(6):1598–1601. doi: 10.1021/ja01008a601. [DOI] [Google Scholar]
- 29.Amberg A, Harvey JS, Czich A, Spirkl H-P, Robinson S, White A, Elder DP. Do carboxylic/sulfonic acid halides really present a mutagenic and carcinogenic risk as impurities in final drug products? Org Process Res Dev. 2015;19(11):1495–1506. doi: 10.1021/acs.oprd.5b00106. [DOI] [Google Scholar]
- 30.Mancuso AJ, Swern D. Activated dimethyl sulfoxide: useful reagents for synthesis. Synthesis. 1981;1981(3):165–185. doi: 10.1055/s-1981-29377. [DOI] [Google Scholar]
- 31.Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environmental Mutagenesis. 1987;9(supplement 9):1–109. doi: 10.1002/em.2860090602. [DOI] [PubMed] [Google Scholar]
- 32.Nikolaides N, Schipor I, Ganem B. Conversion of amines to phospho esters: decyl diethyl phosphate. Org Synth. 1995;72:246. doi: 10.15227/orgsyn.072.0246. [DOI] [Google Scholar]
- 33.Jokelainen K, Siitonen A, Jousimies-Somer H, Nosova T, Heine R, Salaspuro M. In vitro alcohol dehydrogenase-mediated acetaldehyde production by aerobic bacteria representing the normal colonic flora in man. Alcoholism. 1996;20(6):967–972. doi: 10.1111/j.1530-0277.1996.tb01932. [DOI] [PubMed] [Google Scholar]
- 34.Foster RS, Fowkes A, Cayley A, Thresher A, Werner AD, Barber CG, Kocks G, Tennant RE, Williams RV, Kane S, Stalford SA. The importance of expert review to clarify ambiguous situations for (Q) SAR predictions under ICH M7. Genes Environ. 2020;42(1):27. doi: 10.1186/s41021-020-00166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piegorsch WW, Zeiger E. Measuring intra-assay agreement for the Ames Salmonella assay. In: Hothorn L, editor. Statistical methods in toxicology. Lecture notes in medical informatics. Heidelberg: Springer; 1991. pp. 35–41. [Google Scholar]
- 36.Williams RV, DeMarini DM, Stankowski LF, Jr, Escobar PA, Zeiger E, Howe J, Elespuru R, Cross KP. Are all bacterial strains required by OECD mutagenicity test guideline TG471 needed? Mutat Res. 2019;848:503081. doi: 10.1016/j.mrgentox.2019.503081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All Ames data are available in the Supplementary Tables. Materials are not applicable.