Abstract
The commercially available diagnostic tests for Chagas’ disease employ whole extracts or semipurified fractions of Trypanosoma cruzi epimastigotes. Considerable variation in the reproducibility and reliability of these tests has been reported by different research laboratories, mainly due to cross-reactivity with other pathogens and standardization of the reagents. The use of recombinant antigens for the serodiagnosis of Chagas’ disease is recommended to increase the sensitivity and specificity of serological tests. Expressed in Escherichia coli, as fusion products with glutathione S-transferase, six T. cruzi recombinant antigens (H49, JL7, A13, B13, JL8, and 1F8) were evaluated in an enzyme-linked immunosorbent assay for Chagas’ disease. The study was carried out with a panel of 541 serum samples of chagasic and nonchagasic patients from nine countries of Latin America (Argentina, Bolivia, Brazil, Chile, Colombia, El Salvador, Guatemala, Honduras, and Venezuela). The optimal concentration of each recombinant antigen for coating of plates was determined with the help of 125I-labelled recombinant proteins. While the specificity of the epimastigote antigen was 84% because of false positives from leishmaniasis cases, for the recombinant antigens it varied from 96.2 to 99.6%. Recombinant antigens reacted with 79 to 100% of serum samples from chronic chagasic patients. In this way, it is proposed that a mixture of a few T. cruzi recombinant antigens should be employed in a diagnostic kit to minimize individual variation and promote high sensitivity in the diagnosis of Chagas’ disease.
Chagas’ disease is caused by the protozoan parasite Trypanosoma cruzi, widespread on the American continent. It is estimated that 16 to 18 million people are currently infected and that approximately 90 million individuals living in areas where Chagas’ disease is endemic are at risk of contracting T. cruzi infection. T. cruzi is normally transmitted through the feces of infected triatomid bugs. Control of vectorial transmission has been successfully achieved in several Southern Cone countries (26), and blood transfusion is now the main way of acquiring the disease in these countries (25, 30). The seroprevalence of Chagas’ disease in blood donors is about 51% in Santa Cruz (Bolivia), 5.6% in Buenos Aires (Argentina), 0.18 to 12.4% in Chile, 0.7% in Brazil, and 5.3% in Paraguay (5, 30). With increasing migrations of people from areas where Chagas’ disease is endemic to more developed countries there is the possibility that blood transfusion transmission of Chagas’ disease may become a worldwide problem (13, 25).
Detection of anti-T. cruzi antibodies, by serological methods, is still the main method for the diagnosis of Chagas’ disease. The commercially available diagnostic tests are based on the use of the whole extracts or semipurified fractions of the epimastigotes of T. cruzi (the noninfective form of the parasite), with a considerable variation in the reproducibility and reliability of the results obtained with different methods (1). Cross-reactivity to T. cruzi has been observed mainly in patients with leishmaniasis (3) or Trypanosoma rangeli infection (11). Furthermore, some chagasic patients can present negative results by classical serology (17). This scenario clearly indicates the need for better-defined antigens in the serodiagnosis of Chagas’ disease (19).
In recent years, recombinant DNA technology has been used to isolate and characterize T. cruzi antigens which are immunodominant in human infections (4, 6, 7, 10, 14, 21). The evaluation of some defined antigens has shown promising results for the diagnosis of Chagas’ disease (2, 12, 15, 16, 22–24, 31). The first coordinated study to evaluate the diagnostic potential of recombinant antigens was undertaken by the World Health Organization (19). An assessment of 17 recombinant antigens was carried out in a double-blind multicenter study with a limited number of sera that were sent to the participating laboratories. The sera were tested by using the immunoassays developed by each laboratory. This study allowed the ranking of the diagnostic recombinant antigens. In addition, this experience also showed that a given antigen could be assay restricted and while performing well in the laboratory of origin it could be less sensitive and/or specific when applied in alternative assay techniques. Consequently, the need to evaluate the potentiality of some of these antigens by standardized protocols and immunoassays has emerged.
In this direction, the Project of Biotechnology of the Programa Iberoamericano de Ciencia y Tecnologia para el Desarrollo organized a collaborative study for the serological evaluation of 10 recombinant antigens from three laboratories in South America. The reactivities of the recombinant antigens were initially assayed in a reference laboratory by using a phage dot blot immunoassay (15). The data confirmed that several recombinant antigens were specifically recognized by a great majority of chagasic sera and proved that they may constitute the basis of a new generation of diagnostic reagents. However, the phage dot blot immunoassay is not suitable for serodiagnosis under routine conditions, since it requires the preabsorption of human sera with Escherichia coli extracts, to avoid any cross-reaction (15).
To overcome this problem, in the present study six T. cruzi recombinant proteins in fusion with glutathione S-transferase (GST) were expressed in bacteria and used to develop an enzyme-linked immunosorbent assay (ELISA). The selected antigens (H49, JL7, B13, and JL8) are composed of tandem amino acid repeats and showed high sensitivity, specificity, and positive and negative predictive values in previous studies (15, 19). We also included in this study two nonrepetitive antigens, A13 (21) and 1F8 (9). The determination of the diagnostic efficiency of the ELISA for the six recombinant antigens was carried out with 541 serum samples from nine South and Central America countries (Argentina, Brazil, Bolivia, Chile, Colombia, El Salvador, Guatemala, Honduras, and Venezuela). This study reinforces the feasibility and importance of performing studies in order to standardize and improve the generation of new diagnostic reagents for Chagas’ disease serodiagnosis.
MATERIALS AND METHODS
Study population.
Serum samples from 541 individuals were used in this study: 304 from chronic chagasic seropositive patients and 237 from nonchagasic seronegative patients. Sera were collected in nine countries of South and Central America: 37 from Argentina (32 chagasic and 5 nonchagasic), 42 from Bolivia (32 chagasic and 10 nonchagasic), 178 from Brazil (38 chagasic and 140 nonchagasic), 49 from Chile (29 chagasic and 20 nonchagasic), 46 from Colombia (26 chagasic and 20 nonchagasic), 49 from El Salvador (49 chagasic), 46 from Guatemala (36 chagasic and 10 nonchagasic), 47 from Honduras (32 chagasic and 15 nonchagasic), and 47 from Venezuela (30 chagasic and 17 nonchagasic). The 140 nonchagasic Brazilian patients included (i) 50 blood donors from an area where Chagas’ disease is endemic; (ii) 50 patients with unrelated diseases, as defined by their respective clinical, epidemiological, and serological diagnoses of their respective pathologies (1 patient infected with T. rangeli, 5 with toxoplasmosis, 4 with malaria, 4 with paracoccidioidomycosis, 5 with schistosomiasis, 8 with syphilis, 16 with connective tissue diseases and who were positive for antinuclear antibodies, and 7 with rheumatic fever); and (iii) 40 patients with active visceral leishmaniasis from a region where Chagas’ disease is nonendemic, of whom 85% had samples that cross-reacted with T. cruzi antigens in the conventional serology. Aliquots of all sera were stored in buffered glycerol, pH 7.2 (vol/vol), as a stabilizer for freezer storage at −20°C to avoid protein damage in the freeze-thaw cycles (28).
Recombinant antigens.
The recombinant proteins selected for this study were H49, JL7, A13, B13, JL8, and 1F8 (Table 1). These antigens are similar or identical to ones cloned by other laboratories and that had received different denominations (6, 19; Table 1). Antigens H49 and JL7 are similar to each other and consist, respectively, of 4.5 and 3.6 tandemly arranged repeated sequences of 68 amino acids (4, 14). Antigens B13 (10) and JL8 (14) are composed of tandem repetitions of 12 and 18 amino acids, respectively. Antigen 1F8 (8, 9) is a 24-kDa flagellar calcium-binding protein, and antigen A13 (21) is a polypeptide of about 28.5 kDa.
TABLE 1.
Characteristics of the recombinant antigens
| Antigen | Repeata | Size of the insert (bp) | No. of repeats | Size of the fusion protein (kDa) | Reference |
|---|---|---|---|---|---|
| H49 | 68 | 978 | 4.5 | 63.5 | 4 |
| JL7 | 68 | 747 | 3.6 | 58.0 | 14 |
| B13 | 12 | 600 | 16.6 | 51.5 | 10 |
| JL8 | 14 | 351 | 6.7 | 42.1 | 14 |
| A13 | None | 755 | 56.0 | 21 | |
| 1F8 | None | 600 | 51.5 | 9 |
Number of amino acid repeats.
Subcloning and purification of fusion proteins.
The inserts encoding T. cruzi antigens were isolated from different vectors, subcloned into the EcoRI site of plasmid pGEX-A, and expressed as fusion polypeptides with the Schistosoma japonicum GST (EC 2.5.1.18). The fusion proteins were purified by affinity chromatography on glutathione-agarose beads as previously described (22). The purity and specificity of the recombinant proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with a pool of chagasic sera. Protein contents were quantified in the antigenic preparation by using the Macro-BCA protein assay reagent kit (Pierce).
T. cruzi epimastigote antigen.
Whole antigenic extracts from T. cruzi epimastigotes were prepared as described previously (29). Briefly, fresh epimastigotes, Y strain, grown in a liquid medium (liver infusion tryptose) were incubated in 0.3 N NaOH for 18 h at 4°C. After neutralization with 0.3 N HCl, the cellular lysate was centrifuged at 12,000 × g for 1 min. The protein concentration was estimated in the supernatant by using the Macro-BCA protein assay reagent kit. Aliquots of the epimastigote antigen were stored at −70°C.
ELISA.
ELISAs using the whole extract of epimastigote (epiELISA) and recombinant purified fusion proteins (recELISA) were performed as described previously (29). The optimal concentrations of serum, antigens, and conjugate were determined by checkerboard titration. Aliquots of 50 μl of epimastigote extract (6 μg ml−1) or recombinant antigens were incubated in 96-well flat-bottom microtiter plates (Nunc, PolySorp, Roskilde, Denmark) in a 0.05 M carbonate-bicarbonate buffer, pH 9.6, for 18 h at 4°C. The unbound material was discarded, and plates were blocked for 1 h with phosphate-buffered saline-Tween 20 (0.05%) (PBS-T) containing 5% defatted milk (Nestlé). The sera (50 μl) were added, diluted 1:50 (recELISA) or 1:200 (epiELISA) in PBS-T–1% milk, and incubated for 1 h at 37°C. After five washes in PBS-T–5% milk, peroxidase-conjugated goat anti-human immunoglobulin G (IgG) (Sigma), diluted 1:6,000 in PBS, was added, and the mixture was incubated for 1 h at 37°C. After new cycles of washes the immune complexes were revealed by the addition of hydrogen peroxide and O-phenylenediamine dihydrochloride. After 30 min of incubation at 37°C in the dark, the reaction was stopped with 4 N HCl, and the absorbance at 492 nm was determined in a Labsystem Multiskan MS plate ELISA reader. All the experiments were carried out in duplicate and repeated at least twice on different days.
Data analysis.
The figures of samples recorded as optical densities at 492 nm (OD492) were distributed by using computer graphics software. The cutoff values of each antigen in ELISA were calculated as the mean OD492 of sera from 30 blood donors plus 3 standard deviations (SD). Cutoff values were established for each antigen to obtain the highest possible sensitivity. The values of sensitivity and specificity were calculated as described before (15).
RESULTS
Development of recombinant antigen-based serodiagnosis by ELISA.
T. cruzi recombinant antigens were produced as GST fusion proteins and purified by affinity chromatography on gluthatione-agarose beads. Yields of the purified proteins were between 10 and 34 mg per liter of culture. The relative molecular masses of the fusion proteins were as expected from the sizes of the coding regions of the corresponding insert plus 27.5 kDa for the GST (Table 1). All recombinant antigens were specifically recognized by human chronic chagasic antibodies in a Western blotting assay (data not shown).
Control experiments were first performed to check the specificity and sensitivity of the ELISA with each one the recombinant antigens. The ideal concentration of each antigen to coat the microtiter plates was determined by using recombinant antigens labelled with 125I and purified by chromatography on Sephadex G-25 columns. The optimal coating concentration of recombinant protein per well was 15 ng for H49, 100 ng for JL7, 20 ng for A13, 25 ng for B13, 100 ng for JL8, 50 ng for 1F8, and 50 ng for the control GST. The specificity of the antibody binding was confirmed by performing competitive inhibition assays with homologous and heterologous recombinant antigens (data not shown).
Sensitivity and specificity of the recombinant antigens.
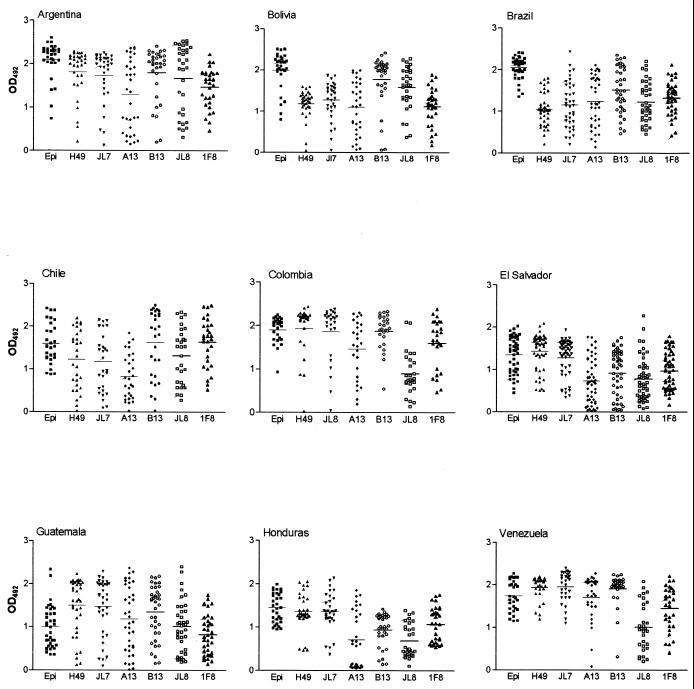
IgG survey in the serum samples of 304 chagasic patients showed that the values of positivity (Table 2 and Fig. 1) varied according to the recombinant antigen and the geographical origin of the patient. The global analysis of 304 chronic chagasic sera (Tables 2 to 4) showed that the lowest and highest positivity values were presented by A13 antigen (87%) and JL7 and 1F8 antigens (99%) (Table 2). The epimastigote antigen (epiELISA) showed the highest OD492 arithmetic mean and sensitivity (mean ± SD; sensitivity) (1.66 ± 0.53 [100%]) followed by the recombinant antigens H49 (1.47 ± 0.57 [97.7%]), B13 (1.47 ± 0.66 [93.4%]), JL7 (1.45 ± 0.59 [97.4%]), 1F8 (1.18 ± 0.53 [99%]), JL8 (1.11 ± 0.64 [93.8%]), and A13 (1.11 ± 0.69 [87.1%]) (Tables 3 and 4; Fig. 1).
TABLE 2.
Percent positivity obtained in recELISA with recombinants proteins and in epiELISA for chronic chagasic patients from different countries
| Country | No. of patients | Positivity (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| H49 | JL7 | A13 | B13 | JL8 | 1F8 | Epimastigote | ||
| Argentina | 32 | 97 | 97 | 88 | 94 | 100 | 100 | 100 |
| Bolivia | 32 | 94 | 97 | 84 | 94 | 100 | 97 | 100 |
| Brazil | 38 | 97 | 97 | 97 | 100 | 100 | 100 | 100 |
| Chile | 29 | 93 | 93 | 97 | 97 | 100 | 100 | 100 |
| Colombia | 26 | 96 | 96 | 100 | 100 | 88 | 100 | 100 |
| El Salvador | 49 | 100 | 100 | 82 | 79 | 82 | 98 | 100 |
| Guatemala | 36 | 97 | 97 | 89 | 95 | 89 | 97 | 100 |
| Honduras | 32 | 100 | 100 | 88 | 91 | 97 | 100 | 100 |
| Venezuela | 30 | 100 | 100 | 97 | 100 | 97 | 100 | 100 |
| TOTAL | 304 | 97 | 99 | 87 | 93 | 94 | 99 | 100 |
FIG. 1.
Distribution of OD492 data for sera from chagasic patients from different countries. The recombinant proteins used in recELISA were H49, JL7, A13, B13, JL8, and 1F8 and in epiELISA the epimastigote extract (Epi). The horizontal line inside the drops for each recombinant antigen represents the arithmetic mean.
TABLE 4.
OD492 obtained by recELISA and epiELISA with sera from chronic chagasic patients from different countries
| Group | Country | OD492 (mean ± SD) for:
|
||||||
|---|---|---|---|---|---|---|---|---|
| H49 | JL7 | A13 | B13 | JL8 | 1F8 | Epimastigote | ||
| 1 | Argentina | 1.82 ± 0.54 | 1.74 ± 0.58 | 1.29 ± 0.80 | 1.79 ± 0.61 | 1.67 ± 0.77 | 1.48 ± 0.44 | 2.10 ± 0.42 |
| Bolivia | 1.18 ± 0.34 | 1.28 ± 0.46 | 1.04 ± 0.63 | 1.77 ± 0.60 | 1.58 ± 0.52 | 1.11 ± 0.44 | 1.99 ± 0.45 | |
| Brazil | 1.05 ± 0.39 | 1.16 ± 0.55 | 1.25 ± 0.60 | 1.51 ± 0.55 | 1.23 ± 0.48 | 1.32 ± 0.39 | 2.05 ± 0.25 | |
| Chile | 1.22 ± 0.68 | 1.17 ± 0.68 | 0.84 ± 0.52 | 1.63 ± 0.77 | 1.32 ± 0.65 | 1.63 ± 0.56 | 1.60 ± 0.47 | |
| 2 | Colombia | 1.94 ± 0.58 | 1.86 ± 0.63 | 1.46 ± 0.66 | 1.82 ± 0.41 | 0.90 ± 0.49 | 1.59 ± 0.57 | 1.90 ± 0.30 |
| Venezuela | 1.94 ± 0.28 | 1.95 ± 0.35 | 1.70 ± 0.52 | 1.91 ± 0.38 | 1.00 ± 0.56 | 1.45 ± 0.51 | 1.74 ± 0.36 | |
| 3 | El Salvador | 1.45 ± 0.42 | 1.30 ± 0.42 | 0.75 ± 0.52 | 0.93 ± 0.53 | 0.79 ± 0.52 | 0.99 ± 0.43 | 1.37 ± 0.40 |
| Guatemala | 1.51 ± 0.64 | 1.48 ± 0.64 | 1.19 ± 0.75 | 1.35 ± 0.61 | 1.01 ± 0.60 | 0.83 ± 0.46 | 1.00 ± 0.49 | |
| Honduras | 1.33 ± 0.46 | 1.33 ± 0.46 | 0.72 ± 0.64 | 0.91 ± 0.40 | 0.67 ± 0.38 | 1.05 ± 0.40 | 1.46 ± 0.34 | |
| Total | 1.47 ± 0.57 | 1.45 ± 0.59 | 1.11 ± 0.69 | 1.47 ± 0.66 | 1.11 ± 0.64 | 1.18 ± 0.53 | 1.66 ± 0.53 | |
TABLE 3.
Diagnostic performance of recELISA and epiELISA for IgG detection
| ELISA | % Detection
|
|||||||
|---|---|---|---|---|---|---|---|---|
| H49 | JL7 | A13 | B13 | JL8 | 1F8 | GST | Epimastigote | |
| Sensitivitya | 97.7 | 97.4 | 87.1 | 93.4 | 93.8 | 99.0 | 4.2 | 100 |
| Specificityb | 100 | 99.5 | 100 | 99.5 | 95.4 | 99.5 | 98.5 | 99.5 |
| Specificityc | 97.5 | 96.6 | 99.6 | 99.2 | 96.2 | 99.6 | 98.7 | 84 |
A total of 304 chronic patients.
A total of 197 nonchagasic patients (excluding those with visceral leishmaniasis).
A total of 237 nonchagasic patients (including those with visceral leishmaniasis).
The specificities of recombinant antigens were analyzed with 237 serum samples from nonchagasic patients, including healthy individuals and individuals with other parasitic or unrelated diseases (see Materials and Methods). The epimastigote antigen displayed a specificity of 99.5% when assayed with 197 nonchagasic sera, excluding patients with leishmaniasis (Table 3). However, when 40 serum samples from individuals with active visceral leishmaniasis were included in this study the specificity of the epimastigote antigen diminished to 84%. These results confirm previous observations on the presence of cross-reacting antigens between T. cruzi and Leishmania spp. (3, 18). The specificity values of the recombinant proteins for the 237 nonchagasic sera, including the leishmaniasis patients, varied from 96.2% (JL8) to 99.2 to 99.6% (B13 and A13-1F8). The recombinant antigens H49 and JL7 reacted weakly with six and seven serum samples from patients with leishmaniasis, respectively. No cross-reaction was detected with antigens 1F8 and JL8. Taken together, our results indicate that the recombinant antigens are more specific for the detection of anti-T. cruzi antibodies than for that of the whole epimastigote antigen.
Control experiments performed with GST, the support protein from S. japonicum present in the recombinant protein, showed a sensitivity of 4.2% (with 304 chronic chagasic sera) and a specificity of 98.7% (with sera from 237 nonchagasic individuals). A previous report indicates that a strong homology exists between GSTs from S. japonicum and Schistosoma mansoni (27). Therefore, we investigated whether sera of individuals infected with S. mansoni, who have a high prevalence in some areas where Chagas’ disease is endemic, reacted with this GST. Interestingly GST did not react with sera from patients with S. mansoni infection. From these results we concluded that the reactivity of the recombinant peptides was specific for the T. cruzi component, since only some chagasic and nonchagasic individuals reacted, at low titers, with GST, the portion of the fusion protein that is not related to T. cruzi (Table 3).
Geographic focus and variations in the performance of the recombinant proteins.
Strain variation and host-related factors (genetic background, history of exposure, etc.) may be responsible for the heterogeneity of recognition of the T. cruzi antigens in the conventional serology. An important question is if these factors could also affect the performance of the recombinant antigens. Data presented in Table 2 show that there is some variation in the positivities of the recombinant antigens. To better investigate this aspect, data obtained with sera from patients in different geographic regions were analyzed independently (Fig. 1 and 2; Tables 2 and 4).
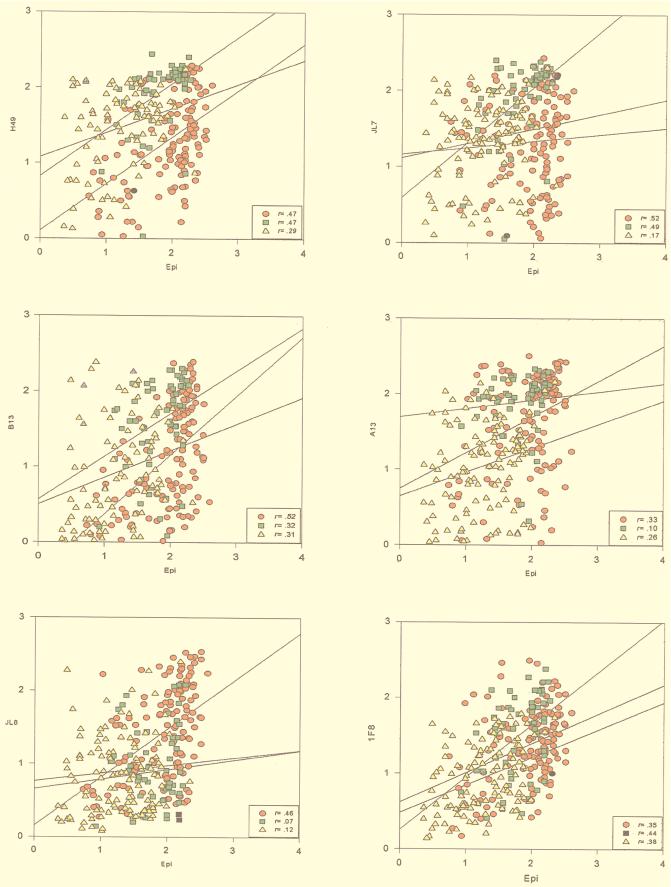
FIG. 2.
Linear regression distribution of OD492 data of recombinant antigens H49, JL7, A13, B13, JL8, and 1F8 and epimastigote extract (Epi) in sera from chagasic patients and correlation coefficient (r) values for group 1 (Argentina, Bolivia, Brazil, and Chile) (circles), group 2 (Colombia and Venezuela) (squares), and group 3 (El Salvador, Guatemala, and Honduras) (triangles).
Figure 1 shows the distribution of OD492 obtained with the epimastigote and recombinant antigens with serum samples from nine Latin American countries. As expected, ODs obtained with epiELISA were higher than those obtained with the recombinant antigens, which is probably due to the presence of a large number of antigenic determinants in the whole parasite extract. Only one exception to this rule was found with serum samples from Guatemala. No differential reactivity of epimastigote and recombinant antigens was observed with serum samples from this country (Fig. 1).
Analysis of linear data dispersion (Fig. 2) and the correlation coefficient (r) were calculated between epimastigote antigen and each recombinant antigen. Data were analyzed by grouping the countries in three groups: group 1 (Argentina, Bolivia, Brazil, and Chile), group 2 (Venezuela and Colombia), and group 3 (El Salvador, Guatemala, and Honduras) (Fig. 2). They suggest the existence of an association between the reactivity pattern of the recombinant antigens and the geographical origin of the serum samples (Fig. 1). The graphic of correlation dispersion between recombinant proteins compared with epimastigote antigen of sera from patients in Southern Cone countries (Argentina, Bolivia, Brazil, and Chile) presented higher r values for all the recombinant antigens. In groups 2 (Venezuela and Colombia) and 3 (El Salvador, Guatemala, and Honduras), the r values varied according to the antigen (Fig. 2).
DISCUSSION
Serological methods are widely accepted for the diagnosis of Chagas’ disease. The commercially available diagnostic tests are based on the whole or semipurified extracts of T. cruzi. The lack of specific and well-characterized antigens prepared under quality-control conditions has introduced a source of variability in the final reagent, and controversial results have been obtained with these reagents (1). To overcome this problem, we evaluated the diagnostic efficiencies of six recombinant antigens produced in the same bacterial expression system. The assays were carried out by the same laboratory with the ELISA, and the relative indices of sensitivity and specificity were evaluated with sera from 304 chagasic and 237 nonchagasic patients, collected in nine countries of Latin America. The OD492 were compared to those obtained with the whole extract of epimastigote forms.
Four recombinant antigens (1F8, H49, JL7, and B13) showed high sensitivities varying from 93.4 to 99%, and the mean OD492 was between 1.18 to 1.47. As expected, high levels of antibodies against repetitive amino acid antigens (H49, JL7, and B13) were found in a large number of individuals living in areas where Chagas’ disease is endemic. The performance of recombinant protein H49 was similar to that of antigen JL7, since they have the same antigenic repeats (4, 14). The sensitivity (99%) and specificity (99.6%) of 1F8 antigen are comparable to those of other repetitive antigens, indicating that chronic chagasic patients also display antibodies against nonrepetitive antigens. In order to improve the specificity of serodiagnostic tests, we believe that the nonrepetitive antigen 1F8 should be included in the multiantigen immunoassay. Available recombinant antigens react with 87 to 99% of the chronic chagasic sera, suggesting that the combination of two or more antigens to build up a multiantigen immunoassay may result in a truly reliable T. cruzi serodiagnostic test.
In this study the epimastigote antigen showed 100% sensitivity and a mean OD492 (± SD) of 1.66 ± 0.53. However, the specificity of the epimastigote antigen was lower (84%) than that of recombinant proteins which displayed specificity values between 96.2% (JL8) and 99.6% (A13, B13, and 1F8). The lower specificity of the epimastigote antigen is mainly due to cross-reacting epitopes between T. cruzi and Leishmania spp. (3, 18, 28). In fact, 34 (of 40) sera from leishmaniasis patients reacted with the epimastigote antigen. From these results, we conclude that one of the major advantages of the recELISA for the serodiagnosis of Chagas’ disease is the lack of cross-reaction with other parasitic diseases such as leishmaniasis.
Individual serum samples reacted to the various recombinant antigens in different ways. The most important variations in the ODs were found with antigens A13 and JL8, which presented coefficient of variation values of 63 and 57, respectively (data not shown). When positivity data obtained in different geographic areas were compared, the variation of recognition by isolated antigens was more marked, e.g., higher ODs were obtained with sera from patients in Colombia and Venezuela (group 2) than those in Southern Cone countries (group 1). An intense variation of the OD data could be observed among different countries, depending on the antigen used (Fig. 1). Strain heterogeneity and host-related factors (genetic background, history of exposure, etc.) may be responsible for the variability of recognition of the recombinant antigens. In fact T. cruzi is not a homogenous population of parasites; it is composed rather of a pool of subpopulations (strains) that present a high heterogeneity in biological parameters and genetic characteristics. It has been suggested that the variability among T. cruzi isolates in conjunction with the immunogenetic features of the human host may be responsible for the large spectrum of clinical manifestations of Chagas’ disease, such as the indeterminate form, the different degrees of cardiomyopathies, and the digestive forms (20).
Serum samples from chagasic patients reacted with, at least, one recombinant antigen, suggesting that a mixture of recombinant antigens could be able to detect anti-T. cruzi antibodies in all the serum samples used in this study. The positivity of a hypothetical antigenic mixture composed of the recombinant peptides H49 or JL7, B13, and 1F8 was calculated as 100%. We believe that the use of a cocktail of recombinant antigens will minimize the individual variation and a high sensitivity will be achieved. Different complementary antigens could be combined in a relatively simple immunoassay, and this combination may lead to the development of a multiantigen diagnostic kit standardization for the routine diagnosis of Chagas’ disease.
ACKNOWLEDGMENTS
We thank the following investigators for the generous gift of human sera: F. Guhl (Universidad de Los Andes, Colombia), J. C. P. Cortez (Bolivia), D. Henriquez (Universidad Simon Bolivar, Venezuela), L. M. Linares (El Salvador), V. Matta (Universidad de San Carlos, Guatemala), C. Ponce and E. Ponce (Lab. Central del Ministerio de Salud, Honduras), and A. Solari (Universidad de Chile, Chile). The statistical analysis was performed by L. P. Lima from Hospital Sírio-Libanes, São Paulo, Brazil.
This work was supported by grants from FAPESP (96/06736-1), CYTED (Ibero American Project of Biotechnology), International Atomic Energy Agency (IAEA), FMUSP-LIM49, and CNPq.
REFERENCES
- 1.Camargo M E, Segura E L, Kagan I G, Souza J M P, Carvalheiro J R, Yanovsky J F, Guimarães M C S. Collaboration on the standardization of Chagas’ disease in the Americas: an appraisal. Bull Pan Am Health Organ. 1986;20:233–244. [PubMed] [Google Scholar]
- 2.Carvalho M R, Krieger M A, Oeleman W, Shikanai-Yasuda M A, Ferreira A W, Pereira J B, Saez-Alquezar A, Dorlhiac-Llacer D F, Chamone D F, Goldenberg S. Chagas’ disease diagnosis: evaluation of several tests in blood bank screening. Transfusion. 1993;33:830–834. doi: 10.1046/j.1537-2995.1993.331094054620.x. [DOI] [PubMed] [Google Scholar]
- 3.Chiller T M, Samudio M, Zoulek G. IgG reactivity with Trypanosoma cruzi and leishmania antigens in sera of patients with Chagas’ disease and leishmaniasis. Am J Trop Med Hyg. 1990;43:650–656. doi: 10.4269/ajtmh.1990.43.650. [DOI] [PubMed] [Google Scholar]
- 4.Cotrim P C, Paranhos G P, Mortara R A, Wanderley J, Rassi A, Camargo M E, Franco da Silveira J. Expression in Escherichia coli of a dominant immunogen of Trypanosoma cruzi recognized by human chagasic sera. J Clin Microbiol. 1990;28:519–524. doi: 10.1128/jcm.28.3.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias J C P, Schofield C J. Controle da transmissão transfusional da doença de Chagas na iniciativa do Cone Sul. Rev Soc Bras Med Trop. 1998;31:373–383. doi: 10.1590/s0037-86821998000400007. [DOI] [PubMed] [Google Scholar]
- 6.Franco da Silveira J. Trypanosoma cruzi recombinant antigens for serodiagnosis. In: Wendel S, Brener Z, Camargo M E, Rassi A, editors. Chagas’ disease (American trypanosomiasis): its impact on transfusion and clinical medicine, ISBT Brazil 92. São Paulo, Brazil: Sociedade Brasileira de Hematologia e Hemoterapia; 1992. pp. 207–218. [Google Scholar]
- 7.Frasch A C C, Cazzulo J J, Aslund L, Pettersson U. Comparison of genes encoding Trypanosoma cruzi antigens. Parasitol Today. 1991;7:148–151. doi: 10.1016/0169-4758(91)90284-u. [DOI] [PubMed] [Google Scholar]
- 8.Godsel L M, Tibbetts R S, Olson C L, Chaudoir B M, Engman D M. Utility of recombinant flagellar calcium-binding protein for serodiagnosis of Trypanosoma cruzi infection. J Clin Microbiol. 1995;33:2082–2085. doi: 10.1128/jcm.33.8.2082-2085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez A, Lerner T J, Huecas M, Sosa-Pineda B, Nogueira N, Lizardi P M. Apparent generation of a segmented mRNA from two separate tandem gene families in Trypanosoma cruzi. Nucleic Acids Res. 1985;13:5789–5803. doi: 10.1093/nar/13.16.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber A, Zingales B. Trypanosoma cruzi: characterization of two recombinant antigens with potential application in the diagnosis of Chagas’ disease. Exp Parasitol. 1993;76:1–12. doi: 10.1006/expr.1993.1001. [DOI] [PubMed] [Google Scholar]
- 11.Guhl F, Hudson L, Marinkelle C J, Jaramillo C A, Bridge D. Clinical Trypanosoma rangeli infection as a complication of Chagas’ disease. Parasitology. 1987;94:475–484. doi: 10.1017/s0031182000055827. [DOI] [PubMed] [Google Scholar]
- 12.Krieger M A, Almeida E, Oelemann W, Lafaille J J, Pereira J B, Krieger H, Carvalho M R, Goldenberg S. Use of recombinant antigens for the accurate immunodiagnosis of Chagas’ disease. Am J Trop Med Hyg. 1992;46:427–434. doi: 10.4269/ajtmh.1992.46.427. [DOI] [PubMed] [Google Scholar]
- 13.Leiby D A, Read E J, Lenes B A, Yund A J, Stumpf R J, Kirchhoff L V, Dodd R Y. Seroepidemiology of Trypanosoma cruzi, etiologic agent of Chagas’ disease, in U.S. blood donors. J Infect Dis. 1997;176:1047–1052. doi: 10.1086/516534. [DOI] [PubMed] [Google Scholar]
- 14.Levin M J, Mesti E A, Benarous R, Levitus G, Schijman A, Levy-Yeyati P, Ruiz A, Kahan A, Rosembaum M B, Torres H N, Segura E L. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas’ heart disease. Am J Trop Med Hyg. 1989;41:530–539. doi: 10.4269/ajtmh.1989.41.530. [DOI] [PubMed] [Google Scholar]
- 15.Levin M J, Franco da Silveira J, Frasch A C C, Camargo M E, Lafon S, Degrave W M, Rangel-Aldao R. Recombinant antigens and Chagas’ disease diagnosis: analysis of a workshop. FEMS Microbiol Immunol. 1991;89:11–20. doi: 10.1111/j.1574-6968.1991.tb04965.x. [DOI] [PubMed] [Google Scholar]
- 16.Lorca M, Gonzalez A, Veloso C, Reyes V, Vergara U. Immunodetection of antibodies in sera from symptomatic and asymptomatic Chilean Chagas’ disease patients with Trypanosoma cruzi recombinant antigens. Am J Trop Med Hyg. 1992;46:44–49. doi: 10.4269/ajtmh.1992.46.44. [DOI] [PubMed] [Google Scholar]
- 17.Luquetti A O. Megaesofago e anticorpos anti-Trypanosoma cruzi. Rev Goiana Med. 1987;33:1–16. [Google Scholar]
- 18.Matsumoto T K, Hoshino-Shimizu S, Nakamura P M, Andrade H F, Jr, Umezawa E S. High resolution of Trypanosoma cruzi amastigote antigen in serodiagnosis of different clinical forms of Chagas’ disease. J Clin Microbiol. 1993;31:1486–1492. doi: 10.1128/jcm.31.6.1486-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncayo A, Luquetti A O. Multicentre double blind study for evaluation of Trypanosoma cruzi defined antigens as diagnostic reagents. Mem Inst Oswaldo Cruz. 1990;85:489–495. doi: 10.1590/s0074-02761990000400020. [DOI] [PubMed] [Google Scholar]
- 20.Montamat E E, de Luca Dóro G M, Gallenaro R H, Sosa R, Blanco A. Characterization of Trypanosoma cruzi populations by zymodemes: correlation with clinical picture. Am J Trop Med Hyg. 1996;55:625–628. doi: 10.4269/ajtmh.1996.55.625. [DOI] [PubMed] [Google Scholar]
- 21.Paranhos G S, Cotrim P C, Mortara R A, Rassi A, Corral R, Freilij H L, Grinstein S, Wanderley J, Camargo M E, Franco da Silveira J. Trypanosoma cruzi: cloning and expression of an antigen recognized by acute and chronic human chagasic sera. Exp Parasitol. 1990;71:284–293. doi: 10.1016/0014-4894(90)90033-9. [DOI] [PubMed] [Google Scholar]
- 22.Paranhos G S, Santos M R M, Cotrim P C, Rassi A, Jolivet M, Camargo M E, Franco da Silveira J. Detection of antibodies in sera from Chagas’ disease patients using a Trypanosoma cruzi immunodominant recombinant antigen. Parasite Immunol. 1994;16:165–169. doi: 10.1111/j.1365-3024.1994.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 23.Pastini A C, Iglesias S R, Carricarte V C, Guerin M E, Sanchez D O, Frasch A C. Immunoassay with recombinant Trypanosoma cruzi antigens potentially useful for screening donated blood and diagnosing Chagas’ disease. Clin Chem. 1994;40:1893–1897. [PubMed] [Google Scholar]
- 24.Peralta J M, Teixeira M D G M, Shreffler W G, Pereira J B, Burns J M, Jr, Sleath P R, Reed S G. Serodiagnosis of Chagas’ disease by enzyme-linked immunosorbent assay using two synthetic peptides as antigens. J Clin Microbiol. 1994;32:971–974. doi: 10.1128/jcm.32.4.971-974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmunis G A. Trypanosoma cruzi, the etiologic agent of Chagas’ disease: status in the blood supply in endemic and nonendemic countries. Transfusion. 1991;31:547–557. doi: 10.1046/j.1537-2995.1991.31691306255.x. [DOI] [PubMed] [Google Scholar]
- 26.Schofield C J, Dujardin J P. Chagas’ disease vector control in Central America. Parasitol Today. 1997;13:141–144. doi: 10.1016/s0169-4758(97)89811-0. [DOI] [PubMed] [Google Scholar]
- 27.Trottein F, Kieny M P, Verwaerde C, Torpier G, Pierce R J, Balloul J M, Schmitt D, Lecocq J, Capron A. Molecular cloning and tissue distribution of a 26 kilodalton Schistosoma mansoni glutathione S-transferase. Mol Biochem Parasitol. 1990;41:35–44. doi: 10.1016/0166-6851(90)90094-3. [DOI] [PubMed] [Google Scholar]
- 28.Umezawa E S, Nascimento M S, Kesper N, Jr, Coura J R, Borges-Pereira J, Junqueira A C V, Camargo M E. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas’ disease. J Clin Microbiol. 1996;34:2143–2147. doi: 10.1128/jcm.34.9.2143-2147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umezawa E S, Shikanai-Yasuda M A, Gruber A, Pereira-Chiocolla V L, Zingales B. Trypanosoma cruzi defined antigens in the serological evaluation of an outbreak of acute Chagas’ disease in Brazil (Catolé do Rocha, Paraíba) Mem Inst Oswaldo Cruz. 1996;91:87–93. doi: 10.1590/s0074-02761996000100015. [DOI] [PubMed] [Google Scholar]
- 30.Wendel S, Gonzaga A L. Chagas’ disease and blood transfusion: a new world problem? Vox Sang. 1993;64:1–12. doi: 10.1111/j.1423-0410.1993.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 31.Zingales B, Gruber A, Ramalho C B, Umezawa E S, Colli W. Use of two recombinant proteins of Trypanosoma cruzi in the serological diagnosis of Chagas’ disease. Mem Inst Oswaldo Cruz. 1990;85:519–522. doi: 10.1590/s0074-02761990000400024. [DOI] [PubMed] [Google Scholar]