Abstract
Background
Coronavirus disease 2019 (COVID-19) is a highly infectious and pathogenic respiratory disease. To date, there is no effective treatment, and there is an urgent need to develop vaccines against the virus. Five coronavirus COVID-19 vaccines have been approved for inoculation in China, with good safety and few adverse reactions.
Case presentation
A 50-year-old woman complained of bilateral blurred vision and visual distortion 5 days after vaccination with the inactivated COVID-19 vaccine. Physical and auxiliary examination showed that she developed bilateral posterior uveitis. The patient was administered local and systemic steroids, and the symptoms were appreciably improved 5 weeks later.
Conclusions
A case of bilateral uveitis after COVID-19 vaccination was reported and indicated that uveitis after vaccination appears transient and responds well to steroids.
Key Words: Coronavirus disease-2019, COVID-19 vaccine, uveitis, adverse reaction
Background
Coronavirus disease 2019 (COVID-19) is a highly infectious and pathogenic respiratory disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(Shereen et al.,2020). It has been declared a pandemic. According to the World Health Organization (WHO), as of 30 August 2021, the number of confirmed COVID-19 cases worldwide reached 216,229,741 with 4,496,681 deaths reported worldwide (WHO coronavirus (COVID19) dashboard). The rapid spread of this disease has prompted researchers to search for an effective and safe COVID-19 vaccine, with the aim of suppressing the pandemic and restoring social and economic activity as soon as possible.
According to the WHO, 299 new COVID-19 vaccines are currently being researched worldwide, of which 114 have entered clinical trials and 185 are undergoing pre-clinical trials (COVID-19 vaccine tracker and landscape). Five COVID-19 vaccines have been used in China, with good immunogenicity and safety (National Health Commission, PRC) (Guidelines for Coronavirus-19 Vaccination, First Edition).
Ocular complications after COVID-19 vaccination have been reported and include central serous retinopathy, acute abducens nerve palsy, and two cases of anterior uveitis (Fowler et al.,2021; Reyes-Capo et al.,2021; Renisi et al.,2021; El Sheikh et al.,2021). One case of anterior uveitis occurred after BNT162b2 inoculation (Renisi et al.,2021) and the other after Sinopharm inoculation (El Sheikh et al.,2021); in both cases, the uveitis was resolved via use of topical steroids. Here, we report a case of bilateral posterior uveitis after inoculation with an inactivated COVID-19 vaccine.
Case Presentation
A 50-year-old woman was admitted to the Department of Ophthalmology, Qilu Hospital, on 31 May 2021 complaining of bilateral blurred vision and visual distortion 5 days after vaccination with an inactivated COVID-19 vaccine. She denied any past disease or medication history. Her best-corrected visual acuity (BCVA) values were 20/33 OD and 20/66 OS. The intraocular pressure (IOP) values were 12 mmHg OD and 14 mmHg OS. No abnormality was observed in the bilateral anterior segments.
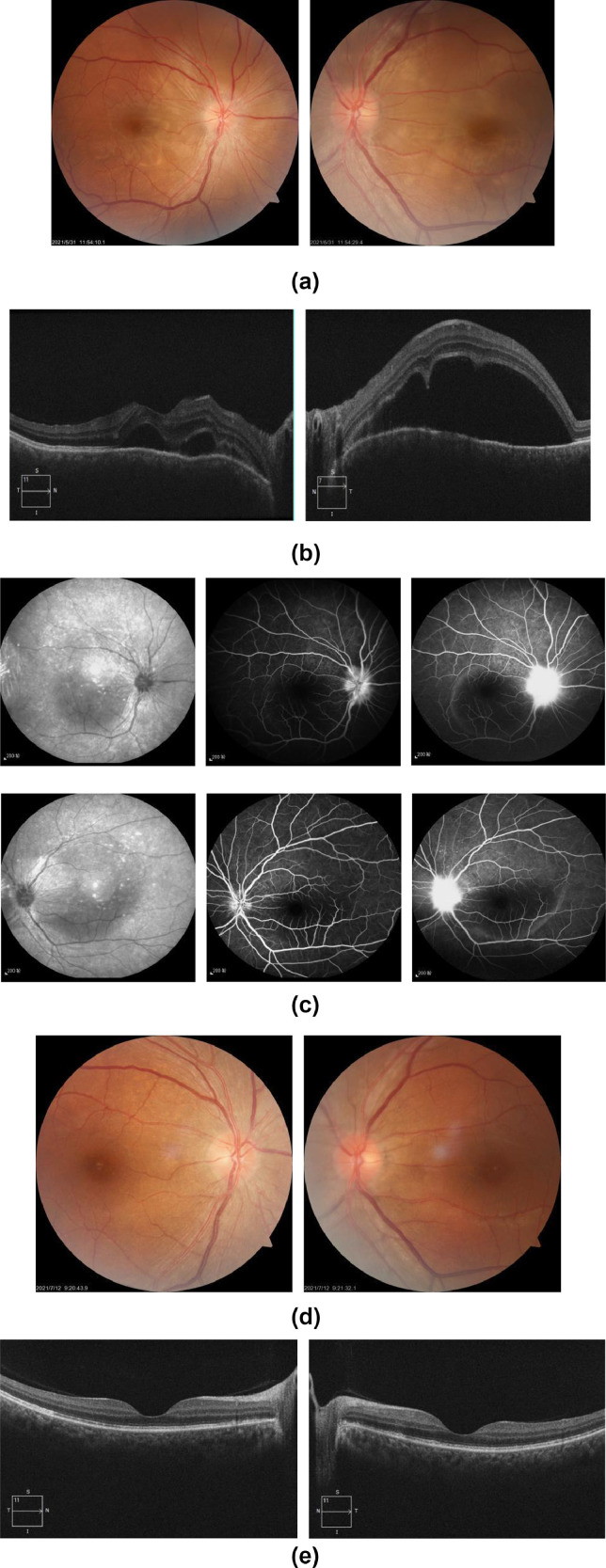
Fundus examination revealed that the optic disc was pale and blurry in the right eye, and the foveal reflex was lost in both eyes (Figure 1. A,B). One week later, her visual acuity decreased to 12/200 (bilateral). Fundus fluorescein angiography (FFA) showed high fluorescence in the optic disc in the early vein stage and fluorescence leakage in the optic disc with circular fluorescence accumulation in the posterior pole in the late stage (Figure 1.C). A diagnosis of bilateral choroiditis was made.
Figure 1.
A) (color online only) Fundus photography: The optic disc of right eye was pale and blurry, the arteries were slightly thin and the foveal reflex was lost; the optic disc of left eye is nearly clear, the foveal reflex was lost. B) Optical coherence tomography (OCT): The RNFL layer of both eyes was elevated with sublaminar fluid accumulation. C) FFA showed high fluorescence in the optic disc in the early vein stage, capillaries of optic disc were tortuous and dilated. In the late stage, fluorescence leakage in the optic disc and circular fluorescence accumulation in the posterior pole can be seen. D) (color online only) Fundus photography showed that the boundary of both eyes became clear. E) OCT: The form of central fovea in both eyes restored to nearly normal.
The results of routine blood, CRP, anti streptolysin O(ASO), antineutrophil cytoplasmic antibody(ANCA) and HLA-B27 were normal. TORCH screening, HIV, syphilis or tuberculosis showed negative results. The patient was administered triamcinolone acetonide (40 mg, periocular injection) and oral prednisone (20 mg once a day). Visual acuity improved to 20/25 OD and 20/20 OS 5 weeks after the application of steroids. Fundus examination showed a clear optic disc, with alleviation of the macular oedema (Figure 1.D, E).
Discussion
Given the high prevalence of COVID-19 and lack of specific, effective pharmaceutical treatments, the need for “herd immunity” is urgent. In China, five novel vaccines have been approved for marketing or emergency use, including three inactivated vaccines, one adenovirus vector vaccine, and one recombinant vaccine.
Ocular complications caused by vaccination are rare. Between 1984 and 2014, there were 289 reported cases of vaccine-induced uveitis, 199 of which occurred in women (Benage and Fraunfelder, 2016). Patient age ranged widely from 2 months to 86 years, with a mean age of 30 years. The median duration from vaccination to the onset of uveitis was 16 days (range, 1 day to 6 years). Thirty-five cases were associated with simultaneous administration of two or more vaccines, and the most common uveitis-associated vaccines were the hepatitis B virus (40.5%), human papillomavirus (HPV) (15.6%), influenza virus (9.7%), Bacillus Calmette–Guérin (7.4%), varicella virus (4.8%), and measles, mumps, and rubella (MMR) (4.8%) vaccines. According to the US Food and Drug Administration Vaccine Adverse Event Reporting System, 204 cases of uveitis after COVID-19 vaccination involved mRNA or adenovirus vector vaccines, whereas no cases involved inactivated vaccinesUnited States Department of Health and Human Services (DHHS) (Vaccine Adverse Event Reporting System).
The pathogenesis of vaccine-induced uveitis remains unclear, molecular mimicry and antigen-antibody reactions are relatively recognised hypotheses (Cunningham et al., 2019; Emmett et al., 2019). Molecular mimicry mainly occurs when an attenuated live vaccine (e.g. the MMR and rhinovirus vaccines) that retains biological activity after inoculation imitates or produces antigens similar to itself, causing autoimmune inflammatory reactions in various parts of the body including the uvea (Minor, 2015). The immune response is usually triggered by the entry of the excipients or adjuvants of the inactivated or subunit vaccine (Emmett et al., 2019) into the body and the subsequent production of IgE, which mediates the release of histamine and other inducers of immediate hypersensitivity (Stone and Brown, 2017). Delayed-type hypersensitivity, which involves CD4+ and CD8+ T-cells, is another important mechanism. In our case, although specific antibody test of the virus was not performed, the vaccination history of the patient, the duration of disease onset, the results of accessory examinations, and the good response to steroids strongly suggest that the uveitis was associated with the vaccine-induced immune response rather than an infection or systemic autoimmune diseases.
The inactivated COVID-19 vaccine in our case was produced by infecting Vero cells with the SARS-CoV-2. After culturing the cells, the virus was harvested, inactivated, concentrated, purified, and absorbed to an adjuvant (aluminum hydroxide) to increase the immunogenicity of the virus. We speculate that the adjuvant caused the uveitis; a strong immune response occurred locally, resulting in damage to the uvea. In a randomized, double-blind phase II trial comprising 742 adults, the most common adverse reactions to an inactivated COVID-19 vaccine were slight pain, itching, redness at the injection site, and slight fatigue and fever; there were no serious events (Che, 2020).
The treatment of uveitis mainly consists of topical steroids, which is suitable for most patients with vaccine-related uveitis (Cunningham and Moorthy, 2020). For patients with severe or refractory uveitis, oral prednisone and immunosuppressive agents may be recommended. The remarkable recovery of our patient after periocular injection of triamcinolone acetonide and administration of oral steroids suggests that ocular inflammation after COVID-19 inoculation is mostly transient and responsive to glucocorticoids. Based on additional medical recommendations, we advised the patient to delay the second vaccination in case the immune response was reactivated.
In conclusion, we reported a rare case of bilateral uveitis after inoculation with a COVID-19 vaccine. The prognosis of the patient suggests that uveitis after vaccination is probably transient and treatable.
Declarations
Ethics approval and consent to participate
Approvals were given by the patient for reporting case details.
Competing interests
The authors declare that they have no competing interests.
Funding
Not applicable.
Authors' contributions
Lijie Pan collected the patient's data and images,and wrote the original manuscript. Yan Cui took part in the diagnosis and treatment of the patient. Yuting Zhang performed the FFA examination of the case. Xinyi Wu was responsible for revising the draft. All authors read and approved the final manuscript.
Acknowledgements
We'd like to express our thanks to the patient and our colleagues.
References
- Benage M, Fraunfelder FW. Vaccine-Associated Uveitis. Mo Med. 2016;113(1):48–52. Jan-Feb PMID: 27039491; PMCID: PMC6139748. [PMC free article] [PubMed] [Google Scholar]
- Che Yanchun, et al. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, ciaa1703. 9 Nov. 2020. Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Moorthy RS. Vaccine-Associated Posterior Uveitis. Retina. 2020;40(4):595–598. doi: 10.1097/IAE.0000000000002816. Apr. [DOI] [PubMed] [Google Scholar]
- ElSheikh RH, Haseeb A, Eleiwa TK, Elhusseiny AM. Acute Uveitis following COVID-19 Vaccination. Ocul Immunol Inflamm. 2021 Aug 11:1–3. doi: 10.1080/09273948.2021.1962917. [DOI] [PubMed] [Google Scholar]
- Cunningham Emmett T., Jr, Moorthy Ramana S., Fraunfelder Frederick W., Zierhut Manfred. Vaccine-Associated Uveitis. Ocular Immunology and Inflammation. 2019;27(4):517–520. doi: 10.1080/09273948.2019.1626188. [DOI] [PubMed] [Google Scholar]
- Fowler N, Mendez Martinez NR, Pallares BV, Maldonado RS. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. Am J Ophthalmol Case Rep. 2021;23 doi: 10.1016/j.ajoc.2021.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479-480:379–392. doi: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- National Health Commission, PRC. Guidelines for Coronavirus-19 Vaccination (First Edition)[EB/OL].(20210329). http://www.nhc.gov.cn/xcs/gzzcwj/202103/c2febfd04fc5498f916b1be080905771.shtml.
- Renisi G, Lombardi A, Stanzione M, Invernizzi A, Bandera A, Gori A. Anterior uveitis onset after bnt162b2 vaccination: is this just a coincidence? Int J Infect Dis. 2021 Jul 18;110:95–97. doi: 10.1016/j.ijid.2021.07.035. [DOI] [PubMed] [Google Scholar]
- Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J AAPOS. 2021 May 24 doi: 10.1016/j.jaapos.2021.05.003. S1091-8531(21)00109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020 Mar 16;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone C, Jr, Brown NJ. Angiotensin-converting Enzyme Inhibitor and Other Drug-associated Angioedema. Immunol Allergy Clin North Am. 2017;37(3):483–495. doi: 10.1016/j.iac.2017.04.006. Aug. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services (DHHS), Public Health Service (PHS), Centers for Disease Control (CDC) /Food and Drug Administration (FDA), Vaccine Adverse Event Reporting System (VAERS) 1990 - 09/03/2021, CDC WONDER On-line Database. Accessed at http://wonder.cdc.gov/vaers.html on Sep 11, 2021 6:38:16 AM
- World Health Organization. WHO coronavirus(COVID19) dashboard[EB/OL]. https://cov-id19.who.int/ (Accessed 30 August 2021)
- World Health Organization. COVID-19 vaccine tracker and landscape [EB/OL]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. (Accessed 7 September 2021)