Abstract
Hsp40s are ubiquitous, conserved proteins which function with molecular chaperones of the Hsp70 class. Sis1 is an essential Hsp40 of the cytosol of Saccharomyces cerevisiae, thought to be required for initiation of translation. We carried out a genetic analysis to determine the regions of Sis1 required to perform its key function(s). A C-terminal truncation of Sis1, removing 231 amino acids but retaining the N-terminal 121 amino acids encompassing the J domain and the glycine-phenylalanine-rich (G-F) region, was able to rescue the inviability of a Δsis1 strain. The yeast cytosol contains other Hsp40s, including Ydj1. To determine which regions carried the critical determinants of Sis1 function, we constructed chimeric genes containing portions of SIS1 and YDJ1. A chimera containing the J domain of Sis1 and the G-F region of Ydj1 could not rescue the lethality of the Δsis1 strain. However, a chimera with the J domain of Ydj1 and the G/F region of Sis1 could rescue the strain’s lethality, indicating that the G-F region is a unique region required for the essential function of Sis1. However, a J domain is also required, as mutants expected to cause a disruption of the interaction of the J domain with Hsp70 are inviable. We conclude that the G-F region, previously thought only to be a linker or spacer region between the J domain and C-terminal regions of Hsp40s, is a critical determinant of Sis1 function.
Hsp70s and Hsp40s (DnaJs) are two families of molecular chaperones which work together in a variety of cellular processes, including protein translation, translocation, and folding, as well as in the regulation of the biological activity of signalling molecules (5). Hsp70s bind unfolded or partially folded polypeptides, preventing aggregation and assisting the folding of these polypeptide substrates (4, 14). The cycle of binding and release of substrate polypeptides to Hsp70 is nucleotide dependent. ATP-bound Hsp70 has a low affinity for substrates, while ADP-bound Hsp70 has a relatively high affinity (30). Therefore, the Hsp40s, which stimulate the weak intrinsic ATPase activity of Hsp70s, play critical roles in regulating substrate binding (23). Some Hsp40s also prevent aggregation by binding unfolded polypeptide substrates and therefore can be considered molecular chaperones in their own right (10). In some cases, Hsp40s may transfer bound substrates to Hsp70 (19).
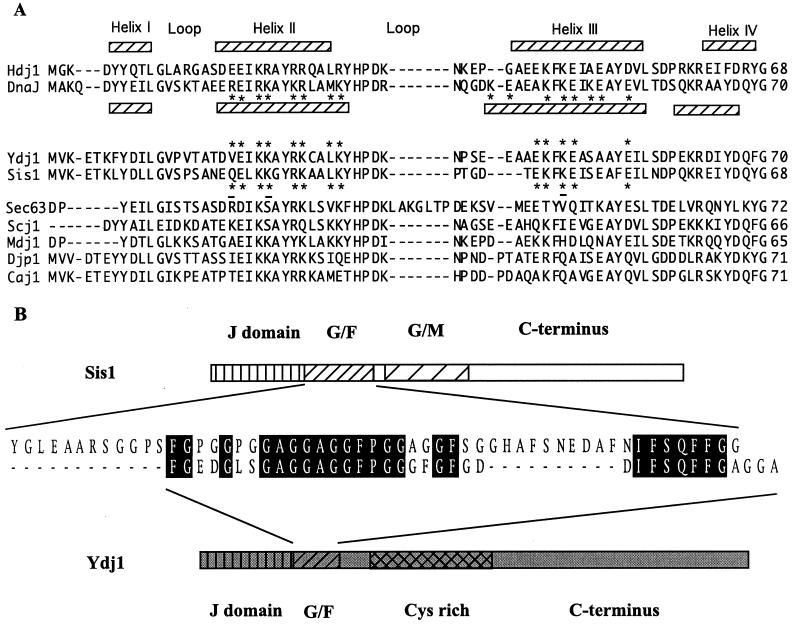
Multiple Hsp40s have been discovered in both prokaryotic and eukaryotic cells. All contain a signature J domain of about 70 amino acids. Genetic and biochemical evidence indicates that the J domain of Hsp40s interacts with the ATPase domain of Hsp70 (9, 18). The structures of the J domains of Escherichia coli DnaJ and Hdj1, a mammalian Hsp40, have been solved by nuclear magnetic resonance (NMR) (16, 25, 27). The tertiary structures of the two different J domains are remarkably similar even though there is only 54% sequence similarity between them. Both consist of four α-helices. Helix II and helix III are antiparallel. Hydrophobic residues on the interior faces of helices I, II, and III form a hydrophobic core which stabilizes the structure. Amino acids on the outer surface of helices II and III and in the loop between them, which contains the highly conserved HPD tripeptide, are thought to be important in determining the affinity and selectivity of the interaction between a particular J domain and its Hsp70 partner (13, 25). Mutations within the HPD tripeptide lead to a loss of both J domain function and interaction with Hsp70 (11, 12, 36, 37, 39).
The Hsp40 class of proteins is divided into three subgroups based on the presence of conserved domains in addition to the J domain (9). Class I Hsp40s have a glycine-phenylalanine-rich (G-F) region adjacent to the N-terminal J domain, followed by a cysteine-rich region which forms a zinc finger motif and a poorly conserved C-terminal region. DnaJ of E. coli and Ydj1 of Saccharomyces cerevisiae are class I Hsp40s. Class II Hsp40s, which include Sis1 of S. cerevisiae and Hdj1 of mammalian cells, lack the zinc finger motif. Class III Hsp40s lack both the G-F region and the zinc finger motif. Thus, the J domain is the only conserved structure among these Hsp40s. While the conserved J domain is involved in interactions with Hsp70s, the polypeptide binding site(s) has been located in the zinc finger and/or poorly conserved C-terminal regions of representatives of class I and II Hsp40s. So far, no class III Hsp40 has been shown to bind unfolded proteins. The function of the G-F regions of class I and II Hsp40s has not been established. It has been proposed that the G-F region is a flexible linker between the J domain and other regions of the type I and II Hsp40s (34) but may be important for interactions with Hsp70s (17, 38).
In the yeast S. cerevisiae, 16 Hsp40s have been identified by their sequence similarities to E. coli DnaJ. Although they have not all been analyzed, some have been localized to major cellular compartments: three in the endoplasmic reticulum (ER) (Sec63, Scj1, and Jem1), three in the mitochondria (Mdj1, Mdj2, and Jac1), and at least four in the cytosol (Ydj1, Sis1, Zuo1, and Djp1) (10, 15, 35, 40, 41) (37a). This report focuses on the yeast cytosolic Hsp40 Sis1. Sis1 and Ydj1, another yeast cytosolic Hsp40, have similar biochemical properties in vitro. Both can stimulate the ATPase activity of the yeast cytosolic Hsp70 Ssa1, can bind unfolded polypeptides, and can function with Ssa1 to refold denatured luciferase (20). However, they appear to carry out different functions in vivo. Ydj1 has been implicated in the folding of proteins and the translocation of proteins into organelles (1, 3, 6, 21). Sis1, on the other hand, appears to be required for the initiation of translation (42). Overexpression of YDJ1 cannot suppress the lethal phenotype of the sis1 disruption mutant; overexpression of SIS1 can only suppress the slow-growth phenotype of the ydj1-null mutant at low temperatures (7, 22) and the translocation defect of a temperature-sensitive ydj1 mutant (6).
To understand the domain structure of Sis1 required for its essential function within the cell, we carried out a genetic analysis. The J domain and G-F region of Sis1 alone are sufficient to support cell growth. A functional J domain is required to maintain cell viability, but the J domain of Ydj1 can substitute. However, the G-F region of Sis1 is specifically required, as the G-F region of Ydj1 cannot substitute for it. We conclude that the unique specificity of some Hsp40s is determined by their G-F regions.
MATERIALS AND METHODS
Yeast strains and culturing methods.
The yeast strains used in this study were as follows: PJ43-2B (MATα, trp1-1 ura3-1 leu2-3,112 his3-11,15 ade2-1 can1-100 GAL2+ met2-Δ1 lys2-Δ2), PJ53 (a/α, rp1-1/trp1-1 ura3-1/ura3-1 leu2-3,112/leu2-3,112 his3-11,15/his3-11,15 ade2-1/ade2-1 can1-100/can1-100 GAL2+/GAL2+ met2-Δ1/met2-Δ1 lys2-Δ2/lys2-Δ2), and WY26 (MATα, trp1-1 ura3-1 leu2-3,112 his3-11,15 ade2-1 can1-100 GAL2+ met2-Δ1 lys2-Δ2 Δsis1::LEU2)/pYW17 (SIS1-YCP50).
The Δsis1::LEU2 mutation in the yeast strain WY26 used in this study contains the LEU2 gene in place of sequence −13 to +1,056 bp of the SIS1 gene. A SmaI-NotI fragment containing the entire LEU2 gene was cloned into the BstZ17I site (13 bp upstream of the ATG of the SIS1 coding region) and the NotI site (introduced immediately before the stop codon) of SIS1 to replace the entire SIS1 coding region. An EcoRI-SalI fragment from this construct containing the upstream and downstream noncoding regions of SIS1 gene with an inserted LEU2 gene in place of the coding region was transformed into the yeast strain PJ53 to make a heterozygous diploid Δsis1 strain by one-step gene replacement (28). This heterozygous diploid Δsis1 strain carrying a wild-type SIS1 gene in YCp50 plasmid (pYW17) was sporulated and dissected to obtain a haploid Δsis1::LEU2 strain (WY26) whose survival is maintained by the pYW17 plasmid.
Yeast cultures were grown on either yeast extract-peptone-dextrose (YEPD) medium containing 2% glucose (31) or synthetic complete medium containing 5-fluoroorotic acid (5-FOA) (32). For drop test, cells were grown to an optical density at 600 nm (OD600) of 0.5 to 1.0. About 0.4 OD600 U of cells was resuspended in 5 ml of sterile water, and a 1:10 serial dilution was prepared. Seven microliters of each dilution was spotted on YEPD or 5-FOA plates which were then incubated at the temperatures indicated for the number of days indicated (see Fig. 1 and 5). To prepare cell lysates to analyze protein expression, about 1.5 OD600 U of cells was resuspended in 100 μl of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer to make cell lysates by beating with glass beads (2), and 12 μl of lysates was subjected to electrophoresis and immunoblot with the indicated antibodies.
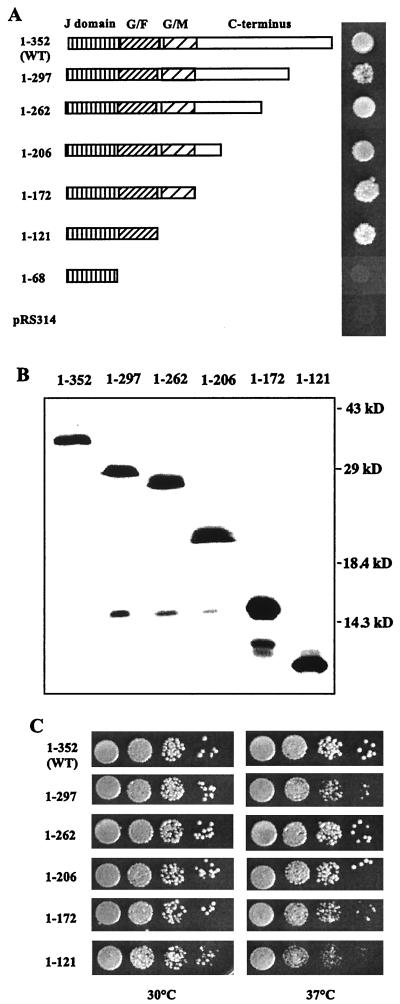
FIG. 1.
Growth phenotype of sis1 truncation mutants. (A) Diagram of Sis1 truncations used in this study and their ability to rescue the lethal phenotype of the Δsis1 strain. All of the sis1-truncated mutants were cloned into the pRS314 plasmid and transformed into the strain WY26. Transformed cells were grown and spotted onto 5-FOA plates (see Materials and Methods) and were incubated at 30°C for 3 days. (B) Immunodetection of mutant Sis1 proteins. Cells surviving on the 5-FOA plates shown in A were grown in YEPD medium. Cell lysates were prepared as described in Materials and Methods and were subjected to SDS-PAGE and immunoblotted using a Sis1-specific antibody. (C) Cell cultures used in panel B were diluted and spotted on YEPD plates. Plates were incubated at the indicated temperatures for 2 days.
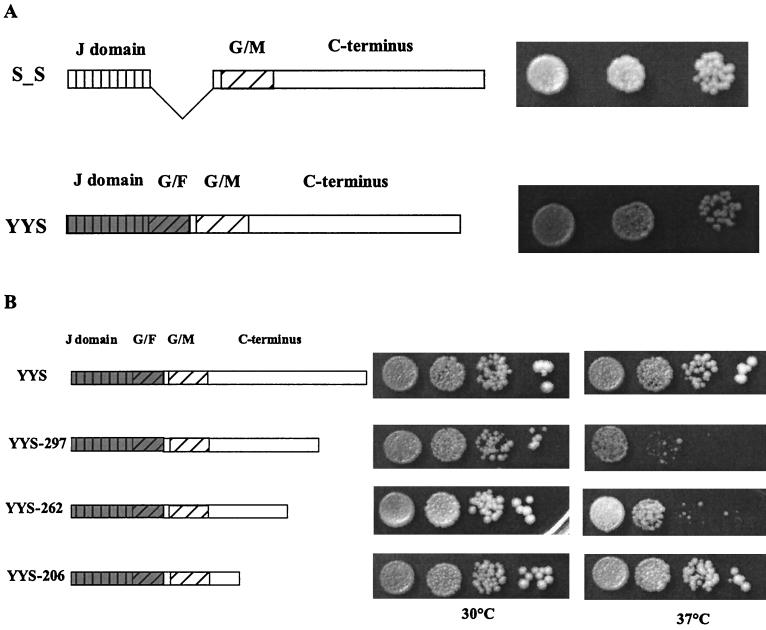
FIG. 5.
The G-F region is dispensable in the context of the full-length protein. (A) Diagram of the G-F region-deleted sis1 mutant SXS and YYS chimeric fusion. Cells carrying these constructs were created for drop test on 5-FOA plates and were incubated at 30°C for 3 days. (B) Full-length and truncated YYS chimeric fusions are shown in the diagram. A similar drop test was performed with cells containing these constructs, and cells were spotted onto YEPD plates which were incubated at the indicated temperatures for 3 days.
PCR mutagenesis of SIS1 gene.
A BamHI-SalI fragment of the SIS1 gene from plasmid CB371 (a generous gift from Kim Ardnt’s lab) was cloned into YCp50 and used as a wild-type plasmid (pYW17, SIS1-YCP50). This fragment was also cloned into plasmid pRS314 (33) to be used as the backbone plasmid (pYW65, SIS1-pRS314) to construct sis1 mutants. An error-prone PCR was carried out in buffer A (10 mM Tris-HCl [pH 9], 1.5 mM MgCl2, 25 mM KCl, and 0.1 mg of gelatin per ml) to synthesize a NsiI-SalI fragment of the SIS1 gene. This mutant PCR fragment was cloned into the pYW65 plasmid to replace the wild-type NsiI-SalI fragment and was transformed into the Δsis1 strain WY26 which carries SIS1-YCP50. Transformants were screened for temperature-sensitive alleles of the SIS1 gene. Three mutants which grew slightly more slowly than the wild-type strain at 38.5°C were obtained from this screen. Sequencing of these mutated genes indicated that they encode three C-terminal truncated fragments of Sis1 containing the first 297, 262, and 206 amino acids, respectively.
To construct truncations 1–68, 1–121, and 1–172, a NotI site was introduced by PCR immediately after amino acid 68, 121, or 172, respectively. Taking advantage of another NotI site which was introduced immediately before the stop codon (see above), NotI fragments containing amino acids 69 to 352, 122 to 352, or 173 to 352 of Sis1 were removed to construct the truncations 1–68, 1–121, and 1–172, respectively. Consequently, truncations 1–68 and 1–172 encode the first 68 and 172 amino acids of Sis1, respectively, plus two extra amino acids (Gly and Arg) at the C terminus, and truncation 1–121 (SS) encodes the first 121 amino acid of Sis1 plus an Arg residue at the C terminus. A similar strategy was used to construct the internal deletion Δ71–121; a XhoI site was introduced immediately prior to amino acid 122. Then a XhoI fragment (from the native XhoI site of the SIS1 gene at codon 69 to this introduced XhoI site) containing amino acids 71 to 121 was removed by molecular cloning to construct a Sis1 protein carrying an internal deletion of the entire G-F region.
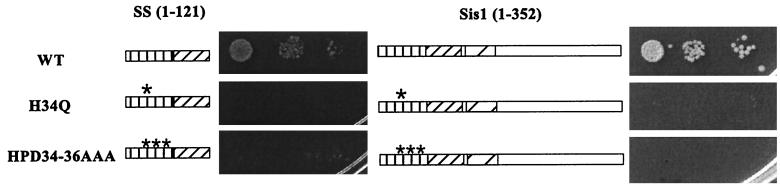
Two-point mutants of SIS1, H34Q, and HPD34-36AAA were constructed by site-directed PCR mutagenesis and were cloned into the pRS314 plasmid. The mutated sequences were synthesized in the oligonucleotide primers and were incorporated into the context of the SIS1 gene.
Construction of Sis1-Ydj1 chimeras.
A Ydj1 truncation which encodes the first 104 amino acids of YDJ1 (YY) was kindly provided by Jill Johnson of this laboratory. Based on the YY construct and the SS (1–121) construct described above, chimeric genes encoding the J domain of Sis1 and the G-F region of Ydj1 (SY) and the J domain of Ydj1 and the G-F region of Sis1 (YS) were constructed. To create these chimeras, a BclI site was introduced into YDJ1 gene (at the end of the J domain) without changing the amino acid sequence by substituting the C residue (+200 bp, A of ATG is counted as 1) with T through PCR to allow swapping the G-F region between SS (containing a native BclI site at the analogous position) and YY at the BclI site. To compare expression levels of YS and YY constructs using antibody against the same epitope, an NcoI site was introduced by PCR at the ATG of either YS or YY to allow addition of an Xpress and polyhistidine tag (XbaI-NcoI fragment of the pRSET B vector; Invitrogen Co., Carlsbad, Calif.) to the N terminus of the YS or YY coding region (encompassed in an NcoI-SalI fragment). This N-terminally tagged YS or YY (XbaI-NcoI-SalI fragment) region was then cloned into the SpeI and SalI sites of the p414GPD vector under the control of the glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter (24). To express the N-terminally tagged YS construction under the control of different promoters, such as the promoters of translation elongation factor 1α (TEF) and alcohol dehydrogenase 1 (ADH), a XbaI-SalI fragment from the above YS-p414GPDHisB construct was moved into p414TEF or p414ADH vectors at the SpeI and SalI sites. Similarly, a XbaI-SalI fragment from the YY-p414GPDHisB was cloned into the SpeI-SalI site of the multicopy 2μm version of the GPD vector, p424GPD, to overexpress the N-terminally tagged YY.
To construct the YYS fusion, an EcoRV site was introduced by PCR into the SIS1 gene close to the end of the G-F region, thereby replacing asparagine 113 with aspartic acid. This construct can rescue the lethal phenotype of the Δsis1 strain as well as the wild-type SIS1 (data not shown). Using this introduced EcoRV site, the glycine-methionine-rich (G-M) C-terminal region of Sis1 (EcoRV-SalI fragment of this SIS1 construct) was fused to the C terminus of the Ydj1 G-F region (a SacI-EcoRV fragment of YDJ1 containing the promoter, J domain, and G-F region of Ydj1) to make a YYS fusion. To make the truncated fusion YYS-292, YYS-262 and YYS-206, the wild-type parts of the YYS construction, were replaced with the NsiI-SalI fragments of SIS1 containing these sis1 truncation mutations.
Protein purification and antibody generation.
Ydj1 was purified as described previously from a T7 expression construct in E. coli (43). Sis1, a generous gift from Tara Beck of this laboratory, was expressed from a T7 expression construct (pET11a-SIS1) in E. coli and was purified by chromatographic separations with Q-Sepharose and hydroxyapatite. Purified Ydj1 and Sis1 were used as immunogens to raise the count of polyclonal antibodies in rabbits.
Quantitation of protein expression by immunoblot.
Yeast lysates from wild-type strain PJ43-2B were prepared as described previously (26). Serial dilutions of purified Ydj1 and Sis1 proteins were used as standards to determine the relative protein levels in the lysate. The cell lysate and purified proteins were run on SDS-PAGE, were transferred to nitrocellulose (Hybond-C; Amersham Corp., Arlington Heights, Ill.), and were immunoblotted for Ydj1 and Sis1 using the ECL detection kit (Amersham). Exposed film (BioMax-AR; Eastman Kodak Co., Rochester, N.Y.) was densitometrically analyzed using Ofoto (Light Source Computer Images, Inc.) and Scan Analysis (Biosoft) software packages. Quantification of the cellular amount of Ydj1 or Sis1 in the cell lysate was achieved by comparison of the densitometric signals from the serial dilutions of purified proteins to those from the cell lysate.
RESULTS
Analysis of domains of Sis1 required for essential functions.
To determine the sequences of Sis1 which play critical roles in its function, a series of C-terminal truncation mutants of SIS1 were isolated (Fig. 1A and Materials and Methods). These constructs were transformed into strain WY26, which contains a complete deletion of the SIS1 coding region on the chromosome (Δsis1::LEU2) and carries a wild-type SIS1 gene on a low-copy-number centromeric plasmid harboring the URA3 gene (SIS1-YCp50) to allow cell viability. To test whether these Sis1 truncations could substitute for the wild-type Sis1, the transformants were spotted onto plates containing 5-FOA to select for cells having lost the URA3-based plasmid carrying the wild-type SIS1 gene (32). As can be seen in Fig. 1A, all of the truncations rescued the Δsis1 strain on 5-FOA plates with the exception of the shortest truncation, containing the first 68 amino acids of the protein. This 68-amino-acid fragment encompassing only the J domain is expressed as a stable protein (data not shown). The ability of all but one of the constructs to rescue indicates that much of the C terminus of Sis1 can be deleted, and the remaining fragments still retain sufficient activity to support growth.
Those transformants which were viable on 5-FOA plates were analyzed by immunoblotting to ensure that the observed growth was caused by truncated Sis1 proteins rather than by the presence of any full-length Sis1 protein resulting from plasmid recombination (Fig. 1B). All truncation mutants showed bands of the expected size and lacked detectable levels of the wild-type protein. These cells were also tested for their ability to grow at a variety of temperatures on nutrient-rich media (Fig. 1C). Strains containing truncations 1–297, 1–262, 1–206, and 1–171, all of which lack part or all of the C-terminal region, grew as well as the wild-type strain at 30°C, and nearly as well as the wild type at 37°C. These results suggest that the C-terminal region (amino acids 172 to 353) of Sis1 may not play an essential role for cell survival, at least under normal laboratory conditions. Interestingly, the strain containing a truncated Sis1 (1–121), retaining only the J domain and the G-F region, allowed growth of the Δsis1 strain at 30 and 37°C, even though such cells grew somewhat more slowly than the wild type. Therefore, we conclude that the J domain and the G-F region of Sis1 are sufficient to carry out the essential function of Sis1 at optimal growth temperatures.
The G-F region of Sis1 is specifically required for its essential function.
The lethal phenotype of the Δsis1 strain can be suppressed by a fragment containing the J domain and the G-F region of Sis1 (Fig. 1C) but not by overexpression of Ydj1 (22) (data not shown). We investigated why Ydj1, which also contains a similar J domain and G-F region, cannot substitute for the absence of Sis1. We reasoned that differential localization caused by the farnesylation signal at the C-terminal end of Ydj1 which results in a portion of Ydj1 being associated with the membrane might be responsible (8). A ydj1 mutant, ydj1-C406S, in which the farnesylation signal CASQ has been changed to SASQ has been shown to have reduced membrane association (8). To test if the inability to rescue Δsis1 was due to farnesylation, ydj1-C406S (a gift from Avrom J. Caplan) was transformed into the Δsis1 strain. This ydj1 mutant was unable to support cell growth (data not shown), suggesting the targeting of the membrane of Ydj1 caused by farnesylation does not prevent Ydj1 from substituting for Sis1.
We then tested if the J domain and G-F region of Ydj1 lacking the C-terminal regions could suppress the lethality of the Δsis1 strain. The Ydj1 truncation containing only the J domain and the G-F region (YY, amino acids 1 to 104) was transformed into the Δsis1 strain (Fig. 2A). The YY truncation did not rescue the Δsis1 strain. This YY construction made functional protein as it rescued the slow-growth phenotype of the Δydj1 strain (16a). These results suggest that a unique structure within the J domain and/or the G-F region of Sis1, which cannot be replaced by the analogous domains of Ydj1, is required to rescue the lethality of the Δsis1 strain.
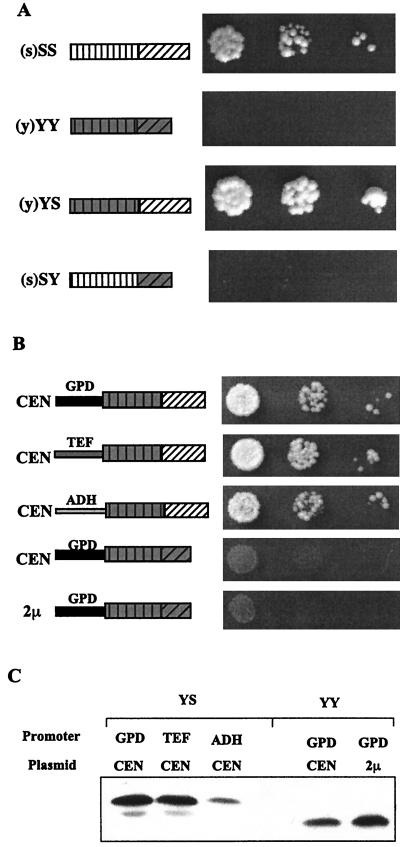
FIG. 2.
Evidence for the importance of the G-F region of Sis1. (A) Truncated fusion constructs containing various combinations of the J domain and G-F region from either Ydj1 or Sis1 are shown in the diagram. The capitalized S or Y stands for domains from Sis1 and Ydj1, respectively. The s or y in parentheses indicates the individual promoter of each construct as being from either SIS1 or YDJ1, respectively. Cell cultures were diluted, spotted on 5-FOA plates, and incubated at 30°C for 4 days. (B) The YS or YY construct was cloned into either a low copy (CEN) or multiple-copy (2μm) plasmid under the control of the GPD, TEF, or ADH promoters to express the proteins tagged with Xpress epitope at different levels. Drop tests were performed as described in panel A using cells carrying these constructs. (C) Cell lysates from cells shown in panel B were separated by SDS-PAGE and were immunoblotted by using the anti-Xpress antibody.
To further dissect whether the J domain or the G-F region from Sis1 is important for the essential function, two fusion proteins were constructed: one in which the J domain from Sis1 was fused to the G-F region of Ydj1 (SY) and one in which the J domain from Ydj1 was fused to the G-F region of Sis1 (YS) (Fig. 2A). These two fusion constructions (YS and SY) were transformed in the Δsis1 strain to test their ability to provide Sis1 function. YS was able to rescue as well as SS, while neither SY nor YY permitted growth (Fig. 2A). This result suggests that it is the G-F region from Sis1 which is specifically required for that protein’s essential function and that the J domain is not responsible for the functional specificity.
Differences in the extent of lethality suppression by the chimeric proteins could possibility be attributed to different expression levels. To eliminate this possibility, we wanted to compare the levels of chimeric proteins in the transformants. Unfortunately, available antibodies against either Sis1 or Ydj1 do not recognize the YS-YY pair equally and thus could not be used for this purpose. To circumvent this problem, the N termini of both YS and YY were tagged with an Xpress epitope (Invitrogen Co.) so that protein expression levels could be detected using anti-Xpress antibody for both proteins without epitope bias. The N-terminally tagged YS and YY were cloned into either low-copy-number (CEN) or multiple-copy (2-μm) plasmids under control of the GPD, TEF, or ADH promoters to allow differential expression levels of each construction. All YS constructs were able to rescue growth of Δsis1 cells (Fig. 2B). However, YY was unable to rescue even when expressed at a higher level than YS (compare lanes 3 and 5 in Fig. 2C). We conclude that the differential abilities of YS and YY to sustain growth of Δsis1 cells represent a functional difference between the two proteins and not merely different levels of expression.
Since overexpression of Sis1 partly suppresses the slow-growth phenotype of Δydj1 cells, but overexpression of Ydj1 does not allow growth of Δsis1 cells at any temperature (7, 22), we wanted to compare the normal levels of expression of these two Hsp40s in wild-type cells. The relative amounts of Ydj1 and Sis1 were determined by immunoblot analysis by using serial dilutions of cell extracts, purified Ydj1, and purified Sis1 as described in Materials and Methods. Ydj1 and Sis1 were detected by immunoblot analysis using antibodies specific to Ydj1 or Sis1, respectively. By comparing the signal from cell extracts with that from purified proteins, we determined the relative amounts of Ydj1 and Sis1 in cell extracts. The results of one experiment are shown in Fig. 3. From the results of a number of experiments, we calculated that Ydj1 is between 10 and 15 times more abundant than Sis1 inside the cell. The fact that the expression of Ydj1 is higher than the expression of Sis1 in vivo, together with the fact that the loss of the farnesylation signal does not affect the rescue of the Δsis1 strain, supports the idea that Sis1 is functionally distinct from Ydj1.
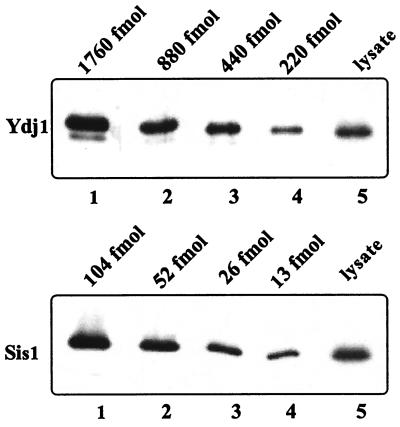
FIG. 3.
Determination of the relative levels of Sis1 and Ydj1 in wild-type cells. Wild-type cells (PJ43-2B) were grown at 30°C in YEPD media to an OD600 of 0.5. Approximately 0.3 OD600 U of cells was collected, and 200-μl cell lysates were made from these cells. Ten microliters of these cell lysates and the indicated amounts of purified Sis1 and Ydj1 were run on SDS-PAGE and immunoblotted with anti-Sis1 and anti-Ydj1 antibodies. Immunoblot signals from cell lysates were compared with those from the predetermined amounts of Sis1 or Ydj1. The cell lysate was calculated to contain about 19 fmol of Sis1 and 285 fmol of Ydj1.
J domain function is required for the rescue of the Δsis1 strain.
Since constructs containing the J domains from either Sis1 or Ydj1 are equally capable of rescuing a SIS1 deletion, we tested whether J domain function is required for Sis1 activity. Two different point mutations, H34Q and HPD34-36AAA, were introduced into the region of SIS1 encoding the highly conserved HPD loop region. Similar mutations in several Hsp40 homologues such, as DnaJ, Ydj1, Sec63, and the large T antigen of simian virus 40, have been shown to disrupt the J domain functions (11, 12, 36, 37, 39). Plasmids containing H34Q or HPD34-36AAA in the context of either the SS truncation or full-length Sis1 were transformed into the Δsis1 strain. None of the constructs were able to rescue the lethality of the Δsis1 strain (Fig. 4), even though the mutant genes expressed stable proteins (data not shown). We conclude that J domain function is required for Sis1 activity.
FIG. 4.
Amino acid changes in the HPD loop disrupt Sis1 function. Diagram of sis1 point mutations H34Q (single star) and HPD34-36AAA (triple stars) in truncated (amino acids 1 to 121) or full-length Sis1 (WT). WY26 cells containing these mutant constructs were diluted and spotted on 5-FOA plates; the plates were incubated at 30°C for 2 days (truncation constructs) and 3 days (full-length constructs), respectively.
Function of the G-F region is redundant to the functions of the G-M and C-terminal regions.
Having determined that the essential Sis1 function requires a functional J domain and G-F region, we wanted to investigate the roles of the G-M and the C-terminal regions for Sis1 function. Since the N-terminal J domain is absolutely required for Sis1 function, we made a deletion mutant in which the segment encoding the G-F region was deleted, but the reading frame was maintained, and we tested the ability of this deletion mutant to support cell growth. This construct (S_S) rescued the Δsis1 strain as well as wild-type Sis1 (Fig. 5A). This rescue indicates that the G-M region and the C-terminal region from Sis1 can substitute for the G-F region of Sis1 to maintain cell survival in the absence of wild-type Sis1. Consistent with this result, a chimeric fusion protein, YYS, in which the J domain and the G-F region from Ydj1 were fused to the G-M and C-terminal regions of Sis1, efficiently rescued growth of the Δsis1 strain (Fig. 5A).
The above results suggest a functional redundancy between the G-F region and the C-terminal 231 amino acids of Sis1. To investigate which sequences within this C-terminal 231 amino acids are minimally required to duplicate the function of the G-F region, we introduced our sis1 C-terminal truncated mutations into the YYS chimeric construct (Fig. 5B). Among the four truncated fusion genes, only YYS-206, YYS-262, and YYS-297 expressed stable proteins (data not shown). As shown in Fig. 5B, these three truncated fusion proteins each allowed growth of the Δsis1 strain. The smallest truncation, YYS-206, which removes amino acids 207 to 352 of the C terminus, allowed wild-type growth rates, while growth of transformants carrying the longer fusions grew more slowly at 37°C. We conclude that the region from amino acid 122 to 206, which includes the G-M region and part of the C-terminal region, is redundant with the G-F region for the essential function of Sis1.
DISCUSSION
In this study, we have analyzed the domains of Sis1 needed for its essential house-keeping roles. Analysis of mutants encoding amino acid substitutions showed the Sis1 J domain to be essential, as is the case with many Hsp40s which have been tested. Our data showing that the J domain of Sis1 plays a critical role is consistent with the previously published observation that a 22-amino-acid deletion within the J domain of Sis1 which includes the conserved HPD sequence resulted in a lethal phenotype (22). Since the J domain has been shown to be the interactive site with the ATPase domain of Hsp70s, these results suggest that the site of interaction of Sis1 with an Hsp70 is essential for its in vivo function. Although the J domain is critical for Sis1 to support cell growth, it is not necessary that the J domain be from Sis1. The J domain from Ydj1 can provide the same essential function, indicating that the J domain structures of these two cytosolic Hsp40s are functionally similar.
As is the case with the J domains of cytosolic Ydj1 and Sis1, the J domains of two ER Hsp40s, Sec63 and Scj1, were found to be interchangeable. However, the J domain of Sis1 could not substitute for the J domain of Sec63 (29). Therefore, it was proposed that the specificity of J domain interaction with Hsp70s is determined by the amino acids on the outside of helices II and III of the J domain, as they were very similar between these two Hsp40s of the ER (9, 25, 29). The Sis1 J domain was able to substitute if three amino acids on the surfaces of helix II or III were altered to more closely resemble those of the ER J domains (29). After modeling structures of Sis1 and Ydj1, we compared the amino acids on the outer surfaces of Sis1 and Ydj1 helices II and III. We found that the sequences of Sis1 and Ydj1 at these locations are more similar to each other than to other Hsp40s (Fig. 6A). Whether these similarities between the Hsp40s of the same cellular compartment (Sec63/Scj1 and Sis1/Ydj1) are simply the result of the concerted evolution of Hsp70s and Hsp40s that function together or whether these similarities play a more critical role is not known at this time.
FIG. 6.
Sequence alignment of the J domains and G-F region. (A) Alignment of the sequences of the J domains of DnaJ, Hdj1, and Hsp40s of S. cerevisiae carried out by the Jotun-Hein method using MegAlign software of the DNA* program package (DNASTAR Inc., Madison, Wis.). Assignments of the helices was based on the NMR structures of Hdj1 (1HDJ) and DnaJ (1XBL) in the PDB from the Brookhaven National Laboratory. The asterisks indicate residues predicted to be on the outside of helix II and III. The residues of Ydj1 and Sis1 were marked with asterisks based on the predicted structures of the proteins modeled from the DnaJ structure using SYBYL molecular modeling software (Tripos Inc., St. Louis, Mo.). The three amino acids of Sis1 which, when replaced with the corresponding residues of Sec63, enable this J domain to substitute for the J domain of Sec63 are underlined. (B) The entire amino acid sequences of Sis1 and Ydj1 were aligned and compared by the same alignment method described for panel A. Only the alignment of the G-F region is shown.
While the J domain is critical and interchangeable between Sis1 and Ydj1, our results indicate that it is the G-F region that differentiates Sis1 from Ydj1. The minimal 121-amino-acid fragment of Sis1 containing only the G-F region and the J domain allows nearly wild-type growth rates at 30°C. The G-F region of Sis1 cannot be replaced by the G-F region of Ydj1, even when such a fusion is expressed at levels higher than the analogous Sis1 fragment. This is a surprising result, as no specific role has been assigned to the G-F region. It has been suggested that the G-F region serves as a linker or spacer region between the J domain and the more C-terminal regions of type I and II Hsp40s (17, 34). However, our results show that in the case of Sis1 the G-F region plays a much more fundamental role in the absence of C-terminal sequences, as it is specifically required.
Recently published results suggest that the interaction of the J domain of Sec63 with the ER Hsp70 BiP broadens the range of peptides which stably bind to BiP and may determine the peptide binding specificity of BiP (23). The G-F region may affect substrate binding of an Hsp70 by directly contacting Hsp70 or by regulating the conformation of the J domain. Recently Huang et al. showed that the NMR structure of the J domain in DnaJ is affected by the G-F region (16). It is possible that different Hsp40 partners are able to differentially affect the specificity of Hsp70 interaction with substrates by causing slightly altered conformations which in turn affect substrate binding affinities. In this regard, it is interesting that the only defect observed for a DnaJ mutant protein deleted for the G-F region was a defect in the targeting of the ς32 protein substrate to Hsp70/DnaK (38).
Previously reported data suggests that both Ydj1 and Sis1 may function with the cytosolic chaperone Ssa. Both are able to stimulate its ATPase activity. Both Sis1 and Ssa are involved in initiation of translation (15a, 42), while Ydj1 and Ssa play roles in protein translocation and folding (1, 3, 6, 21). Specificity of those roles in which Ssa functions could be determined by the Hsp40 partner. Such specificity could be conveyed by structural difference between Sis1 and Ydj1 within the G-F region. Comparison of the G-F sequences between these two Hsp40s indicates that there are two stretches of sequences (of 12 and 11 amino acids, respectively) in the Sis1 G-F region which are absent in the Ydj1 G-F region (Fig. 6B). These two extra stretches, which contain amino acids other than glycine and phenylalanine, might be responsible for the functional difference between the G-F regions of these two Hsp40s.
While the G-F region in conjunction with the J domain is sufficient to supply essential Sis1 function, the role of the G-F region of Sis1 can be carried out by more C-terminal sequences, as indicated by the ability of a protein containing a deletion of the G-F region or replacement of the Sis1 G-F region with the Ydj1 G-F region to allow growth of Δsis1 cells. This functionally redundant region was narrowed down to the segment between amino acids 122 and 206. This region includes the G-M region, which is encompassed within amino acids 122 to 171, and part of the more C-terminal region. Since both the G-F and G-M regions are rich in glycine, we suspect that the G-M region itself is sufficient. However, we were unable to test this idea directly, as the fusion protein containing the J domain and G-F region of Ydj1 linked directly to amino acids 122 to 171 of Sis1 was unstable.
The function of the most C-terminal segment of Sis1 remains unclear. Cells containing a truncated Sis1 in which the entire region between amino acids 207 and 352 has been deleted grow as well as wild-type cells at both 30 and 37°C (as demonstrated by the growth of truncation 1-206 shown in Fig. 1C and the growth of YYS-206 shown in Fig. 5B). However, removing part of the sequence from amino acid 207 to 352, as indicated by truncation YYS-262 and truncations 1-297 and YYS-297, resulted in a slow growth at 37°C (Fig. 1C, 5B). The growth defect caused by these truncations is consistent with previously published results. Four sis1 mutants, including the mutant sis1-85 which has been used for all the genetic analysis of Sis1 function, each having a 22-amino-acid deletion within this region, have either lethal or severe temperature-sensitive phenotypes (22). It is possible that the four internal deletions as well as our partial truncations within this region from amino acid 207 to 353 may produce structural alterations which affect the ability of Sis1 to function normally, while deletion of the entire region is not problematic.
In any case, our analysis of the deletions indicates that the C-terminal region is not required for robust growth under the conditions tested. Interestingly, the C-terminal region of Sis1 containing amino acids 171 to 353 of Sis1 is able to bind unfolded proteins (20). Together with the fact that the 1-172 truncation is able to perform the critical functions of Sis1, this result suggests that the ability to bind unfolded proteins is not an essential aspect of Sis1 function. Although it is still formally possible that the G-F region can bind unfolded polypeptides, to our knowledge no indication of such a function exists.
In summary, we suggest that the J domain and G-F region of Hsp40s of classes I and II may be sufficient for the basic functions of DnaJs in vivo, while the more C-terminal regions are important under suboptimal growth conditions (for example, at the upper limits of the temperature range allowing growth). This idea is supported by the data presented here. In addition, a Ydj1 truncation containing only the J domain and the G-F supports normal growth rates of a Δydj1 strain at 30°C (16a). A similar truncation of DnaJ also permits λ phage replication, albeit at a reduced efficiency (38). The challenge will be to determine the mechanistic role of the G-F region in determining the specificity of its function.
ACKNOWLEDGMENTS
We thank J. Johnson, C. Pfund, and N. Lopez for critical reading of the manuscript, Tara Beck for providing antibodies to Sis1 and Ydj1 and purified Sis1 and Ydj1 proteins, A. Caplan for providing ydj1 mutant C406S, J. Johnson for providing the Ydj1 truncation, and K. Arndt for providing a wild-type SIS1 plasmid.
This work was supported by NIH grant 5RO1 GM31107 to E.A.C.
REFERENCES
- 1.Atencio D P, Yaffe M P. MAS5, a yeast homolog of DnaJ involved in mitochondrial protein import. Mol Cell Biol. 1992;12:283–291. doi: 10.1128/mcb.12.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F, Brent R, Kingston R, Moore D, Seidman J G, Smith J, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1997. [Google Scholar]
- 3.Becker J, Walter W, Yan W, Craig E A. Functional interaction of cytosolic Hsp70 and DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol Cell Biol. 1996;16:4378–4386. doi: 10.1128/mcb.16.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchberger A, Reinstein J, Bukau B. The DnaK chaperone system: mechanism and comparison with other Hsp70 systems. In: Bukau B, editor. Molecular chaperones and folding catalysts: regulation, cellular function and mechanisms. Amsterdam, The Netherlands: Harwood Academic Publishers; 1999. pp. 609–635. [Google Scholar]
- 5.Bukau B, Schmid F X, Buchner J. Assisted protein folding. In: Bukau B, editor. Molecular chaperones and folding catalysts: regulation, cellular function and mechanisms. Amsterdam, The Netherlands: Harwood Academic Publishers; 1999. pp. 3–10. [Google Scholar]
- 6.Caplan A J, Cyr D M, Douglas M G. YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992;71:1143–1155. doi: 10.1016/s0092-8674(05)80063-7. [DOI] [PubMed] [Google Scholar]
- 7.Caplan A J, Douglas M G. Characterization of YDJ1: a yeast homologue of the bacterial DnaJ protein. J Cell Biol. 1991;114:609–621. doi: 10.1083/jcb.114.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan A J, Tsai J, Casey P J, Douglas M G. Farnesylation of YDJ1p is required for function at elevated growth temperatures in S. cerevisiae. J Biol Chem. 1992;267:18890–18895. [PubMed] [Google Scholar]
- 9.Cheetham M, Caplan A. Structure, function and evolution of DnaJ: conservation and adaption of chaperone function. Cell Stress Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:sfaeod>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig E, Yan W, James P. Genetic dissection of the Hsp70 chaperone system of yeast. In: Bukau B, editor. Molecular chaperones and folding catalysts: regulation, cellular function and mechanisms. Amsterdam, The Netherlands: Harwood Academic Publishers; 1999. pp. 139–162. [Google Scholar]
- 11.Dey B, Caplan A J, Boschelli F. The Ydj1 molecular chaperone facilitates formation of active p60v-src in yeast. Mol Biol Cell. 1996;7:91–100. doi: 10.1091/mbc.7.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldheim D, Rothblatt J, Schekman R. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol. 1992;12:3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene M, Maskos K, Landry S. Role of the J-domain in the cooperation of Hsp40 with Hsp70. Proc Natl Acad Sci USA. 1998;95:6108–6113. doi: 10.1073/pnas.95.11.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 15.Hettema E H, Ruigrok C C M, Koerkamp M G, v. d. Berg M, Tabak H F, Distel B, Braakman I. The cytosolic DnaJ-like protein Djp1p is involved specifically in peroxisomal protein import. J Cell Biol. 1998;142:421–434. doi: 10.1083/jcb.142.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Horton, L., and J. Hensold. Personal communication.
- 16.Huang K, Flanagan J M, Prestegard J H. The influence of C-terminal extension on the structure of the “J-domain” in E. coli DnaJ. Protein Sci. 1999;8:203–214. doi: 10.1110/ps.8.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Johnson, J., and E. Craig. Unpublished data.
- 17.Karzai A W, McMacken R. A bipartite signaling mechanism involved in DnaJ-mediated activation of the Escherichia coli DnaK protein. J Biol Chem. 1996;271:11236–11246. doi: 10.1074/jbc.271.19.11236. [DOI] [PubMed] [Google Scholar]
- 18.Kelley W L. The J-domain family and the recruitment of chaperone power. Trends Biochem. 1998;23:222–227. doi: 10.1016/s0968-0004(98)01215-8. [DOI] [PubMed] [Google Scholar]
- 19.Laufen T, Mayer M, Beisel C, Klostermeier D, Mogk A, Reinstein J, Bukau B. Mechanism of regulation of Hsp70 chaperones by DnaJ co-chaperones. Proc Natl Acad Sci USA. 1999;96:5452–5457. doi: 10.1073/pnas.96.10.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Z, Cyr D. Protein folding activity of Hsp70 is modified differently by the Hsp40 co-chaperones Sis1 and Ydj1. J Biol Chem. 1998;273:27824–27830. doi: 10.1074/jbc.273.43.27824. [DOI] [PubMed] [Google Scholar]
- 21.Lu Z, Cyr D M. The conserved carboxyl terminus and zinc finger-like domain of the co-chaperone Ydj1 assist Hsp70 in protein folding. J Biol Chem. 1998;273:5970–5978. doi: 10.1074/jbc.273.10.5970. [DOI] [PubMed] [Google Scholar]
- 22.Luke M, Suttin A, Arndt K. Characterization of SIS1, a Saccharomyces cerevisiae homologue of bacterial dnaJ proteins. J Cell Biol. 1991;114:623–638. doi: 10.1083/jcb.114.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misselwitz B, Staeck O, Rapoport T. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol Cell. 1998;2:593–603. doi: 10.1016/s1097-2765(00)80158-6. [DOI] [PubMed] [Google Scholar]
- 24.Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 25.Pellecchia M, Szyperski T, Wall D, Georgopoulos C, Wuthrich K. NMR structure of the J-domain and the Gly/Phe-rich region of the Escherichia coli DnaJ chaperone. J Mol Biol. 1996;260:236–250. doi: 10.1006/jmbi.1996.0395. [DOI] [PubMed] [Google Scholar]
- 26.Pfund C, Lopez-Hoyo N, Ziegelhoffer T, Schilke B A, Lopez-Buesa P, Walter W A, Wiedmann M, Craig E A. The molecular chaperone SSB from S. cerevisiae is a component of the ribosome-nascent chain complex. EMBO J. 1998;17:3981–3989. doi: 10.1093/emboj/17.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Y Q, Patel D, Hartl F U, McColl D J. Nuclear magnetic resonance solution structure of the human Hsp40 (HDJ-1) J-domain. J Mol Biol. 1996;260:224–235. doi: 10.1006/jmbi.1996.0394. [DOI] [PubMed] [Google Scholar]
- 28.Rothstein R. Targeting, disruption, replacement and allele rescue: intergrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 29.Schlenstedt G, Harris S, Risse B, Lill R, Silver P A. A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interactions with Hsp70s. J Cell Biol. 1995;129:979–988. doi: 10.1083/jcb.129.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmid D, Baici A, Gehring H, Christen P. Kinetics of molecular chaperone action. Science. 1994;263:971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- 31.Sherman F, Fink G R, Hicks J B. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- 32.Sikorski R S, Boeke J D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 33.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver P A, Way J. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell. 1993;74:5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]
- 35.Strain J, Lorenz C R, Bode J, Garland S, Smolen G A, Ta D T, Vickery L E, Culotta V C. Suppressors of superoxide dismutase (SOD1) deficiency in Saccharomyces cerevisiae. J Biol Chem. 1998;273:31138–31144. doi: 10.1074/jbc.273.47.31138. [DOI] [PubMed] [Google Scholar]
- 36.Suh W-C, Burkholder W, Lu C Z, Zhao X, Gottesman M, Gross C. Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc Natl Acad Sci USA. 1998;95:15223–15228. doi: 10.1073/pnas.95.26.15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai J, Douglas M G. A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem. 1996;271:9347–9354. doi: 10.1074/jbc.271.16.9347. [DOI] [PubMed] [Google Scholar]
- 37a.Voisine, C., and E. Craig. Unpublished results.
- 38.Wall D, Zylicz M, Georgopoulos C. The conserved G/F motif of the DnaJ chaperone is necessary for the activation of the substrate binding properties of the DnaK chaperone. J Biol Chem. 1995;270:2139–2144. doi: 10.1074/jbc.270.5.2139. [DOI] [PubMed] [Google Scholar]
- 39.Wall D, Zylicz M, Georgopoulos C. The NH2-terminal 108 amino acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for lambda replication. J Biol Chem. 1994;269:5446–5451. [PubMed] [Google Scholar]
- 40.Westermann B, Neupert W. Mdj2p, a novel DnaJ homolog in the mitochondrial inner membrane of the yeast. J Mol Biol. 1997;272:477–483. doi: 10.1006/jmbi.1997.1267. [DOI] [PubMed] [Google Scholar]
- 41.Yan W, Schilke B, Pfund C, Walter W, Kim S, Craig E A. Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J. 1998;17:4809–4817. doi: 10.1093/emboj/17.16.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong T, Arndt K T. The yeast SIS1 protein, a DnaJ homolog, is required for initiation of translation. Cell. 1993;73:1175–1186. doi: 10.1016/0092-8674(93)90646-8. [DOI] [PubMed] [Google Scholar]
- 43.Ziegelhoffer T, Lopez-Buesa P, Craig E A. The dissociation of ATP from hsp70 of Saccharomyces cerevisiae is stimulated by both Ydj1p and peptide substrates. J Biol Chem. 1995;270:10412–10419. doi: 10.1074/jbc.270.18.10412. [DOI] [PubMed] [Google Scholar]