Abstract
Purpose:
Dedicator of cytokinesis 8 (DOCK8) was reported to have a vital link to immunoregulation. However, the mechanisms by which it drives immune infiltration in cancer remain uncertain. We tried to assess the role of DOCK8 in patients with cancer, especially human papillomavirus (HPV)-positive head and neck squamous cell carcinoma (HNSCC).
Methods:
Data on the expression and survival of DOCK8 in patients with various cancers were analyzed using the Oncomine and TIMER databases. The TIMER database assessed the relationship of DOCK8 with immune infiltration levels and various markers of multiple immune cells. Gene set enrichment analysis revealed tumor-associated biological processes related to DOCK8. ENCODE database was used to explore relevant transcription factors of DOCK8, and a PPI network was constructed using GENEMINIA. The expression and survival role of DOCK8 was confirmed in patients from independent GEO datasets.
Results:
We determined that DOCK8 expression was upregulated or downregulated in various cancers unlike in healthy tissues. A high expression of DOCK8 was significantly correlated with a favorable prognosis in HPV-positive HNSCC and lung adenocarcinoma (LUAD). Furthermore, multivariate Cox regression analysis revealed that DOCK8 was an independent prognostic factor of HPV-positive HNSCC. Additionally, elevated DOCK8 expression was positively correlated with multiple immune cell infiltration levels and immune marker expression associated with particular immune cell subsets. Also, 14 pathways involved in immune activities and carcinogenesis, 22 potential TFs, and co-expression proteins of DOCK8 indicated DOCK8 to be related to tumor-associated biological processes. Ultimately, we verified that DOCK8 is upregulated and confers a favorable overall survival and progression-free survival status in patients with HPV-positive HNSCC.
Conclusion:
These results elucidate that high expression of DOCK8 indicates a favorable prognosis in patients with HPV-positive HNSCC as well as increased microenvironmental immune infiltration levels. It would provide new insights into the prognosis predicting and clinical regimen decision making in patients with HPV-positive HNSCC.
Keywords: DOCK8, HPV-positive head and neck squamous cell carcinoma, prognosis, tumor microenvironment, tumor-infiltrating immune cells
Introduction
Head and neck squamous cell carcinoma (HNSCC), one of the most prevalent malignant tumors, is located at various sites, including the lip and oral cavity, oropharynx, hypopharynx, or larynx.1 Although the treatment of HNSCC has made significant progress in previous decades, its 40%-50% mortality rate is unsatisfactory.2,3 Smoking and alcohol consumption are the classical risk factors for HNSCC. Currently, a substantial and rising proportion of HNSCC caused by infection with human papillomavirus (HPV) is receiving more attention.4 Compared to patients with HPV-negative HNSCC, patients with HPV-positive HNSCC have no evident history of smoking or drinking, are younger, and their disease is more sensitive to radiotherapy and chemotherapy, leading to a better prognosis.5 Therefore, it is important to explore the heterogeneity of the molecular mechanisms and the possibility of precise treatment based on HPV infection status to improve survival rates.6
Over the past decades, the tumor microenvironment (TME) has gained increasing attention. It is a mixture of tumor-infiltrating immune cells (TIICs), endothelial cells, mesenchymal cells, extracellular matrix molecules, and inflammatory mediators.7-9 It has been shown that TIICs provide all the metabolites and factors controlling proliferation, dissemination, dormancy, and drug resistance in tumor cells, suggesting their vital role in the development of tumors, including HNSCC.10 In addition, it has been proven that patients with HPV-positive HNSCC have a better prognosis than those with HPV-negative HNSCC, owing to enhanced immune activation in the TME.11,12 Therefore, with the TME and TIICs in mind, exploring the molecular markers related to infiltrating immune cells may help shed light on the prognosis of the different subtypes of HNSCC.
Dedicator of cytokinesis 8 (DOCK8), located on chromosome 9p24.3, was reported to regulate interstitial migration of dendritic cells as a Cdc42-specific GEF.13 In addition, previous reports have shown that deficiency of DOCK8 in humans is related to severe combined immunodeficiency syndromes, which have the characteristics of allergic inflammation and susceptibility to infections, tumors, and autoimmunity.14-17 Previous studies also found that DOCK8 is required for lymphocyte migration, survival, and immune synapse formation.18,19 These reports highlighted the crucial role of DOCK8 in immune surveillance. Nevertheless, the underlying functions and mechanisms of DOCK8 in HNSCC tumor immunology and progression remain uncertain.
In the present study, we comprehensively analyzed DOCK8 expression in cancer patients in databases such as Oncomine and Tumor Immune Estimation Resource (TIMER) to explore its correlation with the prognosis of several tumors. In addition, we investigated the relationship between DOCK8 and TIICs as well as their classic markers in patients with HPV-positive HNSCC and the relevant transcript factors and co-expression proteins of DOCK8. We verified the differential expression in patients with HPV-positive HNSCC compared to that in patients with HPV-negative HNSCC. We confirmed the prognostic value of DOCK8 expression levels in patients with HPV-positive HNSCC in an independent dataset from the GEO platform. The results of this study revealed the critical role of DOCK8 in HPV-positive HNSCC and provided a potential relationship and an underlying mechanism between DOCK8 and the TME.
Materials and Methods
Oncomine Database Analysis
The Oncomine database (http://www.oncomine.org), an online platform, harbored most malignant human cancer microarrays and provided ways to analyze differentially expressed genes and identify outliers based on the genome-wide expression profiles. According to a P-value of 0.05, fold change of all, and gene ranking of top 10%, we identified the expression levels of DOCK8 in various types of cancers and compared them to those of healthy tissues.
TIMER Analysis
TIMER version 2.0 (https://cistrome.shinyapps.io/timer/), a comprehensive resource for systematic analysis of immune infiltrates across diverse cancer types, was used to estimate the potential association between candidate genes, immune cell infiltration, and clinical parameters. In this study, we used the Diff Exp module to compare DOCK8 mRNA expression between tumors and healthy tissues or different tumor subtypes. Next, the survival module was used to explore the association between DOCK8 expression and overall survival (OS) in several tumors. In addition, we used the gene modules to analyze the correlation of DOCK8 expression with tumor purity and the abundance of immune infiltrates, including B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells. Finally, we applied a correlation module to explore the correlation between DOCK8 expression and the markers of various immune cells in patients with HPV-positive HNSCC.
Gene Set Enrichment Analysis (GSEA)
GSEA was performed using GSEA software (http://www.broadinstitute.org/gsea/). The expression data of 38 patients with HPV-positive HNSCC from the TCGA database were divided into 2 groups according to the median expression value of DOCK8. | NES | >1, nominal P-value <0.05, and false discovery rate (FDR) q < 0.25 were considered statistically significant.
ENCODE and GENEMANIA Analysis
ENCODE Consortium (https://www.encodeproect.org/) is a database for providing elements that act at the protein and RNA levels, and regulatory elements that control cells and circumstances. It also provided the binding of transcription factors (TFs) to specific candidate gene promoter regions based on comprehensive Chip-seqs data or DNA motif binding tendency. To identify the direct TFs targeting the DOCK8 promoter region, we used DOCK8 as the query.
GENEMANIA (http://genemania.org/) is a database for predicting protein-protein physical and functional interactions. It could find related data by inputting genes, including proteins and interactions, pathways, co-expression, colocalization, and protein domain similarity. To identify DOCK8-related proteins, we used DOCK8 as the query.
GEO Database
GEO database (https://www.ncbi.nlm.nih.gov/geo/) is a public repository that harbors microarrays and other forms of high-throughput functional genomics data. In this study, we obtained the expression profiles and clinical data of patients with HPV-positive HNSCC from GSE65858 (n = 270). The data from GSE6585 included the DOCK8 expression profile, OS, and PFS of patients with HPV-positive HNSCC.
Statistical Analysis
Statistical analysis and charting were performed using GraphPad Prism version 8.0 and R programming language (version 3.5.1). The results generated in Oncomine are presented with P-values determined in t-tests, fold changes, and gene ranks. For survival analyses, we used the Kaplan-Meier method to analyze the correlation between variables and survival, and the log-rank test to compare the survival curves. Multivariate Cox analysis was used to evaluate the influence of DOCK8 expression and other clinicopathological factors on survival. The correlation of gene expression was evaluated in the TIMER databases using Spearman’s correlation analysis. A P-value < 0.05 was defined as a statistically significant difference.
Results
Overview of DOCK8 Expression Levels in Various Types of Human Tumors
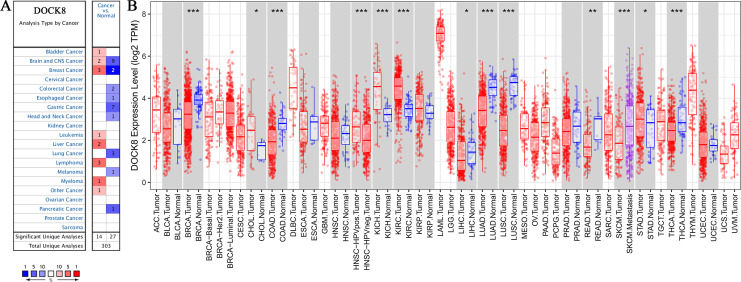
To explore the changes in DOCK8 expression levels in various human tumors compared to those in healthy tissues, we first analyzed the data from the Oncomine database, which included 303 datasets. We compared tumors and healthy tissues to identify DOCK8 expression according to the criteria of P-value less than 0.05, and the threshold gene rank at 10%. As shown in Figure 1A, DOCK8 was significantly upregulated in bladder cancer, leukemia, liver cancer, lymphoma, myeloma, and other cancers (such as embryonal carcinoma and mixed germ cell tumor). Nevertheless, DOCK8 was downregulated in colorectal cancer, esophageal cancer, gastric cancer, head and neck cancer, lung cancer, melanoma, and pancreatic cancer.
Figure 1.
The expression levels of DOCK8 in various human cancers. (A) DOCK8 in different cancers compared to normal tissues in the Oncomine database. (B) The TIMER database detected DOCK8 expression levels of different tumor types in the TCGA database (*P < 0.05, **P < 0.01, ***P < 0.001). TCGA, The Cancer Genome Atlas; TIMER, Tumor Immune Estimation Resource.
To further identify these expression differences between tumors and healthy tissues, we analyzed the malignant tumor mRNA-seq data from TCGA through the TIMER database. DOCK8 was upregulated in cholangiocarcinoma, kidney chromophobe, and stomach adenocarcinoma. At the same time, it was downregulated in LUAD, breast invasive carcinoma, colon adenocarcinoma, HPV-negative HNSCC, liver hepatocellular carcinoma, lung squamous cell carcinoma, rectal adenocarcinoma, and thyroid carcinoma unlike in healthy tissues (Figure 1B). Although the results of the expression of DOCK8 in tumors versus healthy tissues were different, there were common trends in colon adenocarcinoma and lung cancer. In addition, DOCK8 was differentially expressed in HPV-infected and uninfected HNSCC cells. These collective data suggest that different expression levels of DOCK8 in different types or subtypes of tumors might play different roles.
DOCK8 Predicted the Prognosis of Patients With Different Cancers
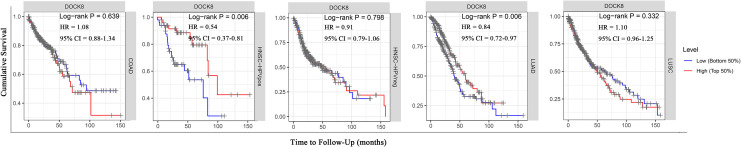
To clarify the role of DOCK8 during tumor progression, we analyzed the relationship between the expression levels of DOCK8 and the prognosis of patients. According to the results of differential expression of DOCK8 in the Oncomine and TIMER databases, patients with colon cancer, HPV-positive head and neck squamous cell carcinoma, HPV-negative head and neck squamous cell carcinoma, lung squamous cell carcinoma, and lung adenocarcinoma were selected for Kaplan-Meier analyses and log-rank analyses. As shown in Figure 2, those with higher expression of DOCK8 had significantly more favorable OS than those with lower expression in patients with HPV-positive HNSCC (P-value = 0.006) and LUAD (P-value = 0.006). However, DOCK8 expression was not significantly associated with colon cancer (P-value = 0.64), HPV-negative HNSCC (P-value = 0.80), and lung squamous cell carcinoma (P-value = 0.33). According to univariate and multivariate COX analysis, for patients with LUAD, both DOCK8 and the clinical stage could be used as independent factors to predict the OS of patients (Table 1, DOCK8 HR: 0.85, 95% CI = 0.73-0.99, Cox P-value = 0.035). High DOCK8 expression was the only independent prognostic factor for patients with HPV-positive HNSCC (Table 1, DOCK8 HR: 0.54, 95% CI = 0.37-0.81, Cox P-value = 0.003). From the information mentioned above, it is suggested that the high expression level of DOCK8 could be considered a favorable prognostic indicator in patients with HPV-positive HNSCC. Therefore, our further study will focus on the role of DOCK8 in patients with HPV-positive HNSCC.
Figure 2.
Comparison of Kaplan-Meier survival curves of DOCK8 high expression versus low expression in patients diagnosed with HNSCC. (A) The relationship between DOCK8 expression and OS in patients diagnosed with colon adenocarcinoma in the TIMER database. (B) The relationship between DOCK8 expression and OS in patients diagnosed with HPV-positive HNSCC in the TIMER database. (C) The relationship between DOCK8 expression and OS in patients diagnosed with HPV-negative HNSCC in the TIMER database. (D) The relationship between DOCK8 expression and OS in patients diagnosed with LUAD in the TIMER database. (E) The relationship between DOCK8 expression and OS in patients diagnosed with lung squamous cell carcinoma in the TIMER database (*P < 0.05, **P < 0.01, ***P < 0.001). OS, overall survival.
Table 1.
Univariate and Multivariate Cox Analysis of DOCK8 Expression for Overall Survival LUAD Patients and HPV-Positive HNSCC Patients From TCGA Database.
| Characteristics | LUAD | HPV-positive HNSCC | ||||
|---|---|---|---|---|---|---|
| β | HR (95% CI) | P value | β | HR (95% CI) | P value | |
| Univariate Cox | ||||||
| Age | 0.01 | 1.01 (0.99-1.02) | 0.33 | 0.01 | 1.00 (0.96-1.06) | 0.96 |
| Gender | 0.06 | 1.07 (0.80-1.42) | 0.67 | −0.19 | 0.72 (0.28-1.89) | 0.51 |
| Stages 2 | 0.88 | 2.42 (1.68-3.48) | 0.00*** | 17.38 | 26415391 (0.00-inf) | 0.99 |
| Stage 3 | 1.28 | 3.58 (2.45-5.24) | 0.00*** | 17.23 | 12217993 (0.00-inf) | 0.99 |
| Stage 4 | 1.34 | 3.83 (2.21-6.64) | 0.00*** | 17.31 | 30590908 (0.00-inf) | 0.99 |
| Purity | 0.44 | 1.55 (0.85-2.82) | 0.15 | −2.07 | 0.29 (0.05-1.56) | 0.15 |
| DOCK8 | −0.18 | 0.84 (0.72-0.97) | 0.02* | −0.62 | 0.54 (0.37-0.81) | 0.03* |
| Multivariate Cox | ||||||
| Stages 2 | 0.88 | 2.42 (1.68-3.49) | 0.00*** | – | – | – |
| Stage 3 | 1.26 | 3.54 (2.42-5.18) | 0.00*** | – | – | – |
| Stage 4 | 1.32 | 3.75 (2.16-6.51) | 0.00*** | – | – | – |
| DOCK8 | −0.17 | 0.85 (0.73-0.99) | 0.035* | −0.62 | 0.54 (0.31-0.95) | 0.03* |
Note: inf means the value was infinity. ***P < 0.001; **P < 0.01; *P < 0.05.
The mRNA Level of DOCK8 Was Positively Associated With Tumor Immune Cell Infiltration
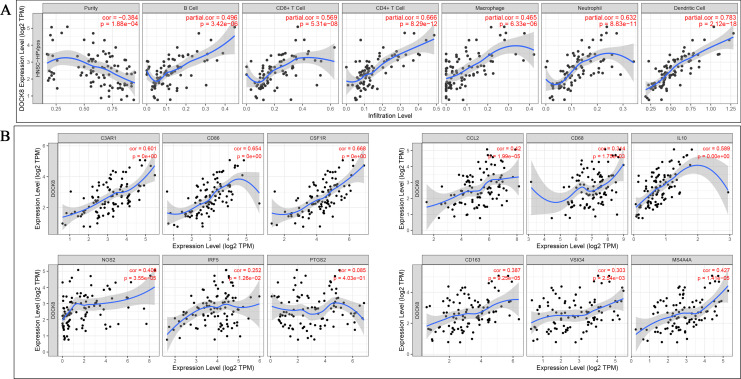
Considering previous reports that DOCK8 is involved in immune diseases and tumor infiltration is closely related to the prognosis of HPV-positive HNSCC, we further investigated whether DOCK8 is related to the level of immune infiltration in HPV-positive HNSCC. It was considered to be strongly correlated after adjusting for purity as Cor >0.5 and P-value < 0.05. The results showed that DOCK8 expression was positively related to 6 types of immune cells, including B cells (r = 0.496, P-value = 3.42e-06), CD8+ T cells (r = 0.596, P-value = 5.31e-08), CD4+ T cells (r = 0.666, P-value = 8.29-12), macrophage neophiles (r = 0.465, P-value = 6.33e-06), neophiles (r = 0.632, P-value = 8.83e-11), and dendritic cells (r = 0.783, P-value = 2.12e-18) (Figure 3A).
Figure 3.
Correlation between DOCK8 expression and immune infiltration in patients with HPV-positive HNSCC. (A) DOCK8 expression was positively correlated with B cell, CD8+ T cells, CD4+ T cells, macrophages, neutrophils, and dendritic cells. (B) DOCK8 expression was positively correlated with markers of macrophage polarization (*P < 0.05, **P < 0.01, ***P < 0.001).
We further examined the association between DOCK8 and the molecular markers of the immune cell subtypes, including monocytes, TAM, macrophages (M1/2), general T cells, CD8 + T cells, B cells, neutrophils, natural killer cells, dendritic cells, Th1/2/17 cells, Tfh cells, Tregs, and T cell exhaustion. The positive correlations between DOCK8 and monocytes (C3AR1, CD86, and CD115), tumor-associated macrophages (CCL2, CD68, IL10), macrophages 1 (INOS, IRF5, COX2 were excluded), and macrophages 2 (CD163, VSIG4, MS4A4A) are shown in Figure 3B. We also examined the correlation between DOCK8 and the above-mentioned immune cell relative markers. The results showed that the expression of DOCK8 is also strongly related to the level of various immune infiltrating cells, with or without adjustment for tumor purity. The results showed that DOCK8 expression positively correlates with Treg and the T cell exhaustion markers (FOXP3, CCR8, STAT5B, TIM-3, PD-1, CTLA4, and LAG3 in HPV-positive HNSCC). Furthermore, significant correlations were found between DOCK8 expression and the regulation of several markers of T helper cells (Th1, Th2, Tfh, and Th17) in HPV-positive HNSCC (Table 2).
Table 2.
Correlation Analysis Between DOCK8 and Related Genes and Markers of Immune Cells in HPV Positive HNSCC via TIMER Database.
| Description | Gene markers | HPV positive HNSCC | |||
|---|---|---|---|---|---|
| None | Purity | ||||
| Cor | P | Cor | P | ||
| CD8+ T cell | CD8A | 0.75 | **** | 0.69 | **** |
| CD8B | 0.61 | **** | 0.59 | **** | |
| T cell (general) | CD3D | 0.68 | **** | 0.61 | **** |
| CD3E | 0.79 | **** | 0.72 | **** | |
| CD2 | 0.76 | **** | 0.71 | **** | |
| B cell | CD19 | 0.68 | **** | 0.60 | **** |
| CD79A | 0.66 | **** | 0.60 | **** | |
| Monocyte | CD86 | 0.65 | **** | 0.57 | **** |
| C3AR1 | 0.60 | **** | 0.54 | **** | |
| CD115 (CSF1R) | 0.67 | **** | 0.62 | **** | |
| TAM | CCL2 | 0.42 | **** | 0.33 | ** |
| CD68 | 0.31 | *** | 0.20 | * | |
| IL10 | 0.59 | **** | 0.52 | **** | |
| M1 Macrophage | INOS (NOS2) | 0.41 | **** | 0.43 | **** |
| IRF5 | 0.25 | * | 0.27 | ** | |
| COX2 (PTGS2) | 0.09 | ns | 0.14 | ns | |
| M2 Macrophage | CD163 | 0.39 | **** | 0.34 | ** |
| VSIG4 | 0.30 | ** | 0.25 | * | |
| MS4A4A | 0.43 | **** | 0.38 | **** | |
| Neutrophils | CD66b (CEACAM8) | 0.10 | ns | 0.11 | ns |
| CD11b (ITGAM) | 0.61 | **** | 0.60 | **** | |
| CCR7 | 0.80 | **** | 0.73 | **** | |
| Natural killer cell | KIR2DL1 | 0.27 | ** | 0.17 | ns |
| KIR2DL3 | 0.32 | ** | 0.25 | * | |
| KIR2DL4 | 0.45 | **** | 0.42 | **** | |
| KIR3DL1 | 0.29 | ** | 0.24 | * | |
| KIR3DL2 | 0.58 | **** | 0.52 | **** | |
| KIR3DL3 | 0.34 | **** | 0.29 | ** | |
| KIR3DS4 | 0.33 | ** | 0.31 | ** | |
| Dendritic cell | HLA-DPB1 | 0.69 | **** | 0.62 | **** |
| HLA-DQB1 | 0.54 | **** | 0.47 | **** | |
| HLA-DRA | 0.72 | **** | 0.68 | **** | |
| HLA-DPA1 | 0.76 | **** | 0.71 | **** | |
| BDCA-1 (CD1C) | 0.58 | **** | 0.51 | **** | |
| BDCA-4 (NRP1) | 0.15 | ns | 0.11 | ns | |
| CD11c (ITGAX) | 0.64 | **** | 0.60 | **** | |
| Th1 | T-bet (TBX21) | 0.75 | **** | 0.69 | **** |
| STAT4 | 0.75 | **** | 0.67 | **** | |
| STAT1 | 0.51 | **** | 0.49 | **** | |
| IFN-γ (IFNG) | 0.58 | **** | 0.50 | **** | |
| TNF-α (TNF) | 0.46 | **** | 0.44 | **** | |
| Th2 | GATA3 | 0.67 | **** | 0.61 | **** |
| STAT6 | 0.39 | **** | 0.47 | **** | |
| STAT5A | 0.56 | ns | 0.55 | **** | |
| IL13 | 0.33 | ** | 0.28 | ** | |
| Tfh | BCL6 | 0.35 | **** | 0.42 | **** |
| IL21 | 0.74 | **** | 0.70 | **** | |
| Th17 | STAT3 | 0.54 | **** | 0.60 | **** |
| IL17A | 0.47 | **** | 0.44 | **** | |
| Treg | FOXP3 | 0.81 | **** | 0.78 | **** |
| CCR8 | 0.75 | **** | 0.72 | **** | |
| STAT5B | 0.51 | **** | 0.47 | **** | |
| TGFβ (TGFB1) | −0.05 | **** | −0.11 | ns | |
| T cell exhaustion | PD-1 (PDCD1) | 0.70 | **** | 0.63 | **** |
| CTLA4 | 0.72 | **** | 0.66 | **** | |
| LAG3 | 0.57 | **** | 0.51 | **** | |
| TIM-3 (HAVCR2) | 0.66 | **** | 0.62 | **** | |
| GZMB | 0.51 | **** | 0.42 | **** | |
Note: P values from Spearman’s correlation analysis. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; ns P > 0.05.
These findings further confirmed the relationship between DOCK8 expression and immune cell infiltration levels, which might explain the prognostic value of DOCK8 in patients with HPV-positive HNSCC.
DOCK8 Was Involved in Immune Activities and Tumorigenesis in Patients With HPV-Positive HNSCC
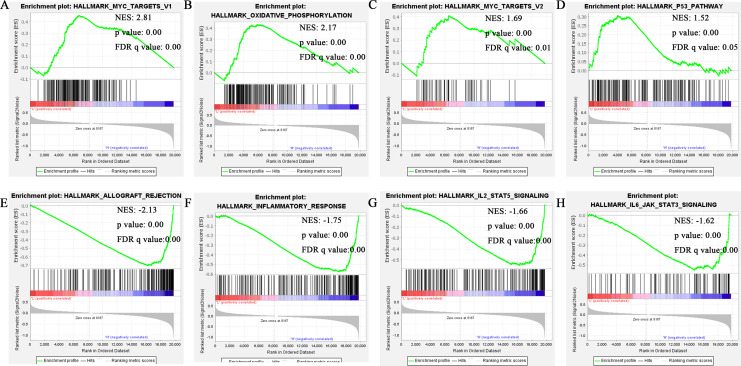
To identify the DOCK8-associated signaling pathways in HPV-positive HNSCC, we conducted a gene set enrichment analysis for patients from the TCGA database. Based on the selection criteria, | NES | >1, nominal P-value <0.05, FDR q-value <0.25, the details of those shown in the top 4 pathways in the DOCK8 low and high groups are listed in Figure 4A-H and Supplementary Table 1.
Figure 4.
Gene set enrichment analysis of DOCK8. (A-D) MYC targets V1, oxidative phosphorylation, MYC targets V2, and P53 pathway were enriched in the DOCK8 low expression group in HPV-positive HNSCC. (E-H) Allograft rejection, inflammatory response, IL2-STAT5 signaling, and IL6 JAK STAT3 signaling were enriched in the DOCK8 high expression group in HPV-positive HNSCC.
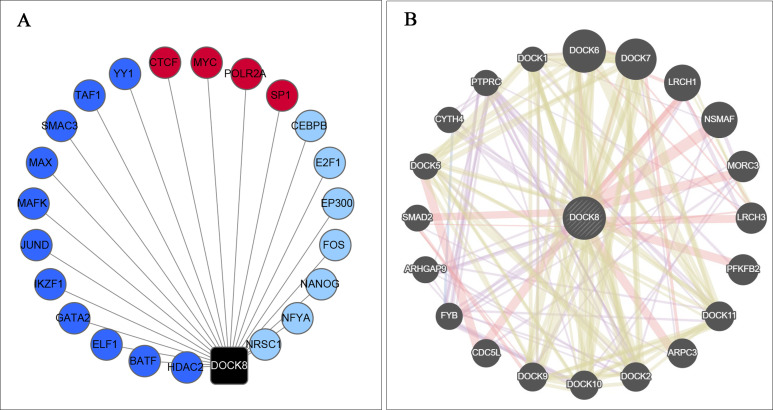
In the occurrence and development of cancer, transcription factors and co-expression proteins play an essential role in the regulatory network. Therefore, the TFs directly targeting the DOCK8 promoter region were examined using ENCODE online tools. As shown in Figure 5A, MAFK, BATF, ELF1, GATA2, HDAC2, IKZF1, JUND, MAX, SMC3, and YY1, which bind to the DOCK8 promoter region, were from existing CHIP-seqs. Meanwhile, CEBPB, E2F1, EP300, FOS, NANOG, NFYA, and NRSC1 were predicted through motif binding preferences. In addition, CTCF, POLR2A, MYC, and SP1 were screened as related transcription factors based on these 2 methods. Furthermore, the co-expression proteins of DOCK8 were revealed by the GENEMANIA database (Figure 5B). The TFs and co-expression proteins engage in immune activities and tumorigenesis. This confirms the role of DOCK8 in leading to a favorable prognosis in patients with HPV-positive HNSCC.
Figure 5.
Analysis of TFs for DOCK8 and co-expressed genes related to DOCK8. (A) The related TFs directly targeting DOCK8. The black dot represents DOCK8; the navy-blue node represents verified TFs bound to the DOCK8 promoter region by CHIP assays; the light node represents the predicted TFs bound to the DOCK8 promoter region, and the red point represents those present in both groups. (B) PPI network of proteins co-expressed with DOCK8. TFs, transcription factors.
The Relationship Between the DOCK8 mRNA Expression Level in Patients With Different HPV Status-HNSCC and Their Prognosis Was Verified
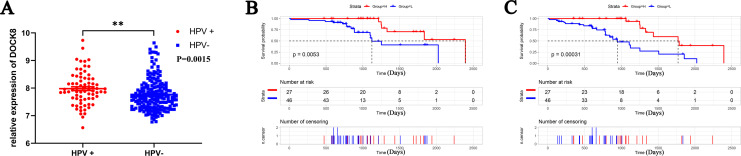
To verify the expression and prognostic value of DOCK8 in patients with HNSCC under different HPV infection statuses, we downloaded and analyzed the data from the GEO dataset (GSE = 65858). A significantly higher expression of DOCK8 mRNA was present in patients with HPV-positive HNSCC (Figure 6A). For prognosis, the Kaplan-Meier curve and log-rank test analyses varied the DOCK8 effect on OS and PFS of patients with HPV-positive HNSCC. Seventy-three patients with HPV-positive HNSCC in the cohort were separated into DOCK8 high-expression and low-expression groups using X-tile plots. As shown in Figure 6B, a higher expression of DOCK8 in patients was significantly associated with favorable overall survival compared to a lower expression (P-value = 0.0053, HR = 0.30, 95% CI = 0.13-0.71). In addition, a high expression level of DOCK8 was significantly associated with a favorable PFS in patients with HPV-positive HNSCC (Figure 6B, P -value = 0.00031, HR = 0.27, 95% CI = 0.13-0.55). Combining the results mentioned above confirmed that DOCK8 was upregulated in HPV-positive HNSCC unlike in HPV-negative HNSCC. High expression of DOCK8 was closely related to a favorable prognosis in patients with HPV-positive HNSCC.
Figure 6.
Validation of the relationship between DOCK8 expression and the clinical information in the GEO dataset (GSE = 65858). (A) DOCK8 was up-regulated in patients with HPV-positive HNSCC unlike those with HPV-negative HNSCC. (B) High DOCK8 expression was related to favorable OS in patients with HPV-positive HNSCC. (C) High DOCK8 expression was related to favorable PFS in patients with HPV-positive HNSCC. (*P < 0.05, **P < 0.01, ***P < 0.001). OS, overall survival; PFS, progression-free survival.
Discussion
In the present study, we observed DOCK8 expression using an extensive bioinformatics examination and found it to be a promising independent prognostic factor in patients with HPV-positive HNSCC. Additionally, this study revealed that DOCK8 expression is positively associated with immune infiltration levels and diverse immune activities in patients with HPV-positive HNSCC, which may be the mechanism by which DOCK8 confers a favorable prognosis. These results indicated that DOCK8 could serve as a candidate biomarker for prognosis and as a novel immune-related therapeutic target.
Analysis of DOCK8 mRNA levels in cancer and healthy tissues in the Oncomine and the TIMER databases revealed that DOCK8 expression differed in different tumors and in healthy tissues, suggesting that there is heterogeneity in DOCK8 expression. In addition, there was variability in the prognostic value in various types of cancers (e.g., HPV-positive HNSCC, HPV-negative HNSCC, and LUAD). These may reflect differences in the underlying causative mechanisms.20,21 Furthermore, multivariate Cox regression analysis of HPV-positive HNSCC and LUAD showed that the expression of DOCK8 could be the only independent prognostic factor for HPV-positive HNSCC rather than LUAD. This suggests that for LUAD, DOCK8 can be combined with traditional clinical features for prognostic evaluation. DOCK8 can be used as a new molecular marker independent of the clinical TNM stage to predict survival outcomes for HPV-positive HNSCC. In summary, the expression DOCK8 provided prognostic information independent of the conventional clinical parameters in HPV-positive HNSCC patients, thus extending the research field of the impact of HPV on their prognosis and providing a potential supplement to the TNM staging system.11
HNSCC is intrinsically an immunosuppressive disease. This necessitates research on the effects of the immune system on the prognosis of HNSCC.11 As an increasingly common treatment method, immune therapy presents different responses due to the degree of tumor lymphocyte infiltration, expression of immune checkpoint proteins, availability of neoantigens, and others.8,12 Studies have demonstrated that TIICs may act as potential efficient prognosticators for patients with HNSCC and included that CD4+ T cells, CD8+ T cells, and M2 macrophages were significantly related to the HNSCC outcome.22-24 In the present study, we found that the DOCK8 expression level was positively associated with B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils, and dendritic cells. This may partly explain why cancer patients with a higher expression of DOCK8 have a better outcome than those with a lower expression. In addition, the correlation observed between DOCK8 and specific immunological marker genes strongly suggested that DOCK8 can regulate immune responses.
Similarly, it was reported that DOCK8 regulates the function of diverse immune cell sub-types, particularly lymphocytes, to drive innate and adaptive immune responses.18 Moreover, DOCK8 expression was weakly correlated with M1 macrophage markers, including NOS2, IRF5, and PTGS2, compared to the strong association with M2 macrophage markers CD163, VSIG4, and MS4A4A. This suggests that DOCK8 potentially plays a role in regulating TAM polarization. Given that active CD8+ cytotoxic T lymphocytes (CTLs) kill tumor cells, and they would be dysfunctional or exhausted after being exposed to TME persistently (TME is linked to exhausted T cells), we examined the relationship between the expression of DOCK8 and exhausted T cells. The DOCK8 expression results positively correlated with Treg, T cell exhaustion, and T helper cells (Th1, Th2, Tfh, and Th17), markers in HPV-positive HNSCC. These correlations could indicate a potential mechanism by which DOCK8 regulates T cell functions in HPV-positive HNSCC and provide potential references for therapy selection in patients with HPV-positive HNSCC. Taken together, these data suggest that DOCK8 plays a vital role in the recruitment and regulation of immune infiltrating cells in HPV-positive HNSCC.
To further investigate the molecular mechanisms underlying the role of DOCK8 in HPV-positive HNSCC, we explored the enriched pathways, potential TFs, and co-expression network of DOCK8 via bioinformatics methods. In view of the hallmarks of DOCK8, it was indicated that DOCK8 is involved in the HPV-positive HNSCC immune microenvironment and tumorigenesis. Similar to our results, previous reports also showed that DOCK8 is involved in the process of Interleukin-2 (IL-2) and downstream transcription factor STAT5 to maintain regulatory T cell homeostasis, competitive fitness, immunological tolerance, and in vivo suppressive function.25-27 In addition, DOCK8-immunodeficient patients have mutable mosaic genomes that can modulate disease phenotype over time, leading to more prolonged survival.28 Also, allograft rejection, inflammatory response, and STAT3 related signaling were significantly associated with DOCK8 expression in various diseases.17,29,30 These findings are consistent with the molecular pathways implicated in HPV-positive HNSCC carcinogenesis.
Moreover, the relevant TFs found in the current study were also demonstrated in HPV-positive HNSCC progression. Mark S. Swanson31 found that Nanog was downregulated in HPV-positive HNSCC unlike in HPV-negative HNSCC. Additionally, CEBPB, EP300, and CTCF were reported to have mutations in HPV-positive HNSCC.32 Moreover, Maura L. Gillison33 showed that EP300 mutations could be a candidate driver event in HPV-positive cancers. These data suggest that DOCK8 may be regulated by these TFs and then participate in tumor progression in patients with HPV-positive HNSCC. This proved the vital role of DOCK8 in patients with HPV-positive HNSCC from another aspect.
To confirm the prognostic value of DOCK8 in HPV-positive HNSCC, we examined DOCK8 expression and its relationship with the outcome of patients diagnosed with HNSCC based on a different HPV status from the GEO dataset. The results of a higher DOCK8 expression in HPV-positive HNSCC than in HPV-negative HNSCC were the same as those from TCGA. Thus, we speculated that patients with low DOCK8 levels were more sensitive to virus infection. HNSCC patients usually die due to recurrence or local lymph node metastasis. So, the PFS of patients is an essential reason for the outcome of HNSCC patients.2,34 Hence, we conducted a Kaplan-Meier analysis on the OS and PFS of patients with HPV-positive HNSCC to identify the role of DOCK8 in PFS. The result showed that a higher DOCK8 expression dramatically predicted better OS and PFS compared to a lower expression. Altogether, we obtained similar results by analyzing multiple databases, which supports the conclusions of our study. Several factors could influence the outcomes of this study. First, the data in this study were from public repositories and published articles. Hence, the quality of data and the number of samples in the databases can influence the study outcomes. Second, the cases in this study were based on retrospective studies. Therefore, the number of cases needs to be supervised continuously and extended to better assess the value of the results in further prospective studies.
In summary, a high expression of DOCK8 was closely correlated with HPV infection status in patients with HNSCC and may improve the prognosis of patients with HPV-positive HNSCC through immune infiltration. In addition, DOCK8 was associated with immune infiltration, several immune relevant pathways, TFs, and proteins closely correlated with DOCK8. The expression changes of DOCK8 and its impact on prognosis were verified in an independent HNSCC dataset.
Conclusion
Taken together, our study revealed the potential role of DOCK8 in tumor immunology and its prognostic value. High DOCK8 expression is associated with higher immune infiltration levels and immune checkpoint expression in HPV-positive HNSCC and hence a favorable prognosis. These findings may provide an immune-based anti-tumor strategy involving tumor microenvironment infiltrates and offers clinical value in directing personalized therapeutic regimen selection for patients with HPV-positive HNSCC. Further studies are needed to confirm these results and reveal the underlying mechanisms.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211011951 for DOCK8 Serves as a Prognostic Biomarker and Is Related to Immune Infiltration in Patients With HPV Positive Head and Neck Squamous Cell Carcinoma by Zeying Zhang, Yandong Bao, Lu Zhou, Yanling Ye, Weineng Fu and Changfu Sun in Cancer Control
Acknowledgments
We thank the TCGA and GEO databases for providing the mRNA and clinical data involved in this study.
Abbreviations
- DOCK8
Dedicator of cytokinesis 8
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomavirus
- LUAD
lung adenocarcinoma
- OS
overall survival
- PFS
progression-free survival
- TFs
transcription factors
- TIMER
tumor Immune Estimation Resource
- TIICs
tumor-infiltrating immune cells
- TME
tumor microenvironment
Authors’ Note: The gene expression and clinical data was obtained from open databases. Therefore, further approval by an ethics committee was not required.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Changfu Sun  https://orcid.org/0000-0001-6568-5416
https://orcid.org/0000-0001-6568-5416
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi:10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (I). Oral Oncol. 2010;46(9):630–635. doi:10.1016/j.oraloncology.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4.Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. doi:10.1093/jnci/djv403 [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Yan B, Lou H, et al. Immunological network analysis in HPV associated head and neck squamous cancer and implications for disease prognosis. Mol Immunol. 2018;96:28–36. doi:10.1016/j.molimm.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Fleming JC, Woo J, Moutasim K, et al. HPV, tumour metabolism and novel target identification in head and neck squamous cell carcinoma. Br J Cancer. 2019;120(3):356–367. doi:10.1038/s41416-018-0364-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig N, Yerneni SS, Razzo BM, Whiteside TL. Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Mol Cancer Res. 2018;16(11):1798–1808. doi:10.1158/1541-7786.MCR-18-0358 [DOI] [PubMed] [Google Scholar]
- 8.Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33(29):3293–3304. doi:10.1200/JCO.2015.61.1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puram SV, Tirosh I, Parikh AS, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171(7):1611–1624. e24. doi:10.1016/j.cell.2017.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Z, Hong M, Chen X, et al. Transcriptome analysis reveals the link between lncRNA-mRNA co-expression network and tumor immune microenvironment and overall survival in head and neck squamous cell carcinoma. BMC Med Genomics. 2020;13(1):57. doi:10.1186/s12920-020-0707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269–282. doi:10.1038/nrc.2018.11 [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi:10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunimura K, Uruno T, Fukui Y. DOCK family proteins: key players in immune surveillance mechanisms. Int Immunol. 2020;32(1):5–15. doi:10.1093/intimm/dxz067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy DL, Zhu G, Li X, et al. Novel pleiotropic risk loci for melanoma and nevus density implicate multiple biological pathways. Nat Commun. 2018;9(1):4774. doi:10.1038/s41467-018-06649-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGhee SA, Chatila TA. DOCK8 immune deficiency as a model for primary cytoskeletal dysfunction. Dis Markers. 2010;29(3-4):151–156. doi:10.3233/DMA-2010-0740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35(2):189–198. doi:10.1007/s10875-014-0126-0 [DOI] [PubMed] [Google Scholar]
- 17.Farmand S, Sundin M. Hyper-IgE syndromes: recent advances in pathogenesis, diagnostics and clinical care. Curr Opin Hematol. 2015;22(1):12–22. doi:10.1097/MOH.0000000000000104 [DOI] [PubMed] [Google Scholar]
- 18.Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14(5):406–411. doi:10.1038/cmi.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randall KL, Chan SS, Ma CS, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208(11):2305–2320. doi:10.1084/jem.20110345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koneva LA, Zhang Y, Virani S, et al. HPV integration in HNSCC correlates with survival outcomes, immune response signatures, and candidate drivers. Mol Cancer Res. 2018;16(1):90–102. doi:10.1158/1541-7786.MCR-17-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cillo AR, Kurten CHL, Tabib T, et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity. 2020;52(1):183–199 e9. doi:10.1016/j.immuni.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y, Qin X. Profiles of immune cell infiltration and their clinical significance in head and neck squamous cell carcinoma. Int Immunopharmacol. 2020;82:106364. doi:10.1016/j.intimp.2020.106364 [DOI] [PubMed] [Google Scholar]
- 23.Liang B, Tao Y, Wang T. Profiles of immune cell infiltration in head and neck squamous carcinoma. Biosci Rep. 2020;40(2): BSR20192724. doi:10.1042/BSR20192724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar AT, Knops A, Swendseid B, et al. Prognostic significance of tumor-associated macrophage content in head and neck squamous cell carcinoma: a meta-analysis. Front Oncol. 2019;9:656. doi:10.3389/fonc.2019.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H, Liu C, Tan H, et al. Hippo kinases Mst1 and Mst2 sense and amplify IL-2R-STAT5 signaling in regulatory T cells to establish stable regulatory activity. Immunity. 20 2018;49(5):899–914 e6. doi:10.1016/j.immuni.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AK, Eken A, Hagin D, et al. DOCK8 regulates fitness and function of regulatory T cells through modulation of IL-2 signaling. JCI Insight. 2017;2(19):e94275. doi:10.1172/jci.insight.94275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen E, Kumari S, Tohme M, et al. DOCK8 enforces immunological tolerance by promoting IL-2 signaling and immune synapse formation in Tregs. JCI Insight. 2017;2(19):e94298. doi:10.1172/jci.insight.94298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing H, Zhang Q, Zhang Y, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014;133(6):1667–1675. doi:10.1016/j.jaci.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuellar-Rodriguez J, Freeman AF, Grossman J, et al. Matched related and unrelated donor hematopoietic stem cell transplantation for DOCK8 deficiency. Biol Blood Marrow Transplant. 2015;21(6):1037–1045. doi:10.1016/j.bbmt.2015.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clay GM, Valadares DG, Graff JW, et al. An anti-inflammatory role for NLRP10 in murine cutaneous leishmaniasis. J Immunol. 2017;199(8):2823–2833. doi:10.4049/jimmunol.1500832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson MS, Kokot N, Sinha UK. The role of HPV in head and neck cancer stem cell formation and tumorigenesis. Cancers (Basel). 2016;8(2):24. doi:10.3390/cancers8020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haft S, Ren S, Xu G, et al. Mutation of chromatin regulators and focal hotspot alterations characterize human papillomavirus-positive oropharyngeal squamous cell carcinoma. Cancer. 2019;125(14):2423–2434. doi:10.1002/cncr.32068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillison ML, Akagi K, Xiao W, et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 2019;29(1):1–17. doi:10.1101/gr.241141.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seppälä M, Pohjola K, Laranne J, et al. High relative density of lymphatic vessels predicts poor survival in tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016;273(12):4515–4524. doi:10.1007/s00405-016-4150-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211011951 for DOCK8 Serves as a Prognostic Biomarker and Is Related to Immune Infiltration in Patients With HPV Positive Head and Neck Squamous Cell Carcinoma by Zeying Zhang, Yandong Bao, Lu Zhou, Yanling Ye, Weineng Fu and Changfu Sun in Cancer Control