Abstract
This systematic review aims to gather primary data from cancer institutions that have implemented changes to cancer service provision amid the COVID-19 outbreak to inform future intervention and health care facility response strategies. A comprehensive literature search was done on Global Health Medline and EMBASE using pertinent key words and MeSH terms relating to COVID-19 and Cancer service provision. A total of 72 articles were selected for inclusion in this systematic review. Following the narrative synthesis that was conducted of the literature, 6 core themes that encompassed common cancer service intervention adopted by institutions were identified: (1) Testing and Tracking, (2) Outreach and Communication, (3) Protection, (4) Social Distancing (5) Treatment Management, (6) Service Restructuring. Since cancer patients are a high-risk population amid the COVID-19 pandemic, these areas of targeted intervention can be used to inform necessary actions in institutions facing similar risks, based on previous learning from numerous cancer centers globally.
Keywords: COVID-19, SARS-CoV-2, cancer, service provision, care organization
Introduction
In December 2019, COVID-19 / Coronavirus / SARS-CoV-2 emerged in Wuhan, China, and has subsequently infected over 53.7 million people and caused over 1.3 million deaths globally (as of 15 November 2020).1 As these numbers are continually increasing, healthcare services worldwide have been subject to immense strain to cater to influx and demands of patients.2 To combat this, service provision in healthcare institutions have subsequently been reorganized in order to cope with COVID-19 related challenges.3
The impacts of the COVID-19 outbreak have been particularly evident for cancer services, with many patients experiencing delays in cancer diagnosis and treatment.4 For example, in the United Kingdom national cancer screening programs have been suspended and patients who are referred may be subject triage before being able to receive treatment.5 Common stressors on healthcare facilities have been due to the shortage of intensive care beds, as well as the inability to protect healthcare staff due to shortages of personal protective equipment (PPE).5
To combat the disruptions, cancer care facilities have had to adopt drastic service configurations in order to maintain timely and effective care.3 These changes have been vital as cancer patients are at high risk of complications from viral infections and are likely to experience adverse outcomes. A Chinese cohort study reported that cancer patients with COVID-19 are at higher risk of severe events, including intensive care unit admission, invasive ventilation and death, compared to patients without cancer (39% vs 8%, p = 0.0003).6 In light of this, the disproportionate vulnerability highlights the need for implementation of effective strategies that safeguard and protect oncology patients during this time.
In summary, the continual fluctuation of caseloads and the evolving nature of the pandemic require flexible and adaptive care to ensure the safety of patients and staff. As a result, there is a need to study and evaluate whether current adaptations to cancer care have been yielding consistent and positive outcomes for health systems worldwide. This systematic literature review aims to gather primary data from cancer institutions that have implemented changes to cancer service provision amid the COVID-19 outbreak to inform future intervention and health care facility response strategies.
Methods and Materials
Search Strategy
A comprehensive literature search was done on Medline, Global Health and EMBASE to identify articles relating to cancer service provision during the COVID-19 pandemic. This was done in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). Grey literature was also included and obtained through snowballing. The search was deconstructed into 3 categories that comprised relevant keywords and MeSH headings relating to (1) COVID-19, (2) Cancer and (3) Service Provision (Table 1). All the relevant articles were identified and screened by 3 authors.
Table 1.
Search Strategy.
| Category | |
|---|---|
| COVID-19 AND |
“Coronavirus,” OR “nCoV*,” OR “2019-nCoV,” OR “COVID*” OR “SARS-CoV*” |
| Cancer AND |
“Cancer” OR “carcinoma” OR “malignancy” OR “metastasis” OR “Neoplasm” |
| Service Provision | “Service” OR “service provision” OR “Care provision” OR “Care” OR “Care organisation” OR “Healthcare provision” |
Inclusion and Exclusion Criteria:
The inclusion and exclusion criteria are outlined in Table 2. Studies were included if they contained primary data on cancer service provision amid the COVID-19 pandemic (Table 2).
Table 2.
Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Exposure: challenges to routine service provision due to COVID-19 pandemic | Published before Jan 2020 |
| Outcome: Mitigating measures being undertaken | Does not contain primary Data |
| Date range: Papers from Jan 2020 to September 2020 | Other Languages |
| In the English language |
Quality Assessment
A quality assessment for the included articles was carried out using the NIH quality assessment tool for the appropriate studies. No articles were excluded based on their quality score.
Data Extraction
All the relevant articles were screened and selected for inclusion by 3 authors and any disagreements were resolved through consensus and vote. Data extracted from the included articles were tabulated and then a narrative synthesis was undertaken to identify key themes in the literature.
Results
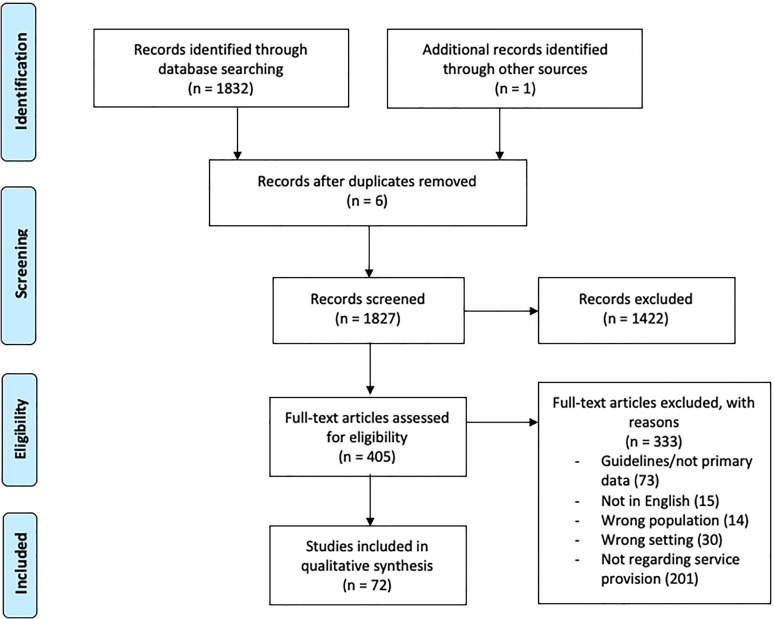
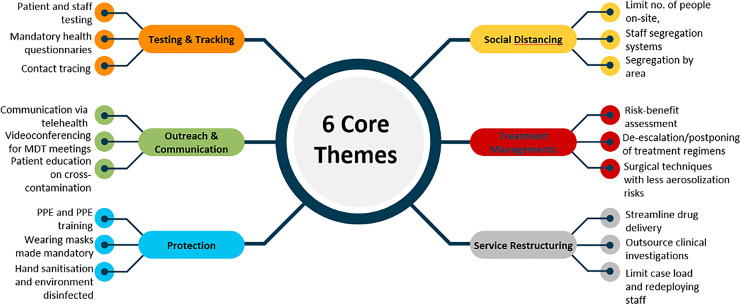
A total of 72 articles were selected for inclusion in this review following screening and duplicate removal (Figure 1). A narrative synthesis was conducted following the analysis of the data to identify recurrent and common themes of intervention that were frequently mentioned. Following this, we categorized the data into 6 themes of core cancer service interventions: (1) Testing and Tracking, (2) Outreach and Communication, (3) Protection, (4) Social Distancing, (5) Treatment Management, (6) Service Restructuring (Table 3). These themes encompass the comprehensive interventions adopted by various cancer departments/institutions during the pandemic. The characteristics of the included articles are summarized in Table 4 and have been explored in a narrative manner in the main text. A large proportion of the studies were conducted in China, Italy, Singapore, United Kingdom (UK) and the United States (US) and included various oncological sub-specialisms.
Figure 1.
Prisma Flow Diagram.
Table 3.
Articles Categorized by Theme.
| Author | Themes | |||||
|---|---|---|---|---|---|---|
| Tracking and Triage | Outreach and Communication | Protection | Social Distancing | Treatment Management | Service Restructuring | |
| Agyapong et al52 | ✓ | |||||
| Ardizzone et al75 | ✓ | |||||
| Baabdullah et al67 | ✓ | |||||
| Batt et al76 | ✓ | |||||
| Blot et al77 | ✓ | |||||
| Brody et al38 | ✓ | ✓ | ||||
| Butler et al19 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Casella et al11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Chiang et al14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cinelli et al53 | ✓ | ✓ | ||||
| Civantos et al9 | ✓ | ✓ | ||||
| Civantos et al49 | ✓ | ✓ | ✓ | |||
| Collins et al17 | ✓ | ✓ | ✓ | ✓ | ||
| Curigliano et al55 | ✓ | ✓ | ✓ | |||
| Czernin et al36 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| de Marinis et al39 | ✓ | ✓ | ✓ | ✓ | ||
| Dharmarajan et al51 | ✓ | |||||
| Elkaddoum et al59 | ✓ | ✓ | ✓ | |||
| Elkin et al31 | ✓ | |||||
| Flannigan et al57 | ✓ | ✓ | ||||
| Fosker26 | ✓ | ✓ | ||||
| Frey et al66 | ✓ | |||||
| Giuliani et al58 | ✓ | |||||
| Gupta et al48 | ✓ | ✓ | ✓ | |||
| Grenda et al54 | ✓ | ✓ | ||||
| Guven et al12 | ✓ | ✓ | ✓ | ✓ | ||
| Harky et al61 | ✓ | ✓ | ✓ | |||
| Indini et al33 | ✓ | ✓ | ✓ |
✓
|
✓ | ✓ |
| Jiang et al60 | ✓ | ✓ | ||||
| Lee et al15 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| Lee et al27 | ✓ | ✓ | ||||
| Lee et al47 | ✓ | ✓ | ✓ | |||
| Lobascio et al56 | ✓ | ✓ | ||||
| Lombe et al68 | ✓ | ✓ | ✓ | |||
| Mei et al21 | ✓ | ✓ | ✓ |
✓
|
✓ | ✓ |
| Mendoza et al23 | ✓ | ✓ | ✓ | |||
| Millar et al65 | ✓ | |||||
| Mirnezami et al73 | ✓ | ✓ | ||||
| Morrison et al69 | ✓ | ✓ | ||||
| Moss et al32 | ✓ | |||||
| Mulvey et al62 | ✓ | ✓ | ||||
| Ngoi et al41 | ✓ | ✓ | ✓ |
✓
|
✓ | ✓ |
| Ning et al46 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Onesti et al24 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Ong et al72 | ✓ | |||||
| Oualla et al22 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Patel et al16 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| Peeters et al63 | ✓ | |||||
| Peng et al43 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Poggio et al74 | ✓ | |||||
| Porzio et al29 | ✓ | ✓ | ✓ | |||
| Press et al18 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| Quarto et al70 | ✓ | ✓ | ✓ | ✓ | ||
| Rathod et al50 | ✓ | ✓ | ||||
| Rodler et al45 | ✓ | ✓ | ✓ |
✓
|
✓ | ✓ |
| Silvestris et al35 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| Tagliamento et al25 | ✓ | ✓ | ||||
| Tan et al7 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| Tey et al10 | ✓ | ✓ |
✓
|
✓ | ||
| Valenza et al37 | ✓ | ✓ | ✓ |
✓
|
||
| Van de Haar et al3 | ✓ | ✓ |
✓
|
✓ | ✓ | |
| van der Lee et al64 | ✓ | |||||
| Vanderpuye et al40 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Wahed et a28 | ✓ | ✓ | ✓ | |||
| Wang et al34 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| Wakefield et al20 | ✓ | ✓ | ✓ | ✓ | ||
| Wei et al71 | ✓ | |||||
| Weisel et al8 | ✓ | ✓ | ✓ |
✓
|
✓ | |
| Wilkinson42 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Wilson30 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Wu et al44 | ✓ | ✓ | ✓ |
✓
|
||
| Yusuf13 | ✓ | ✓ | ✓ | ✓ | ✓ | |
Table 4.
Summary of Included Articles.
| Author | Study Type | Country | Key Adaptations/Intervention service delivery Outcome (in Italic) |
|---|---|---|---|
| Agyapong et al52
|
Report | Canada |
|
| Ardizzone et al75 | Report | USA |
|
| Baabdullah et al67 | Survey | Saudi Arabia |
Outcome: Survey reveals that transition to telemedicine is well accepted by cancer patients |
| Batt et al76 | Prospective cohort study | UK |
|
| Blot et al77 | Article | France | Highlighted role of ethical committee board during COVID-19
|
| Brody et al38 | Cross-sectional (Multi-center survey) | USA & Canada (North America) |
|
| Butler et al19 | Article | UK |
|
| Casella et al11 | Editorial | Italy |
|
| Chiang et al14 | Article | Singapore |
|
| Cinelli et al53 | Letter | Italy |
|
| Civantos et al9 | Report | USA |
|
| Civantos et al49 | Report | USA |
|
| Collins et al17 | Editorial | USA | Urology department in USA:
|
| Curigliano et al55 | Opinions | Italy |
|
| Czernin et al36 | Report | International |
|
| de Marinis et al39 | Retrospective | Italy |
|
| Dharmarajan et al51 | Cross-sectional (survey) | USA |
|
| Elkaddoum et al59 | Article | Lebanon | MDT meetings were carried out virtually using Microsoft Teams Outcomes
|
| Elkin et al31 | Review | USA |
|
| Flannigan et al57 | Editorial | Canada |
|
| Fosker26 | Editorial | Bermuda |
|
| Frey et al66 | Prospective cohort study | USA |
|
| Giuliani et al58 | Editorial | Canada |
|
| Gupta et al48 | Cohort study/report | India |
|
| Grenda et al54 | Article | USA |
|
| Guven et al12 | Short Report | Turkey |
|
| Harky et al.61 | Letter | UK |
|
| Indini et al33 | Cross-sectional (multi-center survey) | Italy | COVID-19 diffusion containment measures:
|
| Jiang et al60 | Review | USA |
|
| Lee et al15 | Perspective | Hong Kong |
|
| Lee et al47 | Editorial | Korea |
|
| Lee et al27 | Prospective cohort study | UK |
|
| Lobascio et al56 | Opinion | Italy |
|
| Lombe et al68 | Article | Zambia |
|
| Mei et al21 | Reportage | China |
|
| Mendoza et al23 | Editorial | Philippines |
|
| Millar et al65 | Survey | UK |
|
| Mirnezami et al73 | Letter | UK |
|
| Morrison et al69 | Article | USA |
|
| Moss et al32 | Prospective cohort studies | UK |
|
| Mulvey et al62 | Opinions | USA |
|
| Ngoi et al41 | Editorial | Singapore |
|
| Ning et al46 | Prospective cohort study | USA |
|
| Onesti et al24 | Survey | International |
|
| Ong et al72 | Letter | Singapore |
|
| Oualla et al22 | Article | Morocco |
|
| Patel et al16 | Perspectives | USA |
|
| Peeters et al63 | Editorial | Belgium | 1. Mobile phone apps were developed to monitor treatment toxicity in patients and identify individuals who are at risk of COVID-19 infections |
| Peng et al43 | Comment | China | 2. Nation-wide program issued each personnel a health QR code showing a 2 tier contagion risks, which was determined by the number of cases in the area of residency. Medical isolation is required for “high-risk” patients, unless in an emergency 3. Face coverings were required in hospitals 4. Temperature monitoring of patients and staff was implemented 5. Visitors were prohibited in the wards 6. Fever clinics were used to screen patients with suspected COVID-19 symptoms. If cancer patients presented to the hospital with fever, they were attended by an infectious disease specialist before they were seen by oncologists 7. Online consultations were performed Home drug deliveries were done 8. Special programming model was used to aid scheduling of radiotherapy to minimize patients’ waiting time at hospital |
| Poggio et al74 | Survey | Italy |
|
| Porzio et al29 | Perspectives | Italy | Transitioned oncological services to home care under a double triage protocol
|
| Press et al18 | Technical Report | USA |
11% monitored for symptoms/high-risk exposure 8% of patients had an alteration in treatment plans Out of 11 affected patients, 7 were cleared and rescheduled for treatment (median delay of 7 days), 4 patients were indefinitely delayed (including 3 COVID-19 cases, 1 of which died) Majority of patients who required monitoring had not yet started treatment (60%), all except one were cleared and rescheduled (median delay of 4.5 days) Out of 6 patients on-treatment requiring evaluation (40%), 5 had treatment interruptions and were rescheduled (median delay of 4 days) |
| Quarto et al70 | Opinion | USA |
|
| Rathod et al50 | Short Communication | Canada | New guidelines were issued based on principles of 4R’s for radiation oncology
|
| Rodler et al45 | Perspective | Germany |
|
| Silvestris et al35 | Report | Italy |
|
| Tagliamento et al25 | Survey | Italy |
|
| Tan et al7 | Editorial | Singapore |
|
| Tey et al10 | Perspective | Singapore |
|
| Valenza et al37 | Observational | Italy |
|
| Van de Haar et al3 | Perspective | European countries | Inpatient:
|
|
|||
| Van der Lee et al64 | Correspondence | Netherlands |
Felt distance due to less non-verbal communication |
| Vanderpuye et al40 | Editorial | Ghana | West Africa
|
| Wahed et al28 | Article | UK |
|
| Wang et al34 | Report | China |
|
| Wakefield et al20 | Report | USA | Management strategies undertaken in radiation oncology in US:
|
| Wei et al71 | Letter/Survey | China | A survey was conducted to assess the radiotherapy implementation status in 74 Chinese hospitals:
|
| Weisel et al8 | Perspectives | Germany |
6 cancer patients and 5 staff members were tested COVID-19 positive |
| Wilkinson42 | Editorial | UK |
|
| Wilson30 | Report (gray literature) | UK |
|
Treatment adaptations
|
|||
| Wu et al44 | Perspectives | China |
|
| Yusuf13 | Editorial | Pakistan |
|
Discussion
The literature highlighted 6 common key areas for focused intervention that were adopted by many cancer institutions. A detailed summary of each theme is described below so that we can better apprehend how such measures enabled cancer care continuity, while also mitigating viral spread and protecting staff and patients.
(1) Testing and Tracking
Testing played a huge role in various studies, with many hospitals enabling patient screening for COVID-19 symptoms upon hospital entry or pre-operatively.3, 7-38 This involved recording body temperature, checking respiratory symptoms, and taking blood tests and nasal swabs.3,10,11,22,33,34,36,39 If the tests results were positive, patients were either disallowed treatment, asked to isolate/quarantine or were directed to COVID-19 outpatient clinics/admitted in to dedicated COVID-19 wards.33,36,40 These interventions helped to prevent the on-site transmission of the virus. In addition, Ngoi et al describes the use of 2 checkpoints within the hospital, where patients and their accompanying visitors had to fill out a health questionnaire and were screened via a thermal scanner for their body temperature (Singapore). This intervention was deemed effective, as results showed that within a 1-month period of adopting this screening method, only 1 person within the hospital was found to be COVID-19 positive out of 70 people tested.41 As part of patient triaging pathway in some cancer centers, symptomatic patients would have to attend fever clinics before their appointments with their oncologists (UK and China).21,42,43
Staff testing was also mentioned in some articles, with Tan et al and Tey et al reporting that all staff temperature readings were taken twice daily to reduce healthcare worker-patient transmission and safeguard patients and staff.7,10,23,42,44,45A dedicated tracer team that monitored all patients under investigation allowed for active tracing of all clinical staff that were at potential exposure risk, through using a patient points-of-contact framework. This allowed for staff to be notified of infection risk immediately and enabled rapid instruction regarding the need for quarantine.46
In addition, contact tracing was highlighted as a tracking method to identify potentially infected patients worldwide.34,42,43,45-47 Wang et al demonstrated that the use of documentation of all contact and travel histories was imperative for permitting visitors into the facility (China).34 Similar contact tracing tactics were deployed in Korea which required mandatory quarantining of COVID-19 patients and any identified personnel who were in close contact with them.47 A nation-wide program was implemented in China where health QR codes were issued to track case numbers in residential and high-risk areas. This large-scale surveillance system was informative for high-risk patients as they could make the decision to shield themselves if residing in a high-risk area.43 The benefits of tracking were also evident in a study conducted by Ning et al who reported that active tracking reduced adverse effects that can occur from treatment delay and workforce incapacitation46
(2) Outreach and Communication
One of the most prominent strategies to ensure the maintenance of care continuity was regular communication between healthcare providers and patients. While use of telehealth and mobile phone technologies has grown in recent years, it has shown to be particularly useful amid the COVID-19 pandemic.
Many studies reported the use of telehealth through videoconferencing, telephone, email, mobile phone apps and text message as a means to monitor and counsel patients.3,7-8,11-12, 15-19,20-22,24,29,30,33-37,39-41,43,45,46,48-65,78 This included screening, symptom checking and side effect monitoring and outpatient care.8,22,29,39,45,52-54 These modalities of communication were significant in enabling better evaluation and follow-up of patients, as well as facilitating patient triaging and contact tracing. This was demonstrated in an Italian paper, where a “Double Triage Protocol” was put into place involving 2 separate telephone interviews for palliative care patients that require home care. The first interview assessed if COVID-19 symptoms were present in the patient, while the second assessed the severity of their symptoms in order to guide the frequency of home visits.29 Additionally, de Marinis et al showed that telehealth can be utilized for clinical application and diagnostics, whereby telematic evaluation was utilized for CT scans.39 The main disadvantage of telehealth for care provision, however, was reduced efficiency as it made appointments longer by an average of 10 minutes.15 Lobascio et al and Peeters et al also highlighted the use of mobile phone apps to help monitor and manage patient treatment toxicity and nutritional status.56,63
In terms of patient acceptability, Van der Lee et al reported interesting patient feedback, with patients showing a preference for virtual consultation due to the ease of accessibility and increased convenience as it reduced the need to travel.64 Conversely, patients also reported feeling psychologically distant with the doctor as there was reduced non-verbal communication and felt that there was less time for reflection following their call.64,65,78 A Canadian cancer center tried to mitigate this through running a trial to provide patients with daily, self-subscribed supportive text messages for extra psychological support.11 In light of this, healthcare providers must be astute of the risks posed on the weakened doctor-patient interactions which could have significant impacts on patients’ quality of life,66,67 Consequently, the psychological impacts of drastic transitioning to tele-oncology should not be overlooked, especially within oncology where adjunctive emotional and holistic care is crucial.
Other forms of patient outreach and education included the dissemination of educational materials through virtual means or via patient information leaflets.18,44,55-57 In Canada, the Princess Margaret cancer center released core education tools. These tools aided cancer patients with low health literacy to find reliable cancer-related patient education materials and a website (pmcancerclasses.ca) was recommended for online cancer classes for patients and families.58 These strategies may be an effective way to improve patient compliance with protective measures and inform them of potential risks.
Effective communication of COVID-19-related risks was emphasized in the literature prior to consenting patients for treatments.19 In West Africa, patients were educated about the possible additional risks of chemotherapy during the pandemic, including the complications associated with contracting COVID-19 and the possibility of experiencing poorer treatment outcomes.40 Similar practice has been adopted in the UK, where doctors have openly educated patients on COVID-19 related risks before and during surgical treatment.19
Videoconferencing was also frequently utilized between healthcare staff and multidisciplinary teams in order to streamline healthcare provision, continue staff training and maintain timely diagnosis and treatment.7,11,17,19,30,33,35,36,40,41,45,49,51 Dharmarajan et al reported that instating virtual multidisciplinary conferencing facilitated MDT evaluation, referral coordination and reduced diagnosis, treatment delays and travel burdens on patients and staff.38 Utilization of this modality was also well accepted among the users as many found it comparable to in-person meetings.51 In Lebanon, virtual MDT also allowed for patient data to become centralized on a single electronic system, which facilitated efficient virtual meetings and allowed for more participants to be included than in normal face-to-face meetings.59 However, one study reported that the duration of MDT case presentations had also increased and there were greater delays in receiving supporting information such as imaging and pathology slides.78 Although speed and efficiency were marginally hindered compared to “business as usual,” videoconferencing should be considered by institutions as a method to reduce unnecessary physical contact between managing team members. In summary, the adoption of telemedicine into modern practice has been well received by both healthcare professionals and patients.26, 44
(3) Protection
This theme encompasses measures that aim to mitigate the spread of the virus to protect both staff and patients. Use of PPE by staff was adopted by almost all institutions.7,11,15-17,19-21,24,30,35,37,40,43,44,46-49,55,68-70 For example, in a head and neck cancer center in the UK, staff were required to wear full PPE intra-operatively and surgical staff were instructed to change clean scrubs after entering hospital.19 Patients and visitors were also sometimes asked to wear masks, especially if they displayed symptoms.7,10,20,21,24,34,36,37,43,45,46,70
While PPE was endorsed as a necessity within many healthcare institutions, some studies reported shortages. For example, in the US, a 35% shortage in protective gear was reported.16,21 Tey et al, however, stated that their institution in Singapore undertook weekly reviews for the medical supplies, a potential way to ensure PPE shortages can be mitigated.10 Similarly, at the National University Cancer Institute of Singapore, to further reduce the use of N95 masks and gowns in cancer wards, all COVID-19 positive patients were admitted to a designated COVID-19 ward.41 Another approach taken in Zambia was the tiering of PPE protocol, which reserved various types of PPE depending on patient type and status.68 Interestingly, Morrison et al from the USA reported extending the use of N95 respirators through reprocessing under UV light radiation and vaporized hydrogen peroxide.69 On the other hand, Philippines and Pakistan ensured that demands were met through increasing the supply of PPE through working with manufacturers and external charities.13,23 To ensure correct use, one study also conducted a refresher course on PPE and Powered Air Purifying Respirator (PAPR) so that staff knew how to use their PPE properly.7
Disinfection was also an important priority for some healthcare centers with hand sanitizers being widely distributed and hand sanitation/washing being made compulsory.11,18,20-22,34,71 Clinical environments and equipment were also frequently sterilized, such as the disinfection treatment of beds and surrounding areas during treatment intervals.18,20,22,24,44,71 Anesthetists also undertook protective measures whereby intubation procedures were conducted via the use of video laryngoscopes with a plastic drape in order to reduce aerosol spread (Head and Neck Cancer Services, Hong Kong).15
(4) Social Distancing
Social distancing measures were put in place to enforce physical distance to help limit contamination and spread. To minimize contact among patients, some facilities enforced limits on the number of patients who were permitted onsite at a given time for clinical visits.16,33 Stringent restrictions on visitation for visitors/caregivers were applied and waiting rooms were closed in centers to reduce unnecessary congregation of people within a confined space.12,14,16,18,20,22,35,40 In some cases, visitors were able to accompany patients, given that health checks were enacted upon arrival.7,10,34,41 To ameliorate the social impacts of this, cancer care providers from Singapore tried to establish communication with family members through conducting remote meetings.14 Another social-distancing measure to avoid physical proximity was adopted in a cancer center in Bermuda, where patients were asked to wait at car-parks until they were collected for their appointment.26 Similarly, in the UK, phlebotomy services adopted similar approach where patients were asked to wait in their cars until their turn.42 Peng et al also described the use of a special programming model in China which aided the scheduling of radiotherapy, in an attempt to minimize waiting time and hence cross-infection risk for patients requiring in-hospital treatment. Similarly, an online booking system was utilized in Wang et al to improve efficiency and limit the number of patients on-site.34
Clinical space reconfiguration was also undertaken, as some centers attempted to modify their physical environment to better facilitate social distancing through freeing up physical space or marking floors and/or seating.11,12,23,26,68 For example, a breast cancer center in Italy reorganized working spaces and schedules in radiology and outpatient clinics to minimize patient flow and increase physical distance.21 Another study also ensured that work desks had a 2-meter separation between them to ensure adequate spacing.10
Staff and healthcare workers were also subject to intervention to minimize staff-staff/patient-staff contact.7,10,14-17,19,20,23,24,36,41,44,47,72 In Singapore, staff-to-staff transmission was avoided by separating areas for mealtimes for different clusters of staff.10 Additionally, in surgical settings, the presence of staff during high-risk procedures was minimized when possible in order to reduce staff-staff contact and protect them from aerosol generating procedures.19 For example, in head and neck surgery, intubation was performed with only essential members of the anesthetic team, while in Hong Kong the number of staff required to be present was reduced preoperatively and intraoperatively.15 In the USA, Patel et al also noted that several Head and Neck cancer centers limited resident participation in high-risk surgeries.16
Staff-to-patient transmission was mitigated through implementing “staff segregation systems,” in which oncological staff were often separated into 2 teams or had back-up staff to ensure care continuity in the case of staff infection and subsequent need for quarantine.7,10,13,17,23,27,30,36,41,72 In Singapore, Ngoi et al discussed the utilization of a “Team-Segregation Pandemic Strategy” where departmental teams were restricted to one ward and cross-transfer of staff between hospital facilities was forbidden to avoid cross-contamination. The outcome for these measures were favorable as within a month of employing staff segregation, only 1 confirmed case of COVID-19 was reported among staff and patients.41 Wu et al also instated the zoning of departments according to different levels of contamination in a center in China.44
Social distancing was additionally enforced through physical isolation of COVID-19 wards and closure of common areas to prevent viral spread (Italy).35 Rodler et al reported the isolation of new patients in a single room upon arrival (Uro-oncology, Germany), while in Mei et al, an isolation ward was created and had an increased prevention level compared to the rest of the hospital so that patients who tested positive could not be visited (Wuhan, China).21, 45 Valenza et al similarly described the creation of a surveillance zone to house COVID-19 patients, whereby isolation rooms were selected based on transfer time and distance from the hospital CT scanner and contained installed video cameras to limit the number of nurse visits (Italy).37 In addition, COVID-19 patients in need of urgent surgery were operated on when no regular cases were underway in a dedicated COVID-19 theater.37
Attempts were also made to instate COVID-19 free cancer-dedicated centers/hospitals, in order to segregate them from institutions treating COVID-19 cases to prevent viral spread.10,19,35,42,44,70 In Van der Haar et al, cancer patients were transferred from general hospitals dealing with COVID-19 patients to dedicated cancer centers aiming to stay COVID-19 free.3 However, this study also noted that complete segregation is difficult for cancer centers built within general hospitals as they still ended up treating COVID-19 positive patients.3 It should be noted that factors such as widespread use of PPE and good ventilation are key factors that should be considered in order to mitigate aerosol transmission when segregation measures have been implemented within one building.79
Finally, training staff and patients on social-distancing practice proved to be beneficial in improving behaviors conducive to maintaining physical separation.8 Education on good social-distancing practice may be beneficial in ingraining habits that are essential for staff and patients who have high exposure to the virus due to their clinical surroundings.
(5) Treatment Management
With aims to minimize non-essential hospital visits and additional risks of treatment-induced complications, many health care providers adapted individual treatment plans during the COVID-19 pandemic.8, 21 Careful assessment before treatment continuation/initiation and reviewing of hospitalization proposals were conducted to priorities patients requiring necessary and urgent intervention.19, 35
De-escalation of treatment regimens was commonly adopted by many institutions, with some making changes to treatment type, intervals or dosage.11,13,16,24,25,30,33,36,40-42,73,74 Several healthcare providers routinely considered replacing intravenous regimens with oral or subcutaneous agents and prolonging treatment intervals for intravenous treatment.8,10,34,35,62,73,74 Interestingly, in Germany, this was accompanied by dosage increase in immunotherapy but reduction in chemotherapy to minimize risk of leukopenia.45 In the UK, some patients with hematological cancers were denied stem cell transplantation and received radiotherapy instead, while patients with locally advanced colorectal cancer, were offered a short course of radiotherapy prior to radical surgery rather than the standard long-term chemotherapy prior to radical surgery.42,73 In Italy, oncologists adopted regimens that deviated from orthodox first line therapy for breast cancer in the metastatic setting. Preferred adaptions included switching of oral treatment in patients eligible for chemotherapy administration (n = 139; 84.2%) compared to the standard intravenous agents (n = 26; 15.8%). A significant deviation of treatment therapeutics was also observed, whereby the administration of CDK4/6 inhibitors to endocrine therapy for luminal tumors with less-aggressive characteristics was reduced during the pandemic than before the emergency (n = 92; 55.8% vs n = 132; 80.0%).74
In Tagliamento et al, concerns over the potential interference with immune checkpoint inhibitors (ICI) and SARS-COV-2 was highlighted. Despite this, 97.1% of Italian physicians in the survey reported that they would not deny ICI’s as a treatment option, while 31.7% of the respondents did not modify the choice of the ICI and the schedule of administration in order to reduce the number of hospital visits.25 With the ambiguity surrounding the interaction between ICIs and the pathogenesis of the virus worsening hyperinflammation with cytokine release syndrome, is it essential that parallel efforts are made to reduce the risk of contracting COVID-19 among patients taking this medication.25 Delivery of this drug should be evaluated on a case-by-case basis and should take both efficacy and safety into consideration.25
Similarly, treatment de-escalation was made in radiation oncology and nuclear medicine, in compliance with a proposed the 4R’s system by a Canadian group, including (1) viRtual care, (2) Ration radiation, (3) deFer radiation and (4) hypofRactionate radiation.10,18,36,41,50 Hypofractionating radiation in selected patients (i.e. escalation in dose of radiation, but reduced treatment frequencies) was thought to be a viable strategy to shorten treatment schedules in radiotherapy.18 Interestingly, a retrospective cohort study following 800 cancer patients with symptomatic COVID-19 infection found no significant association between mortality and the receipt of cytotoxic chemotherapy, radiotherapy and immunotherapy. This suggests curative treatment should not be delayed in cancer patients while trying to shield cancer patients from exposure to the virus.27
Curative cancer surgeries were also subject to change, delays or cancelation due to safety concerns or lack of resources.9,38,40,48,66 Surgical oncologists often considered referral to non-surgical options such as radiotherapy or switching to a less risky surgical technique.3,9,15,75 For instance, one study reported that cordectomies were performed for vocal cord malignancy using a sharp technique, instead of the laser technique to prevent aerosolization of viruses.49 In addition, attempts to switch from general anesthesia to local or regional anesthesia were observed during sentinel lymph node biopsies and breast cancer surgery in some UK centers.42,76 In the US, robotic technique was preferred over open surgery when managing urological cancers, while some others reported use of enhanced recovery programs after surgery protocols to minimize hospital stay.14,70 Patients who were also candidates for supportive therapy alone or eligible for alternate non-surgical treatment were excluded from hospitalization.35
In summary, many centers judiciously considered risks and benefits for treatment continuation or initiation for patients, such as treatment-related complications and intensive care availability.3 Although downscaling treatment plans in cancer patients was a significant intervention in this review, Poggio et al expressed their concern over potential undertreatment of cancer patients as a result of these treatment changes.74 The general consensus was that each patient should be assessed on a case-by-case basis by multidisciplinary teams and that delaying treatments for curable cancer was not recommended. Tumor stage, histology, age, treatment type, comorbidities, patients’ general well-being and history of recent pneumonitis were taken into account when assessing the risks and benefits of cancer treatments.39 Documentation of treatment variation into trust databases and regular auditing of clinical activity was also deemed crucial in maintaining standard of care during COVID-19 pandemic (Head and Neck Surgery, UK).19
(6) Service Restructuring
Due to resource scarcity, staff shortages and interruptions to care continuity and accessibility, service provision was often adapted to combat the pressures inflicted on healthcare institutions. Service restructuring through role allocation, outsourcing and patient transfers were common methods used to help mitigate the strain. For example, healthcare providers outsourced investigations (blood tests) or transferred patients externally to private sectors/local providers to help alleviate burdens.3,17,46 Some studies made referrals to family doctors and centers closer to the patient’s home in order to maintain care continuity and conduct follow-ups, while also minimizing patient travel.33,39
Good organization and role allocation of healthcare staff proved to be pivotal in streamlining cancer care.11 A case from the epicenter of the outbreak in Wuhan outlined the redeployment of 50 doctors and nurses to oncology departments who were part of departments that were not in service when cases of COVID-19 started exponentially rising.21 Ong et al also ensured that there was efficient deployment of manpower to maintain high patient loads.72 The importance of COVID-19 dedicated teams in Europe was also highlighted, whereby Valenza et al also described how setting up a “surveillance team” (including one nurse specializing in respiratory care) meant that COVID-19 patients could continuously be monitored (Italy).37 In addition, in the UK, junior doctors also provided effective 24-hours cover for all inpatient areas onsite. This was made possible via an implemented shadow rota to cover sickness or self-isolation absence.30 In the context of surgery, the importance of surgeon selection was also mentioned, with one study reported using only highly experienced surgeons who were selected for airway operations as a means to reduce contact time and risk as they were able to carry out surgeries at a quicker pace.15 These examples demonstrate how assembly of a team with dedicated responsibilities and selection of staff based on their skills and competencies can contribute to increased safety and better quality of care.
In addition, to improve drug delivery, drug-refill clinics were set up to fast-track repeat prescriptions for stable patients who did not require drug reviews in Hong Kong.15 In other countries, home delivery of medications was introduced to minimize patient travel to pharmacies.14,21,41,43,60 One study also reported that patients received a higher volume of drugs at each hospital visit so that patients did not have to keep on returning to replenish their supply.55
Another significant determiner for service restructuring was the need to accommodate demand, maintain care continuity, and address the accumulation of delayed and canceled appointments.22,28,66,69 Service restructuring proved to be essential in mitigating these strains. In Italy, a specific outpatient clinic was set up to resolve treatment-related cutaneous and mucosal adverse events53. This allowed for dedicated care for specific issues, while reducing appointment backlogging. Italian cancer designated hubs were also created to deliver necessary curative treatments in regions significantly hit by the virus (Lombardy) to ensure patients in high-risk areas were also able to access adequate care.55 In Singapore, Ong et al reported that efforts were devoted to review patient lists for clinic sessions in order to decrease outpatient load and defer non-urgent cases.70 Healthcare providers that limited caseloads to 10-15 patients per session and spaced out appointment times also implemented evening and weekend clinical sessions to reduce the backlogging of appointments.7,15 Out-of-hour operations were also adopted to catch up on rescheduled lung cancer surgeries (UK).75
Finally, various institutions formed leadership teams and committees, who were pivotal in making crucial decisions in regard to various aspects of service restructuring in order to minimize patient contact.30 For example, Blot et al highlighted the crucial role played by their ethical committee board in assisting physicians with decision making in clinical dilemmas.77 Additionally, Civantos et al also set up an otolaryngologic triage committee in order to decide patient resource allocation, while in Germany a multidisciplinary leadership task force was created to assess treatment plans on a case-by-case basis.8 ,9 These leadership teams were essential for service restructuring decisions and ensured that care provision could be prioritized and maintained in a safe and efficient way.9
The Utilization of Tele-oncology for Care Provision
Possible financial, social and ethical factors should also be considered as telehealth becomes more integrated into cancer care. In terms of ethics, adequate cybersecurity measures should be in place to ensure patient privacy is protected.78 Financially, positive economic outcomes for telemedicine usage have been reported highlighting the potential cost-effectiveness for healthcare institutions.17 Financial support has been demonstrated by some countries such as the USA, where reimbursement for telephone and video encounters has been allowed.78 However, ambiguity remains in regards to how flow of funds and reimbursement pathways will continue post-pandemic and as well as what constitutes as “chargeable” care.78 Thus, there is a need for financial regulation and renegotiation of funding models to ensure that the financial implications posed on cancer institutions and oncologists can be minimized.
Despite the numerous benefits of telehealth, the social and structural implications associated are numerous. Internet connectivity, possession of digital devices and technological literacy are factors that can lead to digital and socioeconomic divides. As aforementioned, the virtual pivot may also have significant effects on doctor-patient interactions and rapport building, as well as removing the “social, moral and ritual significance” of in-person communication.78 Thus, in order to bridge the digital void, acquisition of communication and technological skills within tele-oncology settings are required.78
In summary, the COVID-19 pandemic has emphasized the benefits of the clinical application of tele-oncology for cancer patients.80,81,82 As many cancer treatments often result in immunosuppression, cancer patients are a prominent risk group in the pandemic due to their increased susceptibility to contracting COVID-19. Thus, a significant benefit identified for tele-oncology is that it reduces the risk of infection through decreasing in-person contact, while maintaining care continuity. Additionally, since previous literature has reported that virtual oncology services are efficient, cost effective and result in good patient satisfaction, the future of tele-oncology is a promising prospect and is likely to be continually adopted post-pandemic in routine clinical care.75,83,84
Broad Considerations for Cancer Care Provision
The COVID-19 pandemic has placed inevitable psychological implications on cancer patients. Ciążyńska et al found that cancer patients often felt stressed due the uncertainty regarding their cancer therapy and the risk of developing COVID-19 symptoms while undergoing treatment.84 Furthermore, patient wellbeing was also affected due to social distancing restrictions, as patients who would have previously taken their family members to hospital appointments were asked to come alone.84 While our review focuses on the operational and organizational adjustments to care provision, cancer institutions must also consider the importance of providing emotional support and addressing mental health issues that are prevalent among cancer patients at this time.
Learning from the pandemic has also highlighted the broad impacts of how governmental policies and societal needs have shaped oncological care. It has also emphasized the need for healthcare systems to be dynamic and flexible in order to mitigate the mid and long-term ripple effects that will be reflected in clinical practice and patient outcomes. Broad challenges on oncological care will include: the effects of unemployment and thus the inability to pay for cancer therapies, access to novel treatments and clinical trials as a result of structural impacts, disparities in patient experience due to wider socioeconomic disparities, and enhanced mental health consequences.78 In light of this, we can foresee that future cancer patient care will be disproportionately affected by the consequences of the COVID-19 pandemic.78
Available Models of Care Delivery for COVID-19 Positive/Negative Patients
COVID-19 Positive Patients
As COVID-19 positive patients may still shed the virus for prolonged periods, care delivery was adjusted in order to maintain cancer care for those requiring it in the interim. For these patients, 2 priorities were prominent within the care models of each institution. Firstly, changes to models of care were made to ensure effective isolation (either at home or in a quarantined area onsite) to minimize viral spread to other patients and personnel while receiving care. While isolation requirements often resulted in the cancelation/delay of treatments, implementation of COVID-19 designated areas in institutions facilitated the continuation of treatment for patients in need of in-patient care. Additionally, since COVID-19 positive were deprived of visitors, models of care should include services that supplement the emotional support that is usually provided through visitation.
Another significant consideration was the type, frequency, and continuation of treatment that was given based on their medical need and severity of cancer. Thus, the opportunity cost of maintaining cancer care in those infected was judiciously considered. The factorization of risks imposed by infection, such as developing severe pneumonia during the disease course and their weakened immune state were significant determinants for the proposed model of care delivery which were decided on an individual basis. The importance of conducting risk-benefit analyses has been particularly emphasised in the context of surgery, due to the substantial risk of mortality and developing postoperative complications in patients with COVID-19.85
COVID-19 Negative Patients
For COVID-19 negative patients, efforts were predominantly centered around reducing the risk of contracting the virus. As a result, models of care delivery and treatment were adjusted to safeguard and mitigate the possible negative health outcomes patients may experience should they get infected at a later time. To reduce this risk, segregation systems were often instated to ensure COVID-19 negative patient safety. For institutions providing care within the same building, staff segregation interventions were instated so that staff working in COVID-19 designated areas would not mix with staff in contact with non-infected patients. In addition, some institutions implemented transfer procedures to relocate patients from general hospitals dealing with COVID-19 cases to COVID-19 free centers so that treatment could resume in low infection risk settings. Similar to patients who tested positive, the risk of COVID-19 exposure was weighed against the risk of not receiving or de-escalating cancer treatments.
In summary, the literature emphasizes the great importance of multidisciplinary leadership teams studying treatment regimens on a case-by-case basis due to the varying cancer types, prognosis, and needs within the cancer patient population. Moreover, due to their vulnerability to infection, institutions should also priorities effective segregation and isolation measures within their care delivery procedures. Based on these priorities, there is a need for models of care to be catered and flexible to patients according to a detailed risk-benefit criterion.
Recommendations
The purpose of the narrative synthesis conducted in this review was to identify key areas that must be targeted in order to mitigate viral spread while maintaining cancer care provision. In doing so, the themes outlined in the literature can be considered by cancer centers or other institutions containing high-risk patient populations when planning and implementing their own interventions amid the viral outbreak. Table 5 and Figure 2 highlights recommendations based on the findings from this review.
Table 5.
Table of Recommendations.
| Testing and Tracking |
|
| Outreach and Communication |
|
| Protection |
|
| Social Distancing |
Patients and Visitors
|
| Treatment Managements |
|
| Service Restructuring |
|
Figure 2.
Summary of Themes.
The utilization of common quality improvement tools, such as logic models (to evaluate the effectiveness of interventions), process mapping (to map out staff workflows and patient journeys) and Plan, Do, Study, Act Cycles (to test interventions and incrementally improve them) are recommended for the planning and assessment of new strategies for cancer service provision. These tools will help ensure that any changes to cancer care are thorough, efficacious and efficient.
Future Research
In search of a better strategy to support cancer patients during the COVID-19 pandemic, research priorities should be directed to the following areas. Firstly, long-term clinical data is required to assess the impact of treatment de-escalation has on patients’ outcomes. Secondly, psychological impacts on cancer patients should also be examined with both qualitative and quantitative methods to help guide evidence-based interventions aiming to provide adjunctive holistic and emotional care. Thirdly, though many healthcare institutions have shared their ideas on possible adjustments to be made on an organizational level, quality improvement data is still limited. Sharing of this information should be encouraged as it could provide a point of reference for recommended intervention implementation when managing future health crises. Finally, as we anticipate further consequential impacts on cancer care post-pandemic, it is imperative that efforts are made to better understand the mid- and long-term implications imposed on clinicians and patients. In order to strengthen the evidence-base, qualitative methods should be employed to aid primary data collection through surveys/interviews with relevant stakeholders to better comprehend the impacts of COVID-19 on the oncological landscape.
Limitations
Majority of the articles included in this review were geographically based in high-income/upper-middle income countries. Consequently, relevant learning from low-resource settings may be limited. This was evident in our search, as the majority of screened studies were from China, Italy, Singapore and the United States.
In addition, our search strategy only included publications in the English language, meaning that the review may be subject to geographical bias. Thus, learning from non-English speaking countries (such as high death toll countries like Italy) may have also been forfeited as it can be assumed that a large proportion of studies would have been published in their native language.
While descriptions of interventions were plentiful in the literature, many studies did not report outcomes on whether the strategies were effective in mitigating spread of the virus and facilitating care provision. Therefore, we cannot be certain on how effective the interventions were in yielding positive outcomes and reducing the number of COVID-19 cases in cancer patients and healthcare workers.
Since this review only provides a descriptive list of interventions adopted globally (which were largely adopted due to recommendations encouraged by governments and scientific organizations e.g. testing and triage), it is difficult to form a uniform summary of cancer care changes as different countries have experienced different COVID-19-related challenges. Thus, due to the varying healthcare system contexts and burdens, we are unable to provide definitive recommendations for cancer care provision since there is no one-size fits all solution.
Conclusion
Cancer patients are a high-risk population amid the COVID-19 pandemic. As a result, extensive planning must be undertaken to protect this population from infection and COVID-19-related risks, while also maintaining their cancer treatment and care. Many institutions have adopted various strategies to safeguard their patients and staff and streamline service provision, however the extent of success of these interventions is still unknown. This systematic review provides an updated summary of the evidence-base and presents 6 core themes of targeted intervention commonly adopted within numerous cancer institutions globally. The themes can be used as a tool to inform future interventions that can be implemented by healthcare institutions facing similar risks amid the COVID-19 outbreak.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study did not require ethics approval because the study did not contain human or animal trials.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Chun Ming Chiu  https://orcid.org/0000-0001-8335-0409
https://orcid.org/0000-0001-8335-0409
Thomas Ho Lai Yau  https://orcid.org/0000-0001-5510-6214
https://orcid.org/0000-0001-5510-6214
Amer Harky, MRCS, MSc  https://orcid.org/0000-0001-5507-5841
https://orcid.org/0000-0001-5507-5841
References
- 1.World Health Organization. COVID-19 Weekly Epidemiological Update. WHO; 2020. [Google Scholar]
- 2.Emanuel EJ, Persad G, Upshur R. et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049–2055. doi:10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 3.van de Haar J, Hoes LR, Coles CE. et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665–671. doi:10.1038/s41591-020-0874-8 [DOI] [PubMed] [Google Scholar]
- 4.Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172(11):756–758. doi:10.7326/M20-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21(5):629–630. doi:10.1016/S1470-2045(20)30217-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R. et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi:10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan BF, Tuan JKL, Yap SP, Ho SZ, Wang MLC. Managing the COVID-19 pandemic as a National Radiation Oncology Centre in Singapore. Clin Oncol. 2020;32(7):e155–e159. doi:10.1016/j.clon.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisel KC, Morgner-Miehlke A, Petersen C. et al. Implications of SARS-CoV-2 infection and COVID-19 crisis on clinical cancer care: report of the university cancer center Hamburg. Oncol Res Treat. 2020;43(6):307–313. doi:10.1159/000508272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Civantos FJ, Leibowitz JM, Arnold DJ. et al. Ethical surgical triage of head and neck cancer patients during the COVID-19 pandemic. Head and Neck. 2020;42(7):1423–1447. doi:10.1002/hed.26229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tey J, Ho S, Choo BA. et al. Navigating the challenges of the COVID-19 outbreak: perspectives from the radiation oncology service in Singapore. Radiother Oncol. 2020;148:189–193. doi:10.1016/j.radonc.2020.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casella D, Fusario D, Cassetti D. et al. The patient’s pathway for breast cancer in the COVID-19 era: an Italian single-center experience. Breast J. 2020;26(8):1589–1592. doi:10.1111/tbj.13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guven DC, Aktas BY, Aksun MS. et al. COVID-19 pandemic: changes in cancer admissions. BMJ Support Palliat Care. 2020;bmjspcare–2020-002468. doi:10.1136/bmjspcare-2020-002468 [DOI] [PubMed] [Google Scholar]
- 13.Yusuf A. Cancer care in the time of COVID-19—a perspective from Pakistan. Ecancermedicalscience. 2020;14:1026. doi:10.3332/ECANCER.2020.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang J, Yang VS, Han S. et al. Minimizing transmission of COVID-19 while delivering optimal cancer care in a National Cancer Centre. J Cancer Policy. 2020;25:100241. doi:10.1016/j.jcpo.2020.100241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AKF, Cho RHW, Lau EHL. et al. Mitigation of head and neck cancer service disruption during COVID-19 in Hong Kong through telehealth and multi-institutional collaboration. Head Neck. 2020;42(7):1454–1459. doi:10.1002/hed.26226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel RJ, Kejner A, McMullen C. Early institutional head and neck oncologic and microvascular surgery practice patterns across the United States during the SARS-CoV-2 (COVID19) pandemic. Head Neck. 2020;42(6):1168–1172. doi:10.1002/hed.26189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins PM, Madden A, O’Connell C. et al. Urological service provision during the COVID-19 period: the experience from an Irish tertiary centre. Ir J Med Sci. 2020;1–6. doi:10.1007/s11845-020-02352-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Press RH, Hasan S, Chhabra AM, Choi JI, Simone CB. Quantifying the impact of COVID-19 on cancer patients: a technical report of patient experience during the COVID-19 pandemic at a high-volume radiation oncology proton center in New York city. Cureus. 2020;12(4): e7873. doi:10.7759/cureus.7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler D, Davies-Husband C, Dhanda J. et al. Head and neck oncological ablation and reconstruction in the COVID-19 era—our experience to date. Br J Oral Maxillofac Surg. 2020;58(8):1008–1013. doi:10.1016/j.bjoms.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakefield DV, Sanders T, Wilson E. et al. Initial impact and operational responses to the COVID-19 pandemic by American Radiation Oncology practices. Int J Radiat Oncol Biol Phys. 2020;108(2):356–361. doi:10.1016/j.ijrobp.2020.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei H, Dong X, Wang Y, Tang L, Hu Y. Managing patients with cancer during the COVID-19 pandemic: frontline experience from Wuhan. Lancet Oncol. 2020;21(5):634–636. doi:10.1016/S1470-2045(20)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oualla K, Nouiakh L, Acharfi N. et al. How is Morocco reacting to COVID-19 crisis in anticancer centers? Cancer Control. 2020;27(3):1073274820941973. doi:10.1177/1073274820941973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendoza MJL, Tan HNC, Hernandez ARB. et al. Medical oncology care amidst the COVID-19 pandemic at the National University hospital in the Philippines. Ecancermedicalscience. 2020;14:1066. doi:10.3332/ECANCER.2020.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onesti CE, Rugo HS, Generali D. et al. Oncological care organisation during COVID-19 outbreak. ESMO Open. 2020;5(4):e000853. doi:10.1136/esmoopen-2020-000853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagliamento M, Spagnolo F, Poggio F. et al. Italian survey on managing immune checkpoint inhibitors in oncology during COVID-19 outbreak. Eur J Clin Invest. 2020;50(9):e13315. doi:10.1111/eci.13315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fosker C. COVID-19 and cancer care in Bermuda. Lancet Oncol. 2020;21(6):761–762. doi:10.1016/S1470-2045(20)30272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee LYW, Cazier JB, Angelis V. et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi:10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahed S, Chmelo J, Navidi M, Hayes N, Phillips AW, Immanuel A. Delivering esophago-gastric cancer care during the COVID-19 pandemic in the United Kingdom: a surgical perspective. Dis esophagus Off J Int Soc Dis Esophagus. 2020;33(9):doaa091. doi:10.1093/dote/doaa091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porzio G, Cortellini A, Bruera E. et al. Home care for cancer patients during COVID-19 pandemic: the double triage protocol. J Pain Symptom Manage. 2020;60(1): e5–e7. doi:10.1016/j.jpainsymman.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson T. Maintaining a cancer service in the midst of the COVID-19 pandemic: a single centre experience. NICE. Published online 2020. Accessed December 21, 2020. https://www.nice.org.uk/sharedlearning/maintaining-a-cancer-service-in-the-midst-of-the-covid-19-pandemic-a-single-centre-experience
- 31.Elkin E, Viele C, Schumacher K. et al. A COVID-19 screening tool for oncology telephone triage. Support Care Cancer. 2020;1–6. doi:10.1007/s00520-020-05713-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moss C, Dolly S, Russell B. et al. One piece of the jigsaw for the cancer recovery strategy: prevalence of COVID-19 in patients with cancer. Cancer Control. 2020;27(3):1073274820950844. doi:10.1177/1073274820950844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Indini A, Aschele C, Cavanna L. et al. Reorganisation of medical oncology departments during the novel coronavirus disease-19 pandemic: a nationwide Italian survey. Eur J Cancer. 2020;132:17–23. doi:10.1016/j.ejca.2020.03.024 [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Wang J, He J. Active and effective measures for the care of patients with cancer during the COVID-19 spread in China. JAMA Oncol. 2020;6(5):631–632. doi:10.1001/jamaoncol.2020.1198 [DOI] [PubMed] [Google Scholar]
- 35.Silvestris N, Moschetta A, Paradiso A, Delvino A.COVID-19 pandemic and the crisis of health systems: the experience of the Apulia cancer network and of the comprehensive cancer center Istituto Tumori “Giovanni Paolo II” of bari. Int J Environ Res Public Health. 2020;17(8):2763. doi:10.3390/ijerph17082763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czernin J, Fanti S, Meyer PT. et al. Nuclear medicine operations in the times of COVID-19: strategies, precautions, and experiences. J Nucl Med. 2020;61(5):626–629. doi:10.2967/jnumed.120.245738 [DOI] [PubMed] [Google Scholar]
- 37.Valenza F, Papagni G, Marchianò A. et al. Response of a comprehensive cancer center to the COVID-19 pandemic: the experience of the Fondazione IRCCS–Istituto Nazionale dei Tumori di Milano. Tumori J. 2020;300891620923790. doi:10.1177/0300891620923790 [DOI] [PubMed] [Google Scholar]
- 38.Brody RM, Albergotti WG, Shimunov D, Nicolli E, Harris BN, Bur AM. Changes in head and neck oncologic practice during the COVID-19 pandemic. Head Neck. 2020;42(7):1448–1453. doi:10.1002/hed.26233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Marinis F, Attili I, Morganti S. et al. Results of multilevel containment measures to better protect lung cancer patients from COVID-19: the IEO model. Front Oncol. 2020;10:665. doi:10.3389/fonc.2020.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderpuye V, Elhassan MMA, Simonds H. Preparedness for COVID-19 in the oncology community in Africa. Lancet Oncol. 2020;21(5):621–622. doi:10.1016/S1470-2045(20)30220-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngoi N. A segregated-team model to maintain cancer care during the COVID-19 outbreak at an academic center in Singapore. Ann Oncol. 2020;31(7):840–843. doi:10.1016/j.annonc.2020.03.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson E. How cancer services are fighting to counter covid-19’s impact. BMJ. 2020;370:m2747. doi:10.1136/bmj.m2747 [DOI] [PubMed] [Google Scholar]
- 43.Peng L, Yang JS, Stebbing J. Lessons to Europe from China for cancer treatment during the COVID-19 pandemic. Br J Cancer. 2020;123(1):7–8. doi:10.1038/s41416-020-0856-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, Zheng D, Liu Y, Hu D, Wei W, Han G. Radiation therapy care during a major outbreak of COVID-19 in Wuhan. Adv Radiat Oncol. 2020;5(4):531–533. doi:10.1016/j.adro.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodler S, Apfelbeck M, Stief C, Heinemann V, Casuscelli J.Lessons from the coronavirus disease 2019 pandemic: will virtual patient management reshape URO-oncology in Germany? Eur J Cancer. 2020;132:136–140. doi:10.1016/j.ejca.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ning MS, McAleer MF, Jeter MD. et al. Mitigating the impact of COVID-19 on oncology: clinical and operational lessons from a prospective radiation oncology cohort tested for COVID-19. Radiother Oncol. 2020;148:252–257. doi:10.1016/j.radonc.2020.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Lim AR, Kim MJ. et al. Innovative countermeasures can maintain cancer care continuity during the coronavirus disease-2019 pandemic in Korea. Eur J Cancer. 2020;136:69–75. doi:10.1016/j.ejca.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A, Arora V, Nair D. et al. Status and strategies for the management of head and neck cancer during COVID-19 pandemic: Indian Scenario. Head Neck. 2020;42(7):1460–1465. doi:10.1002/hed.26227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Civantos AM, Carey RM, Lichtenstein GR, Lukens JN, Cohen RB, Rassekh CH. Care of immunocompromised patients with head and neck cancer during the COVID-19 pandemic: two challenging and informative clinical cases. Head Neck. 2020;42(6):1131–1136. doi:10.1002/hed.26165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rathod S, Dubey A, Bashir B. et al. Bracing for impact with new 4R’s in the COVID-19 pandemic—a provincial thoracic radiation oncology consensus. Radiother Oncol. 2020;149:124–127. doi:10.1016/j.radonc.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dharmarajan H, Anderson JL, Kim S. et al. Transition to a virtual multidisciplinary tumor board during the COVID-19 pandemic: University of Pittsburgh experience. In: Head Neck. 2020;42(6):1310–1316. doi:10.1002/hed.26195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agyapong VIO, Hrabok M, Shalaby R. et al. Closing the COVID-19 psychological treatment gap for cancer patients in Alberta: protocol for the implementation and evaluation of text4Hope-cancer care. JMIR Res Protoc. 2020;9(8):e20240. doi:10.2196/20240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cinelli E, Fabbrocini G, Fattore D, Marasca C, Damiani G, Annunziata MC. Safe distance, safe patients! Therapeutic management of oncological patients affected by cutaneous and mucosal adverse events during the COVID-19 pandemic: an Italian experience. Support Care Cancer. 2020;28(9):3991–3993. doi:10.1007/s00520-020-05563-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grenda TR, Whang S, Evans NR. Transitioning a surgery practice to telehealth during COVID-19. Ann Surg. 2020;272(2):e168–e169. doi:10.1097/SLA.0000000000004008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curigliano G.How to guarantee the best of care to patients with cancer during the covid-19 epidemic: the Italian Experience. Oncologist. 2020;25(6):463–467. doi:10.1634/theoncologist.2020-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lobascio F, Caccialanza R, Monaco T. et al. Providing nutritional care to cancer patients during the COVID-19 pandemic: an Italian perspective. Support Care Cancer. 2020;28(9):3987–3989. doi:10.1007/s00520-020-05557-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flannigan R, Sundar M, Weller S. et al. Pearls to pivoting a multidisciplinary prostate cancer survivorship program during the COVID-19 pandemic. Eur Urol Oncol. 2020;3(4):397–399. doi:10.1016/j.euo.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giuliani M, Papadakos T, Papadakos J. Propelling a new Era of patient education into practice—cancer care post–COVID-19. Int J Radiat Oncol Biol Phys. 2020;108(2):404–406. doi:10.1016/j.ijrobp.2020.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elkaddoum R, Kourie HR, Kassis NE. et al. Treating cancer patients in times of COVID-19 pandemic: a virtual women cancers multidisciplinary meeting experience. Bull Cancer. 2020;107(7-8):738–740. doi:10.1016/j.bulcan.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang CY, El-Kouri NT, Elliot D. et al. Telehealth for cancer care in veterans: opportunities and challenges revealed by COVID. JCO Oncol Pract. 2020;17(1):22–29. doi:10.1200/op.20.00520 [DOI] [PubMed] [Google Scholar]
- 61.Harky A, Chiu CM, Yau THL, Lai SHD. Cancer patient care during COVID-19. Cancer Cell. 2020;37(6):749–750. doi:10.1016/j.ccell.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulvey TM, Jacobson JO. Covid-19 and cancer care: ensuring safety while transforming care delivery. J Clin Oncol. 2020;38(28):3248–3251. doi:10.1200/JCO.20.01474 [DOI] [PubMed] [Google Scholar]
- 63.Peeters M, Peeters M, Van Dam P. et al. Prescreening for COVID-19 in patients receiving cancer treatment using a patient-reported outcome platform. ESMO Open. 2020;5(3):e000817. doi:10.1136/esmoopen-2020-000817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Lee ML, Schellekens MPJ. Bridging the distance: continuing psycho-oncological care via video-consults during the COVID-19 pandemic. Psychooncology. 2020;29(9):1421–1423. doi:10.1002/pon.5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Millar C, Campbell S, Fisher P, Hutton J, Morgan A, Cherry MG. Cancer and COVID-19: patients’ and psychologists’ reflections regarding psycho-oncology service changes. Psychooncology. 2020;29(9):1402–1403. doi:10.1002/pon.5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frey MK, Ellis AE, Zeligs K. et al. Impact of the coronavirus disease 2019 pandemic on the quality of life for women with ovarian cancer. Am J Obstet Gynecol. 2020;223(5):725.e1–725.e9. doi:10.1016/j.ajog.2020.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baabdullah M, Zeeneldin A, Alabdulwahab A. et al. Optimizing the communication with cancer patients during the COVID-19 pandemic: patient perspectives. Patient Prefer Adherence. 2020;14:1205–1212. doi:10.2147/PPA.S263022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lombe DC, Mwaba CK, Msadabwe SC. et al. Zambia’s National Cancer Centre response to the COVID-19 pandemic—an opportunity for improved care. Ecancermedicalscience. 2020;14:1051. doi:10.3332/ECANCER.2020.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrison DR, Gentile C, McCammon S, Buczek E.Head and neck oncologic surgery in the COVID-19 pandemic: our experience in a deep south tertiary care center. Head Neck. 2020;42(7):1471–1476. doi:10.1002/hed.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quarto G, Grimaldi G, Castaldo L. et al. Avoiding disruption of timely surgical management of genitourinary cancers during the early phase of the COVID-19 pandemic. BJU Int. 2020;126(4):425–427. doi:10.1111/bju.15174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei W, Jiang H, Chen W. et al. How should we implement radiotherapy for cancer patients in China during the endemic period of COVID-19? Radiother Oncol. 2020;147:100–102. doi:10.1016/j.radonc.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ong CAJ, Lim HJ, Tan JWS. et al. Facilitating timely cancer care in a surgical oncology subspecialty unit during the pandemic and recovery phase of the COVID era. Asian J Surg. 2020;43(9):965–966. doi:10.1016/j.asjsur.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mirnezami R, Knowles J, Kar A, Glynne-Jones R. Preoperative radiotherapy for locally advanced rectal cancer during and after the COVID-19 pandemic. Br J Surg. 2020;107(8):e263. doi:10.1002/bjs.11725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poggio F, Tagliamento M, Di Maio M. et al. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities. JCO Oncol Pract. 2020;16(11):e1304–e1314. doi:10.1200/op.20.00297 [DOI] [PubMed] [Google Scholar]
- 75.Ardizzone L, Barber T, Drebin J. et al. Cancer surgery and COVID19. Ann Surg Oncol. Published online 2020. doi:10.1245/s10434-020-08462-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Batt J, Cook N, Nadeem M, Sahu A. Dilutional local anaesthetic techniques in oncoplastic breast surgery and potential benefits during the COVID-19 pandemic and beyond. J Perioper Pract. 2020;30(9):277–282. doi:10.1177/1750458920944080 [DOI] [PubMed] [Google Scholar]
- 77.Blot F, Dumont SN, Vigouret-Viant L. et al. Ethical issues related to the COVID-19 pandemic in patients with cancer: Experience and organisations in a French comprehensive cancer centre. BMJ Support Palliat Care. 2020;bmjspcare–2020-002504. doi:10.1136/bmjspcare-2020-002504 [DOI] [PubMed] [Google Scholar]
- 78.Broom A, Kenny K, Page A. et al. The Paradoxical effects of COVID-19 on cancer care: current context and potential lasting impacts. Clin Cancer Res. 2020;26(22):5809–5813. doi:10.1158/1078-0432.ccr-20-2989 [DOI] [PubMed] [Google Scholar]
- 79.Tang S, Mao Y, Jones RM. et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ Int. 2020;144:106039. doi:10.1016/j.envint.2020.106039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirke MM, Shaikh SA, Harky A. Tele-oncology in the COVID-19 era: The way forward? Trends in Cancer. 2020;6(7):547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shirke MM, Shaikh SA, Harky A. Implications of telemedicine in oncology during the COVID-19 pandemic. Acta Bio Medica Atenei Parmensis. 2020;91(3):e2020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leung MST, Lin SG, Chow J, Harky A. COVID‐19 and oncology: Service transformation during pandemic. Cancer Med. 2020;9(19):7161–7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis GD, Hatch SS, Wiederhold LR, Swanson TA. Long-term institutional experience with telemedicine services for radiation oncology: a potential model for long-term utilization. Adv Radiat Oncol. 2020;5(4):780–782. doi:10.1016/j.adro.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ciążyńska M, Pabianek M, Szczepaniak K. et al. Quality of life of cancer patients during coronavirus disease (COVID-19) pandemic. Psychooncology. 2020;29(9):1377–1379. doi:10.1002/pon.5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nepogodiev D, Bhangu A, Glasbey JC, Li E, Omar OM, Simoes JF. et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. The Lancet. 2020;396(10243):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]