Abstract
Background:
Treatment options for advanced gastric esophageal cancer are quite limited. Chemotherapy is unavoidable at certain stages, and research on targeted therapies has mostly failed. The advent of immunotherapy has brought hope for the treatment of advanced gastric esophageal cancer. The aim of the study was to analyze the safety of anti-PD-1/PD-L1 immunotherapy and the long-term survival of patients who were diagnosed as gastric esophageal cancer and received anti-PD-1/PD-L1 immunotherapy.
Method:
Studies on anti-PD-1/PD-L1 immunotherapy of advanced gastric esophageal cancer published before February 1, 2020 were searched online. The survival (e.g. 6-month overall survival, 12-month overall survival (OS), progression-free survival (PFS), objective response rates (ORR)) and adverse effects of immunotherapy were compared to that of control therapy (physician’s choice of therapy).
Results:
After screening 185 studies, 4 comparative cohort studies which reported the long-term survival of patients receiving immunotherapy were included. Compared to control group, the 12-month survival (OR = 1.67, 95% CI: 1.31 to 2.12, P < 0.0001) and 18-month survival (OR = 1.98, 95% CI: 1.39 to 2.81, P = 0.0001) were significantly longer in immunotherapy group. The 3-month survival rate (OR = 1.05, 95% CI: 0.36 to 3.06, P = 0.92) and 18-month survival rate (OR = 1.44, 95% CI: 0.98 to 2.12, P = 0.07) were not significantly different between immunotherapy group and control group. The ORR were not significantly different between immunotherapy group and control group (OR = 1.54, 95% CI: 0.65 to 3.66, P = 0.01). Meta-analysis pointed out that in the PD-L1 CPS ≥10 sub group population, the immunotherapy could obviously benefit the patients in tumor response rates (OR = 3.80, 95% CI: 1.89 to 7.61, P = 0.0002).
Conclusion:
For the treatment of advanced gastric esophageal cancer, the therapeutic efficacy of anti-PD-1/PD-L1 immunotherapy was superior to that of chemotherapy or palliative care.
Keywords: PD-1, PD-L1, gastric esophageal cancer, survival, immonotherapy
Introduction
Gastric esophageal cancer is anatomically subdivided into tumors of the stomach, esophagus, and tumors of the gastroesophageal junction (GEJ). Globally, gastric esophageal cancer is one of the main causes of death. According to the estimated numbers of new cases of invasive cancer in the United States in 2019, the new number of esophageal cancer cases will reach to 17650, and the number of gastric cancer cases will be 27510.1
The clinical symptoms of early esophageal cancer are not obvious. Most patients with esophageal cancer are locally advanced or have distant metastases at the time of diagnosis. According to a study from the Surveillance, Epidemiology, and End Results (SEER) database, the median overall survival of esophageal cancers was 9 months with an overall 5-year survival rate of 15.5% in USA population from 1970 to 2000.2 The advanced disease also reached 40% of patient when diagnosed.2,3 As for gastric cancer, it has been reported that one third of patients were diagnosed with advanced disease at initial diagnosis, meaning no chance for radical surgery.4,5 Because advanced esophageal and gastric cancers have lost the opportunity for surgical treatment, systemic therapy (chemotherapy or targeted therapy) is mainly used.6-8 However, the effects of chemotherapy and targeted therapy have been limited, and the recurrence rate and metastasis rate are high, resulting in poor overall prognosis for patients with gastric cancer and esophageal cancer, with a 5-year survival rate of 15% to 25%. Based on the above treatment situation, targeted drugs have been tried in gastroesophageal cancer in recent years. EGFR has been shown to be up-regulated in 30-90% of esophageal cancer patients9 and 27%-64% of gastric cancer patients,10 respectively. However, all the clinical trials of monoclonal antibody and tyrosine kinase inhibitors targeted to EGFR did not gave us the positive outcome, such as cetuximab,11,12 panitumumab,13 nimotuzumab,14 gefitinib and erlotinib.15,16 Trastuzumab and ramulizumab have clear efficacy in adenocarcinoma, but lack of clinical evidence in esophageal squamous cell carcinoma.
The application of new immunotherapy agents in gastric esophageal cancer opens a new chapter of prognosis. Malignant tumors have the characteristic of evading immune surveillance, and the mechanism is derived from the lack of antigen expression of tumor cells or the establishment of an immune tolerance environment. Although gastrointestinal tumors are not traditionally immunogenic malignancies, several studies have confirmed that the number of infiltrating lymphocytes surrounding tumors is closely related to tumor progression and prognosis.17-19 In recent years, immune checkpoint inhibitors developed for the programed death receptor 1 / programed death ligand 1 (PD-1 / PD-L1) signaling pathway has been used in melanoma, non-small cell lung cancer (NSCLC), and digestive system tumors.20-22 Compared to traditional treatment, the continuous treatment response brought by anti-PD-1 treatment is very “amazing.” Therefore, anti-PD-1 immunotherapy is a promising new direction for gastrointestinal tumors. In this meta-analysis and literature review, we tried to analyze the safety of the anti-PD-1/PD-L1 immunotherapy in gastric esophageal cancer and the survival of patients comprehensively.
Methods
Literature Search Strategy
The current systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.23 Initial screening was performed independently by 2 authors independently. We screened the PubMed, Embase, and the Cochrane database of systematic reviews from inception to February 2020 for meta-analyses or systematic reviews of observational studies investigating effects of the anti-PD-1/PD-L1 immune checkpoint therapy in stomach esophagus cancer. The search terms utilized for anti-PD-1/PD-L1 immune checkpoint therapy versus control therapy in stomach esophagus cancer included “esophagogastric junction carcinoma,” “EJC,” “esophageal cancer,” “gastric cancer,” “immunotherapy” and “PD-1.” By fully reading the title and abstract, we have eliminated duplicate literatures. For the rest of the literatures, we read the full-text to assess the eligibility. In addition, we also manually searched related references by literature for other potential related articles.
Inclusion and Exclusion Criteria
Studies were included if the studies compare the therapeutic efficacy and complications of patients receiving anti-PD-1/PD-L1 immune checkpoint therapy or other therapy. The studies should meet the following criteria: (1) randomized clinical trials; (2) metastatic or advanced adenocarcinoma of the stomach esophagus; (3) after first-line therapy, and (4) comparable data of overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and side effects was available. Excluded were articles reporting the clinical trial was designed for first-line therapy or perioperative treatment, the immunotherapy was combined with chemotherapy or doublet, articles lack of survival data or adverse effects, literatures of review type, and articles in non-English languages.
Assessment of the Randomized Clinical Trials Quality
The modified Jadad scale was used to assess the quality of the included studies.24,25 The scale focuses on the 4 factors: (1) randomization, (2) generation of random sequences, (3) blinding, and (4) withdrawals and dropouts. Evaluation of the literatures was scored independently by 2 authors. Generally, 1-3 points are considered low quality and 4-7 points are considered high.
Data Extraction Method
Two authors independently extracted the data from the included studies. The information included the following outcomes: name of trial, publication time and research period, design of the trial, immunotherapy methods, control therapy, and sample size), the therapeutic efficacy (overall survival and progression-free survival), and related side effects.
Statistical Analysis
The meta-analysis was conducted according to the Cochrane handbook for systematic reviews of interventions, and the forest figure were performed using Review Manager 5.3 software (The Cochrane Collaboration, Oxford, UK). In the current study, all the categorical variables were discontinuous. Forest plots were drawn by Review Manager 5.3 software automatically. Odds ratio (OR), P value and 95% confidence intervals (CI) were used to assess whether the differences in results were significant. It was used to assess the multivariate HRs and to obtain summary HRs to compare the PFS and OS, P value less than 0.05 was considered as significant. For heterogeneity evaluation, we used the I2 to evaluate the included studies. If the I2 was lower than 50 percents, the statistical analysis was considered as no significant heterogeneity; if the I2 was higher or equal to 50 percents, the statistical analysis was considered as heterogeneity. Calculation mode was switched between fixed-effects model (FEM) and random-effects model (REM). If there was no significant heterogeneity, FEM was used; otherwise, REM would be used to handle the data.
Results
Search Results
Flow diagram (Figure 1) showed the literature screening process for this study. We totally identified 219 studies from online database and manually search. After rough screening, 117 duplications articles were excluded and 102 studies left for further search. Next, the titles and abstracts of every article were further screened thoroughly in 35 studies. Additionally, 14 articles were found that there were no comparable data of anti PD-1 therapy and control group and were excluded, 8 studies were excluded because the inconsistent purpose, 9 studies were excluded due to lack of full text or the comparative data. Finally, 4 randomized clinical studies reporting the survival and complications of advanced gastric esophageal cancer patients who were assigned to immunotherapy or control group.26-29 The basic characteristics of the included clinical trials were summarized in Table 1.
Figure 1.
Flow diagram depicting the strategies of systematic review and meta-analyses.
Table 1.
Published Articles Reporting on Immunotherapy Versus Chemotherapy or Placebo for the Treatment of Advanced Gastric Esophageal Cancer.
| Clinical trial | Year (study period) | Study design | No.of patients | Included patients | Immunotherapy group | Control group |
|---|---|---|---|---|---|---|
| JAVELIN Gastric 300 | 2018/(2015-2017) | P, M | 371 | metastatic GC/GEJC | avelumab 10 mg/kg, every 2 weeks | physician’s choice of chemotherapy |
| KEYNOTE-061 | 2018/(2015-2016) | P, M | 592 | metastatic GC/GEJC | pembrolizumab 200 mg, every 3 weeks | standard-dose paclitaxel |
| ATTRACTION-2 | 2017/(2014-2016) | P, M | 493 | metastatic GC/GEJC | Nivolumab 3 mg/kg, every 2 weeks | placebo |
| KEYNOTE-181 | NA | P, M | 628 | metastatic GEJC/EC | pembrolizumab 200 mg, every 3 weeks |
physician’s choice of chemotherapy |
P Prospective; M Multicentre; GC Gastric cancer; GEJC Gastro-esophageal junction cancer; EC Esophageal cancer; NA Not available.
The Methodological Quality
All of the included clinical trials were randomized, prospective and multicenter designed. The result showed acceptable quality (Jadad score >4) for all of the included studies. Two trials reached 7 stars and the other 2 trials reached 5 stars. The details of the assessment are shown in Table 2.
Table 2.
The Jadad Scale.
| Clinical trails | JAVELIN Gastric 300 | KEYNOTE-061 | ATTRACTION-2 | KEYNOTE-181 |
|---|---|---|---|---|
| Radomlization | 2 | 2 | 2 | 2 |
| Concealment of allocation | 0 | 2 | 2 | 0 |
| Double blinding | 2 | 2 | 2 | 2 |
| Withdrawals and dropouts | 1 | 1 | 1 | 1 |
| Jadad scorea | 5 | 7 | 7 | 5 |
Note: aMethodological quality of meditative movements studies reviewed using Jadad scoring criteria. Ttotal score is 7. Score 1 to 3 considered as low quality; score 4 to 7 considerd as high quality.
The Long-Term Survival of Patients With Immunotherapy
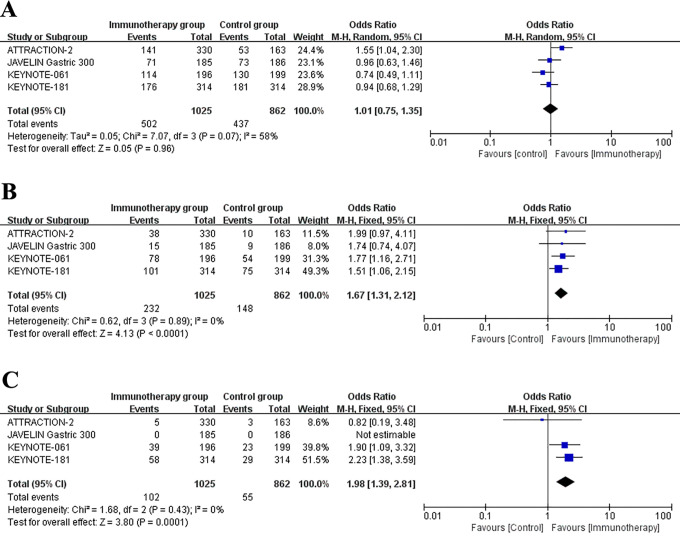
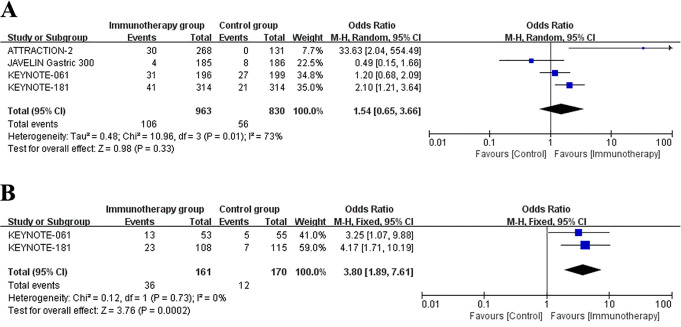
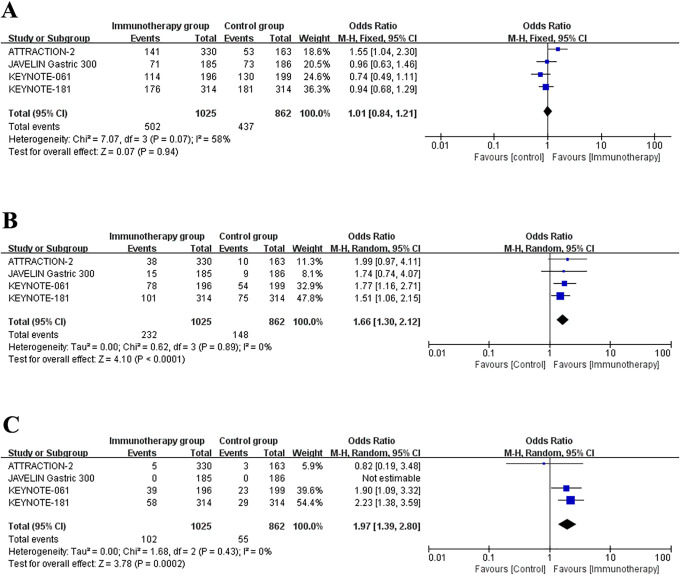
The survival data of patients who underwent immunotherapy or other therapies for advanced gastric esophageal cancer patients was showed in Table 3. The 6-month, 12-month and 18-month OS rate of patients were recorded in all studies. Besides, these studies also reported the PFS data. Figure 2 represented the meta-analysis of OS rates after data integration. Immunotherapy group was not significantly differed with control group in the 6-month OS rates (Figure 2A) (OR = 1.01, 95% CI: 0.75 to 1.35, P = 0.96). Next, compared to control group, the 12-month survival (OR = 1.67, 95% CI: 1.31 to 2.12, P < 0.0001) (Figure 2B) and 18-month survival (OR = 1.98, 95% CI: 1.39 to 2.81, P = 0.0001) (Figure 2C) were significantly longer in immunotherapy group. Analysis of the data of PFS revealed that the 3-month survival rates (OR = 1.05, 95% CI: 0.36 to 3.06, P = 0.92) (Figure 3A) and 18-month survival rates (OR = 1.44, 95% CI: 0.98 to 2.12, P = 0.07) (Figure 3B) were not significantly different between immunotherapy group and control group.
Table 3.
Survival of Patients Who Underwent Immunotherapy or Other Therapy for Advanced Gastric Esophageal Cancer Patients.
| Clinical trial | Groups | 6-month OS rate(%) | 12-month OS rate(%) | 18-month OS rate(%) | 3-month PFS rate (%) |
12-month PFS rate (%) | Median OS (month) | Median PFS (month) |
|---|---|---|---|---|---|---|---|---|
| JAVELIN Gastric 300 | Immunotherapy group | 41% | 8.1% | 0.0% | 19.1% | 1.1% | 4.6 | 1.4 |
| Control group | 45% | 4.8% | 0.0% | 39.2% | 1.1% | 5.0 | 2.7 | |
| KEYNOTE-061 | Immunotherapy group | 58.2% | 40% | 26% | 45% | 14% | 9.1 | 1.5 |
| Control group | 65.3% | 27% | 15% | 70% | 9% | 8.3 | 4.1 | |
| ATTRACTION-2 | Immunotherapy group | 46.1% | 26.2% | 16.2% | 30.5% | 7.6% | 5.26 | 1.61 |
| Control group | 34.7% | 10.9% | 5.0% | 10.5% | 1.5% | 4.14 | 1.45 | |
| KEYNOTE-181 | Immunotherapy group | 56% | 32% | 18.5% | 55% | 12% | 7.1 | 2.1 |
| Control group | 58% | 24% | 9.2% | 38% | 10% | 7.1 | 3.4 |
OS overall survival; PFS progression-free survival.
Figure 2.
Forest plot of the overall survival rates: (A) 6-month survival rates. (B) 12-month survival rates (C) 18-month survival rates. Horizontal lines represent 95% confidence intervals (CIs). M-H, Mantel-Haenszel; df, degrees of freedom.
Figure 3.
Forest plot of the progression-free survival rates: (A) 3-month PFS rates. (B) 18-month PFS rates. Horizontal lines represent 95% confidence intervals (CIs). M-H, Mantel-Haenszel; df, degrees of freedom.
The Confirmed Responses Rates of Patients With Immunotherapy and Subgroup Analysis
The second endpoint of this study was objective response rate (ORR), the percentage of patients whose tumors have shrunk to a certain amount and maintained for a certain period of time, including complete and partial response rates. All the 4 studies recorded the data of ORR. Meta-analysis of ORR was showed in Figure 4A. The result showed that the ORR were not significantly different between the immunotherapy group and control group (OR = 1.54, 95% CI: 0.65 to 3.66, P = 0.01).
Figure 4.
Forest plot of the objective response rates: (A) Objective response rates in all included patients. (B) Subgroup analysis of objective response ratesin PD-L1 CPS expression ≥10 patients. Horizontal lines represent 95% confidence intervals (CIs). M-H, Mantel-Haenszel; df, degrees of freedom.
Next, we evaluated the ORR in PD-L1 CPS ≥10 subgroup population (Figure 4B). Only KEYNOTE 061 and KEYNOTE 181 trails reported the related data.27,29 Meta-analysis pointed out that in the PD-L1 CPS ≥10 sub group population, the immunotherapy could significantly benefit the patients in tumor response rate (OR = 3.80, 95% CI: 1.89 to 7.61, P = 0.0002).Unfortunately, we lack data of ORR in the subgroup population with PD-1 CPS less than 10.
The Safety Evaluation of Anti-PD-1/PD-L1 Immunotherapy
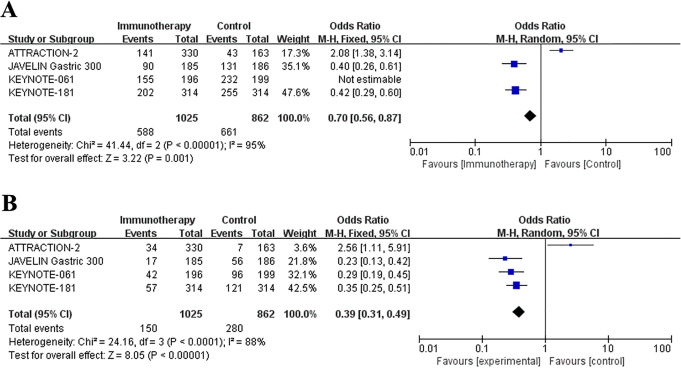
The main adverse effects data were extracted from included trials and were summarized in Table 4. Common side effects reported in the clinical trials including fatigue, decreased appetite, nausea, diarrhea, anemia, alopecia, neuropathy peripheral, and elevated ALT/AST/GGT. Severe adverse events were defined as adverse events of grade ≥3. In our meta-analysis, the data integration showed that the occurrence of adverse effects was fewer in immunotherapy group compared with control group, no matter the all grades adverse effects (OR = 0.43, 95% CI: 0.33 to 0.57, P < 0.0001) (Figure 5A) or severe adverse events (OR = 0.70, 95% CI: 0.56 to 0.87, P < 0.0001) (Figure 5B).
Table 4.
Adverse Events.
| Clinical trial | Groups | Adverse events | Fatigue | Decreased appetite | Nausea | Diarrhea | Anemia | Alopecia | Neuropathy peripheral | Neutrophil count decreased | Peripheral sensory neuropathy | Elevated ALT | Elevated AST | Elevated GGT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade ≥3 | Overall | ||||||||||||||
| JAVELIN Gastric 300 | I group | 17(9.2%) | 90(48.9%) | 11(6%) | 6(3.3%) | 12(6.5%) | 11(6.0%) | 1(0.5%) | 0(0%) | 0(0%) | 0(0%) | NA | 6(3.3%) | 7(3.8%) | 4(2.2) |
| C group | 56(31.6%) | 131(74.0%) | 18(10.2%) | 24(13.6%) | 50(28.2%) | 47(26.6%) | 24(13.6%) | 25(14.1%) | 6(3.4%) | 37(20.9%) | NA | 7(4.0%) | 6(3.4%) | 2(1.1) | |
| KEYNOTE-061 | I group | 42(14.0%) | 155(53.0%) | 35(12%) | 24(8%) | 17(6%) | 16(5%) | 10(3%) | 1(<1%) | 1(<1%) | 0(0%) | 0(0%) | NA | NA | NA |
| C group | 96(35.0%) | 232(84.0%) | 64(23%) | 43(16%) | 50(18%) | 38(14%) | 39(14%) | 111(40%) | 40(14%) | 35(13%) | 35(13%) | NA | NA | NA | |
| ATTRACTION-2 | I group | 34(10%) | 141(43%) | 18(5%) | NA | 14(4%) | 23(7%) | NA | NA | NA | NA | NA | 7(2%) | 11(3%) | NA |
| C group | 7(4%) | 43(27%) | 9(6%) | NA | 4(2%) | 3(2%) | NA | NA | NA | NA | NA | 1(1%) | 3(2%) | NA | |
| KEYNOTE-181 | I group | 57(18.2%) | 202(64.3%) | 37(11.8%) | NA | 22(7%) | 17(5.4%) | 8(2.5%) | NA | NA | NA | NA | NA | NA | NA |
| C group | 121(40.9%) | 255(86.1%) | 61(20.6%) | NA | 64(21.6%) | 60(20.3%) | 85(28.7%) | NA | NA | NA | NA | NA | NA | NA | |
I immunotherapy; C control; NA not available; ALT alanine aminotransferase; AST aspartate aminotransferase; GGT c-glutamyltransferase.
Figure 5.
Forest plots of the treatment-associated adverse effects: (A) All grades adverse effects. (B) Adverse effects of grade ≥3. Horizontal lines represent 95% confidence intervals (CIs). M-H, Mantel-Haenszel; df, degrees of freedom.
Publication Bias
In order to detect the publication bias, the funnel plot of the studies reporting a 6-month OS rate was generated (Figure 6). Visually inspect the shape of the funnel chart, which showed that the funnel chart was relatively symmetrical and normally distributed. From the funnel plot, no obvious publication bias was appeared in current study.
Figure 6.
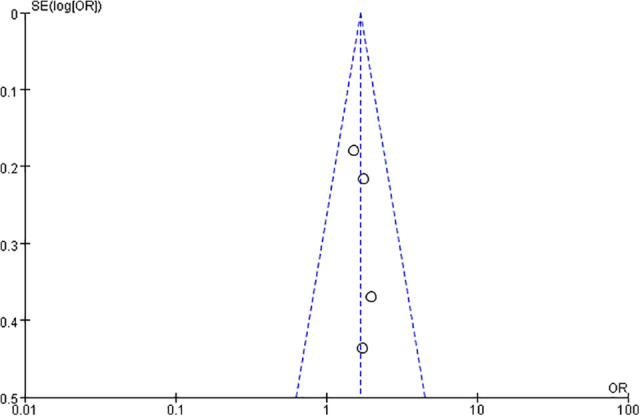
Funnel plot for publication bias.
Sensitivity Analysis
We used different models to analyze the same results for the included studies (The long-term survival of patients with immunotherapy) (Figure 7). For the data previously analyzed using the random effects model, we adopted the fixed effects model; conversely, for the data previously analyzed using the fixed effects model, we adopted the random effects model analysis. After using different model analysis, compared with the previous Meta analysis results, the results did not change much, indicating that the sensitivity of this study is low and the results are more robust and reliable.
Figure 7.
Sensitivity analysis results. Different analysis model used in Forest plot of the overall survival rates: (A) 6-month survival rates. (B) 12-month survival rates (C) 18-month survival rates. Horizontal lines represent 95% confidence intervals (CIs). M-H, Mantel-Haenszel; df, degrees of freedom.
Discussion
The immune checkpoint signaling pathway is mainly composed of CTLA-4 pathway and PD-1/PD-L1 pathway.30 PD-1 is a negative costimulatory receptor mainly expressed on activated T cells, which binds to its ligands PD-L1 and PD-L2 After inhibiting the function of effect or T cells, many types of tumor cells can increase the expression of PD-L1 to achieve the purpose of immune escape. At present, a variety of targeted drugs targeting the PD-1/PD-L pathway have successfully entered clinical trials.31 Some drugs including nivolumab and pembrolizumab have been approved by the FDA for marketing, and have obtained corresponding indications in melanoma and non-small cell lung cancer.32-34 In the field of gastric cancer, a number of clinical studies are currently underway or completed.27,28 This study evaluated the role of related targeted drugs targeting PD-1/PD-L1 pathway in gastroesophageal cancer through meta-analysis. In review of 4 randomized controlled trials, we investigated the prognostic value and safety of anti-PD-1/PD-L1 immunotherapy in metastatic gastroesophageal cancer, based on the relationship with OS, PFS, ORR and side effects. We found that the anti-PD-1/PD-L1 immunotherapy could bring benefits for long-term survival, with less adverse side effects.
The aim of immunotherapy is empowering the body’s natural immune response by facilitating the targeting and destruction of cancer cells.9 Several studies have reported that the engagement of PD-1 receptor and its ligand PD-L1 could down-regulate T-cell mediated immune responses.35-37 Cancer cells are known to evade host immune system defense by activating the PD-1 / PD-L1. With such a theoretical basis, some early preclinical studies applied PD-1/PD-L1 inhibition as a potential mechanism for cancer immunotherapy.36,38 Anti-PD-1/PD-L1 treatment, also known as checkpoint inhibition, has recently been particularly popular in many tumor treatment fields.39-43
This study focused on the safety and efficacy of immune checkpoint inhibitors in gastroesophageal cancer. Because of the non-specific symptoms in the early stage, gastric esophageal cancer patients are often diagnosed with advanced disease and the comprehensive treatment is not effective. Currently, clinical studies on immune checkpoint inhibitors in gastric esophageal cancer have been widely launched worldwide. CheckMate-032 was a phase I / II study of PD-1 inhibitor nivolumab monotherapy or combined with ipilimumab in advanced gastric cancer, esophageal cancer, and esophagogastric junction cancer patients that failed previous chemotherapy.44 The ORR in the nivolumab monotherapy group was 14%, and the 1-year survival rate was 35%. KEYNOTE-012 explored the pembrolizumab monotherapy in patients with positive PD-L1 expression who failed multi-line treatment, the ORR reached 22% and the median OS was 11 months, with a tolerable adverse effect.45 In this meta-analysis, we altogether analyzed 4 randomized, open-label, phase 3 studies. The KEYNOTE-061 study results showed that pembrolizumab was not superior with second-line chemotherapy in OS for advanced gastric or gastro-oesophageal junction cancer (9.1 months vs 8.3 month, 95% CI 0.66-1.03).27 The JAVELIN Gastric 300 also gave out a negative conclusion that single-agent avelumab could not benefit the patients in the third-line setting compared with chemotherapy (OS 4.6 vs 5.0 months, 95% CI 0.9-1.4).28 On the other hand, ATTRACTION-02 showed that compared to the placebo group, nivolumabcould reduced the risk of death by 38% in advanced gastric or gastro-oesophageal junction cancer; the 2-year OS rate was 3 times that of the placebo group. Based on the results of the ATTRACTION-02 study, nivolumab was approved for the treatment of advanced gastric cancer that progresses after standard chemotherapy in Japan, Korea, Singapore, Taiwan, China and other regions.26 Based on the results of current clinical studies, the efficacy of immunotherapy in the post-line treatment of gastroesophageal cancer is controversial. Our meta-analysis concluded that the immunotherapy group was superior to the control group in terms of long-term survival. The 6-month OS rates between immunotherapy and control group were not significantly different, but the immunotherapy group was superior in the 12-month and the 18-month OS rate. Current researches showed that the role of immunotherapy in the treatment of advanced gastric esophageal cancer was still controversial. Different PD-1 /PD-L1 inhibitors appeared to have inconsistent effects in different studies. Therefore, it is also one of the clinical research hotspots to find the superior population of immunotherapy. The results of the Phase II study of KEYNOTE-158 reported by the French National Research Center Marabelle et al. demonstrated the clinical benefit of pembrolizumab among patients with previously treated unresectable or metastatic non-colorectal cancer with high microsatellite instability (MSI-H)/ DNA mismatch repair (dMMR).46 Patients with dMMR have a high somatic mutation rate, which means a high tumor mutation load (TMB). This high mutation load results in a large number of neoantigens. The cancer-specific neoantigens can generate antitumor cytotoxic T-cells inside tumors.47-49 We found that the general population in our study did not benefit from immunotherapy in respect of ODD. Due to the limited available data, we only performed a subgroup analysis on the ORR based on PD-L1 expression (CPS ≥10). The result showed that the immunotherapy could obviously benefit the patients in tumor response rates (OR = 3.80, 95% CI: 1.89 to 7.61, P = 0.0002). As we all know, not all the patients are sensitive to immunotherapy. Which one could be the best marker to evaluate the efficacy of immunotherapyamongPD-1/PD-L1 expression, dMMR, and TMB? A number of meta-analyses have suggested that the expression level of PD-L1may represent a poor prognostic marker in gastrointestinal cancer.50-52 More large-scale multicenter clinical studies are needed to confirm it in the future.
Our study also analyzed the adverse effects between immunotherapy and control group. In terms of adverse reactions, immunotherapy has obvious advantages. Regardless of overall adverse reactions or severe adverse reactions, the incidence in the immunotherapy group decreased significantly. Antitumor chemotherapeutic drugs used in clinical practice have various degrees of toxic and side effects, and some serious toxic side effects are directly related with the dose or use of drugs. The toxic side effects of chemotherapy drugs are mainly blood cell reduction (white blood cells, platelets, hemoglobin, granulocytes, etc.), gastrointestinal reactions (nausea, vomiting, diarrhea, and constipation), abnormal liver function, abnormal renal function, cardiac toxicity, abnormal neurological function, etc.53-55 Compared to traditional chemotherapy, immunotherapy has generally less side effects. However, a series of side effects brought by immunotherapy have a uniform name, called “immune-related adverse events, irAE.” IrAE has just begun to be recognized by clinicians and we lack experience in even finding and handling. In most clinical studies, these side effects are graded 1 to 2, usually within the range that patients can tolerate, and many of them are transient side effects. And most of these reactions did not need special treatment; even if they need to be treated, they can recover quickly after simple symptomatic treatment. More importantly, these side effects do not affect the effectiveness of the treatment.56,57
This study also has some limitations. As the data of TMB, MSI and TCR diversity could not be extracted from the included literature; in-depth analysis was not possible in this regard. In the future, it is hoped that a more in-depth stratified analysis can be conducted to identify the groups who benefit from immunotherapy.
Conclusion
In the current study, we revealed that, for the treatment of advanced gastric esophageal cancer, the therapeutic efficacies of anti-PD-1/PD-L1 immunotherapy were superior to that of chemotherapy or palliative care, given the result that the long-term survival rates were significantly higher. Besides, the PD-L1 expression CPS ≥10 subgroup patients seemed to benefit more significantly than the total population. The side effects incidence rates were lower in the immunotherapy group. Our results suggested that anti-PD-1/PD-L1 immunotherapy could be the new choice after first-line chemotherapy in second-line or later management for advanced gastric esophageal cancer patients. Due to the small number of cases included in this study, large sample RCTs is still needed.
Footnotes
Authors’ Note: ZC and KC gave contributions to conception and design. ZC and XW reviewed the literature and designed the article structure. ZC, KC, and LY contributed to the acquisition and analysis of data. ZC and XW gave interpretation of data. ZC and KC were major contributors in writing the manuscript. LY revised and edited the manuscript critically for important intellectual content. ZC, KC and LY gave final approval of the version to be published.
Author Contribution: Ke Chen, Xiao Wang, these authors contributed equally to the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study did not require an ethical board approval because it did not contain human or animal trials.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Natural Science Foundation of China (grant number, 81802623).
ORCID iDs: Ke Chen  https://orcid.org/0000-0003-3148-9921
https://orcid.org/0000-0003-3148-9921
Zheling Chen  https://orcid.org/0000-0002-3073-1811
https://orcid.org/0000-0002-3073-1811
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31(6):1141–1146. doi:10.1111/jgh.13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Corpo M, Schlottmann F, Strassle PD, Nurczyk K, Patti MG. Treatment modalities for esophageal adenocarcinoma in the United States: trends and survival outcomes. J Laparoendoscopic Adv Surg Tech P A. 2019;29(8):989–994. doi:10.1089/lap.2019.0350 [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- 5.Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388(10060):2606. doi:10.1016/S0140-6736(16)32226-7 [DOI] [PubMed] [Google Scholar]
- 6.van den Ende T, Smyth E, Hulshof M, van Laarhoven HWM. Chemotherapy and novel targeted therapies for operable esophageal and gastroesophageal junctional cancer. Best Pract Res Clin Gastroenterol. 2018;36-37:45–52. doi:10.1016/j.bpg.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 7.Ku GY. Systemic therapy for esophageal cancer: chemotherapy. Chin Clin Oncol. 2017;6(5):49. doi:10.21037/cco.2017.07.06 [DOI] [PubMed] [Google Scholar]
- 8.Maeda O, Ando Y. Recent progress of chemotherapy and biomarkers for gastroesophageal cancer. World J Gastrointest Oncol. 2019;11(7):518–526. doi:10.4251/wjgo.v11.i7.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barsouk A, Rawla P, Hadjinicolaou AV, Aluru JS, Barsouk A. Targeted therapies and immunotherapies in the treatment of esophageal cancers. Med Sci. 2019;7(10):100. doi:10.3390/medsci7100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arienti C, Pignatta S, Tesei A. Epidermal growth factor receptor family and its role in gastric cancer. Front Oncol. 2019;9. doi:10.3389/fonc.2019.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lordick F, Kang Y-K, Chung H-C, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–499. doi:10.1016/s1470-2045(13)70102-5 [DOI] [PubMed] [Google Scholar]
- 12.Huang Z-H, Ma X-W, Zhang J, Li X, Lai N-L, Zhang S-X. Cetuximab for esophageal cancer: an updated meta-analysis of randomized controlled trials. BMC Cancer. 2018;18(1). doi:10.1186/s12885-018-5040-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):481–489. doi:10.1016/s1470-2045(13)70096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Jia J, Lu M, et al. Nimotuzumab plus paclitaxel and cisplatin as a 1st-line treatment for esophageal cancer: long term follow-up of a phase II study. J Cancer. 2019;10(6):1409–1416. doi:10.7150/jca.28659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15(8):894–904. doi:10.1016/s1470-2045(14)70024-5 [DOI] [PubMed] [Google Scholar]
- 16.Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24(30):4922–4927. doi:10.1200/jco.2006.07.1316 [DOI] [PubMed] [Google Scholar]
- 17.Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–415. doi:10.1007/s10120-016-0631-3 [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Li M, Bo C, et al. Prognostic significance of the lymphocyte-to-monocyte ratio and the tumor-infiltrating lymphocyte to tumor-associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2017;66(3):343–354. doi:10.1007/s00262-016-1931-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson MC, Warfvinge CF, Fristedt R, et al. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. 2017;8(42):72108–72126. doi:10.18632/oncotarget.19437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Zhang S, Xu D, et al. Incidence risk of PD-1/PD-L1 related diarrhea in non-small cell lung cancer (NSCLC) patients: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:3957–3969. doi:10.2147/CMAR.S202756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribas A, Kirkwood JM, Flaherty KT. Anti-PD-1 antibody treatment for melanoma. Lancet Oncol. 2018;19(5):e219. doi:10.1016/S1470-2045(18)30202-X [DOI] [PubMed] [Google Scholar]
- 22.Akin Telli T, Bregni G, Camera S, Deleporte A, Hendlisz A, Sclafani F. PD-1 and PD-L1 inhibitors in oesophago-gastric cancers. Cancer Lett. 2020;469:142–150. doi:10.1016/j.canlet.2019.10.036 [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi:10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175. doi:10.2522/ptj.20070147 [DOI] [PubMed] [Google Scholar]
- 25.Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatric Cogn Disord. 2001;12(3):232–236. doi:10.1159/000051263 [DOI] [PubMed] [Google Scholar]
- 26.Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/s0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 27.Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi:10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 28.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–2060. doi:10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah MA, Adenis A, Enzinger PC, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: the phase 3 KEYNOTE-181 study. J Clin Oncol. 2019;37(4_suppl). [Google Scholar]
- 30.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. doi:10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8. doi:10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi:10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 33.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi:10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 34.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 35.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Ann Rev Immunol. 2005;23:515–548. doi:10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- 37.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi:10.1016/s1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- 38.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi:10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 39.Berger JR. PD-1 inhibition: a novel approach to the treatment of progressive multifocal leukoencephalopathy. Ann Transl Med. 2019;7(Suppl 8):S281. doi:10.21037/atm.2019.11.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umemoto K, Togashi Y, Arai Y, et al. The potential application of PD-1 blockade therapy for early-stage biliary tract cancer. Int Immunol. 2020;32(4):273–281. doi:10.1093/intimm/dxz080 [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Ma Q, Yao R, Liu J. Current status and development of anti-PD-1/PD-L1 immunotherapy for lung cancer. Int Immunopharmacol. 2020;79:106088. doi:10.1016/j.intimp.2019.106088 [DOI] [PubMed] [Google Scholar]
- 42.Zhang B, Wang X, Li Q, et al. Efficacy of irinotecan-based chemotherapy after exposure to an anti-PD-1 antibody in patients with advanced esophageal squamous cell carcinoma. Chin J Cancer Res. 2019;31(6):910–917. doi:10.21147/j.issn.1000-9604.2019.06.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Chen L, Zhao Y, Yin H, Ma H, He M. Safety and efficacy in relapsed or refractory classic Hodgkin’s lymphoma treated with PD-1 inhibitors: a meta-analysis of 9 prospective clinical trials. BioMed Res Int. 2019;2019:9283860. doi:10.1155/2019/9283860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun DS, Kim JJ, Ko YH. CheckMate-032 study: promising efficacy with nivolumab-based immunotherapy in pretreated esophagogastric cancer. J Thorac Dis. 2019;11(S3):S394–S395. doi:10.21037/jtd.2018.12.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–726. doi:10.1016/S1470-2045(16)00175-3 [DOI] [PubMed] [Google Scholar]
- 46.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi:10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cimen Bozkus C, Roudko V, Finnigan JP, et al. Immune checkpoint blockade enhances shared neoantigen-induced T-cell immunity directed against mutated calreticulin in myeloproliferative neoplasms. Cancer Discov. 2019;9(9):1192–1207. doi:10.1158/2159-8290.CD-18-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nonomura C, Otsuka M, Kondou R, et al. Identification of a neoantigen epitope in a melanoma patient with good response to anti-PD-1 antibody therapy. Immunol Lett. 2019;208:52–59. doi:10.1016/j.imlet.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 49.Tondini E, Arakelian T, Oosterhuis K, et al. A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control. Oncoimmunology. 2019;8(11):1652539. doi:10.1080/2162402X.2019.1652539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PloS One. 2017;12(8):e0182692. doi:10.1371/journal.pone.0182692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Huang B, Chen L, et al. The expression status and prognostic significance of programmed cell death 1 ligand 1 in gastrointestinal tract cancer: a systematic review and meta-analysis. Onco Targets Ther. 2015;8:2617. doi:10.2147/ott.s91025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu H-X, Zhao L-P, Zhan S-H, et al. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with esophageal squamous cell carcinoma: a meta-analysis. J Thorac Dis. 2016;8(11):3197–3204. doi:10.21037/jtd.2016.11.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanmartin O, Beato C, Suh-Oh HJ, et al. Clinical management of cutaneous adverse events in patients on chemotherapy: a national consensus statement by the Spanish academy of dermatology and venereology and the Spanish society of medical oncology. Actas Dermosifiliogr. 2019;110(6):448–459. Manejo clinico de los eventos adversos cutaneos en pacientes tratados con quimioterapia: consenso nacional de la Academia Espanola de Dermatologia y Venereologia y de la Sociedad Espanola de Oncologia Medica. doi:10.1016/j.ad.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 54.Jovenin N, Eche-Gass A, Chèze S, et al. Nausées-vomissements induits par les traitements anti-cancéreux (NVITAC): quelle prise en charge en 2018? Mise à jour du référentiel AFSOS. Bull Cancer. 2019;106(5):497–509. doi:10.1016/j.bulcan.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 55.Voutsadakis IA. Clinical tools for chemotherapy toxicity prediction and survival in geriatric cancer patients. J Chemother. 2018;30(5):266–279. doi:10.1080/1120009x.2018.1475442 [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal S. Adverse effects of immuno-oncology drugs—Awareness, diagnosis, and management: a literature review of immune-mediated adverse events. Indian J Cancer. 2019;56(5):10. doi:10.4103/ijc.IJC_448_19 [DOI] [PubMed] [Google Scholar]
- 57.Urwyler P, Earnshaw I, Bermudez M, et al. Mechanisms of checkpoint inhibition induced adverse events. Clin Exp Immunol. 2020;200(2):141–154. doi:10.1111/cei.13421 [DOI] [PMC free article] [PubMed] [Google Scholar]