Abstract
Background:
Small cell carcinoma of the esophagus is a rare malignant tumor. We aimed to explore the chemotherapeutic efficacy on the prognosis of patients with small cell carcinoma of the esophagus who received radiotherapy.
Methods:
To identify the population of interest, Surveillance, Epidemiology, and End Results data from 1996 to 2016 were chosen. Univariate and multivariate analyses were used to probe into prognosis factors. Multivariate Cox regression analysis was conducted to identify factors related to overall survival and cancer-specific survival.
Results:
Overall, data from 162 patients were analyzed in this study. Tumor size (P = 0.014), T staging (P = 0.028), and chemotherapy (P < 0.001) were independent prognostic factors affecting overall survival. Patients with regional disease (hazard ratio = 5.435, P < 0.001) and distant metastasis (hazard ratio = 2.183, P < 0.001) who received radiotherapy alone had worse survival than those receiving chemoradiotherapy. Tumor size (P = 0.004) and chemotherapy (P < 0.001) were independent prognostic factors affecting cancer-specific survival. Tumor size was an independent factor affecting cancer-specific survival for patients receiving chemoradiation.
Conclusions:
Age, T staging, tumor size, primary site, and chemotherapy are independent prognosis factors affecting overall survival and cancer-specific survival in patients with small cell carcinoma of the esophagus who receive radiotherapy. Chemotherapy might further improve cancer-specific survival in patients with small cell carcinoma of the esophagus receiving radiotherapy at all stages.
Keywords: esophageal carcinoma, small cell carcinoma, chemotherapy, radiotherapy, prognosis
Introduction
Small cell carcinoma of the esophagus (SCCE) is the most common small cell carcinoma of the digestive tract, but it accounts for only 0.05% to 2.40% of malignant esophageal tumors.1,2 Because the biological behavior of SCCE is more aggressive than that of squamous cell carcinoma (SCC), the prognosis is significantly worse than that of SCC.
Because the histological manifestations, genetic changes, and highly invasive biological characteristics of SCCE are similar to those of small cell lung cancer (SCLC), the therapeutic regimen of SCCE is mostly based on the treatment model of SCLC.3-5 Chemoradiotherapy is the standard treatment modality for SCLC. Most SCCE patients receive radiotherapy.6,7 However, there is little evidence of the effect of chemotherapy in SCCE patients receiving radiotherapy, and therefore this combination is still controversial.6,8 This study retrieved SCCE patient data from the Surveillance, Epidemiology, and End Results (SEER) database and investigated the chemotherapeutic efficacy on the long-term prognosis of SCCE patients receiving radiotherapy.
Materials and Methods
Patient Selection
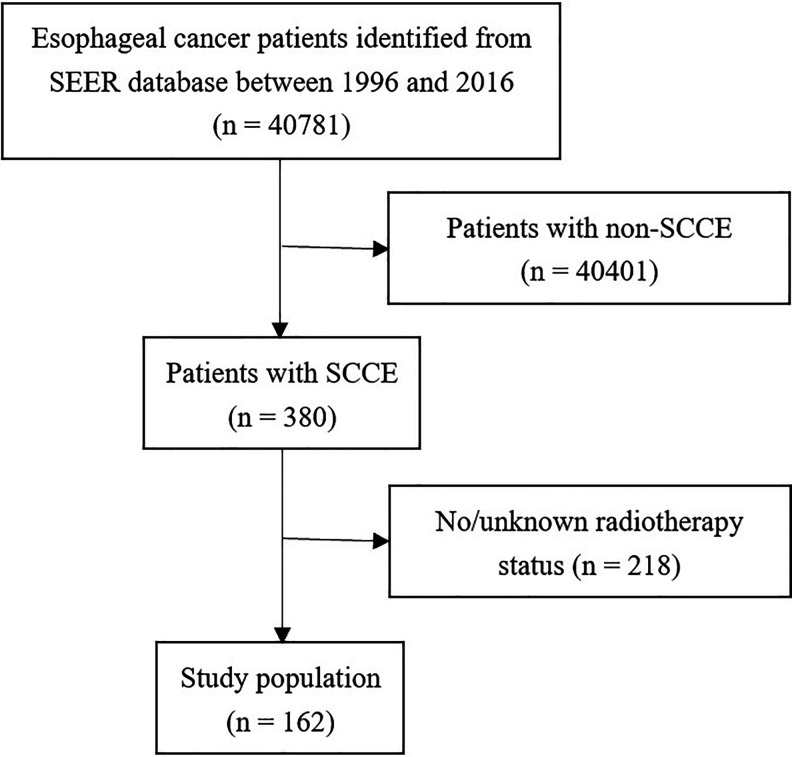
SEER*Stat software (SEER*Stat, v8.3.6) was used to search the data of patients with SCCE (ICD-0-3: codes 8832/8833) between 1996 and 2016. The inclusion criteria were as follows: (1) a pathological diagnosis of primary SCCE; (2) received radiotherapy; (3) clear chemotherapy information; and (4) a report of overall survival (OS) and cancer-specific survival (CSS) time. Figure 1 shows the patient selection process.
Figure 1.
Flow chart of the search protocol and study design (SCCE: small cell carcinoma of the esophagus).
Statistical Methods
The Chi-square test was conducted to analyze the baseline characteristics of SCCE and non-SCCE (NSCCE) cases. The Kaplan-Meier method was conducted to create survival curves and calculate the survival rate. Univariate analysis was performed using Cox proportional risk model, and factors with P < 0.1 were included in the multivariate analysis. Cox regression for multivariate analysis was performed on patients who received combined chemotherapy and radiotherapy, and the stratification was performed according to different stages. SPSS 23.0 (SPSS Inc., USA) was used for statistical analysis.
Results
Baseline Characteristics
40,781 esophageal cancer patients were screened from the SEER database, of which 380 (0.93%) were SCCE. The proportion of patients with distant metastasis at diagnosis in the SCCE group was higher than the NSCCE group (51.6% vs 33.5%; P = 0.045). The percentage of patients undergoing surgery in the SCCE group was lower than the NSCCE group (8.6% vs 24.6%; P = 0.004). There were no significant differences in the distributions of race, gender, radiotherapy administration, or chemotherapy administration between the 2 group (P > 0.05 for all) (Table 1).
Table 1.
Characteristics of SCCE Patients From the SEER Database.
| SCCE | Non-SCCE | χ2 | P value | |
|---|---|---|---|---|
| Age | 18.36 | <0.001 | ||
| <70 years | 191(50.26%) | 8634(21.37%) | ||
| ≥70 years | 189(49.74%) | 31767(78.63%) | ||
| Race | 2.36 | 0.31 | ||
| White | 302(79.47%) | 27453(86.61%) | ||
| Black | 52(13.68%) | 2576(8.13%) | ||
| Other | 26(6.84%) | 1668(5.26%) | ||
| Sex | ||||
| Male | 235(61.84%) | 22573(71.06%) | 1.82 | 0.23 |
| Female | 145(38.16%) | 9194(28.94%) | ||
| Stage | 8.03 | 0.04 | ||
| Localized | 64(16.84%) | 15411(21.89%) | ||
| Regional | 66(17.37%) | 21054(29.91%) | ||
| Distant | 196(51.58%) | 23613(33.54%) | ||
| Unknown | 54(14.21%) | 10323(14.66%) | ||
| Surgery | ||||
| Yes | 32(8.42%) | 17307(24.58%) | 9.07 | 0.004 |
| No/Unknown | 338(91.95%) | 53094(75.41%) | ||
| Chemotherapy | ||||
| Yes | 259(68.16%) | 38759(55.06%) | 3.57 | 0.08 |
| No/Unknown | 121(31.84%) | 31642(44.94%) | ||
| Radiotherapy | ||||
| Yes | 162(42.63%) | 36873(52.38%) | 1.62 | 0.26 |
| No/Unknown | 218(57.37%) | 33528(47.62%) |
SCCE, small-cell carcinoma of the esophagus; SEER, Surveillance, Epidemiology, and End Results.
Chemotherapy was more common among patients with regional disease (regional: 83.3% vs localized: 16.7%, P = 0.009), patients who received radiotherapy (radiotherapy: 86.4% vs no radiotherapy: 13.6%, P < 0.001), and patients who purchased insurance (insurance: 69.5% vs no insurance: 30.5%, P = 0.006).
Prognostic Factors Affecting Overall Survival
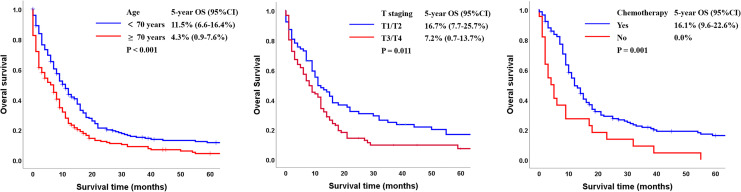
The 5-year OS rate of the cohort was 8.0% (95% confidence interval [CI]: 5.1-10.9). Kaplan-Meier analysis showed that patients younger than 70 had more beneficial prognoses than those older than 70 (11.5% vs 4.3%, P < 0.001; Figure 2A). The 5-year OS rates were 16.7% among patients with T1/T2 and 7.2% among patients with T3/T4 (P = 0.011, Figure 2B). Patients who received chemotherapy had a more satisfying prognosis than patients who did not (OS: 16.1% vs 0%, P = 0.001; Figure 2C).
Figure 2.
Kaplan-Meier analysis of overall survival according to (A) age (<70 years vs ≥70 years); (B) T staging (T1/T2 vs T3/T4); and (C) chemotherapy (Yes vs No) (CI, confidence interval; OS: overall survival).
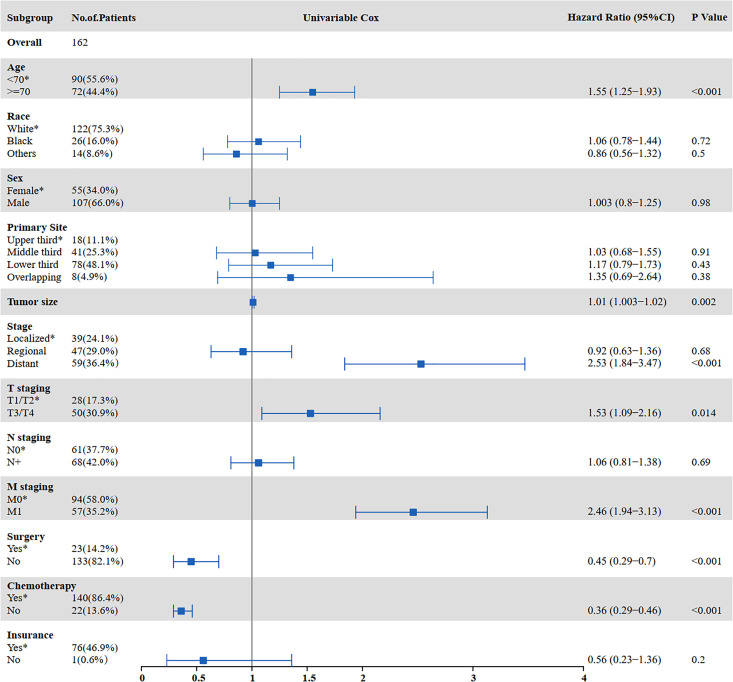
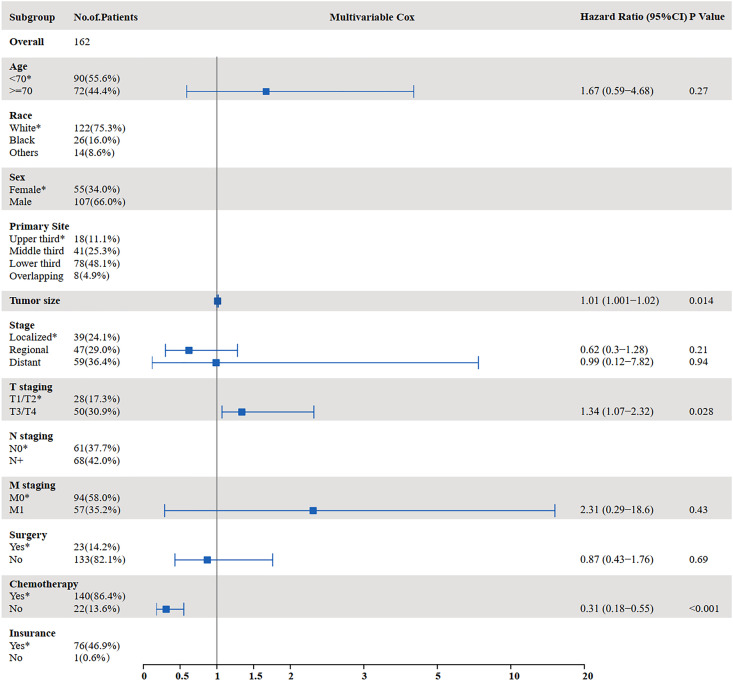
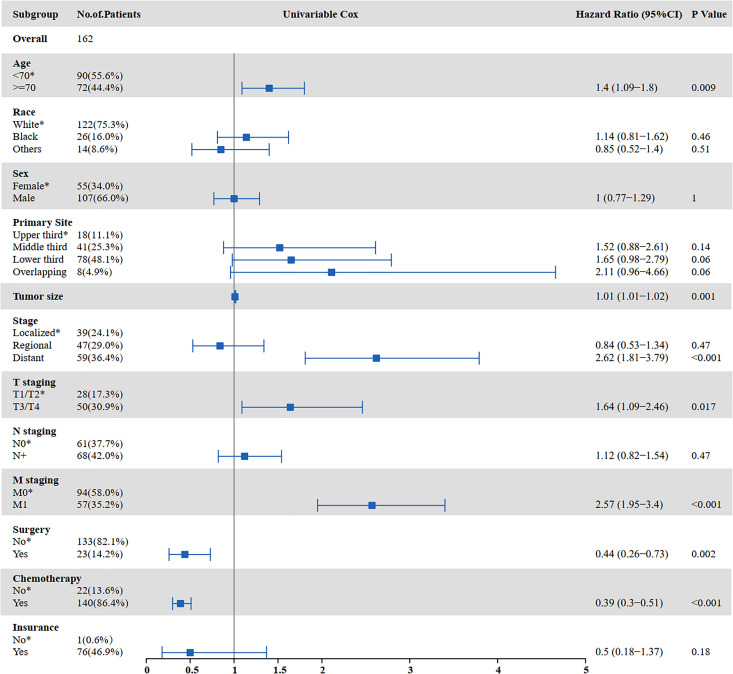
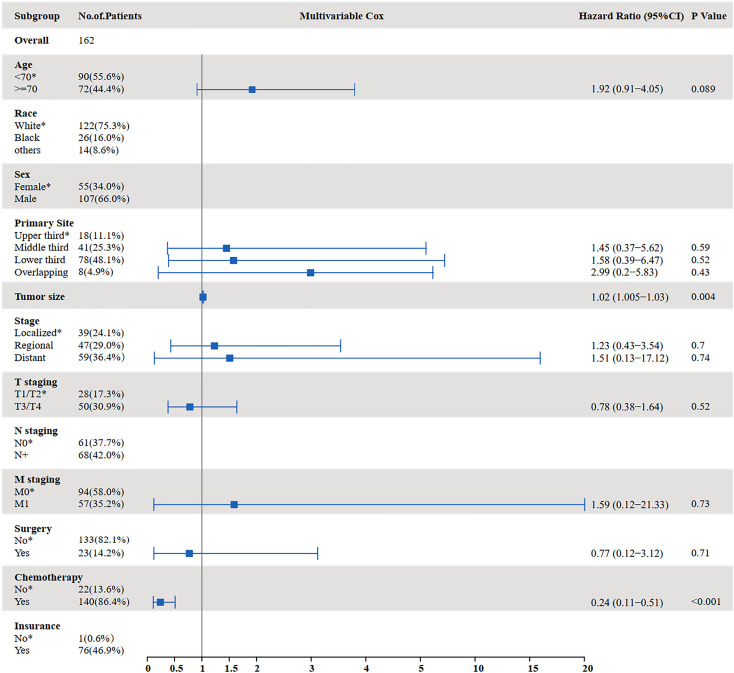
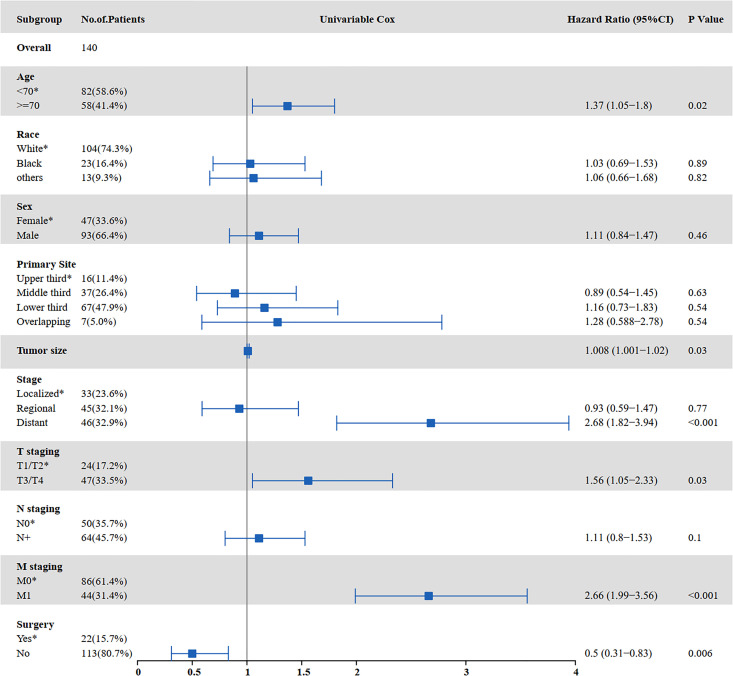
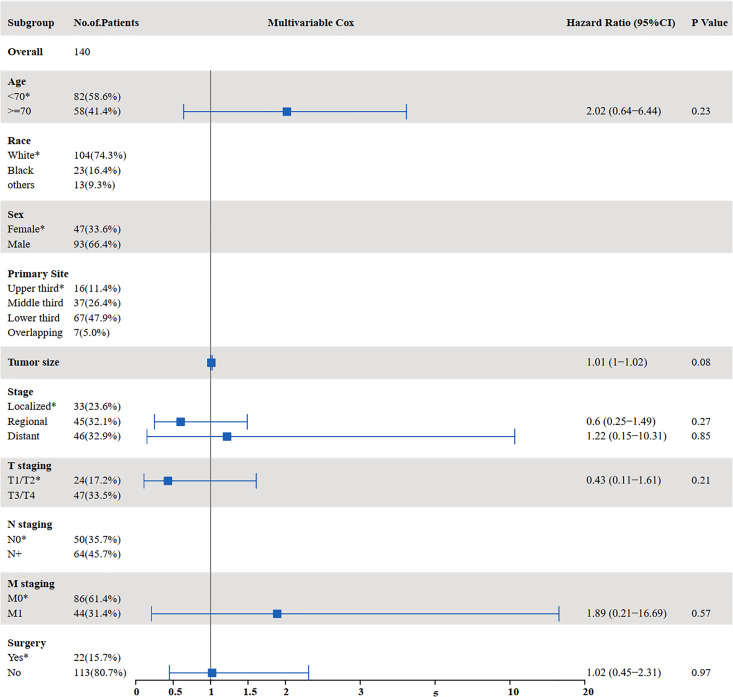
The results of the univariate and multivariate analyses to identify risk factors for OS are shown in Figures 3 and 4. Univariate analysis showed that age ≥70 years (P = 0.000), tumor size (P = 0.002), distant metastasis (P < 0.001), T staging (P = 0.014), M staging (P < 0.001), surgery (P < 0.001), and chemotherapy (P < 0.001) were prognostic factors affecting OS in SCCE patients receiving radiotherapy. Multivariate analysis showed that tumor size (P = 0.014), T staging (P = 0.028), and chemotherapy (P < 0.001) were independent prognostic factors affecting OS in patients receiving radiotherapy.
Figure 3.
Univariate analysis for OS in patients with SCCE who received radiotherapy (OS: overall survival; SCCE: small cell carcinoma of the esophagus).
Figure 4.
Multivariate analysis for OS in patients with SCCE who received radiotherapy (OS: overall survival; SCCE: small cell carcinoma of the esophagus).
Prognostic Factors Affecting Cancer-Specific Survival
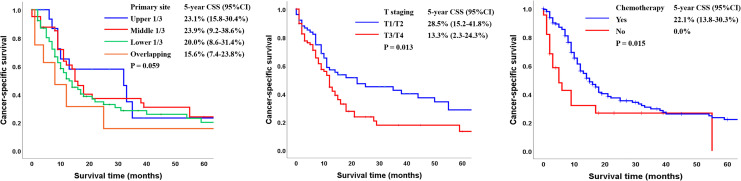
The 5-year CSS rate of the entire cohort was 14.9% (95% CI: 13.2-28.4). Except for patients without clear primary site information, patients with tumors in the upper and middle 1/3 of the esophagus had better outcomes than patients who had tumors in the lower 1/3 of the esophagus or overlapping tumors (CSS: 23.1% vs 23.9% vs 20.0% vs 15.6%; P = 0.059; Figure 5A). The 5-year CSS rates were 28.5% among patients with T1/T2 cancer and 13.3% among patients with T3/T4 cancer (P = 0.016, Figure 5B). Combining chemotherapy to radiotherapy was related to a statistically significant improvement in CSS in comparison with radiotherapy alone (CSS: 22.1% vs 0%, P = 0.015; Figure 5C).
Figure 5.
Kaplan-Meier analysis of cancer-specific survival according to (A) primary site (upper third vs middle third vs lower third vs overlapping); (B) T staging (T1/T2 vs T3/T4); and (C) chemotherapy (Yes vs No) (CSS: cancer-specific survival).
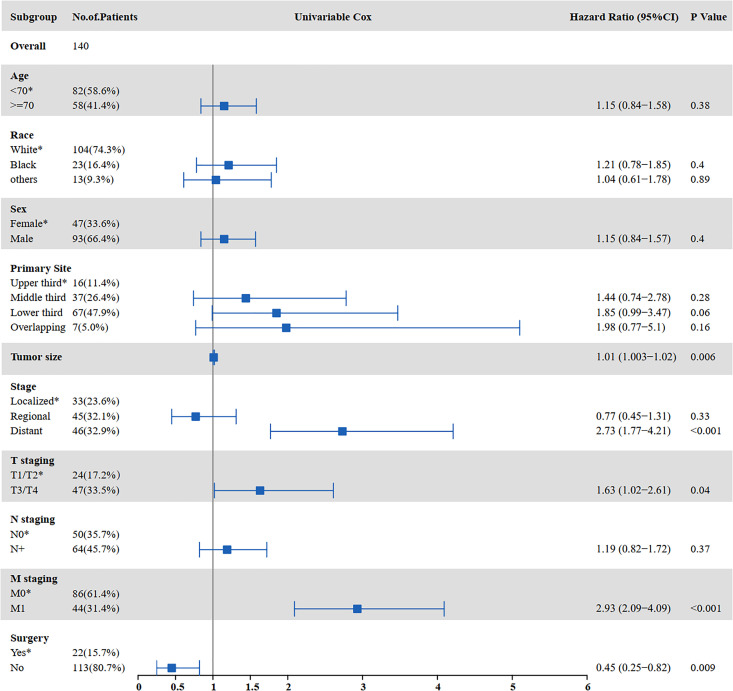
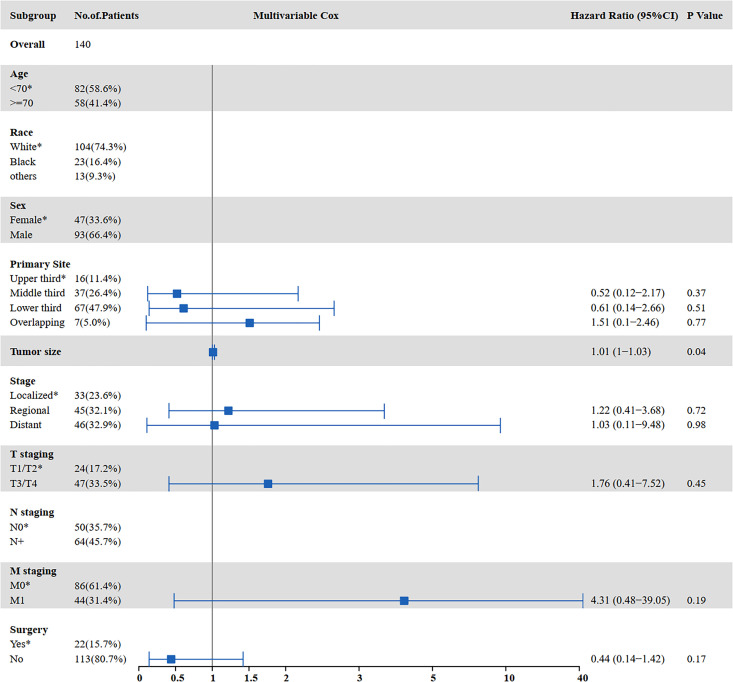
The results of the univariate and multivariate analyses for factors influencing CSS are shown in Figure 6 and Figure 7. Univariate analysis indicated that tumor size (P = 0.001), distant metastasis (P < 0.001), T staging (P = 0.017), M staging (P < 0.001), surgery (P = 0.002), and chemotherapy (P < 0.001) were prognostic factors affecting CSS in SCCE patients receiving radiotherapy. Multivariate analysis showed that tumor size (P = 0.004) and chemotherapy (P < 0.001) were independent prognostic factors affecting CSS in SCCE patients receiving radiotherapy.
Figure 6.
Univariate analysis for CSS in patients with SCCE who received radiotherapy (CSS: cancer-specific survival; SCCE: small cell carcinoma of the esophagus).
Figure 7.
Multivariate analysis for CSS in patients with SCCE who received radiotherapy (CSS: cancer-specific survival; SCCE: small cell carcinoma of the esophagus).
The Effect of Chemotherapy in Patients With Different Stages
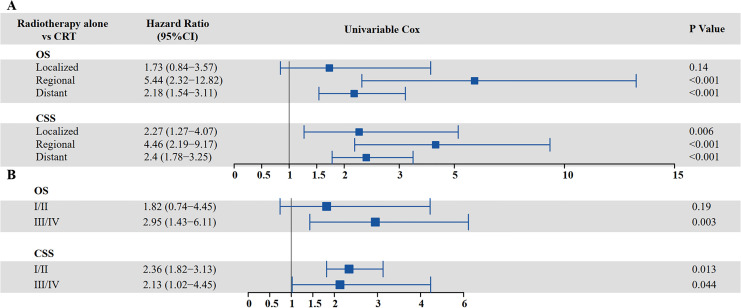
The survival analysis of adding chemotherapy to radiotherapy in patients with different stages of SCCE is shown in Figure 8.
Figure 8.
Cox regression for multivariate analysis of adding chemotherapy to radiotherapy based on different stages of SCCE (A) VALSG stage (B) AJCC staging (CSS: cancer-specific survival; OS: overall survival; SCCE: small cell carcinoma of the esophagus; VALSG: Veterans’ Administration Lung Study Group).
SCCE patients with regional disease (hazard ratio [HR] = 5.435, 95% CI: 2.315-12.821, P < 0.001) and distant metastasis (HR = 2.183, 95% CI: 1.536-3.106, P < 0.001) had worse OS if they received only radiotherapy. In addition, chemotherapy improved the CSS in SCCE patients receiving radiotherapy regardless of stage. Patients with localized disease (HR = 2.268, 95% CI: 1.269-4.065; P = 0.006), regional disease (HR = 4.464, 95% CI: 2.188-9.174; P < 0.001), and distant metastasis (HR = 2.404, 95% CI: 1.776-3.247; P < 0.001) who did not receive chemotherapy had a higher risk of tumor-related death than those who received chemotherapy (Figure 8A). Overall, patients with stage Ⅲ/Ⅳ SCCE, according to AJCC criteria, had worse OS if they received only radiotherapy (HR = 2.95, 95% CI: 1.43-6.11, P = 0.003). Patients with stage Ⅰ/Ⅱ (HR = 2.36, 95% CI: 1.82-3.11; P = 0.013) and stage Ⅲ/Ⅳ (HR = 2.13, 95% CI: 1.02-4.45; P = 0.044) who did not receive chemotherapy were at a higher risk of tumor-related death than those who received chemotherapy (Figure 8B).
Prognostic Factors in SCCE Patients Receiving Both Radiotherapy and Chemotherapy
Univariate analysis showed that age ≥70 years (P = 0.022), tumor size (P = 0.029), distant metastasis (P < 0.001), T staging (P = 0.030), M staging (P < 0.001), and surgery (P = 0.006) were associated with OS among patients receiving chemoradiation. There were no independent prognostic factors affecting OS among these patients (Figure 9 and Figure 10). Univariate analysis indicated that tumor size (P = 0.006), distant metastasis (P < 0.001), T staging (P = 0.042), M staging (P < 0.001), and surgery (P = 0.009) were prognostic factors affecting CSS among patients receiving both radiotherapy and chemotherapy (Figure 11). Multivariate analysis indicated that tumor size (P = 0.043) was an independent prognostic factor affecting CSS in this group (HR = 1.012; 95% CI: 1.000 -1.024; P = 0.048) (Figure 12).
Figure 9.
Univariate analysis for OS in patients with SCCE who received chemotherapy and radiotherapy (OS: overall survival; SCCE: small cell carcinoma of the esophagus).
Figure 10.
Multivariate analysis for OS in patients with SCCE who received chemotherapy and radiotherapy (OS: overall survival; SCCE: small cell carcinoma of the esophagus).
Figure 11.
Univariate analysis for CSS in patients with SCCE who received chemotherapy and radiotherapy (CSS: cancer-specific survival; SCCE: small cell carcinoma of the esophagus).
Figure 12.
Multivariate analysis for CSS in patients with SCCE who received chemotherapy and radiotherapy (CSS: cancer-specific survival; SCCE: small cell carcinoma of the esophagus).
Discussion
SCCE has a low incidence, and, as such, there is still no standard treatment regimen. The current SCCE treatment strategy refers to that for SCLC. This study is the first large-sample retrospective analysis of the clinical effect of chemoradiotherapy on the long-term outcome of SCCE patients receiving radiotherapy. The results showed that adding chemotherapy improves both the OS (17.4% vs 0%, P < 0.001) and CSS (35.9% vs 0%, P = 0.004) of patients receiving radiotherapy. The CSS of SCCE patients receiving radiotherapy was improved with the addition of chemotherapy, regardless of stage. Patients with localized disease (HR = 2.268, 95% CI: 1.269-4.065; P = 0.006), regional disease (HR = 4.464, 95% CI: 2.188-9.174; P < 0.001), and distant metastasis (HR = 2.404, 95% CI: 1.776-3.247; P < 0.001) who didn’t receive chemotherapy had a higher risk of tumor-related death than those who received chemotherapy.
Table 2 summarizes the literature regarding prognostic factors in patients with SCCE. Some studies have tried to investigate the role of chemotherapy in the treatment of SCCE. Nemoto et al analyzed 20 patients with limited-stage SCCE (LS-SCCE) and found that the median survival time (MST) differed significantly between patients treated with 2 or more and patients treated with one or no courses of chemotherapy (11.2 months vs 6.7 months; P = 0.023).9 In a study of 40 SCCE patients with SCCE, among whom 11 received chemotherapy and 29 did not receive chemotherapy, the researchers found that the MSTs were 28 months and 13 months, respectively (P = 0.013).10 Ding et al analyzed the outcomes of different treatments in patients with LS-SCCE and found that patients receiving chemotherapy yielded a 5-year survival rates of 27.2% while patients who did not receive chemotherapy had a 5-year survival rates of 0%.6 Wong et al analyzed the clinical manifestation, therapeutic regimen, and prognosis of patients with SCCE and found that chemotherapy alone (p = 0.003) was related with worse OS compared to chemoradiation.11 A total of 42 SCCE patients underwent radiotherapy had been analyzed in a previous study and the result showed that the survival time was longer in patients who also received chemotherapy (HR = 0.204, P = 0.046).12 In the present study, a better prognosis was observed in patients who received chemotherapy than in patients who did not (OS: 16.1% vs 0%, P = 0.001; CSS: 22.1% vs 0%, P = 0.015). Therefore, we suggest the addition of chemotherapy to radiotherapy among SCCE patients.
Table 2.
Review of Recent Studies on Prognostic Factors in Patients With SCCE.
| Author | Year | Sample size | Median age (years) | MST (month) | 5-year OS (%) | Prognostic factors |
|---|---|---|---|---|---|---|
| Our study | 2020 | 162 | 65-69 | 11 | 8.0 | Age, T staging, tumor size, primary site, and chemotherapy |
| Xiao Q et al19 | 2019 | 137 | 59.3 | 12 | 11.9 | VALSG stage, N stage, and multimodal treatment |
| Chen BQ et al12 | 2019 | 42 | 55 | 12.9 | 13.9 | ECOG PS, lesion length, chemotherapy, and dose of RT |
| Jeene PM et al15 | 2019 | 58 | 55 | 16 | N/A | Chemotherapy cycles |
| Xu L et al13 | 2017 | 152 | 61 | 28 | 8.2 | Treatment modality and N stage |
| Song YQ et al14 | 2015 | 352 | N/A | 8 | N/A | Stage, age, year, and RT |
| Gao R et al18 | 2014 | 225 | 59.5 | 19 (LD) 9 (ED) |
N/A | Chemotherapy |
| Meng MB et al16 | 2013 | 127 | N/A | 21.0 | N/A | Tumor location and type of treatment |
| Ding J et al6 | 2013 | 106 | 58 | N/A | N/A | Chemotherapy |
| Kukar M et al17 | 2013 | 387 | 68 | N/A | N/A | Age, gender, race, and stage |
| Chen SB et al10 | 2011 | 40 | 57 | 13.0 | 10.5 | Operation and chemotherapy |
ECOG: Eastern Cooperative Oncology Group; ED: extensive disease; F: female; LD: limited disease; N/A: Not available; M: male; PS: performance status; RT: radiotherapy; SCCE: small cell carcinoma of the esophagus; VALSG: Veterans’ Administration Lung Study Group.
Tumor size has been found to be associated to prognosis. Xu et al found that SCCE patients with lesions > 6 cm had an poorer MST than patients with lesions 2-6 cm and those with lesions < 2 cm.13 42 SCCE patients treated with radiotherapy had been analyzed and the researcher found that the OS was significantly longer in patients with smaller tumors.12 Our results indicated that tumor size was an independent factor affecting CSS (HR = 1.012, P = 0.008).
Age and T staging have been proposed as factors associated with oncological outcomes. Song et al found that age was related with OS (0.05 < P < 0.1), but there was no relation with CSS.14 Xu et al reported that patients with T1 SCCE had a longer survival time than those with T2, T3, and T4 SCCE (MST: 39 months vs 36 months vs 21 months vs 7 months, P < 0.001), whereas age (≤60 years vs >60 years) was uncorrelated with survival time (P > 0.05).13 Jeene analyzed 55 patients receiving radiotherapy and indicated that T staging had no significant correlation with survival.15 Our study showed that patients <70 years old had a better outcome than those ≥ 70 years old (OS: 11.5% vs 4.3%, P < 0.001), and age was an independent prognostic factor affecting OS. The discrepancy with the findings of Xu et al are likely because we divided patients into multiple age groups, and therefore the difference between groups was more obvious. T staging was also an independent prognostic factor for OS in this study. The 5-year OS rates were 16.7% among patients with T1/T2 and 7.2% among patients with T3/T4 (P = 0.011). In addition, the 5-year CSS rates were 28.5% among patients with T1/T2 and 13.3% among patients with T3/T4 (P = 0.013).
The primary site can also be relevant to the prognosis. Meng et al examined 127 SCCE patients and found that tumor location (upper third of the esophagus) was an poor prognosis factor of OS.16 Xu et al found that SCCE patients with tumors in the middle 1/3 of the esophagus had more satisfying MST than that of patients with tumors in the upper or lower esophagus (32 months vs 17 months vs 23 months, P = 0.029).13 Similarly, in our study, patients with tumors in the middle and lower 1/3 of the esophagus had better outcomes than patients with tumors in the lower 1/3 of the esophagus or overlapping tumors (CSS: 23.1% vs 23.9% vs 20.0% vs 15.6%; P = 0.059).
Advanced stage tends to be related to unfavorable prognosis. Kukar et al indicated that distant metastasis was associated with poor survival (HR = 2.72).17 Gao et al evaluated 124 patients of limited disease (LD) and 88 patients of extensive disease (ED) and found that the MST was obviously prolonged by chemotherapy for LD cases (20 months vs 10 months, P < 0.01), whereas this superiority was not seen in ED patients.18 Nevertheless, our results showed that radiotherapy alone led to significantly worse survival than chemoradiotherapy in patients with distant metastasis (HR = 2.183 and 2.404, respectively, P < 0.001). Xiao et al revealed that patients with regional (MST 16.0 months vs 8.0 months, P = 0.003) and extensive (MST 11.0 months vs 5.0 months, P < 0.001) disease had a longer survival time from chemotherapy, but no benefit were seen in patients with localized disease.19 In the present study, patients with regional disease (HR = 5.435, 95% CI: 2.315-12.821, P < 0.001) and distant metastasis (HR = 2.183, 95% CI: 1.536-3.106, P < 0.001) had shorter OS if they received only radiotherapy. In addition, chemotherapy could improve CSS in all-stage SCCE patients receiving radiotherapy. The reason for the discrepancy may be that our sample size is larger than those in previous studies.
Based on the favorable results of Impower133, adding atezolizumab, a PD-L1–targeted immune checkpoint inhibitor, to a platinum and etoposide chemotherapeutic regimen has been recommended as the preferred first-line systemic therapy option for patients with extensive-stage SCLC.20 In addition, both pembrolizumab and nivolumab have achieved favorable efficacy in esophageal cancer 1. We were not able to evaluate the effects of immunotherapy in our cohort, as the treatment details were not available through the SEER database. However, the possibility of clinical benefit from immunotherapy might be worth exploring.
There are some limitations in this study. First, as the number and the control for selection bias is inadequate and limited, the results obtained in this study are exploratory. Second, considering that the SEER database does not contain information on specific chemotherapy regimens, it is significative to carry out further large-scale prospective studies of the effect of different chemotherapy regimens on survival in SCCE patients. Finally, due to the limitation of the database and the lack of pathological features of the tumor, there is no exploration of the mechanism behind our observations.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Authors’ Note: Tao Li and Sijia Chen contributed equally to the manuscript. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The data analyzed and used in this study was obtained from Surveillance, Epidemiology, and End Results (SEER) database in accordance with the SEER data use agreement (ID: 13522-Nov2018). Therefore, this study did not require approval of ethical board.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Joint Funds for the Innovation of Science and Technology, Fujian Province (grant number 2017Y9074) and the Scientific Research Foundation for Youth Talent of the Xiamen Cancer Center and the First Affiliated Hospital of Xiamen University (grant number ZLYYB201801).
ORCID iD: Jinluan Li, MD, PhD  https://orcid.org/0000-0002-3533-898X
https://orcid.org/0000-0002-3533-898X
References
- 1.Ji A, Jin R, Zhang R, Li H. Primary small cell carcinoma of the esophagus: progression in the last decade. Ann Trans Med. 2020;8(7):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard S, O’Regan K, Jagannathan J, et al. Extrapulmonary small cell carcinoma: a pictorial review. AJR. Am J Roentgenol. 2011;197(3):W392. [DOI] [PubMed] [Google Scholar]
- 3.Purwar P, Jiwnani S, Karimundackal G, Pramesh CS. Management of esophageal small cell carcinoma. Ann Thorac Surg. 2015;99(4):1488. [DOI] [PubMed] [Google Scholar]
- 4.Raja S, Rice TW, Rajeswaran J, Zhong J, Blackstone EH. Esophageal small-cell cancer: study of a rare disease. Dis Esophagus. 2013;26(7):690–695. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Wang F, Chen S, et al. Detailed analysis of prognostic factors in primary esophageal small cell carcinoma. Ann Thorac Surg. 2014;97(6):1975–1981. [DOI] [PubMed] [Google Scholar]
- 6.Ding J, Ji J, Zhu W, et al. A retrospective study of different treatments of limited-stage small-cell esophageal carcinoma and associated prognostic factor analysis. Dis Esophagus. 2013;26(7):696–702. [DOI] [PubMed] [Google Scholar]
- 7.Meng M, Wang H, Zaorsky N, et al. Multimodality therapy is recommended for limited-stage combined small cell esophageal carcinoma. Oncotargets Ther. 2015;(8):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Li D, Xu M. Combined chemotherapy and surgery in primary small cell carcinoma of the esophagus. Minerva Chir. 2015;70(2):69. [PubMed] [Google Scholar]
- 9.Nemoto K, Zhao HJ, Goto T, et al. Radiation therapy for limited-stage small-cell esophageal cancer. Am J Clin Oncol. 2002;25(4):404–407. [DOI] [PubMed] [Google Scholar]
- 10.Chen SB, Yang JS, Yang WP, et al. Treatment and prognosis of limited disease primary small cell carcinoma of esophagus. Dis Esophagus. 2011;24(2):114–119. [DOI] [PubMed] [Google Scholar]
- 11.Wong AT, Shao M, Rineer J, et al. Treatment and survival outcomes of small cell carcinoma of the esophagus: an analysis of the National Cancer Data Base. Dis Esophagus. 2017;30(2):1–5. [DOI] [PubMed] [Google Scholar]
- 12.Chen B, Yang H, Ma H, et al. Radiotherapy for small cell carcinoma of the esophagus: outcomes and prognostic factors from a retrospective study. Radiat Oncol. 2019;14(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Li Y, Liu X, et al. Treatment strategies and prognostic factors of limited-stage primary small cell carcinoma of the esophagus. J Thorac Oncol. 2017;12(12):1834–1844. [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Wang W, Tao G, et al. Survival benefit of radiotherapy to patients with small cell esophagus carcinoma: an analysis of Surveillance Epidemiology and End Results (SEER) data. Oncotarget. 2016;7(13):15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeene PM, Geijsen ED, Muijs CT, et al. Small cell carcinoma of the esophagus. Am J Clin Oncol. 2019;42(6):534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng M, Zaorsky NG, Jiang C, et al. Radiotherapy and chemotherapy are associated with improved outcomes over surgery and chemotherapy in the management of limited-stage small cell esophageal carcinoma. Radiother Oncol. 2013;106(3):317–322. [DOI] [PubMed] [Google Scholar]
- 17.Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol. 2013;20(13):4239–4244. [DOI] [PubMed] [Google Scholar]
- 18.Gao R, Zhang Y, Wen XP, Fu J, Zhang GJ. Chemotherapy with cisplatin or carboplatin in combination with etoposide for small-cell esophageal cancer: a systemic analysis of case series. Dis Esophagus. 2014;27(8):764–769. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Q, Xiao H, Ouyang S, et al. Primary small cell carcinoma of the esophagus: comparison between a Chinese cohort and Surveillance, Epidemiology, and End Results (SEER) data. Cancer Med-Us. 2019;8(3):1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N En J Med. 2018;379(23):2220–2229. [DOI] [PubMed] [Google Scholar]