Abstract
Background
It is inconclusive whether R1 margin determined by postoperative pathological examination indicates worse long-term survival in gastric cancer (GC) patients after curative intent resection (CIR). Hence, we aimed to systematically pool the conflicting evidence to fill this gap.
Methods
The present study was performed according to the published protocol and Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Published studies examining the impact of microscopic margin status on overall survival (OS) and 5-year OS rate in GC were systematically searched in PubMed, Embase, and Cochrane Library databases. RevMan 5.3 was used to conduct statistical analysis, and the Grading of Recommendations, Assessment, Development, and Evaluations approach was used to assess the certainty of evidence for each outcome.
Results
Twenty-three retrospective cohort studies including 19 992 patients were analyzed. The pooled hazard ratio for OS of 14 studies was 2.06 (95% confidence interval [CI]: 1.61–2.65, low certainty), indicating that R1 margin predicted inferior OS. Subgroup and sensitivity analyses upheld the statistical stability of this finding. The pooled odds ratio (OR) of 14 studies was .21 (95% CI: .17–.26, moderate certainty), demonstrating that the presence of R1 margins was associated with a poorer 5-year OS rate. Sensitivity analyses and most of the subgroup analyses confirmed this finding, except the “esophagogastric junction (EGJ) cancers” subgroup, which included two studies with a pooled OR of .41 (95% CI: .10–1.61).
Conclusion
R1 margin detected by pathological examination might exhibit a high correlation with poorer OS and 5-year OS rate in GC (except EGJ cancers) patients who underwent CIR. To figure out the effect of R1 margin on survival of different stages and histological types need prospective studies with large sample sizes and standardized methods. What is the best treatment for R1 margin patients also need more in-depth and special research.
Keywords: gastric cancer, surgery, surgical margin, survival, meta-analysis
Introduction
Gastric cancer is the second leading cause of death among all cancer types, causing 782 685 deaths worldwide in 2018. The incidence of GC has significantly increased, with approximately 1 033 701 new cases every year.1 Although adjuvant therapies such as chemoradiotherapy (CRT), chemotherapy (CT), and immunotherapy can improve the survival of GC patients to some extent, radical surgery is still considered the first-line treatment.2-4 Curative resection with microscopically negative (R0) margins (no cancer cells identified at the resection margin on pathological examination) has been accepted as the most effective treatment based on the surgical philosophy, as even a minimal number of remaining cancer cells would develop recurrences.5,6 Although the impact of microscopically positive (R1) margins (cancer cells present at the resection margin on pathological examination) on survival in GC has been discussed in many studies, inconsistent conclusions have been reported.2,5,7-16 Kim et al15 and Postlewait et al12 found that R1 margin was not independently associated with survival, while Woo et al12 and Nagata et al5 revealed that it boded ill for survival. Bickenbach et al10 reported that R1 margin was an independent predictor of worse survival but not in patients with more than three positive nodes or T3–4 disease.
Raziee et al17 performed a systematic review examining positive resection margins of GC by mainly exploring their predictive factors and impact on survival. However, the review did not distinguish R1 margins from macroscopically positive (R2) margins (tumor tissue seen at the resection margin on gross examination by the naked eye) and did not perform a quantitative analysis. Another systematic review focusing on the management of R1 margins revealed that re-operation of R1 margins might be technically demanding and the operative mortality risk should be balanced with the benefits of re-operation, but the impact of further therapies (either surgical or oncological) on the outcomes in patients with positive margins is unknown.18 Quantitative evidence regarding whether R1 margin has a negative impact on long-term survival in GC is limited. This aroused our interest in performing a systematic review and meta-analysis to address this question.
The study population and patient exposure were clearly defined and limited in the inclusion criteria of our study to reduce confounders. R2 margin is most frequently observed in palliative intent resection19 wherein there are many factors deadlier than the margin status that affect survival.9 Thus, we considered only patients who underwent curative intent resection (CIR) and excluded those who underwent palliative intent surgery. Moreover, studies comparing the survival of patients with R2 and R0 margins and that did not distinguish between the R1 and R2 margins were excluded.20-24 Microscopic margin status is sometimes different between intraoperative frozen sections and postoperative pathological examination.25 In many cases, R0 margin confirmed by frozen sections can be ruled out by pathological examination. Therefore, we considered only studies that determined the final margin status by pathological examination and grouped patients into R1 or R0 arm only according to their pathological examinations, regardless of the margin status in the frozen sections.
To the best of our knowledge, the present study is the first meta-analysis to explore whether R1 margin determined by postoperative pathological examination indicates worse long-term survival in GC patients after CIR.
Materials and Methods
The present study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Table S1).26 The study protocol was pre-specified and registered in the International Prospective Register of Systematic Reviews under the code CRD42020165110. The study protocol was published elsewhere.27
Inclusion and exclusion criteria
Studies were deemed eligible according to the PICOS approach as follows.
P: Patients who underwent CIR for treating GC
I/E: R1 margin, which indicated that cancer cells confirmed by pathological examination were identified at the linear, circular, proximal, or distal resection margins
C: R0 margin confirmed by pathological examination
O: Time-to-event overall survival (OS) (primary outcome), 5-year OS rate (secondary outcome), or relevant data to estimate these parameters
S: Studies with randomized controlled, cohort, or case-control designs with a follow-up period of 60 months
The exclusion criteria were as follows: (1) letters to the editor, abstract publications, case reports, mechanism studies, reviews, commentaries, perspectives, and editorials; (2) studies with overlapping data; and (3) studies investigating endoscopic submucosal dissection and endoscopic mucosal resection for GC.
Literature Search
The literature search was conducted in two stages.1 Bibliographic database searches: A systematic search of PubMed, Embase, and Cochrane Central Register of Controlled Trials databases was performed from their inception to April 2020 without language restriction. The details of the PubMed database search strategy and syntax are shown in Table S2.2 Search through other sources: we manually searched the references of relevant articles to identify eligible studies.
Data Extraction and Quality Assessment
Data extraction for the included studies was conducted by two independent reviewers (blinded for peer-review) using the standardized electronic data extraction form listed in Table S3. The following data were extracted from all eligible studies: first author, year of publication, study design, study period, country, proportion of male patients, age (mean or median), follow-up (mean or median), sample size, R1 margin rate, R0 margin rate, tumor size, tumor site, tumor stage, histological grade, type of surgery, lymphadenectomy, neoadjuvant or adjuvant treatments, and survival outcomes. If outcomes were reported in multiple datasets, the one with the greatest number of adjusted confounders was used. Two reviewers (blinded for peer-review) independently assessed the methodological quality and risk of bias of the included studies using the Risk Of Bias In the Non-randomized Studies of Interventions (ROBINS-I) tool.28 All disputes during the process of data extraction and methodological quality assessment were arbitrated by a third team member (blinded for peer-review).
Dealing with Missing Data
For time-to-event OS, when a hazard ratio (HR) and its upper or lower limit of 95% confidence interval (CI) were provided by a trial, we calculated the lnHR (the natural logarithm of HR) and its standard error and then merged the overall HRs. When the aforementioned data were incomplete, some or all of the variables among lnHR, the observed log rank minus expected events (O-E), the log rank variance, and the lnHR variance were estimated by indirect methods.29 When these indirect methods were not effective, the necessary statistics were generated from the Kaplan–Meier curves.29,30 When a study failed to provide the necessary statistics using the aforementioned methods, it was omitted from the pooling process for HRs. To estimate pooled odds ratios (ORs) for dichotomous outcomes (5-year OS rate), we recorded data regarding the total number of participants and the incidence of events in each arm of every trial. When these data in the full text were incomplete, the authors were contacted for more details and studies that failed to provide the necessary data were excluded from our analysis.
GRADE Certainty Assessment
Two reviewers (blinded for peer-review) independently assessed the certainty (quality of evidence) of each outcome using the five Grading of Recommendations, Assessment, Development and Evaluations (GRADE) considerations. The certainty was analyzed in terms of five downgrade considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) and three upgrade considerations (large magnitude of effect, dose-response relation, and plausible confounders or biases). Subsequently, it was rated as high, moderate, low, or very low.31-38 Additionally, a “summary of findings” table was compiled for each outcome using the GRADEpro software (GRADEpro GDT 2015). Disagreements during this process were resolved through team discussion.
Statistical Analysis
The pooled estimates of the association between margin status and survival outcomes were calculated in the meta-analysis. An observed HR > 1 or OR < 1 indicated a poorer time-to-event OS or 5-year OS rate, respectively, in patients with R1 margins. When multiple HRs were presented in a study, the HR adjusted for the greatest number of confounders was selected.39 Heterogeneity among studies was measured using Cochran’s Q statistics and by estimating the I2 value. I2 <50% or ≥50% indicated low or high heterogeneity, respectively. A fixed-effects model was used when heterogeneity was low; otherwise, a random effects model was selected. Subgroup analyses were performed according to a predetermined plan. A leave-one-out sensitivity analysis was also performed to evaluate the robustness of the results. Publication bias was assessed using funnel plots. All statistical tests in this meta-analysis were performed using the RevMan software, version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). All statistical tests were two-tailed, and statistical significance was set at P < .05.
Results
Search Results
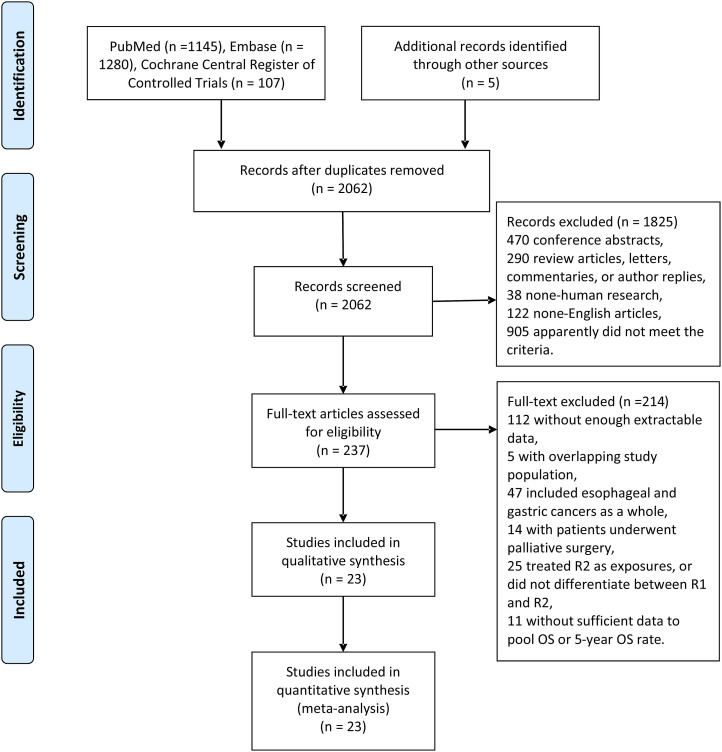
A flow chart of the selection process is depicted in Figure 1. The search strategy yielded 2537 potential studies. According to the exclusion criteria, we excluded 2300 duplicate or irrelevant studies by screening their titles and abstracts. The full texts of 237 studies were assessed, and 214 studies were excluded due to various objective reasons, for instance, 86 studies were excluded as they did not meet the “exposure” or “population” criteria, as were 11 without sufficient data to estimate the pooled relative effect sizes. Finally, 23 studies were included in the meta-analysis (Table 1).
Figure 1.
Flow chart illustrating the study selection.
Table 1.
Baseline Characteristics of all Studies Included in the Meta-Analysis.
| Author | Year | Study design | Region | Period | Median age (year) | Median follow-up (m) | Sample size (n) | R1 (n) (%) | R0 (n) (%) | Adjuvant therapy (n) (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Koumarianou | 2019 | Retrospective/single center | Greece | 2006-2010 | 65.0 | 114.8 | 125 | 25 (20.0) | 100 (80.0) | CT: 123 (75.0) |
| RT: 138 (84.1) | ||||||||||
| Kim | R1: CT: 14 (25.9) | |||||||||
| 2017 | Retrospective/multicenter | USA | 2000-2013 | 65.2 | N/A | 716 | 54 (7.5) | 662 (92.5) | CRT: 26 (48.1) | |
| R0: CT: 126 (19.0) | ||||||||||
| CRT: 227 (34.3) | ||||||||||
| Zhang | 2017 | Retrospective/single center | China | 2003-2009 | N/A | N/A | 633 | 77 (12.2) | 556 (87.8) | N/A |
| Schoenfeld | R1: CT: 9 (47.4) | |||||||||
| 2016 | Retrospective/multicenter | USA | 1998-2010 | 60.0 | 42.0 | 91 | 19 (20.9) | 72 (79.1) | RT: 19 (100) | |
| R0: CT: 24 (33.3) | ||||||||||
| RT: 72 (100) | ||||||||||
| Liang | 2015 | Retrospective/single center | China | 2003-2008 | 62.0 | N/A | 1025 | 75 (7.3) | 950 (92.7) | R1: CT: 34 (45.3) |
| R0: CT: 322 (33.9) | ||||||||||
| Postlewait | R1: CT: 9 (81.8) | |||||||||
| 2015 | Retrospective/multicenter | USA | 2000-2012 | 64.4 | 27.2 (R0) | 162 | 11 (6.8) | 151 (93.2) | RT: 9 (81.8) | |
| R0: CT: 74 (55.2) | ||||||||||
| RT: 46 (35.1) | ||||||||||
| Kim | 2014 | Retrospective/single center | Korea | 1992-2010 | Mean: 55.7 (R1), 56.8 (R0) | 88.0 | 1888 | 17 (.9) | 1871 (99.1) | N/A |
| Canyilmaz | 2014 | Retrospective/single center | Turkey | 2001-2014 | 55.0 | 22.5 (mean) | 193 | 30 (15.5) | 163 (84.5) | R1: CRT: 30 (100) |
| R0: CRT: 163 (100) | ||||||||||
| Roberts | 2014 | Retrospective/single center | Jamaica | 2000-2010 | 67.0 | N/A | 79 | 13 (16.5) | 66 (83.5) | N/A |
| Chen | 2013 | Retrospective/single center | China | 1996-2008 | 60.0 (R1), 59.0 (R0) | at least 3 years | 122 | 72 (59.0) | 50 (41.0) | N/A |
| Gierej | 2012 | Retrospective/single center | Poland | 1994-2001 | 61.6 | N/A | 158 | 22 (14.0) | 136 (86.0) | N/A |
| Bilici | 2012 | Retrospective/single center | Turkey | 2000-2009 | 57.0 | 28.5 | 148 | 14 (9.5) | 134 (90.5) | R1: CRT: 14 (100) |
| R0: CRT: 134 (100) | ||||||||||
| Nagata | 2011 | Retrospective/single center | Japan | 1997-2009 | Mean: 68.1 (R1), 63.7 (R0) | 35.9 | 824 | 23 (2.8) | 801 (97.2) | R1: CT: 10 (43.5) |
| Wang | 2009 | Retrospective/single center | Taiwan | 1994-2004 | Mean:61.6 (R1), 62.2 (R0) | 28.6 | 1565 | 129 (8.2) | 1436 (91.8) | N/A |
| Sun | 2009 | Retrospective/single center | China | 1980-2006 | Mean: 58.0 (R1), 57.7(R0) | 39.0 | 2269 | 110 (4.8) | 2159 (95.2) | N/A |
| Johansson | 2008 | Retrospective/single center | Sweden | 1990-2001 | Mean: 64.7 | to death or at least 5-years | 120 | 20 (16.7) | 100 (83.3) | N/A |
| An | 2008 | Retrospective/single center | Korea | 1995-2005 | N/A | 68.2 | 4147 | 383 (9.2) | 3764 (90.8) | CT: 1191 (28.4) |
| CRT: 1065 (25.4) | ||||||||||
| Morgagni | 2008 | Retrospective/multicenter | Italy | 1988-2001 | N/A | 69.0 | 2084 | 89 (4.3) | 1995 (95.7) | N/A |
| Cho | 2007 | Retrospective/single center | Korea | 1987-2002 | 58.0 (R1), 57.0 (R0) | 26.0 (R1) | 2732 | 49 (1.8) | 2683 (98.2) | R1: CT: 43 (87.8) |
| Mariette | 2003 | Retrospective/single center | France | N/A | 62.0 (R1), 61.0 (R0) | 20.5 | 94 | 8 (8.5) | 86 (91.5) | CT: 11 (11.5) |
| Jakl | 1995 | Retrospective/single center | Austria | 1970-1992 | 67.0 | N/A | 108 | 20 (18.5) | 88 (81.5) | R1: CT/RT: 0 |
| R0: CT/RT: 0 | ||||||||||
| Hallissey | 1993 | Retrospective/single center | UK | N/A | N/A | to death or at least 5 years | 424 | 55 (13.0) | 369 (87.0) | N/A |
| Hockey | 1984 | Retrospective/multicenter | UK | N/A | N/A | N/A | 285 | 46 (16.1) | 239 (83.9) | N/A |
Abbreviations: N/A, not available; CT, chemotherapy; RT, radiotherapy; CRT, chemoradiotherapy.
Study Characteristics and Quality Assessments
The characteristics of the included studies are summarized in Table 1. All enrolled studies were retrospective cohort studies with median follow-up duration varying from 20.5 to 114.8 months. A total of 19 992 patients (range: 79 to 4147) who underwent CIR for GC treatment were included. Among these, 6.8% (range: .9% to 59.0%) were reported to have R1 margins. Among the 23 studies, nine were conducted in Europe, nine in Asia, three in North America, one in Australia, and one in Latin America. All enrolled studies were published in English. Nine studies reported time-to-event OS, nine reported 5-year OS rates, and five reported both (Table S4: detailed characteristics of the included studies). Twelve studies5,7,3,12,15,16,20,40-44 reported the proportion of patients receiving adjuvant therapies. Among these, six studies3,11,15,16,42,43 reported the proportions in R1 and R0 groups separately (Table 1). The results of the risk of bias assessment using the ROBINS-I tool showed that the overall risk of bias was low for 21 studies and moderate for two studies (Table S5).
Overall Survival
Twelve studies3,12,15,16,17,22,43–45,47–49 reported HRs, and the necessary statistics were extracted from the Kaplan–Meier curves in two studies.8,9 Adding these, 14 studies were selected to estimate pooled HR. With inter-study heterogeneity (I2 = 84.0%, P-value of Cochran’s Q test <.00001), a random effects model was used and the pooled HR was 2.06 (95% CI: 1.61–2.65, low certainty) (Figure 2(A), Table 2 and Table 3), which indicated that R1 margin predicted inferior OS.
Figure 2.
Forest plots of the studies to evaluate the impact of R1 margin on survival of patients with gastric cancer. (A) Overall survival (OS), (B) 5-year OS rate.
Table 2.
Summary of Meta-Analysis Results and Subgroup Analyses.
| Analysis specification | Studies (n) | Study heterogeneity | Effects model | Pooled estimates [95% CI] | P value | |
|---|---|---|---|---|---|---|
| I2 (%) | P heterogeneity | |||||
| OS | HR | |||||
| Overall | 14 | 84 | <.00001 | Random | 2.06 [1.61, 2.65] | <.00001 |
| 1. Geographical region | ||||||
| Asia | 5 | 85 | <.0001 | Random | 2.27 [1.73, 2.97] | <.00001 |
| other regions | 9 | 80 | <.00001 | 2.00 [1.30, 3.08] | .001 | |
| 2. Tumor stage | ||||||
| AGC | 2 | 75 | .05 | Random | 2.10 [1.49, 2.97] | <.0001 |
| 3. Tumor site | ||||||
| Proximal cancers | 2 | 0 | .44 | Fixed | 1.85 [1.17, 2.93] | .009 |
| 4. Data extracted from | ||||||
| multivariate analyses | 11 | 81 | <.00001 | Random | 1.95 [1.47, 2.59] | <.00001 |
| univariate analyses | 3 | 78 | .01 | 2.59 [1.79, 3.74] | <.00001 | |
| 5. Lymphadenectomy | ||||||
| ≤D1 | 1 | N/A | N/A | Fixed | 2.42 [1.03, 5.73] | .04 |
| ≥D2 | 1 | N/A | N/A | 2.41 [1.94, 3.00] | <.00001 | |
| 5-year OS rate | OR | |||||
| Overall | 14 | 42 | .05 | Fixed | .21 [.17, .26] | <.00001 |
| 1. Geographical region | ||||||
| Asia | 7 | 70 | .003 | Random | .21 [.13, .34] | <.00001 |
| other regions | 7 | 0 | .87 | .24 [.17, .34] | <.00001 | |
| 2. Tumor stage | ||||||
| EGC | 3 | 59 | .09 | Random | .16 [.03, .94] | .04 |
| AGC | 4 | 0 | .67 | .26 [.18, .36] | <.00001 | |
| 3. Tumor site | ||||||
| Proximal cancers | 2 | 0 | .97 | Fixed | .41 [.10, 1.61] | .20 |
| 4. Lymphadenectomy | ||||||
| ≤D1 | 1 | N/A | N/A | Fixed | .21 [.10, .43] | <.0001 |
| ≥D2 | 4 | 0 | .60 | .30 [.22, .40] | <.00001 | |
Abbreviations: OS, overall survival; 5-year OS rate, 5-year overall survival rate; CI, confidence interval; HR, hazard ratio; OR, odds ratio; EGC, early gastric cancer; AGC, advanced gastric cancer; N/A, not applicable.
Table 3.
Summary of Findings for OS and 5-Year OS Rate.
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) |
|---|---|---|---|
| OS assessed with: HR follow-up: more than 5 years | HR 2.06 (1.61 to 2.64) | 15526 (14 non-randomized studies) | Lowa,b |
| 5-year OS rate assessed with: OR follow-up: more than 5 years | OR .21 (.17 to .26) | 13726 (14 observational studies) | Moderatec,d |
Abbreviations: OS, overall survival; 5-year OS rate, 5-year overall survival rate; CI, confidence interval; HR, hazard ratio; OR, odds ratio.
aThere was heterogeneity among studies, and little overlap between confidence intervals without reasonable explanations.
bThe funnel plot showed obvious asymmetry.
cIndividual studies had been shown to have serious risk of bias in the at intervention and post-intervention stages.
dThe funnel plot showed obvious asymmetry.
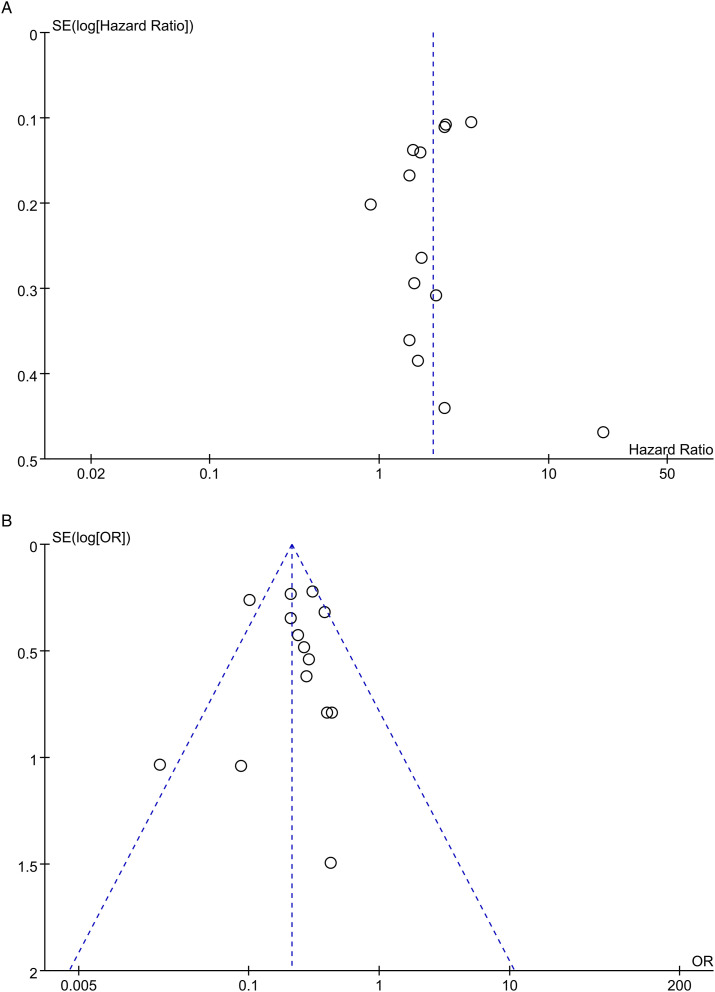
Subgroup analyses are shown in Table 2 and Figures S1–5. The results of all subgroup analyses suggested that R1 margin was a prognostic factor despite heterogeneity among some subgroups. Sensitivity analyses showed that the pooled HRs did not substantially vary, ranging from 1.88 (95% CI: 1.51–2.33) to 2.21 (95% CI: 1.75–2.78). However, the asymmetrical funnel plot indicated a potential publication bias in the included studies (Figure 3(A)).
Figure 3.
Funnel plots for evaluating the publication bias. Each point represents a separate study for the indicated association. (A) overall survival (OS); (B) 5-year OS rate. HR, hazard ratio; OR, odds ratio.
Five-Year OS Rate
The 5-year OS rates of the two groups were reported in 14 studies.2,5,7-9,3,40-42,46,48-51 As there was no apparent inter-study heterogeneity (I2 = 42.0%, P-value of Cochran Q test: .05), a fixed-effects model was selected. The pooled result (OR = .21, 95% CI: .17–.26, moderate certainty; Figure 2(B), Table 2, and Table 3) indicated that the presence of R1 margin was associated with a poorer 5-year OS rate.
Subgroup analyses were conducted (Table 2 and Figures S6–S9) and most of them confirmed that the R1 margin was associated with a lower 5-year OS rate, except the “proximal cancers” subgroup, which included two studies that reported a pooled OR of .41 (95% CI: .10–1.61). In sensitivity analyses, the pooled ORs ranged from .19 (95% CI: .15-.24) to .25 (95% CI: .20–.31), demonstrating that the results did not change significantly. Asymmetry in the funnel plot indicated a potential publication bias (Figure 3(B)).
Discussion
The management of positive surgical margins is a dilemma, considering the operative risk of a second surgery or a more extensive primary resection and potential technical difficulties.17,18 Cascinu et al52 suggested that a re-operation should be considered in patients with N0 stage disease if achieving a tumor-free resection line seems feasible. The authors also suggested that patients with positive nodes should be monitored closely without a need for aggressive surgical approach because a significant difference in survival conferred by positive and negative margins was observed only in patients with negative nodes on definitive histological examination (pT2–pT3 N0 stage disease) in their study. Nagata et al5 concluded that histological margin status is always significant in GC patients undergoing gastrectomy and re-operation for early GC in patients with R1 margins is recommended as R1 margins were associated with worse survival outcomes in these patients. It is inconclusive whether R1 margins indicate worse long-term survival in GC patients after CIR. Thus, before weighing the risks and benefits of re-operation as well as other aggressive treatments, evidence is needed to confirm the effect of R1 margin on prognosis.
Until a prospective randomized controlled trial (RCT) is conducted, the findings from a meta-analysis of retrospective studies are the best evidence available.53 However, designing a prospective experimental trial on this topic would be difficult considering the concomitant ethical issues.54 According to the plan in its protocol,27 the present study should have enrolled both eligible RCTs and non-randomized studies (NRSs) and pooled the outcomes from these two types of studies. However, after study retrieval and screening, only NRSs (specifically, cohort studies) met the inclusion criteria. Moreover, outcomes in the present study should have included time-to-event disease-free survival (DFS) and the 5-year DFS rate, but only one eligible study43 reported DFS and none of the studies reported the 5-year DFS rate. Hence, we analyzed only the pooled estimates for time-to-event OS and 5-year OS rate. These two points were the major compromises we made in the formal study when compared with the protocol.
Twenty-three cohort studies with a large sample size of 19 992 patients were included in this meta-analysis. The results of this study demonstrated that largely, R1 margins were associated with poorer survival than R0 margins. However, based on the GRADE approach, low certainty in terms of OS and moderate certainty in terms of the 5-year OS rate should be considered while interpreting these results. The funnel plots for both outcomes were asymmetrical, indicating that publication bias was strongly suspected, which downgraded the GRADE certainties. For the 5-year OS rate, another consideration for the downgrade was those two studies41,51 had a serious risk of bias in the intervention and post-intervention stages. Meanwhile, the large pooled effect with no plausible confounders upgraded the quality of evidence for the 5-year OS rate by one level (Table 3).
Positive margins were associated with more aggressive tumor biology, including larger tumor size, deeper wall penetration (T stage), more extensive gastric involvement, greater number of involved nodes, advanced stage, diffuse histology type, higher Borrmann type, and lymphatic vessel involvement.55 In 11 of the included studies, the HRs for OS were collected from multivariate analyses that included tumor stage, tumor size, lymph node metastasis, age, and histological classification as covariates and R1 margin status was found to be an independent prognostic factor. The subgroup analysis of these 11 studies showed that the pooled HR was 1.95 (95% CI: 1.47–2.59), which was consistent with the overall results (Table 2). Admittedly, the covariates considered in the multivariate analysis of each study were not exactly the same, resulting in the pooled HR being affected by these confounders and consequently reducing the GRADE level of evidence for OS.
Sensitivity analyses showed that the pooled estimates for both outcomes were always positive and supported the R0 margin. For OS, all subgroup analyses suggested that R1 margin was an adverse prognostic factor. For the 5-year OS rate, most subgroup analyses confirmed that R1 margin had a negative impact, except the “tumor site” subgroup, which contained two studies,40,41 including only patients with esophagogastric junction (EGJ) cancer. The pooled OR of these two studies was .41 (95% CI: .10–1.61), which probably revealed that R1 margin had no definite effect on the 5-year OS rate of these patients. This is consistent with the opinion of many researchers who suggest that EGJ cancer should be regarded as a separate neoplasm due to its distinct prognostic and pathological features.56 Cumulatively, the prognostic value of R1 margin was not affected as the results were not significantly altered by most of the corresponding subgroup and sensitivity analyses, indicating the relative robustness of the results.
Six enrolled studies reported the proportions of patients receiving adjuvant therapy in the R1 and R0 groups separately (Table 1). In two of these studies, all patients in both the groups underwent postoperative CRT. Patients in the study by Bilici et al42 underwent fluorouracil and fludarabine-based CRT. The authors reported that both the 3- and 5-year OS rates of the R1 group were significantly lower. Patients in Canyilmaz et al’s study43 received fluorouracil and leucovorin-based CRT. The authors reported no significant difference in OS between the groups (HR: 1.6, 95% CI: .9–2.6), but the DFS in the R1 group was worse (HR: 2.1, 95% CI: 1.2–3.8). In the remaining four studies, the proportions of patients receiving adjuvant therapy were higher in the R1 group. Among these studies, the study by Liang et al3 confirmed that the 5-year OS rate of the R1 group was significantly worse. The remaining three studies12,15,16 reported the OS and yielded invalid HRs, suggesting that the OS of the R1 group was not worse than that of the R0 group. Considering this conflicting evidence, it is difficult to know whether postoperative adjuvant therapy for patients with R1 margins can improve their prognosis to the level of patients with R0 margins or which postoperative adjuvant therapy is the most suitable approach for these patients, and these need to be verified by more in-depth research.
Recommendations from previous studies regarding the re-operation of positive margins also vary.55 Some authors believe that patients with positive margins should only be watched closely,57 while others recommend re-resection for all patients, irrespective of their stage.8 In case of early GC, authors frequently advised re-operation but few of them reported data on re-resected patients.58 For locally advanced GC, the indication for re-operation is generally evaluated on the basis of nodal stage. Kim et al59 found that R1 margin lost its prognostic impact in multivariate analysis in all but in patients with less than five positive nodes disease when D2 or D3 lymphadenectomy was performed and thus hypothesized re-resection for such patients. Squires et al25 resected 48 patients with R1 margins and all achieved R0 resection, these patients showed a lower local recurrence rate, but the survival was not better than that of patients without re-resection. At present, there is still no evidence to guide surgeons on whether to perform re-operation to the R1 margins. This present study confirmed the negative effect of R1 margin on the long-term prognosis of GC patients and indicated the potential therapeutic value on R1 margin. However, what are the most suitable treatments for this group of patients still need more in-depth and special research attention.
Despite a robust methodological process and important findings, the present study has some limitations. All the enrolled studies were retrospective in nature. Thus, several intrinsic drawbacks such as recall and selection bias might be a concern, which made our meta-analysis sensitive to potential confounding variables. Second, we planned to estimate the pooled effect sizes for DFS and 5-year DFS rate besides OS and the 5-year OS rate. However, as only one eligible study reported DFS and none of the studies reported the 5-year DFS rate, this idea was not implemented. Third, substantial heterogeneity observed in some of the subgroups might be due to the differences in baseline characteristics of patients, such as the locations of R1 margins, tumor size, TMN stage, histological type, and the technical differences associated with the surgeons. However, no further subgroup analysis of these factors can be performed at present. Fourth, the addition of adjuvant CRT or CT may be an important consideration for patients with R1 margins or advanced disease because most of the enrolled studies did not report adjuvant therapy in detail and given that the six studies that separately reported the proportions of patients receiving adjuvant therapy in the R1 and R0 groups differed greatly in terms of CRT or CT regimens. Hence, adjuvant therapy might be another potentially important source of bias; however, it cannot be addressed further in this review. In addition, inconsistencies without reasonable excuses and strongly suspected publication bias downgraded the GRADE certainties for OS, which led it to be rated as low certainty.
Several important strengths of this study should be noted. First, we followed the established and published protocol to implement this study, which made the entire research process more transparent, reasonable, and rigorous. Second, we adopted stringent inclusion and exclusion criteria, which excluded patients with R2 margins, with R1 margins that were not confirmed by pathological examination, or who had undergone palliative surgery, thus reducing the confounders. Moreover, the present study indicated a consistent survival advantage associated with R0 margins in a large sample through a series of subgroup analyses and sensitivity analyses, which upheld the statistical stability of this finding. All the included studies directly compared the effects of these two exposure factors on OS or the 5-year OS rate in similar populations, thus avoiding the interference of indirectness on the quality of evidence. Finally, we applied the GRADE approach to rate the certainty of evidence and presented a “summary of findings” table highlighting certainty of evidence.
Conclusion
The present meta-analysis suggested that R1 margins detected by histopathological examination might exhibit a high correlation with poorer OS and 5-year OS rates in GC patients (except in those with EGJ cancers) who underwent CIR. Surgeons should focus on achieving R0 margins to optimize long-term survival whenever possible. To figure out the effect of R1 margin on GC survival (especially DFS) of different stages and histological types needs some prospective studies with large sample sizes and standardized methods. In addition, what are the most suitable treatment for R1 margin patients also need more in-depth and special research attention.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211043665 for Impact of Surgical Margin Status on Survival in Gastric Cancer: A Systematic Review and Meta-Analysis by Zhiyuan Jiang, Chunyu Liu, Zhaolun Cai, Chaoyong Shen, Yuan Yin, Xiaonan Yin, Zhou Zhao, Mingchun Mu, Yiqiong Yin and Bo Zhang in Cancer Control
Author Contributions: The original idea of this research was conceived by ZB. JZY and LCY drafted this manuscript. CZL, SCY, YXN, ZZ, and MMC participated in developing the eligibility criteria, search strategy, data extraction methods and data summary plan. JZY, LCY, CZL, and YY accomplished searching for studies, extracting and analyzing the data, and assessing risk of bias and quality of evidence. ZB and YYQ supervised the work. All authors approved the final version of this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Project Sichuan Science and Technology Support Program (Grant No. 2020YFS0234 and 2020YFS0233) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18034).
Supplementary Material: Supplementary material for this article is available online.
ORCID iDs
Zhiyuan Jiang https://orcid.org/0000-0003-2483-3413
Zhaolun Cai https://orcid.org/0000-0002-3706-6703
Zhou Zhao https://orcid.org/0000-0001-7349-7159
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen J-D, Yang X-P, Shen J-G, Hu W-X, Yuan X-M, Wang L-B. Prognostic improvement of reexcision for positive resection margins in patients with advanced gastric cancer. Eur J Surg Oncol. 2013;39(3):229-234. doi: 10.1016/j.ejso.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Liang Y, Ding X, Wang X, et al. Prognostic value of surgical margin status in gastric cancer patients. ANZ J Surg. 2015;85(9):678-684. doi: 10.1111/ans.12515. [DOI] [PubMed] [Google Scholar]
- 4.Cai Z, Yin Y, Yin Y, et al. Comparative effectiveness of adjuvant treatments for resected gastric cancer: a network meta-analysis. Gastric Cancer. 2018;21(6):1031-1040. doi: 10.1007/s10120-018-0831-0. [DOI] [PubMed] [Google Scholar]
- 5.Nagata T, Ichikawa D, Komatsu S, et al. Prognostic impact of microscopic positive margin in gastric cancer patients. J Surg Oncol. 2011;104(6):592-597. doi: 10.1002/jso.22022. [DOI] [PubMed] [Google Scholar]
- 6.Cai Z, Yin Y, Shen C, et al. Comparative effectiveness of preoperative, postoperative and perioperative treatments for resectable gastric cancer: a network meta-analysis of the literature from the past 20 years. Surg Oncol. 2018;27(3):563-574. doi: 10.1016/j.suronc.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Cho BC, Jeung HC, Choi HJ, et al. Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. J Surg Oncol. 2007;95(6):461-468. doi: 10.1002/jso.20731. [DOI] [PubMed] [Google Scholar]
- 8.Wang S-Y, Yeh C-N, Lee H-L, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol. 2009;16(10):2738-2743. doi: 10.1245/s10434-009-0616-0. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, Li D-m., Wang Z-n., et al. Prognostic significance of microscopic positive margins for gastric cancer patients with potentially curative resection. Ann Surg Oncol. 2009;16(11):3028-3037. doi: 10.1245/s10434-009-0624-0. [DOI] [PubMed] [Google Scholar]
- 10.Bickenbach KA, Gonen M, Strong V, Brennan MF, Coit DG. Association of positive transection margins with gastric cancer survival and local recurrence. Ann Surg Oncol. 2013;20(8):2663-2668. doi: 10.1245/s10434-013-2950-5. [DOI] [PubMed] [Google Scholar]
- 11.Woo J-W, Ryu KW, Park JY, et al. Prognostic impact of microscopic tumor involved resection margin in advanced gastric cancer patients after gastric resection. World J Surg. 2014;38(2):439-446. doi: 10.1007/s00268-013-2301-5. [DOI] [PubMed] [Google Scholar]
- 12.Postlewait LM, Squires MH, 3rd, Kooby DA, et al. The importance of the proximal resection margin distance for proximal gastric adenocarcinoma: a multi-institutional study of the US Gastric Cancer Collaborative. J Surg Oncol. 2015;112(2):203-207. doi: 10.1002/jso.23971. [DOI] [PubMed] [Google Scholar]
- 13.Bissolati M, Desio M, Rosa F, et al. Risk factor analysis for involvement of resection margins in gastric and esophagogastric junction cancer: an Italian multicenter study. Gastric Cancer. 2017;20(1):70-82. doi: 10.1007/s10120-015-0589-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim BS, Oh ST, Yook JH, Kim HS, Lee IS, Kim BS. Appropriate gastrectomy resection margins for early gastric carcinoma. J Surg Oncol. 2014;109(3):198-201. doi: 10.1002/jso.23483. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Squires MH, Poultsides GA, et al. Impact of lymph node ratio in selecting patients with resected gastric cancer for adjuvant therapy. Surgery. 2017;162(2):285-294. doi: 10.1016/j.surg.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfeld JD, Wo JY, Mamon HJ, et al. The impact of positive margins on outcome among patients with gastric cancer treated with radiation. Am J Clin Oncol. 2016;39(3):243-247. doi: 10.1097/coc.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 17.Raziee HR, Cardoso R, Seevaratnam R, et al. Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric Cancer. 2012;15(1):116-124. doi: 10.1007/s10120-011-0112-7. [DOI] [PubMed] [Google Scholar]
- 18.Aurello P, Magistri P, Nigri G, et al. Surgical management of microscopic positive resection margin after gastrectomy for gastric cancer: a systematic review of gastric R1 management. Anticancer Res. 2014;34(11):6283-6288. [PubMed] [Google Scholar]
- 19.Postlewait LM, Maithel SK. The importance of surgical margins in gastric cancer. J Surg Oncol. 2016;113(3):277-282. doi: 10.1002/jso.24110. [DOI] [PubMed] [Google Scholar]
- 20.An JY, Kang TH, Choi MG, Noh JH, Sohn TS, Kim S. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg. 2008;12(8):1364-1369. doi: 10.1007/s11605-008-0516-9. [DOI] [PubMed] [Google Scholar]
- 21.Dicken BJ, Saunders LD, Jhangri GS, et al. Gastric cancer: establishing predictors of biologic behavior with use of population-based data. Ann Surg Oncol. 2004;11(6):629-635. doi: 10.1245/aso.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Johansson J, Djerf P, Öberg S, et al. Two different surgical approaches in the treatment of adenocarcinoma at the gastroesophageal junction. World J Surg. 2008;32(6):1013-1020. doi: 10.1007/s00268-008-9470-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim J-H, Jang Y-J, Park S-S, et al. Surgical outcomes and prognostic factors for T4 gastric cancers. Asian J Surg. 2009;32(4):198-204. doi: 10.1016/s1015-9584(09)60395-x. [DOI] [PubMed] [Google Scholar]
- 24.Samson S, Escovidal LA, Yrastorza SG, Veneracion RG, Nerves MY. Re-study of gastric cancer: analysis of outcome. World J Surg. 2002;26(4):428-433. doi: 10.1007/s00268-001-0243-9. [DOI] [PubMed] [Google Scholar]
- 25.Squires MH, 3rd, Kooby DA, Pawlik TM, et al. Utility of the proximal margin frozen section for resection of gastric adenocarcinoma: a 7-Institution Study of the US Gastric Cancer Collaborative. Ann Surg Oncol. 2014;21(13):4202-4210. doi: 10.1245/s10434-014-3834-z. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Z, Cai Z, Yin Y, et al. Impact of surgical margin status on the survival outcome after surgical resection of gastric cancer: a protocol for systematic review and meta-analysis. BMJ Open. 2020;10(11):e040282. doi: 10.1136/bmjopen-2020-040282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815-2834. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence-imprecision. J Clin Epidemiol. 2011;64(12):1283-1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence-indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol. 2011;64(12):1294-1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence-publication bias. J Clin Epidemiol. 2011;64(12):1277-1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311-1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence-study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407-415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Kien C, Gartlehner G, Kaminski-Hartenthaler A, et al. GRADE-Leitlinien: 9. Heraufstufen der Qualität der Evidenz. Z Evid Fortbild Qual Gesundhwes. 2013;107(3):249-255. doi: 10.1016/j.zefq.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Hunink MGM, Wong JB. Meta-analysis of failure-time data with adjustment for covariates. Med Decis Making. 1994;14(1):59-70. doi: 10.1177/0272989x9401400108. [DOI] [PubMed] [Google Scholar]
- 40.Jasmine Jakl R, Miholic J, Koller R, Markis E, Wolner E. Prognostic factors in adenocarcinoma of the cardia. Am J Surg. 1995;169(3):316-319. doi: 10.1016/s0002-9610(99)80166-4. [DOI] [PubMed] [Google Scholar]
- 41.Mariette C, Castel B, Balon JM, Van Seuningen I, Triboulet JP. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol. 2003;29(7):588-593. doi: 10.1016/s0748-7983(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 42.Bilici M, Tekin SB, Kandaz M, Cayir K, Erteki MV, Kiziltunc Ozmen H. The evaluation of the results of adjuvant chemoradiotherapy in patients with gastric cancer: results from a single center in eastern anatolia. Mide kanserli hastalarda adjuvan kemoradyoterapi sonuclari{dotless}ni{dotless}n degerlendirilmesi: Dogu anadolu'dan tek merkez sonuclari{dotless}. Turk J Med Sci. 2012;42(2):329-336. doi: 10.3906/sag-1003-708. [DOI] [Google Scholar]
- 43.Canyilmaz E, Soydemir G, Serdar L, et al. Evaluation of prognostic factors and survival results in gastric carcinoma: single center experience from Northeast Turkey. Int J Clin Exp Med. 2014;7(9):2656-2666. [PMC free article] [PubMed] [Google Scholar]
- 44.Koumarianou A, Krivan S, Machairas N, et al. Ten-year survival outcomes of patients with potentially resectable gastric cancer: impact of clinicopathologic and treatment-related risk factors. Ann Gastroenterol. 2019;32(1):99-106. doi: 10.20524/aog.2018.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Wang B, Huang G, et al. Overexpression of HER-2 protein is a high-risk factor for patients with surgically-resected stage T3 gastric adenocarcinoma. Clin Lab. 2017;63(1):115-125. doi: 10.7754/Clin.Lab.2016.160704. [DOI] [PubMed] [Google Scholar]
- 46.Kim MG, Lee J-H, Ha TK, Kwon SJ. The distance of proximal resection margin dose not significantly influence on the prognosis of gastric cancer patients after curative resection. Ann Surg Treat Res. 2014;87(5):223-231. doi: 10.4174/astr.2014.87.5.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts PO, Plummer J, Leake P-A, et al. Pathological factors affecting gastric adenocarcinoma survival in a Caribbean population from 2000-2010. World J Gastrointest Surg. 2014;6(6):94-100. doi: 10.4240/wjgs.v6.i6.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gierej P, Radziszewski J. Risk factors and survival of gastric cancer patients following curative stomach resection: analysis of a homogeneous population of patients in Warsaw, Poland. Viszeralmedizin. 2012;28(3):211-215. doi: 10.1159/000339333. [DOI] [Google Scholar]
- 49.Morgagni P, Garcea D, Marrelli D, et al. Resection line involvement after gastric cancer surgery: clinical outcome in nonsurgically retreated patients. World J Surg. 2008;32(12):2661-2667. doi: 10.1007/s00268-008-9747-x. [DOI] [PubMed] [Google Scholar]
- 50.Hallissey MT, Jewkes AJ, Dunn JA, Ward L, Fielding JWL. Resection-line involvement in gastric cancer: a continuing problem. Br J Surg. 2005;80(11):1418-1420. doi: 10.1002/bjs.1800801121. [DOI] [PubMed] [Google Scholar]
- 51.Hockey MS, Fielding JWL, Kelly KA. Resection line disease in stomach cancer. BMJ. 1984;289(6445):601-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cascinu S, Giordani P, Catalano V, Agostinelli R, Catalano G. Resection-line involvement in gastric cancer patients undergoing curative resections: implications for clinical management. Jpn J Clin Oncol. 1999;29(6):291-293. doi: 10.1093/jjco/29.6.291. [DOI] [PubMed] [Google Scholar]
- 53.Hong X, Li T, Ling F, et al. Impact of surgical margin status on the outcome of bladder cancer treated by radical cystectomy: a meta-analysis. Oncotarget. 2017;8(10):17258-17269. doi: 10.18632/oncotarget.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M-x, Bi X-y, Li Z-y, et al. Impaction of surgical margin status on the survival outcome after surgical resection of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Surg Res. 2016;203(1):163-173. doi: 10.1016/j.jss.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Raziee HR, Cardoso R, Seevaratnam R, et al. Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric Cancer. 2013;15(suppl 1):S116-S124. doi: 10.1007/s10120-011-0112-7. [DOI] [PubMed] [Google Scholar]
- 56.Mariette C, Castel B, Toursel H, Fabre S, Balon JM, Triboulet J-P. Surgical management of and long-term survival after adenocarcinoma of the cardia. Br J Surg. 2002;89(9):1156-1163. doi: 10.1046/j.1365-2168.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- 57.Papachristou DN, Agnanti N, D'Agostino H, Fortner JG. Histologically positive esophageal margin in the surgical treatment of gastric cancer. Am J Surg. 1980;139(5):711-713. doi: 10.1016/0002-9610(80)90369-4. [DOI] [PubMed] [Google Scholar]
- 58.Morgagni P, La Barba G, Colciago E, Vittimberga G, Ercolani G. Resection line involvement after gastric cancer treatment: handle with care. Updates Surg. 2018;70(2):213-223. doi: 10.1007/s13304-018-0552-2. [DOI] [PubMed] [Google Scholar]
- 59.Kim S, Karpeh MS, Klimstra DS, Leung D, Brennan MF. Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg. 1999;3(1):24-33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211043665 for Impact of Surgical Margin Status on Survival in Gastric Cancer: A Systematic Review and Meta-Analysis by Zhiyuan Jiang, Chunyu Liu, Zhaolun Cai, Chaoyong Shen, Yuan Yin, Xiaonan Yin, Zhou Zhao, Mingchun Mu, Yiqiong Yin and Bo Zhang in Cancer Control