Abstract
Despite widespread belief in the extinction burst as a common occurrence, relatively little empirical work has focused directly on the phenomenon. In order to provide additional data on the topic, we report re-analyses of published extinction-control groups from our laboratory following training with a variety of schedules and reinforcers. In addition, two prospective experiments were conducted in which rats responded for food on FR 5 or FR 1 schedules prior to a within-session transition to extinction. The results of these re-analyses and experiments suggest that the obtained prevalence of the extinction burst was considerably greater when response rates in the first minute of the transition to extinction were considered as compared to when session-wide response rates were considered. In addition, when reinforcement time was included in baseline response-rate calculations, the obtained prevalence of the extinction burst was higher than when reinforcement time was omitted. These findings highlight the importance of measurement and definitional issues in the obtained prevalence of the extinction burst. Further, a closer alignment of such issues across basic and applied research would be desirable in terms of the development of future theories describing the processes giving rise to the extinction burst.
Keywords: extinction, extinction burst, reinforcement time, consumption, ratio schedules
The procedure of operant extinction involves the discontinuation of reinforcement for a previously acquired response (e.g., Lattal et al., 2013). Although extinction can effectively reduce levels of behavior, its use in clinical settings has often been associated with undesirable side effects (e.g., Ducharme & Van Houten, 1994; Lerman & Iwata, 1996). One such effect is a transient increase in response rate that is purported to occur following the onset of extinction (i.e., the extinction burst). Although the extinction burst has been invoked frequently in the literature, there is a surprising lack of available empirical data on the phenomenon. Indeed, in their discussions of the extinction burst, numerous prominent textbooks show only hypothetical data (e.g., Cooper et al., 2020; Miltenberger, 2008), or no data whatsoever (e.g., Malott & Suarez, 2004; Martin & Pear, 2015, Miller, 2006). Despite the fact that the extinction burst is commonly described as a negative side effect of clinical extinction procedures (e.g., Cooper et al., 2020; Ducharme & Van Houten, 1994; Iwata et al., 1990; Lerman & Iwata, 1996; Lerman et al., 1996), only a handful of experiments have sought to study the phenomenon specifically. As such, the conditions potentially giving rise to the extinction burst remain poorly understood.
Existing data on the extinction burst have come most frequently from basic research that mentions the effect only in passing or from applied studies examining methods to prevent its occurrence. For example, several basic investigations with non-human subjects have reported extinction bursts following reinforcement on small fixed-ratio (FR) schedules (i.e., FR 1 to FR 5) using food (e.g., Niyuhire et al., 2007; Salamone et al., 1995; Ward et al., 2007), nicotine (Donny et al., 1995), or cocaine reinforcers (Schramm-Sapyta et al., 2006; Ward et al., 2009). None of these studies, however, examined factors governing the occurrence of the extinction burst. Conversely, some applied studies have shown that the extinction burst might be reduced or avoided when other treatments are used in conjunction with extinction. These treatments include antecedent manipulations (Fisher et al., 2018; Pace et al., 1993; Zarcone et al., 1993), non-contingent reinforcement (NCR; Vollmer et al., 1998), and differential reinforcement of alternative behavior (Lerman & Iwata, 1995; Lerman et al., 1999). In an examination of the prevalence of the extinction burst in clinical cases in which they defined an extinction burst as “…an increase in responding during any of the first three treatment sessions above that observed during all of the last five baseline sessions”, Lerman and Iwata (1995, p. 93) and Lerman et al. (1999) found evidence of the extinction burst in 35.7 – 62% of cases when extinction alone was used as a treatment. When extinction was combined with other treatments, they found that only 12 – 15% of cases showed an extinction burst.
The prevalence of the extinction burst has been mixed in the few basic studies where it has been specifically examined. For example, Harris et al. (2007) and Pushparaj et al. (2012), analyzed data from rats trained to self-administer nicotine according to small FR schedules (FR 3 and 5, respectively) and found that 52% and 38% of subjects, respectively, showed an extinction burst. In contrast, in a different condition Pushparaj et al. (2012) found that 100% of 31 additional rats trained on an FR 5 schedule showed extinction bursts when food had been the reinforcer. However, neither these studies nor the basic studies described above have used the definition of Lerman and colleagues. Instead, such basic examinations have defined the extinction burst in a variety of ways including increases in mean session-wide response rates (e.g., Niyuhire et al., 2007; Pushparaj et al., 2012; Ward et al., 2007; 2009), within-session inter-response time distributions (e.g., Salamone et al., 1995), or proportional and or numerical increases in response rate on a variety of different temporal scales (e.g., Donny et al., 1995; Harris et al., 2007; Katz & Lattal, 2020, Lattal et al., 2020; Pushparaj et al., 2012; Schramm-Sapyta et al., 2006). Further, many basic studies have also restricted their definitions of the extinction burst to only the first session of extinction (e.g., Donny et al., 1995; Harris, et al., 2007; Niyuhire et al., 2007; Pushparaj et al., 2012; Salamone et al., 1995; Schramm-Saptya et al., 2006; Ward et al., 2009).
A recent series of experiments by Lattal and colleagues (Lattal et al., 2020; Katz & Lattal, 2020) has shown that the prevalence of the extinction burst may change depending on how it is defined and measured. In Experiment 1, Lattal et al. (2020) examined the prevalence of the extinction burst in pigeons after training with high variable-ratio (VR) schedules (ranging from 60 – 120) and yoked interval schedules. In both Experiments 2 and 3 rats were trained according to variable-interval (VI) 30-s schedules prior to extinction. In all three experiments, there was no evidence of an extinction burst according to the definition used by Lerman and colleagues; however, when 1-minute bins of responding during extinction were compared to equal temporal intervals during baseline, there appeared to be relative increases in response rate for a small number of subjects. In Experiment 1, only 44 of 360 (12%) of data points pooled across the nine subjects were higher than during the corresponding baseline condition, and Experiments 2 and 3 revealed a prevalence of two of 20 (10%) each. Similarly, Katz and Lattal (2020) examined the likelihood of the extinction burst in pigeons following a history of reinforcement on a progressively increasing VR schedule (for which the terminal value was VR 20), a conventional VR 20 schedule, and an FR 1 schedule. Like Lattal et al. (2020), Katz and Lattal (2020) found no evidence of extinction bursts according to the definition of Lerman and colleagues, but when 1-min bins of responding were considered, the prevalence of transient increases in response rate during extinction increased to at most 39.5%. Likewise, a clinical study conducted by Woods and Borrero (2019) reported a small difference in the frequency of bursts during pediatric food refusal based on their analyses. When analyzing within-session response rates, four of ten patients showed an extinction burst, whereas only three of ten did so when session-wide response rates were considered.
An additional consideration raised by Katz and Lattal (2020, 2021) is that time associated with reinforcement consumption or other reinforcement-related behavior might contribute to the extinction burst. Such behavior would be expected to occupy time during baseline, but its elimination during extinction could lead to an apparent increase in response rates. Several earlier examinations of behavior following the implementation of extinction have also noted the potential effects of the removal of reinforcement-related time on response rates (e.g., Boren, 1961; Keller & Schoenfeld, 1950; Skinner, 1979, see also Katz & Lattal, 2021 for discussion). For example, Boren (1961) commented that response rates during conditioning with rats “…include the time to pick up the food pellet and perhaps to eat it” and further, that, “…the rate at the end of training may be due in part to the proportion of time occupied by food-getting behavior rather than differences in rate while responding” (p. 305). Although Boren’s (1961) experiment was not specifically concerned with the extinction burst, he also suggests that “Since no pellets [are] delivered during extinction, the initial rate during the first extinction session provides a rate measure which, although it may be complicated by other factors, is free of food-getting behavior” (p. 305).
The impact of the inclusion of reinforcement-related time on response rate calculations in applied research has been illustrated in some studies examining the effects of noncontingent-reinforcement (i.e., NCR). Both Carr et al. (1998) and Roscoe et al. (2003) showed that target response rates decreased as the magnitude of NCR increased. However, Roscoe et al. showed this was only the case when reinforcement consumption time was included in the time used to calculate overall response rates, as is customary in such applied studies. When reinforcement consumption time was excluded from response rate calculations, as is more typical in contemporary basic research (see Lattal, 1991 for discussion), response rates during the larger-magnitude condition were as high or higher than during their small-magnitude condition. When reinforcement consumption time is included in baseline response-rate calculations, larger or more frequent reinforcement might lead to reduced rates of target behavior as a result of an increase in allocation of the available time to reinforcement consumption and away from the target behavior (see also Fisher et al., 1999). With respect to the extinction burst, when all time including reinforcement-consumption time is included in baseline response-rate calculations, the elimination of reinforcement during extinction could result in a shift to relatively more of the overall available session time to target responding. Thus, it seems plausible that the inclusion of reinforcement time in baseline response rate calculations could make it more likely that an extinction burst would be observed. Nevertheless, despite apparent differences in how contemporary basic and applied researchers tend to treat reinforcement time in response-rate calculations, the potential impact of inclusion versus exclusion of such time on the extinction burst has never been examined directly in either domain.
In summary, based on the existing state of knowledge, it remains difficult to discern when one might reasonably expect an extinction burst to occur or not. Existing studies vary on many dimensions including, but not limited to, how the extinction burst is defined, baseline schedule of reinforcement, duration of baseline, session duration, reinforcer type, and the inclusion versus exclusion of reinforcement time in response-rate calculations. In summary, there appears to be a considerable need for additional empirical data related to the extinction burst. Thus, in order to further assess the prevalence of the extinction burst and to explore the above definitional and measurement issues further, we begin by re-examining previously published datasets from extinction control groups in our laboratory. This re-examination provided data from transitions to extinction for lever pressing of rats previously maintained by various schedules of reinforcement and by a variety of reinforcers.
Reanalysis of Existing Extinction Control Groups
The data came from 45 male Long-Evans rats that served as subjects in extinction-control groups in five different previously published experiments (Browning & Shahan, 2018; Craig et al., 2016, 2017; Craig & Shahan, 2016; Nall et al., 2018). Rats ranged from 71 to 140 days old at the start of their respective experiments, and all but five were experimentally naïve. Although there were many differences between these experiments, some of which are detailed in Table 1, all subjects included in the present analyses experienced a baseline condition in which lever pressing was reinforced according to some schedule of reinforcement and then extinguished. In Craig and Shahan (2016), food restricted rats earned single food pellets according to either a VI 15-s (n = 5) or VI 60-s (n = 5) schedule of reinforcement during baseline. For these rats, during reinforcement deliveries the chamber houselight and all other stimuli were turned off and the food aperture light was illuminated for 3 s. In Craig et al. (2017), food-restricted rats also earned single food pellets according to a VI 60-s schedule during baseline (n = 9), however, these rats experienced a reinforcement delivery period in which the feeder light was illuminated for 10 seconds while all other stimuli were turned off. In Browning and Shahan (2018), free-feeding rats (n = 11) earned dippers of 32% sucrose solution according to an FR 3 schedule in baseline. During reinforcement deliveries, all stimuli were turned off and the dipper aperture light was illuminated for 4 s. Lastly, in Craig et al. (2016) and Nall et al. (2018) food restricted rats (n = 15) earned 0.3 mg/kg intravenous infusions of cocaine hydrochloride according to a VR 20 schedule in baseline. During cocaine infusions, all stimuli were turned off and the chamber remained in a blackout for 45 s. In all experiments, stimulus conditions in the chamber remained unchanged with the transition to extinction, but lever pressing had no effect.
Table 1.
Summary of relevant experimental parameters for each study included in reanalysis of datasets.
| Study | n | Food restriction | Reinforcer | Schedule | Reinforcement time (s) | Session time (min) |
|---|---|---|---|---|---|---|
| Craig & Shahan, 2016 | 5 | 80% | food | VI 15 s | 3 | 30 |
| Craig & Shahan, 2016 | 5 | 80% | food | VI 60 s | 3 | 30 |
| Craig et al., 2017 | 9 | 80% | food | VI 60 s | 10 | 30 |
| Craig et al., 2016 | 5 | 80% | cocaine | VR 20 | 45 | 45 |
| Nall et al., 2018 | 10 | 80% | cocaine | VR 20 | 45 | 45 |
| Browning & Shahan, 2018 | 11 | free feed | sucrose | FR 3 | 4 | 25 |
First, data from these studies were analyzed for the prevalence of the extinction burst at the session-wide level based on the definition provided by Lerman and colleagues. Specifically, response rates during the first three extinction sessions were compared to the maximum of response rates during the final five baseline sessions. Further, due to the possibility that reinforcement consumption time may impact the prevalence of the extinction burst, we also analyzed response rates during baseline sessions when reinforcer delivery time was included versus excluded in baseline response-rate calculations. When reinforcement delivery time was included, any responses made to the target lever during the reinforcer delivery period were also included in the rate calculation. Second, given the findings of previous authors showing transitory rate increases during extinction on smaller, within-session timescales (e.g., Donny et al., 1995; Harris et al., 2007; Lattal et al., 2020; Katz & Lattal, 2020; Pushparaj et al., 2012; Salamone et al., 1995; Woods & Borrero, 2019), we also compared responding in the first minute of each of the last five baseline sessions to responding in the first minute of the first three extinction sessions. Following the analyses of Katz and Lattal (2020), in both the session-wide and 1st-minute analyses, we also considered both the means and maximums of response rates in the final five baseline sessions, both with and without reinforcement delivery time included in the rate calculations.
Figure 1 shows the results of these re-analyses and gives the prevalence (% of rats) of extinction bursts when both session-wide and 1st-minute response rates were considered. The left and right columns show session-wide and 1st-minute prevalence, respectively. The prevalence when response rates were calculated without reinforcement time during baseline are depicted by the white bars and the prevalence with all reinforcement time and responses included are depicted by the shaded bars. In nearly all cases, whether based on session-wide or 1st-minute responding, and regardless of whether reinforcement consumption time was included in rate calculations or not, the prevalence of the extinction burst was greater when extinction responding was compared to baseline means rather than the maximum baseline rate. Extinction burst prevalence was also greater in most cases when only the 1st-minute rate of responding was considered rather than session-wide responding. Finally, when reinforcement time was included in response rate calculations the prevalence was greater than when this time was excluded from rate calculations for all cases except the VI 15 group from Craig and Shahan (2016). Further, of the two groups of rats trained on a VI 60-s schedule in baseline (Craig et al., 2017; Craig & Shahan, 2016), the group with the longer reinforcement time (i.e., 10 s versus 3 s) showed a greater prevalence when reinforcement time was included.
Figure 1.
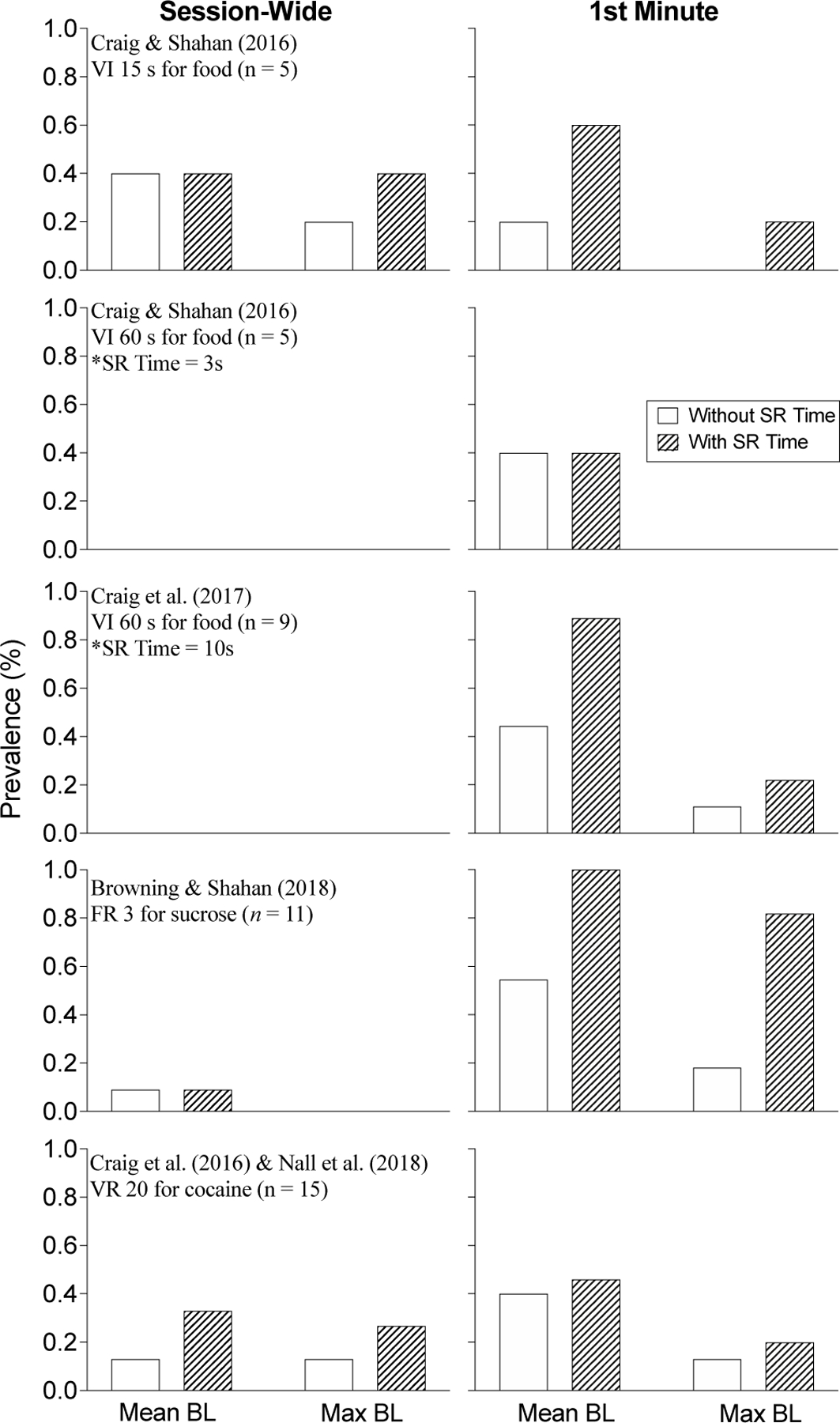
The prevalence (% of subjects) of the extinction burst from reanalysis of extinction control groups in previously published datasets. Left and right columns display the prevalence of bursts when response rates during extinction were calculated for the full session and in the 1st minute of each session only, respectively. White bars show prevalence when reinforcement consumption time was omitted from baseline rate calculations. Shaded bars show prevalence when reinforcement consumption time and responses during food were included in rate calculations.
In summary, the most stringent analysis (i.e., the maximum session-wide rate with reinforcement consumption time excluded from the last five baseline sessions) revealed a total prevalence of three of 45 (6.67%) rats across all five datasets. Conversely, the most lenient analysis (i.e., the mean 1st-minute rate with reinforcement consumption time included) revealed a total prevalence of 31 of 45 (68.9%). These analyses further highlight how measurement and definitional decisions can considerably change the overall obtained prevalence of the extinction burst. Although there was relatively little evidence for the extinction burst at the session-wide level, prevalence was generally greater following all training conditions if response rates during only the first minute of exposure to extinction was considered. Further, the inclusion of reinforcement time in response rate calculations in baseline always increased the obtained prevalence of the extinction burst. In our reanalysis, we found the highest prevalence of the extinction burst following training on an FR 3 schedule (from Browning & Shahan, 2018), regardless of the measurement approach, but especially when reinforcement time was included in baseline calculations. It is noteworthy that the rats in Browning and Shahan were the only rats in the reanalysis that were not food restricted and that were responding for a sucrose solution. Nevertheless, the relatively higher prevalence of the extinction burst for rats responding on a lower FR schedule is consistent with Pushparaj et al. (2012) who reported that 100% of their 31 rats trained with an FR 5 for food showed an extinction burst based within-session responding (i.e., the first five minutes only) compared to mean rates in a similar period across the final three baseline sessions. Notably, Pushparaj et al. included a very long (i.e., 1 min) reinforcement consumption-time blackout in their baseline response-rate calculations which may have impacted their assessment of extinction burst prevalence. Given these considerations with both Browning and Shahan (2018) and Pushparaj et al. (2012), Experiment 1 was designed to further examine the prevalence of the extinction burst based on the variety of definitional and measurement approaches described in the reanalysis above with food-restricted rats following training on a FR 5 schedule for food.
EXPERIMENT 1
The purpose of this experiment was to examine the prevalence of the extinction burst following baseline training according to an FR 5 schedule. In our re-analyses, we found that the group trained with an FR 3 schedule showed the highest prevalence of any of the groups in the first minute of extinction when reinforcement delivery time was included in the baseline rate calculations. Thus, we examined the extinction burst both including and excluding reinforcement time in baseline response rate calculations. Also, as in the re-analyses above, both the mean and maximum baseline response rates were used as the comparator in analyses of prevalence of the extinction burst at both the whole-session and first-minute level of analysis. Further, in order to avoid the impact of any potential systematic differences in response rates at the beginning of sessions (e.g., see McSweeney & Roll, 1993) on the appearance of the extinction burst (see Katz & Lattal, 2021, for discussion), a within-session transition to extinction was used.
Method
Subjects
Ten experimentally naïve male Long-Evans rats (Charles River, Portage, MI) were used. Two rats were excluded due to pellet dispenser failures during the baseline phase. Rats were 71–90 days old upon arrival and were maintained at 80% of their free-feeding weights. Rats were individually housed with free access to water in a temperature-controlled colony room with a 12:12 hour light/dark cycle (lights on at 7:00 AM). Care of animals and all procedures were approved by Utah State University’s Institutional Animal Care and Use Committee.
Apparatus
Ten identical modular Med Associates (St. Albans, VT) operant chambers were used in the experiment. These chambers measured 30 cm X 24 cm X 21 cm and were housed in sound and light attenuating cubicles. Each chamber had aluminum panels on the front and back walls, with Plexiglas walls on each side and the ceiling. On the front panel of each chamber were two retractable levers with stimulus lights above them. Levers were positioned equidistantly on either side of a centered food pellet receptacle that was illuminated with deliveries of 45-mg food pellets (Bio Serv, Flemington, NJ). A house light on the opposite end of the chamber was used for general illumination. The timing of experimental events and data collection were controlled by Med-PC IV (Med Associates) software run on a computer in an adjacent control room.
Procedure
All experimental sessions took place once daily at approximately the same time each day during the light cycle, seven days per week. With the exception of magazine training and extinction sessions, all sessions ended after a specified number of reinforcers were earned to control for the number of reinforcers earned across subjects.
Training.
Prior to the start of operant sessions, rats were trained to consume food pellets from the food aperture for three, 30-min magazine training sessions. During these sessions, the levers were retracted, and the house light and stimulus lights remained off. Food pellets were delivered response-independently according to a variable-time (VT) 60-s schedule with 10 intervals generated by the constant-probability progression of Fleshler and Hoffman (1962). Each food delivery consisted of a single pellet accompanied by an audible click and illumination of the feeder for 3 s. Following the final session of magazine training, operant training sessions began. Sessions during this phase began with illumination of the house light and insertion of a response lever (left-right counterbalanced across subjects), with the stimulus light above it also illuminated. Food pellets were delivered at first according to an FR 1 schedule such that each response made to the lever in the presence of the lever stimulus light was reinforced. During pellet delivery, both the house light and the lever stimulus light were darkened and the food aperture was illuminated for 3 s (i.e., reinforcement time). Any responses made to the lever during this time were recorded but had no consequences. On the first day of training rats were allowed to earn 50 food pellets before the session terminated. This requirement, as well as the ratio requirement, was increased each day such that by the fifth and final day of training all rats were allowed to earn 125 food pellets according to an FR 5 schedule of reinforcement before the session terminated.
Baseline.
Following the final training session, baseline sessions began. As in training, the lever was inserted and the house light and the light above the lever were illuminated. Responses to the lever produced food pellets according to an FR 5 schedule of reinforcement. Each baseline session continued until a rat had earned 150 food pellets. This phase lasted for 15 sessions.
Extinction.
As in baseline, all sessions during this phase began with illumination of the house light and lever stimulus light as well as insertion of the lever. The first extinction session was unique in that during the initial part of the session rats were allowed to earn food pellets according to the FR-5 schedule just as during baseline sessions. Extinction started after the delivery of the 25th reinforcer and continued for 10 minutes following the last reinforcer delivery (the approximate duration of baseline sessions). All subsequent extinction sessions were 10 minutes and lever presses had no consequences throughout the entire session. Including the first extinction session with the within-session transition, this phase lasted for 12 sessions.
Results
Figure 2 shows session-wide response rates for individual subjects in the final five baseline sessions and the first three sessions of extinction. Because the first extinction session began in the same manner as baseline sessions until 25 reinforcers were earned, all responses and time before the first 25 reinforcers were excluded from response-rate calculations in both baseline and the first extinction session. The black data paths represent response rates calculated excluding the 3-s reinforcement delivery period, and the white data paths represent response rates when calculated both with reinforcement time included, as well as any responses made to the lever during this period. The solid black and dotted horizontal lines show mean baseline response rates without or with reinforcement time included, respectively. Zero of the eight rats showed a session-wide increase in lever pressing upon the transition to extinction relative to their response rates during baseline, regardless of the inclusion versus exclusion of reinforcement delivery time or whether or mean or maximum response rates were used as the relevant comparison.
Figure 2.
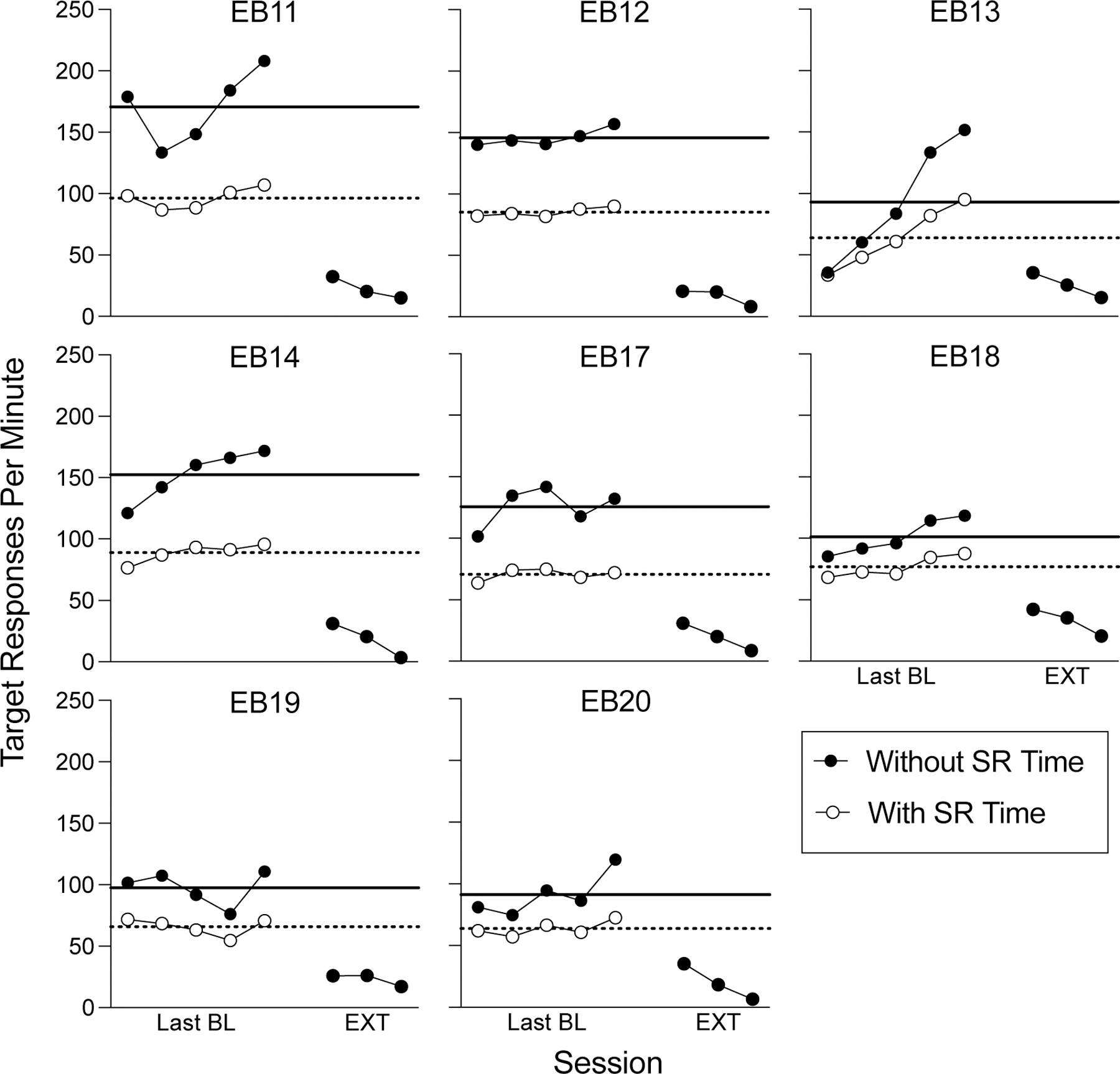
Session-wide resp/min as a function of baseline sessions of FR-5 reinforcement and the first three extinction sessions in Experiment 1. The black data paths show response rates when the 3-s blackout periods during reinforcer deliveries were omitted. The white data show response rates when food delivery time as well as any responses made to the lever during the 3-s blackout period were included in the rate calculation. The solid black and dotted horizontal lines show the mean response rate without or with reinforcement time included, respectively. All data points exclude the first 25 reinforcers from each session, with the exception of extinction days 2 and 3, where no reinforcers were present throughout the entirety of the session.
Figure 3 shows response rates for the 1st-minute of each of the final five baseline sessions and in the first three extinction sessions. All details of the figure are as in Figure 2, with the exception that individual data points are representative of the 1st minute of responding subsequent to the first 25 reinforcer deliveries (including for baseline sessions). Based on this analysis, during the first extinction session two of eight rats (EB18, EB20) showed an increase in response rate relative to their respective mean rate during the last five baseline sessions when reinforcement time was excluded from the calculation of baseline response rates. This number increased to five of eight rats (EB11, EB13, EB17, EB18, EB20) when reinforcement time was included in baseline response rate calculations. When maximum baseline rates were used as the relevant comparison, only one of eight rats (EB18) showed a small increase in response rates when reinforcement time was excluded, and this number increased to three of eight (EB11, EB17, EB18) when reinforcement time was included.
Figure 3.
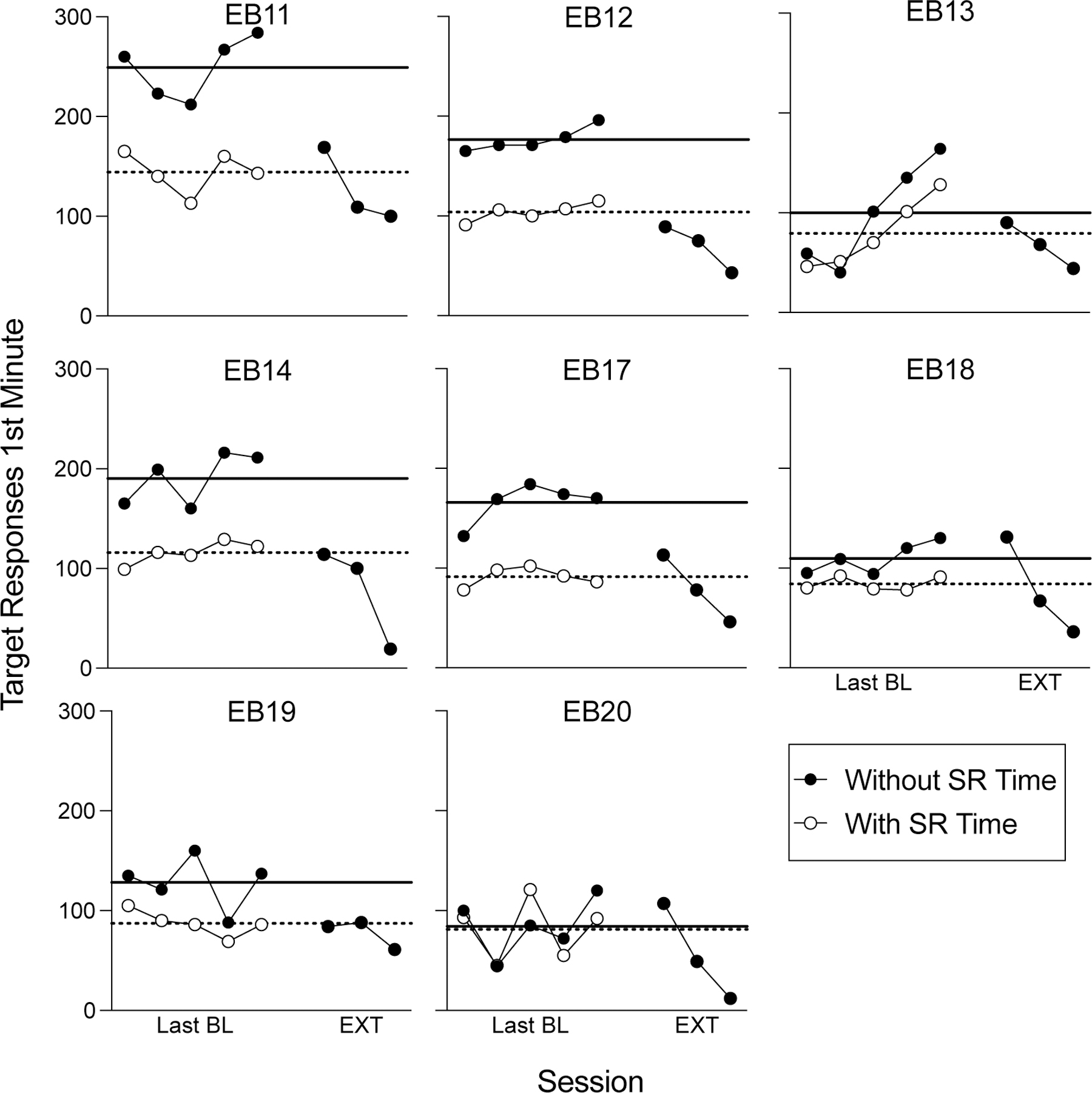
1st-minute responses as a function of baseline sessions of FR-5 reinforcement and the first three extinction sessions in Experiment 1. The black data paths show response rates when the 3-s blackout periods during reinforcer deliveries were omitted. The white data show response rates when food delivery time as well as any responses made to the lever during the 3-s blackout period were included in the rate calculation. The solid black and dotted horizontal lines show the mean response rate without or with reinforcement time included, respectively. All data points exclude the first 25 reinforcers from each session, with the exception of extinction days 2 and 3, where no reinforcers were present throughout the entirety of the session.
Discussion
The purpose of this experiment was to generate additional data assessing the prevalence of the extinction burst after training with a low-value FR schedule and to further examine the impact of measurement considerations on its prevalence. On a session-wide level, zero of eight rats showed an increase in response rate following the implementation of extinction within-session, regardless of whether reinforcement consumption time was included in response rate calculations or not and whether or not baseline mean or maximum response rates were used as the relevant comparison. As in previous studies, we saw more evidence of an extinction burst when responding during only the first minute following the transition to extinction was considered (Katz & Lattal, 2020; Lattal et al., 2020; Woods & Borrero, 2019). Our analyses also compared the difference between within-session analyses when calculated with or without reinforcer consumption time included and showed that inclusion of this time in baseline response rate calculations can increase the apparent prevalence of an extinction burst in the first minute of exposure to extinction.
However, despite the fact that both studies employed an FR-5 schedule of food reinforcement during baseline, there remains a discrepancy in prevalence between the present experiment and the experiment conducted by Pushparaj et al. (2012). An extinction burst was defined by Pushparaj et al. as “…responding during the first 5-min interval of the first extinction session being greater than the first 5-min intervals of the last three self-administration sessions” (p. 425). Our most similar level of analysis (i.e., mean 1st-minute rate from the final five baseline sessions when reinforcement time was included) revealed a prevalence of 62.5%, whereas Pushparaj et al. reported a prevalence of 100%. Notably, Pushparaj et al. included a 1-min blackout period following each reinforcer delivery (as compared to 3 s here) and included this time in their baseline response-rate calculations. However, Pushparaj et al. did not examine response rates without inclusion of reinforcement time, so its potential contribution to their obtained prevalence remains unknown.
The present findings are consistent with those of Lattal and colleagues and provide additional support for the notion that the extinction burst may occur relatively infrequently (especially at a session-wide level) with intermittent reinforcement schedules. However, the possibility exists that the extinction burst may be more common after training on FR 1 schedules, as such schedules are most commonly associated with cursory mentions of the extinction burst in both basic research (e.g., Katz & Lattal, 2020, Schramm-Sapyta et al., 2006, Ward et al., 2007, 2009) and clinical settings (e.g., Crossman et al., 2009; Goh & Iwata, 1994; Iwata et al., 1990; Pace et al., 1993; Zarcone et al., 1993). Thus, Experiment 2 examined the prevalence of the extinction burst following training on an FR-1 schedule using procedures and analyses like those employed in Experiment 1.
EXPERIMENT 2
The weight of the currently available evidence suggests that the extinction burst may be most prevalent following training on an FR-1 schedule (e.g., Katz & Lattal, 2020, Salamone et al., 1995; Schramm-Sapyta et al., 2006; Ward et al., 2007, 2009). Further, FR 1 schedules were the most commonly used schedule included in the extinction burst re-analyses conducted by Lerman and colleagues (see Lattal et al., 2020; Katz & Lattal, 2020). Nevertheless, only one study to date has specifically examined the effects of extinction following FR 1 reinforcement with the goal of assessing the prevalence of the extinction burst. Katz and Lattal (2020) found little to no evidence of the extinction burst with pigeons following FR-1 reinforcement on a session-wide level. In contrast, when 1-minute bins of responding were compared across baseline and extinction conditions they found the prevalence of the extinction burst to be as high as 24.2%. However, Katz and Lattal’s experiment only included a sample of five pigeons (one of which had previous exposure to the experimental conditions). Further, neither they nor any other study has specifically examined the impact of inclusion of reinforcement time in baseline response rate calculations on the extinction burst following FR-1 reinforcement. Because consummatory behavior occurs following each response on an FR-1 schedule, such reinforcement consumption time may have a relatively greater suppressive effect on baseline response rates than when more intermittent reinforcement schedules are arranged in baseline (see also Katz & Lattal, 2021 for discussion). Therefore, Experiment 2 was designed to be a partial replication of the FR-1 reinforcement condition in Katz and Lattal (2020) with a larger sample size using naïve rats as subjects. The variety of definitional and measurement approaches used in our reanalysis and in Experiment 1 were also employed here.
Method
Subjects and Apparatus
Ten experimentally naïve male Long-Evans rats (Charles River, Portage MI) were used in the experiment. Rats were 71–90 days old upon arrival and were maintained under the same conditions as described in Experiment 1. Ten modular Med Associates (St. Albans, VT) operant chambers identical to those described in Experiment 1 were used.
Procedure
All rats first underwent three sessions of magazine training, and then were transitioned into operant training sessions. As in Experiment 1, rats were given five days of training where the amount of food required to terminate the session increased from 50 pellets the first day to 125 pellets on the final day of training. The only procedural difference from the previous experiment was that instead of increasing the ratio requirement each day during training, responding was reinforced according to an FR 1 schedule throughout all of the baseline phase. All other procedures including the within-session transition to extinction were identical to those used in Experiment 1.
Results
Figure 4 shows response rates for individual subjects in the final five baseline sessions and the first three sessions of extinction. Response rates for all sessions (with the exception of extinction sessions two and three) also exclude the first 25 reinforcers and time to earn them as described for Experiment 1. As for the figures in Experiment 1, the black data paths represent response rates when calculated without the 3-s blackout period during food deliveries, and the white data paths represent response rates when calculated both with the 3-s reinforcement time and any responses during that time. The solid black and dotted lines represent the mean baseline rates of these two data paths, respectively. As in Experiment 1, zero of the ten rats showed a session-wide increase in responding upon the transition to extinction relative to their response rates during baseline, regardless of whether mean or maximum baseline response rates were used as the comparator and regardless of whether or not reinforcement time was included in calculations.
Figure 4.
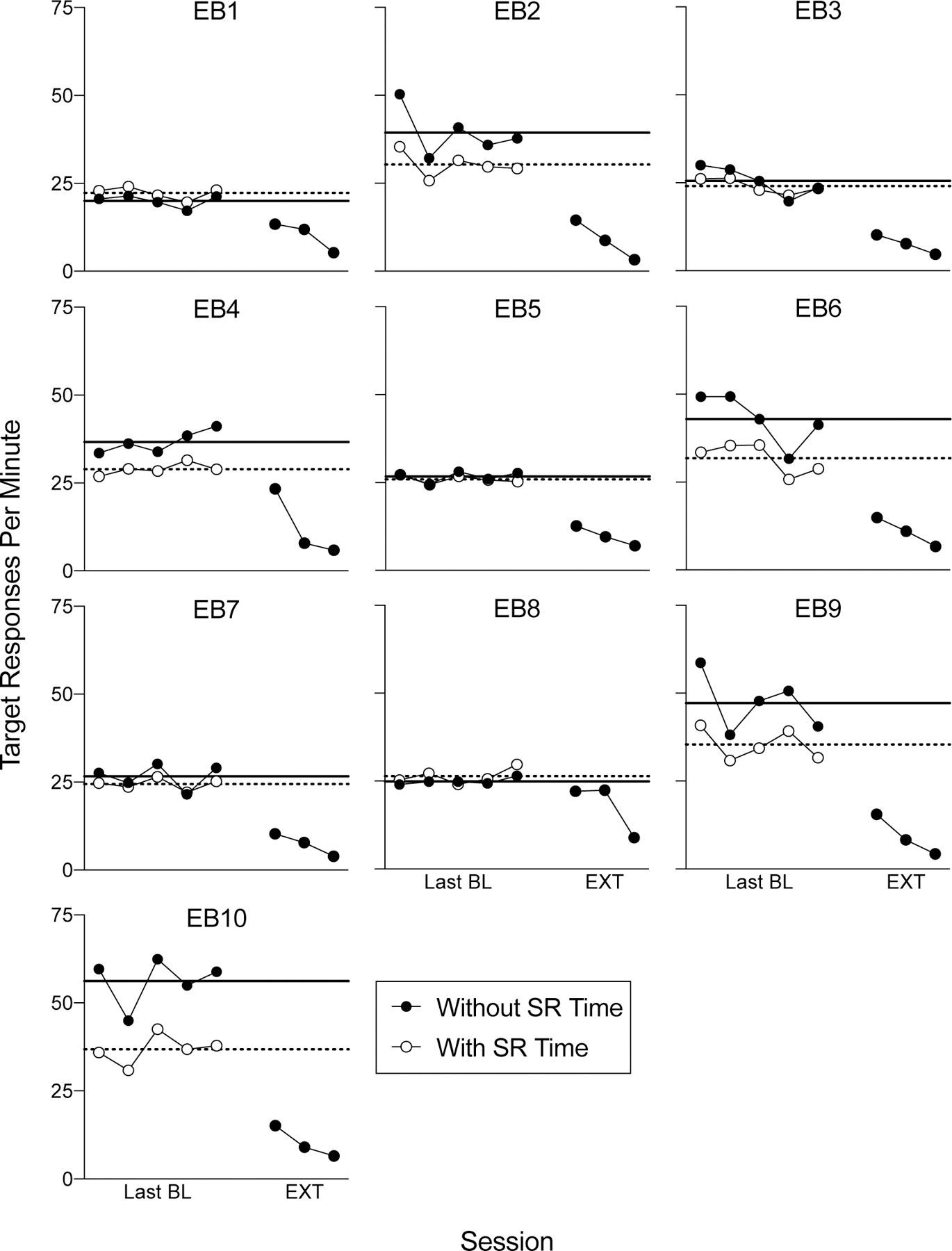
Session-wide resp/min as a function of sessions of FR-1 reinforcement and the first three extinction sessions in Experiment 2. All other details are as in Figure 2.
Figure 5 shows response rates for the first minute following the transition to extinction (i.e., starting immediately after the 25th reinforcer delivery). For the final five baseline sessions, comparable data are presented for the first minute following the 25th reinforcer delivery. All details in the figure are the same as in Figure 4, except that only response rates from the first minute are shown. Based on this analysis, five of ten rats (EB1, EB2, EB4, EB5, and EB8) exceeded their mean baseline 1st-minute response rate when reinforcement time was excluded from rate calculations. But, response rates for EB2 and EB5 were only marginally higher than the baseline mean and only in the first session of extinction. When reinforcement consumption time was included in rate calculations, response rates for nine of ten rats exceeded the mean baseline rate in one or more session of extinction (only rat EB6 was the exception). When maximum baseline response rates were used as the comparator and calculation of response rates excluded reinforcement time, three of ten rats (EB1, EB4, and EB8) showed an increase in responding in the first minute. When reinforcement consumption time was included in baseline rate calculations, five of ten rats (EB1, EB2, EB4, EB8, and EB9) showed an increase in response rates above the maximum baseline rate.
Figure 5.
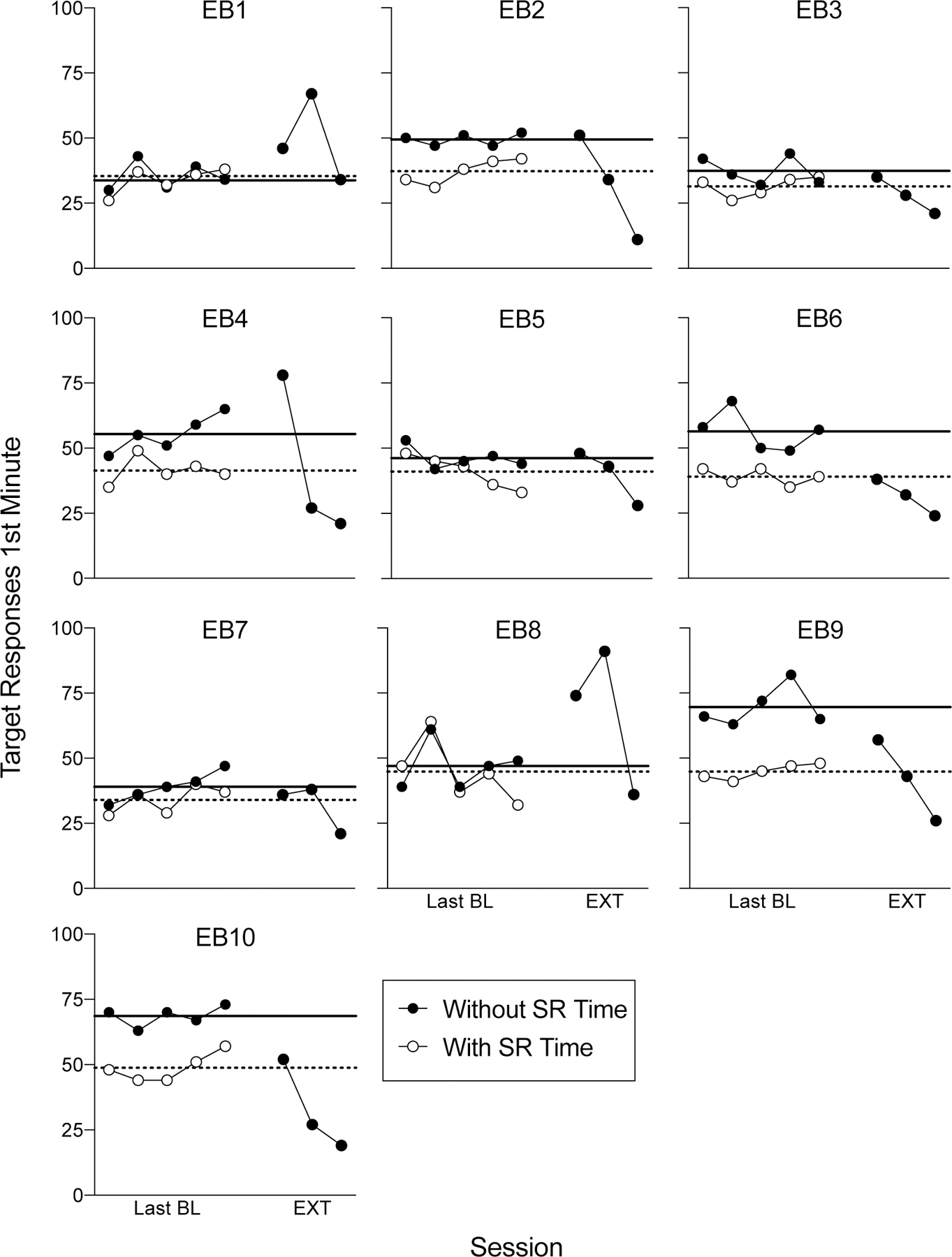
1st-minute responses as a function of sessions of FR-1 reinforcement and the first three extinction sessions in Experiment 2. All other details are as in Figure 3.
Discussion
The purpose of this experiment was to examine the prevalence of the extinction burst following baseline training on an FR-1 schedule. Similar to Experiment 1, there was no session-wide increase in responding above maximum nor mean baseline response rates upon the transition to extinction for any of the ten rats, regardless of whether reinforcement time was included in response rate calculations or not. When only the first minute of responding following the transition to extinction was considered, our analyses showed an increase in response rates relative to the mean baseline rate for five of ten or nine of ten rats, depending on whether or not reinforcement time was excluded or included in rate calculations, respectively. Further, when reinforcement time was excluded from baseline response rate calculations, response rates for three of ten rats in the first minute of extinction exceeded that their maximum baseline first-minute rates. With reinforcement time included, five of ten rats exceeded their maximum 1st-minute baseline rate.
This experiment further emphasizes the importance of taking into consideration how the definition of an extinction burst (i.e., an increase relative to mean or maximum baseline response rate and the timeframe over which response rates are examined) may contribute to its reported prevalence. In addition, this experiment further directly highlights how the inclusion or exclusion of reinforcer consumption time in baseline response rates may affect conclusions about whether or not an extinction burst occurs. These data correspond with the accounts of previous authors with respect to the distinctive qualities of FR-1 reinforcement schedules (e.g., Boren, 1961; Katz & Lattal, 2020, 2021; Keller & Schoenfeld, 1950; Skinner, 1979). Because reinforcer consumption time occurs following every response on an FR 1 schedule, the removal of such time has the potential to affect response rates relatively more than for intermittent-schedule arrangements.
General Discussion
The present experiments and reanalysis of previously published datasets were intended to further examine the prevalence of the extinction burst. Our re-analyses of previously published data from extinction control groups in our laboratory (Browning & Shahan, 2018; Craig et al., 2016, 2017; Craig & Shahan, 2016; Nall et al., 2018) highlighted two important considerations with respect to the obtained prevalence of the extinction burst. First, the definition of the extinction burst (i.e., session-wide versus first-minute increases in response rate) may impact its prevalence. As illustrated in Figure 1, in nearly all cases the prevalence of the extinction burst was greater when 1st-minute response rates during baseline and extinction conditions were compared relative to session-wide response rates. Second, when reinforcement time was included in response rate calculations, the prevalence of the extinction burst was greater than the prevalence when reinforcement time was omitted. Consistent with these findings, both variables had a similar impact on the prevalence of the extinction burst in Experiments 1 and 2.
Figure 6 shows a summary of the prevalence of the extinction burst obtained in Experiments 1 and 2 and how it depended on definitional and measurement decisions. The two upper and lower panels show Experiment 1 (FR 5) and Experiment 2 (FR 1), respectively, and the left and right panels show extinction burst prevalence according to session-wide and 1st-minute response rates, respectively. Lastly, the white and shaded bars depict extinction burst prevalence without or with reinforcement consumption time included in response rate calculations, respectively. Neither experiment showed evidence of the extinction burst at the session-wide level of analysis, regardless of definitional and measurement considerations. Conversely, when responding during only the first minute following the transition to extinction was considered, the overall prevalence increased for both experiments. Further, the prevalence of the extinction burst was greater when mean response rates from the final five baseline sessions were used as the comparator rather than the maximum rate from these sessions. Finally, when reinforcement time was included in rate calculations, the prevalence of the extinction burst was always greater than when it was excluded from such calculations. Overall, these findings correspond with previous research suggesting that the extinction burst is likely to be most apparent based on samples of time smaller than an entire session (e.g., Donny et al., 1995; Harris et al., 2007; Katz & Lattal., 2020; Lattal et al., 2020; Pushparaj et al., 2012; Salamone et al., 1995; Schramm-Sapyta et al., 2006; Woods & Borrero, 2019). Thus, when it occurs, the extinction burst appears to be a relatively short-lived phenomenon.
Figure 6.
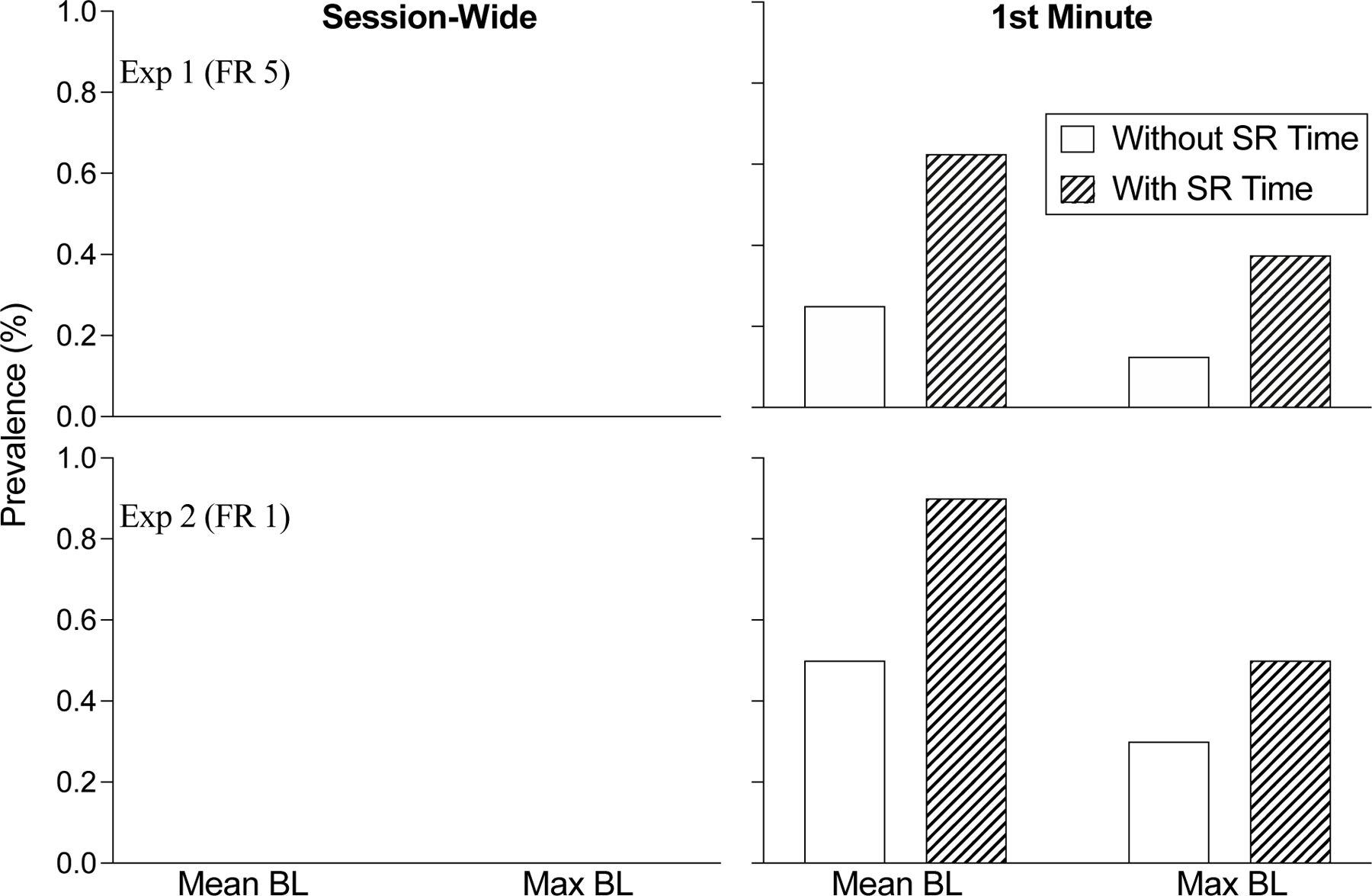
The prevalence (% of subjects) of the extinction burst from Experiment 1 (FR 5) and Experiment 2 (FR 1). Left and right columns display the prevalence of bursts when response rates during extinction were calculated for the full session and in the 1st minute of each session only, respectively. White bars show prevalence when reinforcement consumption time was omitted from baseline rate calculations. Shaded bars show prevalence when reinforcement consumption time and responses during food were included in rate calculations.
Also consistent with previous research is the finding that the extinction burst is more prevalent when response rates during extinction are compared to mean rather than maximum baseline response rates. For example, Katz and Lattal (2020) found the prevalence of extinction bursts to be 38% when extinction responding was compared to the average of the final five baseline sessions, but only 22% when compared to the maximum response rate from these same sessions. This raises the question of whether an increase in response rate during extinction above the average baseline rate is meaningful given the variability in response rate across baseline sessions (see Katz & Lattal, 2021 for discussion). In our opinion, an increase in response rates that does not exceed the variability in a representative sample of baseline responding is less likely to represent a meaningful change in behavior as compared to an increase the exceeds the maximum response rate obtained from the same sample. Nevertheless, even if maximum baseline response rates are to be used as the comparator, questions remain about how many baseline sessions should be used in the comparison and by how much the maximum rate must be exceeded.
Although in clinical examinations an extinction burst has been defined only on a session-wide level (e.g., Lerman & Iwata, 1995; Lerman et al., 1999), it seems difficult to argue that only more prolonged periods of an increase in response rate should be deemed relevant. Take for example, an instance in which there is patient referred to treatment for severe self-injurious behavior (SIB). At the beginning of the first extinction session, this patient experiences a bout of SIB lasting for only one minute before decreasing to low levels, but the entire treatment session lasts for ten minutes. Given this scenario, it is likely that the rapid rate of SIB at the onset of the session would be washed out in time, and thus would not satisfy the criteria to consider it an extinction burst according to a session-wide definition. However, is such an episode of SIB clinically relevant only if it meets some predetermined and arbitrary criterion, or are all instances of SIB relevant irrespective of their duration? Further, when it comes to questions of the prevalence of the extinction burst, it seems differences in definitional criteria have only served to make things more confusing and disjointed in the experimental literature. Recently, Katz and Lattal (2021) argued: “…the pursuit of a single, uniform, and precise definition of the extinction burst is misguided” (p. 10), and we agree. This is because, as the authors rightly note, the extinction burst can only be defined in the context of other variables such as baseline reinforcement conditions, how and when extinction conditions are implemented, and measurement considerations like the temporal window over which response rates are calculated and compared across conditions. The term extinction burst, is descriptive rather than technical, which leaves the definition of such a term open to debate (Vollmer, 2001). Although such definitions may allow one to point to an example of an extinction burst when it occurs, they offer little help in furthering our understanding of the phenomenon. As Staddon (1993) noted: “…if we do not know the law, we have no business being rigid about verbal definitions! Insisting on a term before we have an accepted theory is putting the cart before the horse” (p. 441). Thus, we suggest that a more systematic and thorough program of investigation will be needed before it is possible to provide a meaningful definition of the extinction burst. Such a program should be directed at revealing the conditions that do and do not give rise to temporary increases in responding with the onset of extinction and should strive for a process-oriented and theoretically grounded definition of the phenomenon.
Another issue highlighted by our analyses is how the inclusion versus exclusion of reinforcement time in baseline response rate calculations affects the prevalence of the extinction burst. As noted above, at least in contemporary basic research, this time is typically excluded from response rate calculations (e.g., see Doughty & Richards, 2002; Lattal, 1991; Mazur, 1983). In contrast, applied research typically includes all time, including reinforcement consumption time in response rate calculations (e.g., see Carr et al., 1998; Fisher et al., 1999; Roscoe et al., 2003). As illustrated by our analyses, the decision to include or exclude this time in baseline response rate calculations can change the likelihood that one concludes that an extinction burst has occurred or not. Although we have focused on reinforcement consumption time above, it should be noted response rates in baseline conditions might be reduced by any number of activities or effects related to reinforcer deliveries or their aftereffects. For example, Katz and Lattal (2020, Experiment 2) found that including post-reinforcement pauses associated with a VR 20 schedule in response rate calculations served to decrease baseline response rates and increase the obtained prevalence of the extinction burst. This outcome led the authors to conclude such “…increases in response rate during extinction that classically have been referred to as extinction bursts in fact may be artifacts of the absence of reinforcer deliveries during extinction” (p. 33). Although one could certainly view response rate increases from a baseline rate that is “suppressed” as a result of consumption or otherwise engagement with a reinforcer or its aftereffects as artifactual, we are becoming convinced otherwise given real-world and clinical considerations1. For example, it is likely that reinforcement of a child’s potentially dangerous problem behavior by caregivers results from the fact that access to the reinforcer serves to at least temporarily suppress the problem behavior while the child is engaged with the reinforcer (e.g., access to an iPad). Although problem behavior may be suppressed by consumption/engagement with the reinforcer when it is present, the future likelihood of the problem behavior is also likely increased as a result of the contingency between problem behavior and access to the reinforcer. When extinction is later implemented for the problem behavior, the contingency between the problem behavior and access to the reinforcer is broken and, in the longer run there will likely be a reduction of problem behavior. However, in the shorter run, the suppressive effect of consuming/engaging with the reinforcer is removed and problem behavior is likely to temporarily increase when considered with respect to actual clock time. From a clinical perspective, even temporary increases in potentially dangerous behavior are no artifact, and both clinical interventions and theoretical treatments of the extinction burst should take them into account. Inclusion of all available time and responses in a pre-extinction baseline in the basic laboratory might permit a closer alignment of basic research and theories with such real-world and clinical considerations.
A final question raised by the present data is what gives rise to the variability in the occurrence of the extinction burst across individual subjects? For example, in Experiment 2 where reinforcement was arranged on an FR-1 schedule, even at the first-minute level of analysis with reinforcement time included, the extinction burst occurred for only 50% of rats when the maximum baseline rate was used as the comparator. Although no direct empirical work has been conducted on the topic, there have been suggestions in the literature that different types of reinforcers might differentially generate an extinction burst. Lattal et al. (2020) suggested that different types of drug reinforcers (i.e., cocaine versus nicotine) might differentially produce an extinction burst, but they noted that this was speculative. Lerman et al. (1999) found the prevalence of extinction bursts to be greater for cases in which SIB was maintained by social negative reinforcement compared to cases in which SIB was maintained by social positive reinforcement. In the present reanalysis, we found that the extinction burst in the first minute of extinction was most prevalent for rats that were not food restricted and responding for a sucrose solution. However, these were also the only rats in the reanalysis for which responding was previously maintained on a small FR requirement. Similarly, although the studies examined in our reanalysis above employed a variety of different reinforcers, it is difficult to make direct comparisons across them because they also differed in terms of baseline schedules. Regardless, given the potential relevance of reinforcement consumption/engagement time on the extinction burst, variables such as motivational conditions, reinforcer quality, duration, magnitude, and individual differences related to more extended reinforcement engagement time or aftereffects seem worthy of further investigation.
Acknowledgments
This work was funded by grant R01HD093734 (TAS) from the Eunice K. Shriver National Institute of Child Health and Human Development and was completed in partial fulfillment of the first author’s 2nd year project in the Ph.D. program in the Department of Psychology at Utah State University. The authors thank Greg Madden and Katie Brown for their comments and suggestions.
Footnotes
Thanks to Wayne Fisher for insightful discussions on this issue
References
- Boren JJ (1961). Resistance to extinction as a function of the fixed ratio. Journal of Experimental Psychology, 61(4), 304–308. [Google Scholar]
- Browning KO, & Shahan TA (2018). Renewal of extinguished operant behavior following changes in social context. Journal of the Experimental Analysis of Behavior, 110(3), 430–439. [DOI] [PubMed] [Google Scholar]
- Carr JE, Bailey JS, Scott CL, Lucker KD, & Weil TM (1998). On the effects of noncontingent delivery of differing magnitudes of reinforcement. Journal of Applied Behavior Analysis, 31, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JO, Heron TE, & Heward WL (2020). Applied behavior analysis (3rd ed.). Upper Hoboken, NJ: Pearson. [Google Scholar]
- Craig AR, & Shahan TA (2016). Behavioral momentum theory fails to account for the effects of reinforcement rate on resurgence. Journal of the Experimental Analysis of Behavior, 105(3), 375–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AR, Browning KO, Nall RW, Marshall CM, & Shahan TA (2017). Resurgence and alternative reinforcer magnitude. Journal of the Experimental Analysis of Behavior, 107(2), 218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AR, Nall RW, Madden GJ, & Shahan TA (2016). Higher rate alternative non-drug reinforcement produces faster suppression of cocaine seeking but more resurgence when removed. Behavioural Brain Research, 306, 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman AM, Sullivan MW, Hitchcock DM, & Lewis M. (2009). When frustration is repeated: Behavioral and emotion responses during extinction over time. Emotion, 9(1), 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, & Brown C. (1995). Nicotine self-administration in rats. Psychopharmacology, 122(4), 390–394. [DOI] [PubMed] [Google Scholar]
- Doughty AH, & Richards JB (2002). Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. Journal of the Experimental Analysis of Behavior, 78(1), 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme JM & Van Houten R. (1994). Operant Extinction in the Treatment of Severe Maladaptive Behavior. Behavior Modification, 18(2), 139–170. [DOI] [PubMed] [Google Scholar]
- Fisher WW, Greer BD, Mitteer DR, Fuhrman AM, Romani PW, & Zangrillo AN (2018). Further evaluation of differential exposure to establishing operations during functional communication training. Journal of Applied Behavior Analysis, 51(2), 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher WW, Thomspon RH, DeLeon IG, Piazza CC, Kuhn DE, Rodriguez-Catter V, & Adelinis JD (1999). Noncontingent reinforcement: effects of satiation versus choice responding. Research in Developmental Disabilities, 20(6), 411–427. [DOI] [PubMed] [Google Scholar]
- Fleshler M, & Hoffman HS (1962). A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior, 5(4), 529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh H, & Iwata BA (1994). Behavioral persistence and variability during extinction of self-injury maintained by escape. Journal of Applied Behavior Analysis, 27(1), 173–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, & LeSage MG (2007). Prevalence, magnitude, and correlates of an extinction burst in drug-seeking behavior in rats trained to self-administer nicotine during unlimited access (23 h/day) sessions. Psychopharmacology, 194, 395–402. [DOI] [PubMed] [Google Scholar]
- Iwata BA, Pace GM, Kalsher MJ, Cowdery GE, & Cataldo MF (1990). Experimental analysis and extinction of self-injurious escape behavior. Journal of Applied Behavior Analysis, 23(1), 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, & Lattal KA (2020). An experimental analysis of the extinction-induced response burst. Journal of the Experimental Analysis of Behavior, 114(1), 24–46. [DOI] [PubMed] [Google Scholar]
- Katz BR, & Lattal KA (2021). What is an extinction burst?: A case study in the analysis of transitional behavior. Journal of the Experimental Analysis of Behavior, 115(1), 129–140. [DOI] [PubMed] [Google Scholar]
- Keller FS & Schoenfeld WN (1950). Principles of psychology: A systematic text in the science of behavior. Appleton-Century-Crofts. [Google Scholar]
- Lattal KA (1991). Scheduling positive reinforcers. In Iversen IH & Lattal KA (Eds.), Experimental Analysis of Behavior (Part 1, p. 87–134). New York: Elsevier. [Google Scholar]
- Lattal KA, Kuroda T, & Cook JE (2020). Early extinction effects following intermittent reinforcement: little evidence of extinction bursts. Journal of the Experimental Analysis of Behavior, 114(1), 47–59. [DOI] [PubMed] [Google Scholar]
- Lattal KA, Peter C St., & Escobar R. (2013). Operant extinction: Elimination and generation of behavior. In Madden GJ, Dube WV, Hackenberg TD, Hanley GP, & Lattal KA (Eds.), APA handbook of behavior analysis, vol 2: translating principles into practice (pp. 77–107). Washington, DC: APA. [Google Scholar]
- Lerman DC & Iwata BA (1995). Prevalence of the extinction burst and its attenuation during treatment. Journal of Applied Behavior Analysis, 28(1), 93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman DC & Iwata BA (1996). Developing a technology for the use of operant extinction in clinical settings: An examination of basic and applied research. Journal of Applied Behavior Analysis, 29(3), 345–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman DC, Iwata BA, Shore BA, & Kahng S. (1996). Responding maintained by intermittent reinforcement: implications for the use of extinction with problem behaviors in clinical settings. Journal of Applied Behavior Analysis, 29(2), 153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman DC, Iwata BA, & Wallace MD (1999). Side effects of extinction: Prevalence of bursting and aggression during the treatment of self-injurious behavior. Journal of Applied Behavior Analysis, 32(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malott RW, & Suarez EA (2004). Principles of Behavior (5th ed.). Upper Saddle River, NJ: Pearson. [Google Scholar]
- Martin G, & Pear JJ (2015). Behavior modification: What it is and how to do it. New York, NY: Routledge. [Google Scholar]
- Mazur JE (1983). Steady-state performance on fixed-, mixed-, and random-ratio schedules. Journal of the Experimental Analysis of Behavior, 39(2), 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, & Roll JM (1993). Responding changes systematically within sessions during conditioning procedures. Journal of the Experimental Analysis of Behavior, 60(3), 621–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LK (2006). Principles of everyday behavior analysis (4th ed.). Belmont, CA: Wadsworth Cengage Learning. [Google Scholar]
- Miltenberger RG (2008). Behavior modification: Principles and procedures (4th ed.). Belmont, CA: Wadsworth Cengage Learning. [Google Scholar]
- Nall RW, Craig AR, Browning KO, & Shahan TA (2018). Longer treatment with alternative non-drug reinforcement fails to reduce resurgence of cocaine or alcohol seeking in rats. Behavioural Brain Research, 341, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, & Lichtman AH (2007). The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology, 191(2), 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace GM, Iwata BA, Cowdery GE, Andree PJ, & McIntyre T. (1993). Stimulus (instructional) fading during extinction of self-injurious escape behavior. Journal of Applied Behavior Analysis, 26(2), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushparaj A, Pryslawsky Y, Forget B, Yan Y, & Le Foll B. (2012). Extinction bursts in rats trained to self-administer nicotine or food in 1-h daily sessions. American Journal of Translational Research, 4(4), 422–431. [PMC free article] [PubMed] [Google Scholar]
- Roscoe EM, Iwata BA, & Rand MS (2003). Effects of reinforcer consumption and magnitude on response rates during noncontingent reinforcement. Journal of Applied Behavior Analysis, 36(4), 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Kurth P, McCullough LD, Sokolowski JD (1995). The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: contrasts with the effects of extinction. Pharmacology, Biochemistry, and Behavior, 50(3), 437–443. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Olsen CM, & Winder DG (2006). Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. Neuropsychopharmacology, 31(7), 1444–1451. [DOI] [PubMed] [Google Scholar]
- Skinner BF (1979). The shaping of a behaviorist. Knopf.
- Staddon JER (1993). The conventional wisdom of behavior analysis. Journal of the Experimental Analysis of Behavior, 60(2), 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer TR (2001). Review of Miltenberger’s Behavior Modification: Principles and Procedures (2nd ed.). Journal of Applied Behavior Analysis, 34(4), 551–557. [Google Scholar]
- Vollmer TR, Progar PR, Lalli JS, Van Camp CM, Sierp BJ, Wright CS, … & Eisenschink KJ (1998). Fixed-time schedules attenuate extinction-induced phenomena in the treatment of severe aberrant behavior. Journal of Applied Behavior Analysis, 31(4), 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, & Walker EA (2009). The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug and Alcohol Dependence, 105(3), 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Walker EA, & Dykstra LA (2007). Effect of cannabinoid CB1 receptor antagonist SR141714A and CB1 receptor knockout on cue-induced reinstatement of Ensure® and corn-oil seeking in mice. Neuropsychopharmacology, 32(12), 2592–2600. [DOI] [PubMed] [Google Scholar]
- Woods JN, & Borrero CSW (2019). Examining extinction bursts in the treatment of pediatric food refusal. Behavioral Interventions, 34(3), 307–322. [Google Scholar]
- Zarcone JR, Iwata BA, Vollmer TR, Jagtiani S, Smith RG, & Mazaleski JL (1993). Extinction of self-injurious behavior with and without instructional fading. Journal of Applied Behavior Analysis, 26(3), 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]


