Abstract
Drug-induced liver injury (DILI) is a leading cause of termination in drug development programs and removal of drugs from the market; this is partially due to the inability to identify patients who are at risk1. In this study, we developed a polygenic risk score (PRS) for DILI by aggregating effects of numerous genome-wide loci identified from previous large-scale genome-wide association studies2. The PRS predicted the susceptibility to DILI in patients treated with fasiglifam, amoxicillin–clavulanate or flucloxacillin and in primary hepatocytes and stem cell-derived organoids from multiple donors treated with over ten different drugs. Pathway analysis highlighted processes previously implicated in DILI, including unfolded protein responses and oxidative stress. In silico screening identified compounds that elicit transcriptomic signatures present in hepatocytes from individuals with elevated PRS, supporting mechanistic links and suggesting a novel screen for safety of new drug candidates. This genetic-, cellular-, organoid- and human-scale evidence underscored the polygenic architecture underlying DILI vulnerability at the level of hepatocytes, thus facilitating future mechanistic studies. Moreover, the proposed ‘polygenicity-in-a-dish’ strategy might potentially inform designs of safer, more efficient and robust clinical trials.
PRSs encompassing the cumulative effects of tens of thousands to hundreds of thousands of variants or genome-wide variants can identify individuals at high risk of common diseases3. However, it remains unclear whether a polygenic model can account for susceptibility to relatively rare diseases, such as DILI.
DILI is one of the leading causes of termination in drug development programs and drug withdrawals from the market; in clinical practice, it is also associated with substantial morbidity and mortality1. Genome-wide association studies (GWASs) conducted by the international Drug-Induced Liver Injury Consortium (iDILIC) and the Drug-Induced Liver Injury Network (DILIN) have identified several significant variants associated with DILI due to multiple different drugs (P value from GWAS (PGWAS) < 5 × 10−8)2. Because each variant has modest predictive effect, we revisited GWAS findings to determine whether the PRS, which sums up the effects of numerous variants, informs potential DILI susceptibility in humans. The goal of this study was to develop PRSs using data from previous GWASs (performed by the iDILIC and the DILIN) and to validate their performance characteristics in GWAS data obtained from an independent clinical trial of a hepatotoxic drug, as well as multiple donor-derived organoids and primary hepatocytes treated with a variety of hepatotoxic medications. We also used the derived PRS to identify mechanisms underlying the susceptibility to DILI.
To assess the polygenicity associated with susceptibility to DILI, summary statistics of a GWAS from the iDILIC–DILIN collaboration, which studied cases of DILI due to selected drugs, were used for the discovery data set, including all patients (ALL-DILI GWAS, n = 862), patients with hepatocellular injury (HC-DILI GWAS, n = 474) and patients with cholestatic/mixed injury (CM-DILI GWAS, n = 323), and compared with those of population-matched controls (n = 10,588) (Fig. 1a and Extended Data Fig. 1; see details in ref. 2). In the original report by the iDILIC–DILIN, two loci in the entire cohort (ALL-DILI), one locus in the CM-DILI GWAS and one locus in the HC-DILI GWAS were genome-wide significant (PGWAS < 5 × 10−8)2. In this study, we hypothesized that polygenic architecture informed by the iDILIC–DILIN GWAS would predict the susceptibility of an independent DILI GWAS data set, namely patients who experienced liver injury during the clinical trials of TAK-875 (fasiglifam). As linkage disequilibrium (LD) score regression is inappropriate with the relatively small sample size in the GWAS study4, we sought to investigate the polygenic architecture of DILI susceptibility by comparing two independent DILI GWASs.
Fig. 1 |. Polygenic architecture of DILI.
a, Summary of PRSs and their sources. b, Manhattan plot of P values (logistic regression model, from SNPTEST software) in TAK-875 white GWAS. The blue line is a suggestive cutoff (P <1 × 10−5). c, Polygenic test using CM-DILI GWAS summary statistics. x axis, total number of SNPs, ordered by iDILIC–DILIN GWAS association; y axis, explained variance of TAK-875 DILI phenotype from iDILIC–DILIN GWAS signals; color scale, one-tail P value for the shared genetic aetiology test from PRSice software. The described PGWAS indicates the corresponding thresholds to the x axis. R2, percent of variance explained. d, GARFIELD plot of CM-DILI GWAS summary statistics in ref. 2. Enrichment in FAIRE-seq (formaldehyde-assisted isolation of regulatory elements) annotations in left and genic state in right. e, Enriched experimentally known or related pathways associated with CM-DILI. CM-DILI GWAS summary statistics in ref. 2 were used. The empirical P values were from Pascal software. f, Distribution of PRS in TAK-875 DILI patients (cases, n = 39 individuals) and matched patients treated with TAK-875 without DILI (controls, n = 122 individuals). For each type of PRS, scores were centred on the median values in controls. *P < 0.05 (two-tailed Wilcoxon–Mann–Whitney U test; P = 0.0036 for CM-PRSgw, P = 0.70 for HC-PRSgw, P = 0.27 for ALL-PRSgw and P = 0.029 for CM-PRSgw+). For the box plot, the box represents the first and third quartiles; the center line represents the median; the upper whisker extends from the hinge to the highest value that is within 1.5 × IQR of the hinge; the lower whisker extends from the hinge to the lowest value within 1.5 × IQR of the hinge; and the data beyond the end of the whiskers were plotted as points. ES, embryonic stem; IQR, interquartile range; NS, not significant.
Fasiglifam (TAK-875) is an oral, highly potent and selective FFAR1 (GPR40) agonist, the first of its class tested for glucose-lowering ability in patients with type 2 diabetes5. TAK-875 development was discontinued owing to the rare DILI incidence detected in a phase 3 study6. We recruited 43 patients with TAK-875 DILI (cases) and 129 matched patients who did not experience liver injury when treated with TAK-875 in the clinical trials (controls) for a subsequent GWAS. The TAK-875 cases and controls were not included in the iDILIC–DILIN GWAS. Although an increase in alanine aminotransferase (ALT) levels was observed after treatment with TAK-875 in the patients with DILI, total bilirubin was also increased (Extended Data Fig. 2), consistent with experimental models that have demonstrated an inhibition of bile acid transporters by TAK-875 treatment7. After quality control analysis and genome-wide genotype imputation using populations from 1000 Genomes Phase 3 (1KGP3) as a reference8, TAK-875 DILI cases and 122 controls who were self-reported Caucasians with 13,477,278 autosomal variants and 184,010 non-imputed variants were selected for further study (Extended Data Figs. 1 and 2c–e and Supplementary Table 1). Logistic regression analysis with covariates for population structure yielded no genome-wide significant association signals in the GWAS (Fig. 1b).
After iDILIC–DILIN GWAS summary statistics were clumped by LD in the European population, we performed shared genetic aetiology analysis to determine whether the iDILIC–DILIN GWAS signals correlated with TAK-875 DILI GWAS signals using maximal inclusion of single-nucleotide polymorphisms (SNPs) with PGWAS < 0.5 (Fig. 1c). Interestingly, comparing GWAS results showed that the iDILIC–DILIN GWAS for CM-DILI (Fig. 1c) shared genetic aetiology with TAK-875 DILI more than the iDILIC–DILIN GWAS for ALL- or HC-DILI did (Extended Data Fig. 2f,g), indicating that susceptibility to TAK-875 DILI is genetically linked to CM-DILI susceptibility to many other drugs.
Functional enrichment analysis of summary statistics for iDILIC–DILIN CM-DILI was performed using GARFIELD (GWAS analysis of regulatory or functional information enrichment with LD correction) software using default annotations including more than 1,000 publicly available data sets with regulatory genomic regions9. The strongest enrichment in the epigenetic state was observed in HepG2 cells versus cells of other origin (Fig. 1d, left), suggesting that a substantial genetic susceptibility for CM-DILI resides within the hepatocyte-like cellular context. The strongest enrichment in a genic state was in the 5′ untranslated region (UTR) (Fig. 1d, right). Pascal (pathway scoring algorithm)10 revealed that association signals of CM-DILI GWAS were enriched in experimentally proposed pathways for DILI, including mitochondrial activity, oxidant-induced survival11 and unfolded protein response (UPR)12 (P < 0.05; Fig. 1e and Supplementary Table 2). Thus, genetic annotation of genome-wide CM-DILI GWAS signals reasonably reflect reported molecular and cellular pathways related to human DILI. We hereafter defined the CM-PRSgw (genome-wide PRS) by aggregating 27,740 SNPs in and near hepatocyte-expressed genes with PGWAS < 0.5 for further analysis (see Methods).
Although CM-PRSgw is solely formed by an iDILIC–DILIN study that does not include patients treated with TAK-875, the TAK-875 DILI case samples had a marginally higher CM-PRSgw score distribution than control samples (area under the receiver operating characteristic curve (AUROC) = 0.61 (95% confidence interval (CI): 0.51–0.71; P for two-tailed Wilcoxon–Mann–Whitney U test < 0.05) (Fig. 1f and Extended Data Fig. 3). Interestingly, there was a trend toward an increase in the baseline values of ALT, aspartate transaminase (AST) and total bilirubin as the CM-DILI PRSgw increased in all TAK-875-treated patients (Extended Data Fig. 4). We also found that CM-DILI PRSgw potentially predicted susceptibility to DILI of 167 patients treated with flucloxacillin or 207 patients treated with amoxicillin–clavulanate with a CM-DILI phenotype (AUROC = 0.61 (95% CI: 0.57–0.66) or 0.62 (95% CI: 0.58–0.66), respectively; Extended Data Fig. 5; see Supplementary Note and Supplementary Methods). These cases were not included in the original CM-DILI PRS determinations2 but were described elsewhere (see ref. 13). These data support that the cholestatic/mixed polygenic score (CM-PRSgw) could predict the potential DILI susceptibility of patients caused by multiple different drugs. Notably, when the CM-DILI GWAS added 157 patients with DILI who were treated with flucloxacillin and 320 patients with DILI who were treated with amoxicillin–clavulanate (Fig. 1a), the GWAS signals were also enriched in plausible biological pathways and hepatocyte-like context (Extended Data Fig. 6 and Supplementary Table 3), and its derived PRS, named CM-PRSgw+, was similar to the above in terms of distinguishing TAK-875 DILI cases from controls (Fig. 1f and Extended Data Fig. 3; see Supplementary Note).
Next, we investigated whether the two different PRSs could stratify DILI vulnerability under CM-DILI conditions using both a scored human hepatocyte model and human liver bud organoids (HLOs) derived from induced pluripotent stem cells (iPSCs) (Fig. 2a). Primary human hepatocytes (PHHs)—the gold standard for toxicology testing (and a fully matured adult cell type)—were randomly selected from vendors (Supplementary Table 4). Multi-donor iPSCs were obtained from the cell bank, the Coriell Institute and the European Bank for Induced Pluripotent Stem Cells (Supplementary Table 5). Whole-genome genotypes of purchased PHHs and iPSCs from healthy European donors were determined by SNP array to calculate their CM-PRSgw and CM-PRSgw+ (see Methods). PHHs (21 donors) and iPSC-HLOs (five donors) in this study were selected after a battery of quality control experiments (Extended Data Figs. 7 and 8). For the modeling of the CM-DILI phenotypes with the iPSCs, we adopted modified protocols of previously reported vascularized three-dimensional iPSC-HLOs14. All the donor iPSCs successfully generated HLOs with consistent expression of liver-associated proteins (albumin and bile salt export pump (BSEP)) and CYP3A4 activity (Extended Data Fig. 7a–d). HLOs that were exposed to cholyl-lysyl-fluorescein (CLF), a fluorescently labeled bile acid, displayed prominent accumulation of bile acids under cyclosporin A (CsA) treatment, followed by massive cell death (Extended Data Fig. 7e,f). Hereafter, we used this as a model for assessing CM-DILI by iPSC-HLOs. For the modeling of the CM-DILI phenotypes from PHH cells, we analyzed the viability based on established cholestatic-type DILI protocols15 by pretreatment with lithocholic acid (LCA), a highly toxic secondary bile acid (Extended Data Fig. 9a–f).
Fig. 2 |. Polygenic risk score based human stratification by in vitro multi-drug-induced CM-DILI assays.
a, Workflow of comparison. b, Viability comparison of multi-donor iPSC-HLO models (triangle) and primary human hepatocytes (circle) under multiple DILI drug treatments. See Supplementary Table 4 for donor information per drug. c, Comparison of Pearson correlation coefficients between the survival rate of the liver model (mean value for each donor) and the PRS (PRSgw or PRSgw+) under indicated drugs. *P < 0.01 (two-tailed Welch’s t-test); P = 0.0038 for cholestatic DILI drugs treatment and P = 0.0005 for hepatocellular DILI drugs treatment. For the violin plot, the center point represents the median; the upper whisker extends from the hinge to the highest value that is within 1.5 IQR of the hinge; and the lower whisker extends from the hinge to the lowest value within 1.5 IQR of the hinge. IQR, interquartile range.
Under the cholestatic conditions, we evaluated the toxicity of 12 DILI drugs—cyclosporine, bosentan, troglitazone, diclofenac, flutamide, ketoconazole, carbamazepine, amoxicillin–clavulanate and methapyrilene (drugs that produce DILI with a CM phenotype); and tacrine, acetaminophen and tolcapone (drugs that produce DILI with an hepatocellular phenotype)—to determine if the score-dependent trend is conserved across multiple drugs (Fig. 2a). The correlation patterns between the two different PRSs and cell viability for each drug treatment were plotted (Fig. 2b). CM-PRSgw and the cell death rate under drug treatment were well correlated, and the average Pearson correlation coefficient of each category of drugs used in the experiment was more significant than that of CM-PRSgw+(Fig. 2c). Thus, by examining the correlation comparison using iPSC-HLOs and PHH cells, we found that CM-PRSgw was more strongly correlated with the toxicity evaluation of the DILI drugs than CM-PRSgw+ (Fig. 2b,c). Intriguingly, the five-donor iPSC-HLOs without LCA pretreatment also revealed a correlation between cell viability under CsA treatment and the CM-PRSgw (P < 0.05), whereas CM-PRSgw+ showed no obvious correlation (Extended Data Fig. 9g,h). CM-PRSgw+ was developed from a GWAS of our original cohort plus 477 patients with DILI due to either amoxicillin–clavulanate or flucloxacillin, so that almost half of the cases used to derive CM-PRSgw+ were just due to these two drugs. The fall-off in prediction suggests that CM-PRSgw+ might not be able to identify DILI liability for all drugs in our experimental models. Taken together, these data show that the CM-DILI PRSgw-based stratification approach in the multi-donor PHH- and iPSC-HLO-based assay was more widely applicable than the PRSgw+-based one.
To approach functional pathways associated with CM-DILI risks, we first performed comparative transcriptome analysis of basal expression levels in the cells in culture, regressing out five transcriptome principal components to capture experimental variability16 (Fig. 3a). Unbiased pathway enrichment analysis in ten PHH donors revealed that genes involved in mitochondria and translation were inactivated in the higher CM-PRSgw donors (false discovery rate (FDR) < 0.01; Fig. 3b and Supplementary Table 6). We next determined the CM-PRSgw in 157 donors of human liver samples that had undergone RNA profiling17. There, too, the donors with the highest CM-PRSgw scores appeared to have the same pathways inactivated as we observed in the ten PHH donors (Fig. 3a,b, and Supplementary Table 7; see Methods). In addition, basal expression levels of mitochondrial genes and UPR-related genes were correlated with the CM-DILI PRSgw in the transcriptome analysis of five donor iPSC-HLOs (Fig. 3c). These data suggest that global transcriptional and translational processes might be perturbed in people who are susceptible to CM-DILI, consistent with findings of GWAS signal enrichments in the 5′ UTR region (Fig. 1e).
Fig. 3 |. Mechanistic association studies for CM-DILI vulnerability.
a, Method to find CM-DILI PRSgw-associated pathways. We performed GSEA analysis of CM-DILI PRSgw-associated genes, regressing out five first transcriptome principal components (PCs) to capture experimental variability16. b, Pathway enrichment analysis results for PHH cells and liver tissues. c, Heat map analysis of gene sets involved in UPR (76 genes) and TCA cycle and respiratory electron transport (116 genes) (Reactome pathway database27) in multi-donor iPSC-HLO models. d, Immunostaining of HNF4a and CHOP under the indicated CsA treatment. Representative pictures from two biological replicates. e, XBP1s and KDEL levels under the indicated CsA treatment via immunoblot analysis. GAPDH was used for loading controls. n = 3 independent experiments. f, Live imaging of oxidative stress induction using CellROX reagent (ROX), CLF accumulation and cell death (stained by PI) under the indicated CsA treatment. Representative pictures from three biological replicates. g, Comparison of GSH/GSSG ratio in the iPSC-HLO model between the PRSgw-high donor (45A) and the -low donor (CW10027) upon CsA treatment. *P = 0.017064, a significant difference between the two groups. n = 3, independent experiments. h, GSH/GSSG ratio change by BM treatment in 1383D2-derived iPSC-HLOs under basal condition. n = 2 independent experiments. i, Cell viability upon BM treatment between PRSgw-high and -low donor-derived iPSC-HLOs under CsA 50 μM treatment. A significant difference between the two groups with no treatment was shown (P = 0.0051), whereas a significant diifference was not shown with BM pretreatment (P = 0.1048) or with BM pre-/co-treatment (P = 0.0640). n = 3 independent experiments. All data are shown as mean ± s.d. *P < 0.05 (two-tailed Welch’s t-test). NS. not significant.
On the basis of these pathway extractions, we further investigated CM-DILI vulnerability mechanisms using an iPSC-HLO model focusing on mitochondrial activity and endoplasmic reticulum (ER) stress signals under CsA treatment. Consistently, we observed cholestatic cell death with elevated UPR-associated proteins, massive ROS production and functional mitochondria depletion in iPSC-HLO (Fig. 3d–f and Extended Data Fig. 10). In agreement with this, an antioxidant GSH/GSSG ratio was elevated more under CM-DILI drug treatment in a CM-PRSgw higher donor than a lower donor (Fig. 3g), and this was partially alleviated by bardoxolone methyl (BM), a potent activator of nuclear factor-like-2 factor18 (Fig. 3h). The enhanced antioxidant response induced by BM protects against hepatocyte death in cholestatic conditions (Fig. 3i) even in the CM-PRSgw higher donor. Collectively, ROS activation might be a potential downstream event before hepatocyte death owing to the accumulation of bile acids and a predisposition to transcriptional and translational stress substantiated by PRS.
To verify the connection between CM-DILI vulnerable pathways and CM-DILI drugs, we analyzed the risk score associated transcriptomic signatures in PHH from eight donors under CsA treatment (see Supplementary Table 4) and compared the transcriptome data in Toxicogenomics Project-Genomics Assisted Toxicity Evaluation system (TG-GATEs)19, including 941 distinct conditions of drug-treatment-related changes in transcriptome in PHH (Fig. 4a and Supplementary Table 8). First, the genes highly inactivated in higher CM-PRSgw donors’ PHHs were enriched (FDR < 0.01) in pathways such as respiratory electron transport (that is, inducible mitochondrial toxicity), translational regulation and mRNA processing (Fig. 4b and Supplementary Table 9), in concordance with baseline transcriptome analysis in Fig. 3. To extend these findings, we searched for the drugs that inactivated these DILI vulnerable pathways in TG-GATEs (FDR < 0.01; Supplementary Table 10). Many well-known CM-DILI drugs, such as CsA, flutamide, ketoconazole, ranitidine, sulpiride, valproic acid and azathioprine, decreased levels of genes in these DILI vulnerable pathways (Fig. 4c), supporting our mechanistic findings with CM-DILI-inducible drugs in in vitro multi-donor PHH analysis (Supplementary Table 10). We note that UPR-related genes were also activated in the higher CM-DILI PRSgw PHHs (Fig. 4d), as shown in Fig. 3. These data suggest that in silico toxicity modeling, coupled with in vitro genomic and transcriptomic approaches, might identify compounds with potential for DILI before entering clinical trials.
Fig. 4 |. PrS associated transcriptomic signatures associate with known CM-DILI responses.
a, A schematic representation of the protocol for identifying compounds associated with PRSgw-related transcriptomic pathways (see Results and Methods). b, Heat map analysis of gene sets involved in TCA cycle and respiratory electron transport in eight PHH donors ordered by PRSgw. This pathway was one of the significantly inactivated pathways in higher CM-PRSgw donors (Supplementary Table 9). The core enriched genes in GSEA are shown. c, Network representation of screened gene sets associated with CM-DILI PRSgw (FDR < 0.01). Representative clusters and compounds associated with them are indicated. All of the raw results are shown in Supplementary Tables 9 and 10. d, Gene expression analysis for representative ER stress marker genes in the eight PHH donors under CsA treatment or control conditions (see Methods). **P < 0.01 (two-sided Pearson correlation test; P = 4.7 × 10−3). e, PRSgw informed CM-DILI vulnerable mechanisms, genetic factors and their relationships. Green text, validated events by phenotypic assays; red box, PRS informed mechanisms for CM-DILI.
This is the first unbiased analysis to implicate genetic variation at the level of the hepatocyte contributing to DILI susceptibility, validated in an independent clinical study, as well as unrelated donor-derived organoids and primary hepatocytes. Hepatocyte damage due to an imbalance between formation and clearance of reactive metabolites, steps that are common for many compounds, is likely an upstream event mediating liver injury20. Once hepatocytes are damaged, innate and adaptive immunity play a significant role in driving tissue inflammation and injury21, as supported by identification of human leukocyte antigen (HLA) alleles and haplotypes as DILI risk factors in GWASs (Supplementary Note). However, whereas some of the cases in the iDILIC–DILIN cohort were shown to have a significant HLA association2, the overall HLA contribution in the entire cohort was relatively limited. This is similar to TAK-875 DILI, where no HLA association has been detected. The PRS (PRSgw+) derived from the expanded iDILIC–DILIN cohort included mostly flucloxacillin and amoxicillin–clavulanate cases, for which stronger HLA associations have been demonstrated22,23, and was still useful in predicting CM-DILI but not as well as PRSgw. We speculate that this poorer performance might be due to limited contribution of non-HLA risk factors when HLA is the main genetic risk factor. Up to the present, few non-HLA genetic risk factors for any form of DILI have been identified and replicated, thus highlighting the importance of aggregating many variants, each making a small contribution to overall DILI risk.
Our PRSgw for CM-DILI revealed shared DILI predictivity across a variety of drugs independent of their individual chemical characteristics that are considered important24. This indicates that the makeup of our polygenic scores relates to intra-hepatocyte mechanisms leading to hepatotoxicity. For example, both UPR and oxidative stress have been reportedly associated with DILI pathogenesis and cause cell death due to experimental cholestasis using various drugs such as flucloxacillin, levofloxacin, diclofenac and carbamazepine25,26. Consistently, our in vitro polygenic score-based approach, coupled with genomic, transcriptomic and phenotypic approaches, indicated that diverse biological pathways, including UPR and oxidative stress responses in hepatocytes, contribute to CM-DILI susceptibility (Fig. 4e). These findings can also provide fundamental mechanisms defining CM-DILI and might ultimately contribute to the design of safer, efficient and more robust clinical trials. More broadly, the proposed ‘polygenicity-in-a-dish’ strategy is a powerful approach to investigate and interrogate highly complicated pathogenesis in humans with minimal confounding factors.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41591-020-1023-0.
Methods
iDILIC–DILIN GWAS summary statistics.
iDILIC–DILIN GWAS summary statistics (SNP name, minor allele, odds ratio for the minor allele and the P value) were kindly provided by researchers in these consortia2. GWASs were conducted using three types of cohorts—patients with DILI (ALL-DILI, n = 862), patients with hepatocellular injury (HC-DILI, n = 474) and patients with cholestatic/mixed injury (CM-DILI, n = 323)—against population-matched controls (n = 10,588). We used biallelic and unambiguous SNPs catalogued in snpdb147 and included in the 1000 Genomes Project Phase 3 (1KGP3) imputation reference panel (Minimac3 website)28. The odds ratios of iDILIC–DILIN GWAS summary statistics were aligned toward the alternative allele in the reference panel. Finally, 4,392,401 SNPs in ALL-DILI, 4,392,226 SNPs in CM-DILI and 4,393,472 SNPs in HC-DILI were selected (referred to as processed iDILIC–DILIN GWAS summary statistics).
Collection criteria of controls and of patients receiving TAK-875.
Patients comprised individuals treated with TAK-875 monotherapy or combinatorial therapy. Cases (DILI) were identified as patients receiving TAK-875 and experiencing within 7 d after treatment a rise in serum ALT or AST at least ≥3× the upper limits of normal and at least 2× their baseline value. Otherwise, eligible patients who first met the DILI inclusion criteria more that 7 d after final dosing were excluded from the analysis. Controls were randomly identified and did not experience ALT/AST elevations exceeding 10% from baseline (and not exceeding 3× ULN). Controls were matched in a 3:1 ratio to cases on the basis of study, drug treatment, race and sex.
TAK-875 GWAS.
The study population of the TAK-875 GWAS included patients experiencing DILI (cases) and corresponding matched controls as defined above. Data regarding the patients and controls were collected from TAK-875 phase 2 and 3 clinical studies. All studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice and all applicable local regulatory requirements. All studies, including protocol and informed consent forms, were approved by the institutional review board at each study site. The institutional review board or independent ethics committee for each site was constituted according to the applicable requirements of the participating region and approved the protocol and patient informed consent forms. Before undergoing any study procedures, a signed and dated informed consent form was received from each patient.
We included self-reported Caucasian individuals (case n = 43 and control n = 129) for the GWAS. These individuals were genotyped in a combination of HumanOmni5-Quad BeadChip (Illumina) and Infinium Human Exome-12 v1.2 BeadChip (Illumina). Corresponding manifest files of genotyping arrays, HumanOmni5–4v1_B and HumanExome-12-v1–2-B, respectively, were used. Quality control analysis of the genotyped data checked sample and variant call rate, probe–target duplication, missingness, heterozygosity, Hardy–Weinberg equilibrium, identity by descent, ancestors and minor allele frequency (MAF) using PLINK software (version v1.90b3.44)29 (see Supplementary Methods). The haplotype phase was estimated using Eagle software (version 2.3)30 with the 1KGP3 reference panel (provided on the Minimac3 website, version 5, n = 2,504). Genotypes of autosomal variants were imputed using Minimac3 software (version 2.0.1) with the 1KGP3 imputation reference panel31. Finally, case (n = 39) and control (n = 122) samples with autosomal 13,477,278 variants with a Minimac3 imputation quality (Rsq) ≥0.7 and non-imputed genotyped 184,010 variants were included in the GWAS. GWAS was performed using imputed allele dosages of variants with a MAF >1% and fitted to an additive genetic model of logistic regression with PC1–PC5 in white GWAS, using SNPTEST software (version 2.5.2)32. A Manhattan plot was performed using R package qqman v0.1.2.
Genetic analysis of shared aetiology.
We evaluated shared genetic aetiology between two GWAS summary statistics using PRSice software (version 1.25)33. Common autosomal SNPs between the TAK-875 GWAS panel and the processed iDILIC–DILIN GWAS summary statistics were used. Base summary statistics were pruned from LD-based clumps with parameters recommended in the PRSice software: clump.p1 0.5, clump.p2 0.5 and clump.kb 300 using PLINK software (version v1.90b3.44)29. In this clumping step, the 1KGP3 European super population was used, and 51,052 SNPs for ALL-DILI, 50,029 SNPs for CM-DILI and 50,553 SNPs for HC-DILI were selected. SNPs with odds ratio ≠1 in target GWAS sets were selected. If the β value for the association of SNPs in TAK-875 GWAS was positive infinity (negative infinity), secondary maximum (or minimum) β was replaced with it. Standard errors of data sets were calculated from oddds ratio and P values, using Wald statistics. Finally, variance explained by the base risk score in target GWAS sets and P values were obtained from the PRSice software33. We noted that the increasing trends are important to show the polygenic architecture in DILI, and the levels of variance explained might be high due to the winner’s curse issue. To avoid incorrect conclusions from genetic overlap due to the population stratification problem, we conducted only the summary statistics-based analysis.
PRS analysis.
From the processed iDILIC–DILIN GWAS summary statistics for the European LD-based clumped data, we further selected imputable SNP sets from the 1KGP3 reference panel. PRS was calculated as follows: (β for association) × (number of effect allele for β). The number of alleles was based on the best-guessed genotype upon 1KGP3 imputation with Rsq ≥0.7.
To determine a PRSgw, we considered the cellular context of hepatocytes to observe the phenotype. Hence, we obtained RNA sequencing data of public primary human hepatocytes (SRR4000958)34. Reads from the FastQ files were mapped to the GRCh38.p10 reference sequence, using GENCODE v26 annotation file and STAR software (version 2.5.2b)35, after quality control by FaQCs software (version 1.34)36 with default parameters. FPKM values were obtained using Cufflinks software (version 2.2.1)37, wherein the max-bundle-frags option was adjusted to 109 fragments per locus. Expressed genes (n = 14,576) were considered to have an FPKM greater than 0 in the PHH. Regions 10 kb upstream from the transcription start site to 40 kb downstream of the transcription termination site demarcated the coding sequence. Finally, 28,275 SNPs in ALL-DILI, 27,740 SNPs in CM-DILI and 28,069 SNPs in HC-DILI were selected (PRSgw).
Additionally, we developed CM-DILI PRSgw+ from CM-DILI GWAS summary statistics expanding out the data set to 928 European CM-DILI cases owing to inclusion of 157 flucloxacillin- and 320 amoxicillin–clavulanate-treated patients with DILI who had not been included in the GWAS sets for the CM-DILI PRSgw13. For accurate evaluation of TAK-875 DILI, we used 87,424 SNPs that were clumped by European LD after filtering for imputation accuracy (Rsq ≥0.7) in TAK-875 GWAS sets to maximize the predictive accuracy for TAK-875 GWAS data sets. Next, in scoring PHHs and iPSCs, we used 40,396 SNPs that were clumped by the LD after selecting Hapmap3 SNPs, because the discovery GWAS used a reference panel (Haplotype Reference Consortium) that was different from our available LD data set (1KG European).
GWAS enrichment analysis.
Enrichment analysis was performed using processed iDILIC–DILIN GWAS summary statistics. GWAS analysis of regulatory or functional information enrichment with LD correction (GARFIELD)8 was performed using the default annotation data of the software, using R package garfield v1.0.2 under R 3.3.3. Pathway scoring algorithm (Pascal), integrating GWAS signals from multiple SNPs9, was performed using Pascal software (downloaded on 6 June 2017, from https://www2.unil.ch/cbg/index.php?title=Pascal). For Pascal analysis, we used the Molecular Signature Database (MSigDB) v6 gene sets38,39 as pathway information and default parameters of Pascal: a maximum of 3,000 SNPs were assigned to a gene, and their average GWAS signal was used for gene scoring, followed by pathway empirical P values obtained by their aggregation, considering LD in the European population.
Cell cultures.
PHHs were obtained from vendors (Lonza, KAC and Sekisui Medical) listed in Supplementary Table 4 and cultured in Hepatocyte Plating Medium (Lonza). Hepatocyte Culture Media (Lonza) was used for thawing and daily sub-culturing. PHHs were plated on Lumox plates (Sarstedt) coated with 5 μg cm−2 rat tail type I collagen (Corning) in 0.02 mol L−1 acetic acid. PHHs were plated at 4 × 105 cells per well on a 24-well-type Lumox multi-well plate (Sarstedt) for DNA and RNA extraction and 5 × 104 cells per well on a 96-well-type Lumox multi-well plate for drug sensitivity assays. After 24 h of preculture, cells were treated with LCA or 1% DMSO for 1 h. After washing with phosphate-buffered saline, cells were treated with the indicated drugs for viability assay and transcriptome analysis.
A human iPSC line, 1383D2, was kindly provided by the Center for iPS Cell Research and Application at Kyoto University. Other human iPSC lines (CW10027, 73-B, 75-A, 10-A and 45-A) were obtained from the Coriell Institute or the European Bank for Induced Pluripotent Stem Cells. All iPSCs used in this study had normal karyotypes (data not shown). All iPSCs were cultured on laminin 511 E8-fragment-coated (iMatrix-511, Nippi) dishes in StemFit AK02N (Ajinomoto). Directed differentiation methods for each lineage were described previously13. To generate iPSC-HLOs in vitro, 1.8 × 106 cells per microwell at a ratio of 10:7:1 (human iPSC-hepatic endoderm (HE)/iPSC-endothelial cell (EC)/iPSC-mesenchymal cell (STM)) were resuspended in a mixture of endothelial cell growth medium (Lonza) and HCM containing dexamethasone (0.1 mM; Sigma-Aldrich), oncostatin M (10 ng ml−1; R&D Systems), hepatocyte growth factor (20 ng ml−1; PromoKine) and SingleQuots (Lonza) and plated on six-well plate of the Elplasia micro-space cell culture plate (Kuraray). After 24 h, the generated iPSC-HLOs were harvested via gentle pipetting and transferred into a single-use bioreactor culture system (30 ml; ABLE) and cultured in the same media. Further, after differentiation induction for over 10 d, viability testing and transcriptome after drug addition were performed. Viability testing and transcriptome were performed on iPSC-HLOs after hepatic differentiation for 10 d and more.
Drug treatments.
DILI drug compounds were dissolved in DMSO to a final concentration of 1% in cell culture medium. Each drug was exposed in three doses for 24 or 72 h with or without LCA 1-h pre-treatment. The following DILI drugs used in this study were categorized into two types: CM-DILI drugs—cyclosporin A (Wako), carbamazepine (Tokyo Chemical Industry), ketoconazole (Sigma-Aldrich), troglitazone (Wako), bosentan (Toronto Research Chemicals), flutamide (LKT Laboratories), diclofenac (Wako), amoxicillin–clavulanate (amoxicillin, Wako; clavulanate, Matrix Scientific) and methapyrilene (Toronto Research Chemicals); and HC-DILI drugs—tacrine (Cayman), tolcapone (Tocris Bioscience) and acetaminophen (Toronto Research Chemicals).
Transcriptome analysis.
Total RNA was extracted from cultured cells, using a PureLink RNA Mini Kit (Thermo Fisher Scientific). A cDNA library was generated from 10 ng of total RNA, using a SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific). Amplification, primer digestion and adapter ligation were performed using an Ion AmpliSeq Transcriptome Human Gene Expression Kit (Thermo Fisher Scientific). The cDNA library was purified using Agencourt AMPure XP Reagent (Beckman Coulter) and quantified using an Ion Library TaqMan Quantitation Kit (Thermo Fisher Scientific), followed by dilution to 75 pM with water and pooled equally. Eight samples per pool were sequenced using an Ion 540 Chip Kit (Thermo Fisher Scientific) simultaneously, using IonS5 XL (Life Technologies) and Ion Chef instrument systems (Life Technologies) with Ion 540 Kit-Chef (Life Technologies). All procedures were performed per the manufacturers’ protocols. From the sequencing output, quality control, alignment reads on hg19 (hg19_AmpliSeq_Transcriptome_21K_v1.bed), read counts and the normalization (reads per million) for each gene were obtained using the Torrent ampliSeqRNA plugin (hg19 AmpliSeq Transcriptome ERCC v1) in Torrent Suite Software v5.2.1.
Gene set enrichment analysis (GSEA) for PHH models was performed using GSEA software (v2.2.3, Broad Institute) in the pre-rank mode38, using previously described gene sets. The permutation time of GSEA was set to 10,000 with other default parameters.
In-house whole-genome genotyping and PRS calculation.
DNA from iPSC and PHH cells was extracted using a PureLink Genomic DNA Mini Kit (Thermo Fisher Scientific) per the manufacturer’s protocols. These samples were genotyped using Infinium OmniExpressExome-8 v1.4 BeadChip (Illumina). Corresponding manifest files of the genotyping array were obtained from Illumina. Quality control analysis, haplotype phasing and genotype imputation the genotyped data were performed along with the TAK-875 GWAS. Notably, Hardy–Weinberg equilibrium was not assessed owing to the small sample size; outliers of the known-ancestry cluster were assessed via principal component analysis for SNPs, using Hapmap3 release3 (ref. 40). After genotype imputation using the 1KGP3 reference panel, genotype dosage of variants with a Minimac3 imputation quality (Rsq) ≥0.7 in a batch was used for calculating PRS similar to previously described criteria.
PHH viability assay.
PHH viability was determined using a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega) per the manufacturer’s protocol. After pre-treatment with LCA (Sigma-Aldrich), relative cell viability was determined as a ratio between cell viability of treated LCA-pretreated samples and that of control LCA-pretreated samples. Viability upon LCA treatment was calculated as the ratio of cell viability of control LCA-pretreated samples and that of control non-pretreated (1% DMSO treated) samples. Cell viability was determined for each experimental batch (n = 3 in a condition; n = 1–3 batches from available cryopreserved PHH vials).
iPSC-HLO viability assay.
iPSC-HLO cell viability after drug treatment was determined using a CellTiter-Glo 3D Cell Viability Assay (Promega) per the manufacturer’s protocol. For drug evaluation, over 100 iPSC-HLOs were dispensed per one well of low attached 96-well plate. Raw luminescence data were normalized via bright-field images with Cell3iMager duos (SCREEN Holdings) and dividing luminescence values in the area.
Live imaging.
Indicated molecules or proteins were stained using live cell imaging kits or antibodies, per the manufacturer’s instructions, as follows: dead cells, LIVE/ DEAD Cell Imaging Kit (488/570) (Thermo Fisher Scientific); bile acid transport, CLF (BD Biosciences Discovery Labware); reactive oxygen species, CellROX green reagents (Thermo Fisher Scientific).
Immunostaining.
iPSC-HLOs were fixed in 4% paraformaldehyde (Wako) in phosphate-buffered saline for 15 min. Samples were blocked with donkey serum (Millipore) and probed with primary antibodies against albumin (Sigma-Aldrich), BSEP (Sigma-Aldrich), CD31 (BD Biosciences), CHOP (Santa Cruz Biotechnology) and HNF4a (Santa Cruz Biotechnology) at 4 °C overnight. Samples were probed with secondary antibodies conjugated with Alexa Fluor (Life Technologies) and DAPI (Sigma-Aldrich) for nuclear staining. Images were acquired using LSM 880 with Airy scan (Zeiss).
Immunoblot.
Drug-treated iPSC-HLOs were suspended in Laemmli sample buffer (BioRad). Proteins were resolved via SDS-PAGE and electro-transferred onto PVDF membranes (BioRad). Western blotting was performed per standard protocols, and blotted protein samples were blocked with Blocking One (Nacalai) for 1 h. Samples were incubated with anti-KDEL (Enzo), anti-XBP1-s (Cell Signaling) and anti-GAPDH (Cell Signaling) primary antibodies at 4 °C overnight and probed with horseradish peroxidase-conjugated secondary IgG (Cell Signaling) for 1 h. Signals enhanced by ECL Prime (GE Healthcare) were detected using the ChemiDoc Touch imaging system (BioRad). Protein expression levels were measured by Image Lab software (BioRad).
ELISA.
Human albumin was quantified using a Human Albumin ELISA Quantitation Kit (Bethyl Laboratories) per the manufacturer’s protocols.
CYP3A4 activity.
Cell-based CYP3A4 activity of iPSC-HLOs was measured using P450-Glo CYP3A4 Assay (Promega) per the manufacturer’s protocols. Raw luminescence data were normalized using bright-field images with Cell3iMager duos and dividing luminescence values in the area.
Raw luminescence data were normalized to cell numbers by dividing the P450-Glo Assay values by CellTiter-Glo 3D Cell Viability Assay (Promega).
GSH/GSSG ratio.
Changes in GSH/GSSG, oxidative stress indicator, in iPSC-HLOs under drug treatment were quantified via a luminescence-based GSH/GSSG-Glo assay (Promega) per the manufacturer’s instructions.
Transcriptome analysis of multi-donor liver tissues.
Genotypes and transcriptome data were obtained from ref. 17 via https://www.synapse.org/#!Synapse:syn4492. From the curated SNP array data set including 349,085 variants and 195 donors, we further performed the following quality controls: exclude variants with a genotype call rate less than 95%, a Hardy P value < 10−6 and A/T or G/C SNPs; exclude individuals with non-European ancestry. From the remaining 213,257 variants and 161 donors, we phased and imputed variants as described above and calculated their PRS. From DNA microarray-based transcriptome data sets of liver tissue (40,638 transcripts and 467 donors), we excluded >10% missing donors and ≥1 missing transcripts and aggregated the probe-level data sets into gene-level data sets by replacing with their average (25,015 transcripts and 157 donors remained). From the overlapped 156 donors, we calculated the coefficient effect of PRSgw on each transcript by regressing out the five first transcriptomic principal components to exclude the effects of batch effects in the transcriptome data sets. Pre-ranked GSEA analysis on Reactome gene sets was performed by using the ranking of their t-statistics.
Re-analysis of TG-GATEs.
Transcriptome data of PHHs under drug treatments (2,605 data points including 158 types of drugs, three types of concentration and three types of exposure times) measured by GeneChip Human Genome U133 Plus 2.0 array (Affymetrix) were obtained from the TG-GATEs website (https://toxico.nibiohn.go.jp/english/index.html )19. We carried out normalization of expression levels using Frozen Robust Multiarray Analysis using the R package ‘frma’ (version 1.14.0)8 with default parameters and took average expression levels within replicates. We calculated a signal intensity ratio to each control data for each probe, took an average value among probe sets for each gene and made a gene rank matrix for pre-ranked GSEA analysis.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The AmpliSeq data used in this study are deposited in the Gene Expression Omnibus under accession number GSE152447. The genotype data for TAK-875 DILI GWAS, the related phenotype information and the summary statistics are stored at Takeda Pharmaceutical Company, owing to the ethical approval in this study. These data sets are available upon reasonable request to Takeda Pharmaceutical Company via the corresponding author and after being approved by the Ethics Committee of Takeda Pharmaceutical Company. Web links of publicly available data sets are as follows: transcriptome data of PHH under drug treatments (TG-GATEs), https://toxico.nibiohn.go.jp/english/index.html; transcriptome data of multi-donor liver tissues, https://www.synapse.org/#!Synapse:syn4492; transcriptome data of PHH (SRR4000958); GENCODE (v26), https://www.gencodegenes.org; MSigDB (v6), https://www.gsea-msigdb.org/gsea/msigdb/index.jsp; and 1KGP3 imputation reference panel, https://genome.sph.umich.edu/wiki/Minimac3. Source data are provided with this paper.
Code availability
We used publicly available software and parameters as described in the Methods section for the analysis. The software programs are available from the following URLs: PLINK, https://www.cog-genomics.org/plink2/; Minimac3, https://genome.sph.umich.edu/wiki/Minimac3; SNPTEST, https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html; FaQCs, https://github.com/chienchi/FaQCs; PRSice, http://PRSice.info; STAR, https://github.com/alexdobin/STAR; Cufflinks, http://cole-trapnell-lab.github.io/cufflinks/; GARFIELD, https://www.ebi.ac.uk/birney-srv/GARFIELD/; Pascal, https://www2.unil.ch/cbg/index.php?title=Pascal; GSEA, https://www.gsea-msigdb.org/gsea/; and R, https://cran.r-project.org/.
Extended Data
Extended Data Fig. 1 |. Overview of our polygenicity analysis.
Workflow of shared genetic aetiology analysis was shown. Left: Processing strategy of iDILIC/DILIN GWAS summary statistics (summary data); Right: TAK-875 GWAS procedures.
Extended Data Fig. 2 |. TAK-875 DILI severity and GWAS analysis.
a, Ratio of ALT, AST, and BILT peak values to their basal values. b, Distribution of time from start of drug to DILI onset (days). c, Exclusion criteria of TAK-875 samples based on genetic ancestors. d, The outlier of TAK-875 samples was not observed in Northern Europeans from Utah (CEU), Tuscans from Italy (TSI) and Mexican (MEX) ancestry. e, Quantile-Quantile plot for the TAK-875 GWAS. f, g, Polygenic test using hepatocellular DILI-GWAS summary statistics in (a) and All DILI-GWAS ones in (b). X-axis, the total number of SNPs, ordered by iDILIC/DILIN GWAS association; y-axis, explained the variance of TAK-875 white GWAS; color scale, p-value for the shared genetic aetiology analysis.
Extended Data Fig. 3 |. Distribution and performance of each PRS.
a, Distribution of CM-DILI PRSlimited in TAK-875 DILI patients and the tolerances (see Fig. 1 and Supplementary Text). b, AUROC values and their 95% confidence interval for the indicated PRS. c, Histogram of the indicated PRS.
Extended Data Fig. 4 |. correlation between CM-DILI PRSgw and biomarkers for DILI in TAK-875 treated subjects.
Scatter plot and the linear regression line of clinical laboratory test value in basal state and CM-DILI PRSgw in 172 TAK-875 treated patients (cases and controls). *, p < 0.05; **, p < 0.01, ***, p < 0.001 in coefficients of risk score. ALTB, Basal ALT; ASTB, Basal AST; BILTB, Basal total bilirubin.
Extended Data Fig. 5 |. Predictive accuracy of CM-DILI PRSgw for Flucloxacillin or Amoxicillin-clavulanate DILI.
Distribution of CM-DILI PRSgw and HC-DILI PRSgw in Flucloxacillin or Amoxicillin-clavulanate DILI patients with the indicated DILI type (cholestasis/mixed or hepatocellular). AUROC (95% CI) and P-value of two-tailed Wilcoxon–Mann–Whitney U test were shown.
Extended Data Fig. 6 |. GARFIELD plot of CM-DILI GWAS summary statistics in cirulli et al., 2019.
a, Chromatin state, b, FAIR-seq, c, ENCODE DNase1 footprints, d, Genic annotation.
Extended Data Fig. 7 |. Multi-donor iPSc-HLO cholestatic DILI assays.
a, Bright field images of multi-donor iPSC and iPSC-HLO. b, Immunofluorescence staining of Albumin (ALB), BSEP, CD31 and HNF4a in multi-donor iPSC-HLO. c, ALB production during 24 hours in multi-donor iPSC-HLO. Data represent means ± SD (n = 3). d, CYP3A4 activity. Data represent means ± SD (n = 3). e, CLF accumulation and cell death signal in 1383D2 iPSCs derived iPSC-HLOs under CsA treatment for 24hr and 72hr. f, Viability after 24hr (dotted line) and 72hr (black line) CsA treatment in iPSC-HLO model. The ATP levels were normalized by the area of iPSC-HLO (see Materials and Methods).
Extended Data Fig. 8 |. Transcriptomic expression profiling of PHH cells at basal state.
a-g, Basal expression levels of mRNA were shown by the indicated signature of gene sets. Color scale and sample annotation colors were shown in the bottom right. (a) Liver signature genes1. (b) Signature of gene set characterizing liver sinusoidal endothelial cell (LSEC)2. (c) Signature of gene set characterizing hepatic stellate cell (HSC)2. (d) all drug transport proteins and proteins involved in bile transport and cholestasis (TCP, OATPs, OCT1, OAT2, CNT1, CNT2, ENT1, ENT2, MDR1, MDR3, MRP2, BSEP, BCRP, MATE1, MRP3, MRP4), mRNA coding proteins of N; (e-g) mRNA coding phase 1, 2, and 3 enzymes3. The Z-scores of log2(RPM+1) values were calculated within the indicated samples. Gene with RPM = 0 in all of the samples were excluded. PHH_1day_1/_2, PHH cells after 1 day of culturing (suffix means experimental batch), which was used for assessments of drug-induced transcriptomic change; PHH_2d, PHH cells after 2days of culturing; FLT, fetal liver tissue; ALT, adult liver tissue.
Extended Data Fig. 9 |. reproducibility of drug toxicity assay of PHH cells under LCA pretreatment.
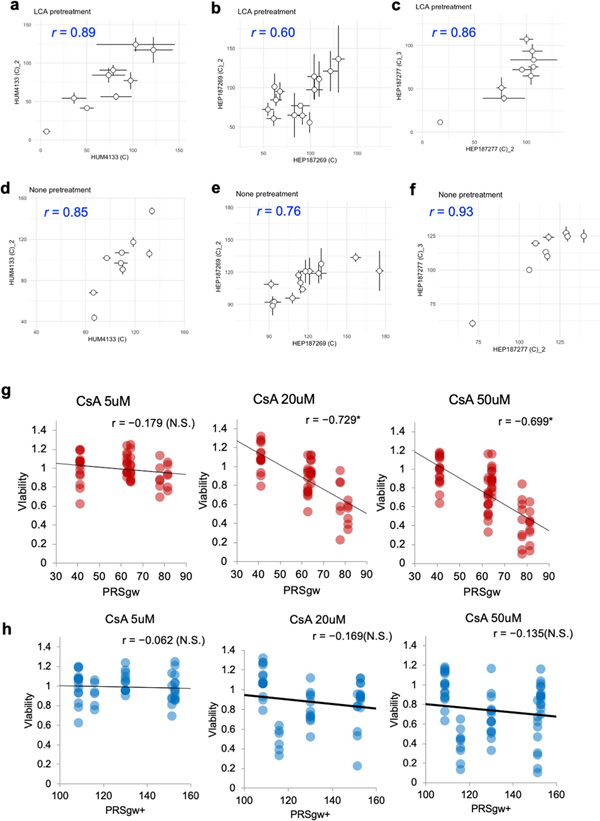
a–c, Drug sensitivity comparison between different days’ experiments under LCA pretreatments. (a) HUM4133. (b) HEP187269. (c) HEP187277. d–f, Drug sensitivity comparison between different days’ experiments under none pretreatments. (d) HUM4133. (e) HEP187269. (f) HEP187277. (g, h) Viability comparison of multi-donor iPSC-HLO models under the indicated CsA treatment without LCA. Pearson’s r for correlation with PRSgw (g) or PRSgw+ h), and its P-value is described. *, p<0.05. We regress mean viability for each donor by PRSgw or PRSgw+ and calculated the P-values.
Extended Data Fig. 10 |. Decreased mitochondria activity by cholestatic DILI drug treatment.
Live staining for TMRM and mitochondria levels (mitotracker) in 1383 iPSC-HLO model under CsA treatment for 72hr.
Supplementary Material
Acknowledgements
We thank S. Yamanaka, S. Izumo and Y. Kajii for their critical comments. We thank T. Kono, K. Araki, K. Enya and H. Kawaguchi for technical and analytical supports and W. L. Thompson for critical reading of the manuscript. The authors thank the Drug Induced Liver Injury Network (DILIN) and the international Drug-Induced Liver Injury Consortium (iDILIC) for providing data included in this paper. The DILIN and iDILIC were not involved in data analyses, manuscript preparation or manuscript review. This study was supported by the T-CiRA Joint Program from Takeda Pharmaceutical Company to T.T. T.T. is a New York Stem Cell Foundation Robertson Investigator and also the recipient of a Cincinnati Children’s Research Foundation grant, National Institutes of Health grant UG3 DK119982, the Dr. Ralph and Marian Falk Medical Research Trust Awards Program, a Takeda Science Foundation award, a Mitsubishi Foundation award and AMED JP19fk0210037, JP19bm0704025, JP19fk0210060, JP19bm0404045 and JSPS JP18H02800, 19K22416. G.P.A. was supported by Medical Research Council: Confidence in Concept, reference number MC_PC_17173.
T.T. received research funding related to this project from Takeda Pharmaceutical Company. M.O., E.K., Y. Nio, T.S., Y.D. and H.A. are employees of Takeda Pharmaceutical Company. P.N. is an employee of Sema4. The remaining authors declare no competing interests.
Footnotes
Competing interests
Extended data is available for this paper at https://doi.org/10.1038/s41591-020-1023-0.
Supplementary information is available for this paper at https://doi.org/10.1038/s41591-020-1023-0 .
Peer review information Kate Gao was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chalasani N et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 148, 1340–1352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicoletti P et al. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology 152, 1078–1089 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khera AV et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 50, 1219–1224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulik-Sullivan B et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burant CF et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379, 1403–1411 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Marcinak JF, Munsaka MS, Watkins PB, Ohira T & Smith N Liver safety of fasiglifam (TAK-875) in patients with type 2 diabetes: review of the global clinical trial experience. Drug Saf. 41, 625–640 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Wolenski FS et al. Fasiglifam (TAK-875) alters bile acid homeostasis in rats and dogs: a potential cause of drug induced liver injury. Toxicol. Sci. 157, 50–61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCall MN, Bolstad BM & Irizarry RA Frozen robust multiarray analysis (fRMA). Biostatistics 11, 242–253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iotchkova V et al. GARFIELD classifies disease-relevant genomic features through integration of functional annotations with association signals. Nat. Genet. 51, 343–353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamparter D, Marbach D, Rueedi R, Kutalik Z & Bergmann S Fast and rigorous computation of gene and pathway scores from SNP-based summary statistics. PLoS Comput. Biol. 12, 1–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kass GEN & Price SC Role of mitochondria in drug-induced cholestatic injury. Clin. Liver Dis. 12, 27–51 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Vatakuti S, Olinga P, Pennings JLA & Groothuis GMM Validation of precision-cut liver slices to study drug-induced cholestasis: a transcriptomics approach. Arch. Toxicol. 91, 1401–1412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirulli ET et al. A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology 156, 1707–1716 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takebe T et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 21, 2661–2670 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Ogimura E, Sekine S & Horie T Bile salt export pump inhibitors are associated with bile acid-dependent drug-induced toxicity in sandwich-cultured hepatocytes. Biochem. Biophys. Res. Commun. 416, 313–317 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Delaneau O et al. Chromatin three-dimensional interactions mediate genetic effects on gene expression. Science 364, eaat8266 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Schadt EE et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 6, 1020–1032 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hybertson BM, Gao B, Bose SK & McCord JM Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Asp. Med. 32, 234–246 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Igarashi Y et al. Open TG-GATEs: a large-scale toxicogenomics database. Nucleic Acids Res. 43, D921–D927 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaliyaperumal K et al. Pharmacogenomics of drug-induced liver injury (DILI): molecular biology to clinical applications. J. Hepatol. 69, 948–957 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Chen M et al. Drug-induced liver injury: interactions between drug properties and host factors. J. Hepatol. 63, 503–514 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Daly AK et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 41, 816–819 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Lucena MI et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology 141, 338–347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: drug-induced liver injury. J. Hepatol. 70, 1222–1261 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Fredriksson L et al. Drug-induced endoplasmic reticulum and oxidative stress responses independently sensitize toward TNFα-mediated hepatotoxicity. Toxicol. Sci. 140, 144–159 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Burban A, Sharanek A, Guguen-Guillouzo C & Guillouzo A Endoplasmic reticulum stress precedes oxidative stress in antibiotic-induced cholestasis a nd cytotoxicity in human hepatocytes. Free Radic. Biol. Med. 115, 166–178 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Fabregat A et al. The reactome pathway knowledgebase. Nucleic Acids Res. 46, D649–D655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 28.Gibbs RA et al. A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CC et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh P-R et al. Reference-based phasing using the haplotype reference consortium panel. Nat. Genet. 48, 1443–1448 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das S et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchini J & Howie B Genotype imputation for genome-wide association studies. Nat. Rev. Genet. 11, 499–511 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Euesden J, Lewis CM & O’Reilly PF PRSice: polygenic risk score software. Bioinformatics 31, 1466–1468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asai A et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development 144, 1056–1064 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo C-C & Chain PSG Rapid evaluation and quality control of next generation sequencing data with FaQCs. BMC Bioinf. 15, 366 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapnell C et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian A et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberzon A et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frazer KA et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The AmpliSeq data used in this study are deposited in the Gene Expression Omnibus under accession number GSE152447. The genotype data for TAK-875 DILI GWAS, the related phenotype information and the summary statistics are stored at Takeda Pharmaceutical Company, owing to the ethical approval in this study. These data sets are available upon reasonable request to Takeda Pharmaceutical Company via the corresponding author and after being approved by the Ethics Committee of Takeda Pharmaceutical Company. Web links of publicly available data sets are as follows: transcriptome data of PHH under drug treatments (TG-GATEs), https://toxico.nibiohn.go.jp/english/index.html; transcriptome data of multi-donor liver tissues, https://www.synapse.org/#!Synapse:syn4492; transcriptome data of PHH (SRR4000958); GENCODE (v26), https://www.gencodegenes.org; MSigDB (v6), https://www.gsea-msigdb.org/gsea/msigdb/index.jsp; and 1KGP3 imputation reference panel, https://genome.sph.umich.edu/wiki/Minimac3. Source data are provided with this paper.