Abstract
Studying the pathophysiology of sepsis still requires animal models, and the mouse remains the most commonly used species. Here we discuss the “cecal slurry” (CS) model of polymicrobial, peritoneal sepsis and compare and contrast it to other commonly used methods. Among the different murine models of sepsis, cecal ligation and puncture (CLP), and not the CS, is often considered the “gold standard” to induce polymicrobial sepsis in laboratory animals. CLP is a well-described model involving a simple surgical procedure that closely mimics the clinical course of intra-abdominal sepsis. However, CLP may not be an option for experiments involving newborn pups, where the cecum is indistinguishable from small bowel, differences in microbiome content, or where surgical procedures/anesthesia exposure needs to be limited. An important alternative method is the cecal slurry (CS) model, involving the intraperitoneal injection of cecal contents from a donor animal into the peritoneal cavity of a recipient animal to induce polymicrobial sepsis. Furthermore, CS is an effective alternative model of intraperitoneal polymicrobial sepsis in adult mice and can now be considered the “gold standard” for experiments in neonatal mice.
Keywords: infection, cecal slurry, sepsis, polymicrobial sepsis, neonatal sepsis, murine sepsis
1). Introduction
Despite significant advances in critical care medicine, sepsis represents a major public health challenge worldwide (1, 2). Although the global epidemiological burden of sepsis is difficult to completely ascertain, the World Health Organization (WHO) estimates that more than 30 million people worldwide are diagnosed with sepsis leading to 6 million deaths per year (3). Sepsis is particularly lethal at the extremes of age (i.e. neonatal period and in older adults). Neonates, particularly pre-term (<37 weeks gestation) and low-birth weight (<2500g) infants are left vulnerable to sepsis due to multiple defects in host protective immunity (4, 5). The incidence of sepsis in neonates and children is estimated to account for 3 million and 1.2 million cases, respectively (6).
Importantly, the management of sepsis and its long-term morbidity is the most expensive medical condition treated in the US healthcare system (7). As sepsis is a substantial patient and financial burden on healthcare systems, it is imperative that we understand its complex pathophysiology so that novel therapeutic targets and effective treatment strategies can be developed. Currently, the use of animal models for the study of sepsis remains an important component of the translational research.
Promising therapeutic strategies to treat sepsis have been developed in preclinical studies using a murine model (mouse; Mus musculus) (8). While it has been argued that animal models do not fully recapitulate the human condition in sepsis (9, 10), it remains an important translational research tool (11, 12). Challenges with using murine models for sepsis research include their reduced responsiveness to microbial products, their different blood leukocyte profile, and the inability to support organ function during critical illness (13). Despite these limitations, murine models of sepsis have been helpful at better understanding individual molecular mechanisms underlying the complex pathophysiology of sepsis. The mouse’s uniform genetic background, availability of various strains, high fecundity, ease of handling, and familiarity within the research community have made it the model of choice (11).
Historically, murine sepsis models relied on the intravenous or intraperitoneal administration of either endotoxin or individual strains of live bacteria (14, 15). These models produced massive non-physiologic increases in most inflammatory mediators associated with hypovolemia and cardiac failure and rapid death in these animals (13). Although these models continue to be used, they are not frequently intended to replicate human sepsis in any but the most unusual circumstances.
More recently, different animal models of intraperitoneal sepsis have been developed, in most of which systemic inflammation is initiated by a local intra-abdominal infection. Among the murine models of intraperitoneal sepsis, cecal ligation and puncture (CLP), is one of the most frequently used and generally accepted as the “gold standard”. CLP is a well understood surgical procedure that is thought to closely resemble the progression and characteristics of the complexity of human sepsis (16). Despite its wide spread use, the CLP model often does not satisfy one critical aspect of sepsis management: source control (17). Despite antibiotic treatment, the presence of a devitalized cecum assures failure of any antibiotic intervention to completely resolve the infection, and involves both abscess formation and chronic inflammation. Complete recovery in this model cannot be assured.
There are a number of alternatives to a CLP, including a cecal stent (colon ascendens stent peritonitis, CASP), inoculation of the peritoneum with standardized amounts of bacteria in fibrin clots or Matrigel™, or administration of fecal contents (13, 18). Cecal stents have the advantage of producing persistent peritonitis, and does not involve a devitalized cecum (19). However, like the CLP model, resolution of the infectious process requires surgical intervention to obtain source control. An infected blood clot eliminates the need for source control, but is technically challenging and difficult to reproduce. Some investigators have used it for single species infections, but this method has not been widely embraced in the research community.
An alternative method to CLP has been proposed involving the intraperitoneal administration of the cecal contents of a euthanized animal, into another animal to induce polymicrobial sepsis (11, 20–22). This offers a number of theoretical and technical advantages. Cecal contents contain not only microbes but also particulate matter that assists with bacterial colonization of the peritoneum. In an untreated animal, bacterial colonies can be recovered transiently from the blood, and persistently in the peritoneum and visceral organs. In contrast to CLP, appropriate antibiotic interventions can completely eliminate mortality and bacterial colonization, and source control is not required.
The cecal slurry (CS) model has become the preferred method by investigators studying sepsis in neonatal mice, as it is an infectious model characterized by bacterial colonization, systemic inflammation, and dose-dependent mortality without surgery, which may be lethal in murine neonatal pups (20, 23–28). This model is designed to more closely replicate the human neonatal condition of necrotizing enterocolitis (NEC), a devastating condition predominantly in preterm neonates. CS has been widely used by numerous laboratories and is now considered the “gold standard” model for murine neonatal sepsis research. Apart from avoiding surgery in newborn mice, CS-induced sepsis can be utilized in a large number of animals from a single CS donor and mimics polymicrobial sepsis often seen in neonatal bowel perforation. Additionally, the technique is acceptably reproducible, involves no surgical manipulation and is easy to perform by researchers. The latter is particularly important as CLP or cecal stent in a neonate would require a level of surgical expertise under a microscope or magnifying glass not present in most laboratories. Additionally, these methods would add significant time constraints to be able to efficiently conduct these experiments in a humane and successful manner under anesthesia.
It is important to note, however, that any peritoneal sepsis model, whether CLP, cecal stent, or CS produces a unique response to severe infection that is both dose dependent and in which the severity of the sepsis can be modified. For example, we evaluated the inflammatory cytokine and genomic response in juvenile mice to a similarly lethal CLP and CS, and found that not only were the patterns of inflammatory cytokine responses different between the two models, but the genomic changes from blood leukocytes were dramatically different, but reproducible (11). Despite a comparable mortality, signaling pathways activated by CLP and CS are different. While the CS model results in early inflammatory response, CLP is characterized by down regulation of T cell activation and increased expression of immune suppressive pathways (11).
2). Materials
All surgical instruments (stainless steel forceps, straight-edge scissors, spatula) should be heat-sterilized as would occur in any surgical procedure. However, because harvesting of the fecal contents is a non-survival procedure and considered ‘dirty’, instruments may be sterilized using a preheated hot bead sterilizer for 10 to 20 seconds (Caution: do not leave instruments longer than 20 seconds as they will become too hot to handle safely and will cause thermal injury).
2.1. Animals
Polymicrobial sepsis induced by CS can be administered in neonatal mice (5–7 days-old), young adult (6–12 weeks) or elderly (>18 weeks) mice, housed under specific pathogen-free conditions. (Note 1)
2.2. Cecal slurry preparation
Cecal slurry (CS) donors: 6–12 weeks old female mice to induce neonatal sepsis and age matched CS donors to induce sepsis in young adult mice or aged mice
CO2 chamber
Straight-edge scissor, forceps and spatula
Sterile 5% dextrose
70% ethanol
Sterile petri dish
15 mL conical polypropylene centrifuge tube
Scale
2.3. Cecal slurry injection
Conventional insulin syringes (1 mL)
Needles 25G × 5/8”, regular bevel
Scale
Heating pad
3). Methods
3.1. Cecal slurry preparation.
Euthanize the CS donor mouse by hypoxia, exposing it to 10–30% CO2 inhalation following AVMA Guidelines for euthanasia of animals until respiration and heartbeat have ceased. An adjunctive physical method (e.g., exsanguination, cervical dislocation) should be used to assure death in an already euthanized animal. (Note 2)
Fix the mouse to a polystyrene foam board and spray the lower abdomen with 70% ethanol. Using a straightedge scissor and a forcep, open the skin and the abdominal musculature to localize the cecum switching to clean instruments in between. Once the abdominal cavity is open, localize the cecum (comma-shaped blind sac-like organ) at the junction of the ileum and the proximal colon. Excise the cecum and place it on a sterile disposable petri dish. (Figure 1)
Using sterile forceps and a spatula, extrude the cecal contents out of the epithelium onto a pre-weighted 15 mL conical polypropylene centrifuge tube.
Weigh the cecal contents and prepare 80–100 mg/mL CS with dextrose 5%.
Vortex the CS solution for 20 – 30 seconds to make a homogenous suspension. (Figure 2)
To ensure maximal effectiveness of the CS, it is recommended to use the solution within 2 hours of preparation. (Note 3)
1.
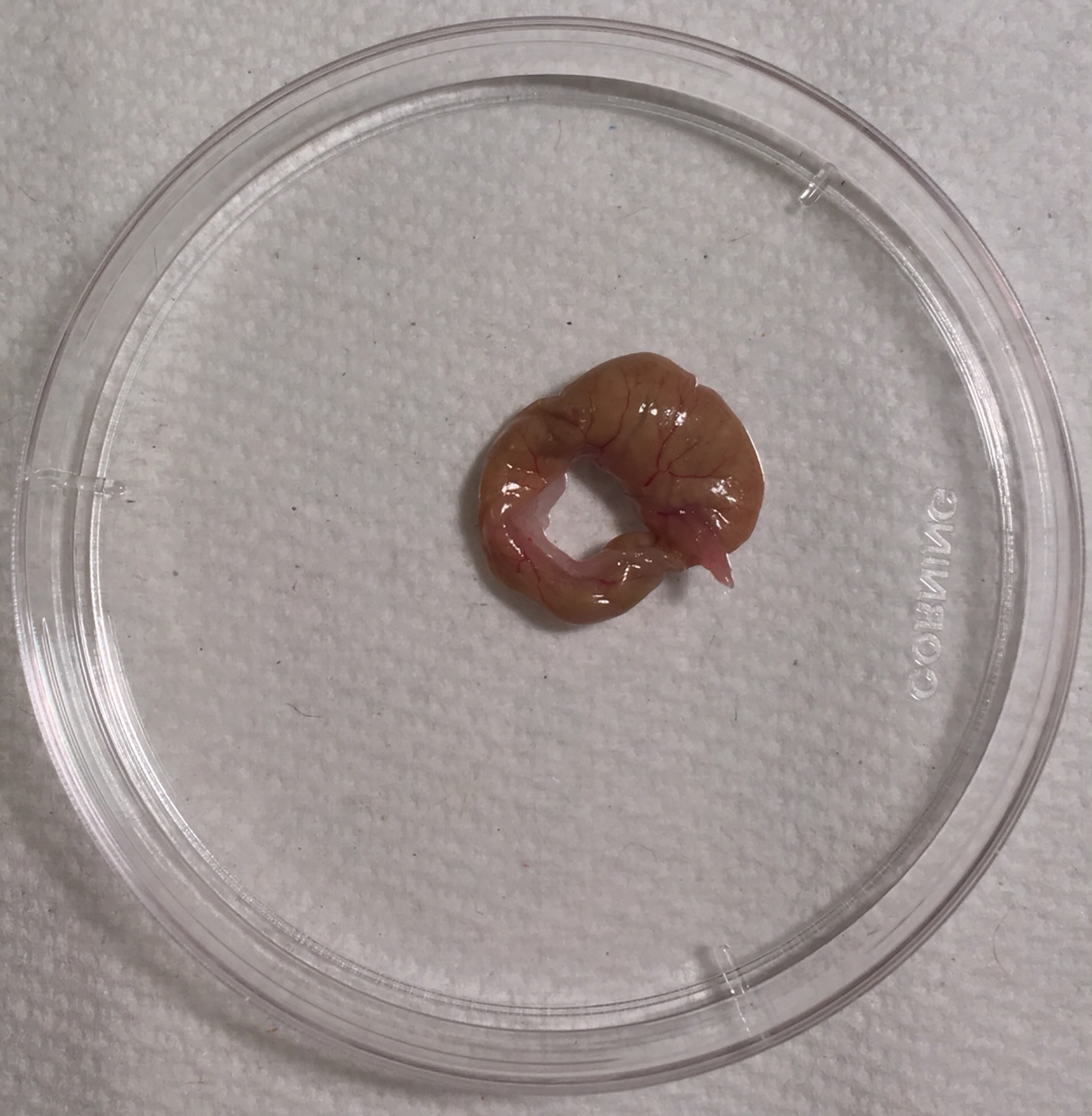
Excised cecum aseptically collected from a mouse donor. Following euthanasia of the CS donor mouse, open the skin and localize the cecum at the junction of the ileum and the proximal colon. Excise the cecum and place it on a sterile disposable petri dish.
2.

Cecal slurry solution (80 mg/mL in 5% dextrose). Extrude the cecal contents out of the epithelium onto a pre-weighted conical polypropylene centrifuge tube, and prepare 80–100 mg/mL CS with dextrose 5%.
3.2. Cecal slurry injection in neonatal mice.
The age of a neonatal mouse can be determined by their physical characteristics during the first week of life and varies according to the strain. To better record the day of birth, pregnant dams may be monitored daily 2–5 days prior delivery. For experiments with neonatal mice, mixed gender pups aged 5–7 days are challenged with CS to induce polymicrobial sepsis.
Separate the litter from the dam by placing them in a new cage. Leave part of the nest on the cage to reduce anxiety to the dam and avoid behavioral changes (e.g. neglect of the pups. (Note 4)
If pups are to receive pre-treatment interventions prior to sepsis mark the tail using a permanent marker. (Note 5)
Weigh each pup to calculate the weight-adjusted dose of the CS solution (80 mg/mL) to induce the required lethality. (Note 6)
Depending on the number of litters to be challenged by CS, fill the insulin syringes with up to 200 – 300 μL of CS solution.
Using the thumb and index fingers, secure the pup by the back of the neck and the lower back.
Insert the needle subcutaneously with the bevel facing up in the lower abdomen (Figure 3). Inject the weight-adjusted dose of CS (1.1–1.3 mg/g BW), waiting 10 seconds to ensure that the correct CS dose is delivered. Carefully withdraw the needle to avoid spillage or injury to the pup. Placing the injected pup on a paper towel will help to determine any CS leakage or bleeding following injection. (Note 7)
Once all pups have been injected and monitored, return the litter to the cage with the dam.
Following CS injection, neonatal mice should be monitored following the local Institutional Animal Care and Use Committee approved protocol. (Note 8) In general, following standard CS dosing, monitor all injected neonatal mice for the next 2 hours to identify any injections-related complications; after that, neonatal mice should be monitored at a minimum every 12 hours to identify post-sepsis morbidities (scattering, absence of milk spot). Scattering is when the mother abandons the pup and it is physically separated from the mother and other pups. Scattering, poor feeding including absence of a milk spot, and/or dam neglect often indicates that the pup is moribund will not survive, and therefore should be euthanized for animal welfare concerns per institutional protocol (these pups are considered non-survivors for the experiment). (Note 4)
3.
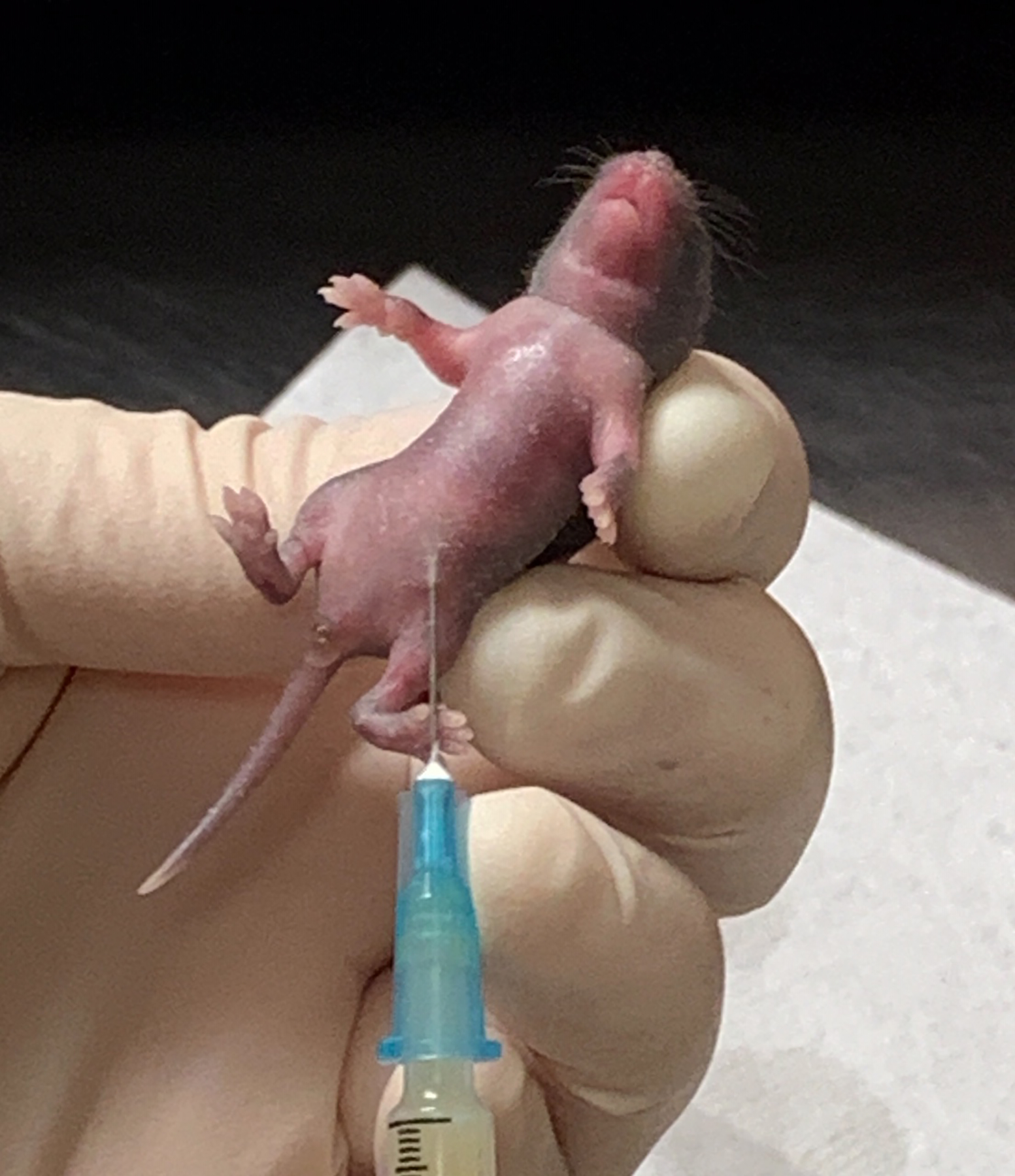
Injection of 1.1 – 1.3 mg/g BW of cecal slurry into a 6-day-old B6 neonatal mouse. Secure the pup by the back of the neck and the lower back and inject the weight-adjusted dose of CS (1.1–1.3 mg/g BW), waiting 10 seconds to ensure that the correct CS dose is delivered.
3.3. Cecal slurry injection in adult mice.
Although CLP is the most widely used model of intra-abdominal sepsis in adult mice, it has been shown that surgical variability and technique may play a significant role in outcome (29). The CS model of sepsis is particularly useful to study early inflammatory components of sepsis or as an alternative model of sepsis for those animals with different cecum size, bacterial flora or animals with deficiency in wound-healing capability (increased sensitivity to surgery).
Gently remove the mouse from the cage and restrain by firmly grasping by the scruff of the neck and lower back (tilt the mouse with its head slightly toward ground).
Weigh each mouse to calculate the weight-adjusted dose of the CS solution (80–100 mg/mL) to induce the required lethality. (Note 6)
Remove the animal from the cage and gently restrain in the head-down position. Tilt the mouse (abdomen side up) with is head slightly toward the ground so that its head is lower than its hind end (in this position any accidental puncture of abdominal organs will be minimized). Prep the site of injection with 70% ethanol.
Inject the weight-adjusted dose of CS in the mouse’s lower right quadrant of the abdomen (just above level of hip). The needle should be inserted with bevel facing “up” at a 30–40° angle to horizontal, to avoid damage to the bladder and other abdominal organs. Plug back on the plunger to ensure negative pressure before injecting the CS, and insert the entire bevel within the abdominal cavity.
Place the injected mouse in a new cage and monitor closely, following the local Institutional Animal Care and Use Committee approved protocol. In adult mice, lethargy, piloerection, hypothermia and hunching posture are examples primary signs of sepsis. Moribund animals (prostrate, unresponsive and unable to right itself) should be euthanized per local protocol to avoid spontaneous death (17). (Note 8)
4). Comments
Treatment of sepsis is a major issue to the U.S health care system due to the high morbidity and mortality, costing billions of dollars annually. Although there are limitations in the animal models of sepsis, they are required for translational research. The CLP murine model of polymicrobial sepsis, initially developed by Chaudry et al. (16) has been widely used over the past years as the “gold standard” model to study the pathophysiology of sepsis in animals. In PubMed alone, more than 3000 articles are found using the key words “cecal ligation and puncture and sepsis”. This is clearly due to the advantages that this model offers to the scientific community focused in the study of host immune reactions to sepsis (simple experimental procedure that has been extensively described).
Although some variations have been made to this model through the years to increase the severity of peritonitis (needle size, more than one puncture), another two models developed to improve CLP reproducibility is the colon ascendens stent peritonitis (CASP) and the implantation models using bacteria-impregnated fibrin clots (30, 31). These two models of peritonitis are different to CLP in terms of pathogenesis and disease progression. While the CLP model is useful to study peritoneal abscess formation accompanied by local peritoneal infection, inflammation and infiltration, the implantation of fibrin clot and CASP models are better options to study enterobacterial peritoneal infection accompanied by bacteremia, SIRS and sepsis progression without the presence of devitalized tissue. (32, 33)
Although these surgical models of sepsis (CLP, fibrin clot and CASP) cover different aspects of sepsis, they do have some important disadvantages for research including experiments using animals with gastrointestinal pathologies, wound-healing deficiencies, different cecum size or shape, and newborn animals. The endotoxemia and bacteria administration models and the intraperitoneal injection of cecal content from a donor rodent (CS model) are non-surgical experimental models of sepsis developed as an alternative to the surgical procedures mentioned before (Table 1).
Table 1.
Experimental models of sepsis
| Non-surgical models of sepsis | Surgical models of sepsis | ||||
|---|---|---|---|---|---|
| Bacteria or endotoxin (LPS) injection | Cecal slurry (CS) injection | Cecal Ligation and Puncture (CLP) | Bacteria and fibrin clot implantation | Colon Ascendens Stent Peritonitis (CASP) | |
| Description | Intraperitoneal or intravenously injection of bacteria or purified LPS | Intraperitoneal injection of cecal contents from a donor | Ligation of a portion of the cecum followed by cecal colostomies via one or more needle punctures | Standardized amounts of bacteria into fibrin clots, implanted into the peritoneal cavity | Insertion of a stent into the ascending colon to allow the stool to continuous flow from the colon into the peritoneal cavity |
| Pros | - Easy procedure - Replicates the physiology of severe sepsis - Highly reproducible - Dose of bacteria or endotoxin can be standardized |
- High reproducibility - No surgical tissue trauma or ischemic tissue - Advantageous in experiments that require large number of animals - Can be performed in neonatal animals with less risk of being neglected by the mother - Standardized polymicrobial inoculum (fix weight-based intraperitoneal fecal injection) |
- Easy procedure - Partly reflects human disease - Extensively studied - Sepsis severity can be adjusted based upon the length of the cecum ligated and the size and number of punctures performed - Better to study peritoneal abscess formation, local inflammation and infiltration |
- Fibrin delays systemic infection - Replicates features of sepsis in humans - Low early mortality - Highly reproducible |
- Mimic human sepsis better than CLP - Better to study bacteremia, SIRS and sepsis - Sepsis severity can be adjusted altering the size of the catheter |
| Cons | - Transient inflammatory response - Intoxication model (septic shock) - Host response limited to gram negative organisms (LPS) or bacteria injected |
- High variability (lethality of the cecal preparation varies between donor batches) | - Surgical trauma - Time consuming - Bacterial dissemination in the peritoneum (more localized inflammation) - High variability - Impossible to control rate and amount of fecal material released |
- Surgical trauma - The use of a single organism in the fibrin clot does not have clinical relevance |
- Time consuming - Low characterized - High variability |
The CS model initially developed to induce sepsis in adult pigs (34), was undertaken to validate it as a model of neonatal sepsis in mice by Wynn et al. (20) This model of sepsis is characterized by bacterial colonization, systemic inflammation, inflammatory-related splenic alterations and a dose-dependent mortality. (20) The main advantage of the CS model being used in the neonatal mice, is that it can be utilized in pups as young as 5 days old mice in place of a surgical model of sepsis (CLP, CASP, fibrin clot). Surgical models of sepsis are not possible to perform in the neonatal mouse due to their body size and the incomplete intestinal development as well as the need for prolonged anesthesia and highly technical surgical expertise). Additionally, CS can be performed with less risk of being neglected by the mother (a common behavior in laboratory rodents). (20, 35) Other non-surgical models of sepsis (LPS or bacteria injection) have inherent limitations. Although LPS is a major component of the outer membrane of gram-negative bacteria, it is a single molecule of the complex pathogen associated molecular patterns (PAMPs) of these organisms. Conversely, this model neglects the host-pathogen interactions of gram-positive bacteria as well as polymicrobial sepsis. Furthermore, bolus administration of LPS has been described as an intoxication model rather than sepsis. (17, 36) Similarly, intraperitoneal or intravenous bacteria injection to induce sepsis in mice have important limitations (10, 13). Large inocula are needed due to the inability of bacteria to replicate in vivo, and in order to overcome the murine host defense (14, 37) In addition, like endotoxicosis, the host early inflammatory response is massively elevated, several logs higher than seen in septic humans.
The lack of clinically predictive animal models remain as an important barrier to develop effective therapeutic strategies to treat sepsis. Antibiotic therapy intervention has been included to mimic the clinical condition and can substantially alter the model of sepsis (38–41); however, the timing of therapeutic interventions, dosage and antibiotic class are inconsistent with the antibiotic therapy in humans. Further, while experimental models of sepsis use a single strain of inbred mice with a single infection, in humans there is a heterogeneous population (genetics, age, disease severity). The CS is an acute model of sepsis developed to induce a systemic inflammatory response in the neonatal mice; antibiotic treatment in the neonatal mice may have a greater impact on the immune system than adult exposure and represent a technical challenge. Additionally, the impact of antibiotics on inflammation in murine models of sepsis, might add additional variables that could hinder the molecular mechanism involved in the poor outcomes to sepsis, therefore it needs to be further investigated.
Although the clinical relevance of sepsis murine models of sepsis are still a topic of debate due to the substantial differences to human responses to sepsis (9, 10), there are biological similarities that may be highlighted as they represent a better option to study the complex pathophysiology of sepsis. As the CS model may be performed in adult and neonatal mice, this model of polymicrobial sepsis is a good option not only to compare neonatal and adult sepsis, but also to develop novel therapeutic agents in sepsis especially designed for these two different populations (23, 24, 42, 43).
5). Notes
Genetics of the mice is important to consider. Although the background of inbred mice is genetically uniform, inbred strains show important variations in their immune response due to genetic mutations and polymorphisms (44). For example, caspase-1/11, IL-18 and PD-1 (B6 background) null mice are more resistant to polymicrobial sepsis induced by CS compared to wild type mice (23, 28, 45, 46). Conversely, CS administration does not affect survival on ASC, NLRP3, TRIF, MyD88 or RAG1-null neonatal mice compared to WT (B6) (24, 45, 47), as well as in C3H/HeJ mice (24).
Following CO2 exposure, mouse death must be confirmed by a combination of criteria including, lack of breathing and heart rate. Follow local Animal Care Guidelines for euthanasia of animals.
Modifications have been described about the CS preparation and long-term storage. CS may be harvested from different donors, pooled, suspended in 5% dextrose, filtered and stored at −80°C in 15% glycerol. Frozen CS should be rapidly thawed and used immediately for intraperitoneal administration to induce sepsis (22, 48).
Maternal neglect and cannibalism are additional challenges aside from the technical difficulties associated with using neonatal mice. Some rodent mothers will reject their pups after being manipulated (smearing the pups with bedding can be helpful before reintroducing the mother). After returning injected pups to the cage with the mother, observe closely the mother’s behavior for the first 10–15 minutes to confirm that it has accepted them back. The milk spot (presence of milk in the neonatal mice stomachs) is an excellent gauge of a healthy pup; therefore, when little or no milk is present then something is wrong with it or the mother has neglected it. The milk spot is clearly visible through their translucent bodies.
Re-apply every 24 hours.
The required lethal dose (LD) of the CS may be standardized by each laboratory to achieve the desired experimental outcomes. Significant variations occur in the intestinal microbiota content of CS donor mice potentially impacting the challenge dose necessary to induce similar LDs from one facility to another.
Neonatal mice that experience CS leakage or bleeding may be excluded from the study. Excluding these animals help to ensure a uniform CS dosage.
In general, post administration analgesics are not recommended after induction of CS sepsis. Although these studies are usually classified as USDA Category (unrelieved pain and distress), use of analgesics are often not helpful and can be detrimental. Opioids in general will depress spontaneous activity and especially feeding. In most cases, local animal care and use committees will permit withholding analgesics as long as there are preset criteria for determining an irreversible moribund state. Physiological or behavioral signs will define humane endpoints. Animals experiencing pain or distress should be euthanized promptly to minimize discomfort (humane endpoint).
Acknowledgments
Supported in part by grants R01 GM097531 (SDL/LLM), P50 GM111152 (LLM), R01 GM113945 (PAE) awarded by the National Institute of General Medical Sciences, USPHS. JCR was partially supported by the Robert H and Kathleen M Axline Fund.
5) References
- 1.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. The Lancet Infectious diseases. 2015;15(5):581–614. [DOI] [PubMed] [Google Scholar]
- 2.Shane AL, Sanchez PJ, Stoll BJ. Neonatal sepsis. Lancet (London, England). 2017;390(10104):1770–80. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. American journal of respiratory and critical care medicine. 2016;193(3):259–72. [DOI] [PubMed] [Google Scholar]
- 4.Wynn JL. Defining neonatal sepsis. Current opinion in pediatrics. 2016;28(2):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raymond SL, Stortz JA, Mira JC, Larson SD, Wynn JL, Moldawer LL. Immunological Defects in Neonatal Sepsis and Potential Therapeutic Approaches. Frontiers in pediatrics. 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. The Lancet Respiratory medicine. 2018;6(3):223–30. [DOI] [PubMed] [Google Scholar]
- 7.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and Costs of Sepsis in the United States-An Analysis Based on Timing of Diagnosis and Severity Level. Critical care medicine. 2018;46(12):1889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink MP. Animal models of sepsis. Virulence. 2014;5(1):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. Journal of leukocyte biology. 2015;98(6):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Szpila BE, et al. Host responses to sepsis vary in different low-lethality murine models. PloS one. 2014;9(5):e94404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall JC, Deitch E, Moldawer LL, Opal S, Redl H, van der Poll T. Preclinical models of shock and sepsis: what can they tell us? Shock (Augusta, Ga). 2005;24Suppl 1:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. Murine Models of Sepsis and Trauma: Can We Bridge the Gap? ILAR journal. 2017;58(1):90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock (Augusta, Ga). 1998;9(1):1–11. [DOI] [PubMed] [Google Scholar]
- 15.Lewis AJ, Seymour CW, Rosengart MR. Current Murine Models of Sepsis. Surgical infections. 2016;17(4):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. The Journal of surgical research. 1980;29(2):189–201. [DOI] [PubMed] [Google Scholar]
- 17.Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon JM, et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): An International Expert Consensus Initiative for Improvement of Animal Modeling in Sepsis. Shock (Augusta, Ga). 2018;50(4):377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemzek JA, Hugunin KM, Opp MR. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comparative medicine. 2008;58(2):120–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Traeger T, Koerner P, Kessler W, Cziupka K, Diedrich S, Busemann A, et al. Colon ascendens stent peritonitis (CASP)--a standardized model for polymicrobial abdominal sepsis. Journal of visualized experiments : JoVE. 2010(46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock (Augusta, Ga). 2007;28(6):675–83. [DOI] [PubMed] [Google Scholar]
- 21.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Cuenca AG, et al. Protective immunity and defects in the neonatal and elderly immune response to sepsis. Journal of immunology (Baltimore, Md : 1950). 2014;192(7):3156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PloS one. 2014;9(12):e115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rincon JC, Cuenca AL, Raymond SL, Mathias B, Nacionales DC, Ungaro R, et al. Adjuvant pretreatment with alum protects neonatal mice in sepsis through myeloid cell activation. Clinical and experimental immunology. 2018;191(3):268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuenca AG, Wynn JL, Kelly-Scumpia KM, Scumpia PO, Vila L, Delano MJ, et al. Critical role for CXC ligand 10/CXC receptor 3 signaling in the murine neonatal response to sepsis. Infection and immunity. 2011;79(7):2746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuenca AG, Cuenca AL, Gentile LF, Efron PA, Islam S, Moldawer LL, et al. Delayed emergency myelopoiesis following polymicrobial sepsis in neonates. Innate immunity. 2015;21(4):386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallon EA, Chun TT, Young WA, Gray C, Ayala A, Heffernan DS. Program Cell Death Receptor-1-Mediated Invariant Natural Killer T-Cell Control of Peritoneal Macrophage Modulates Survival in Neonatal Sepsis. Frontiers in immunology. 2017;8:1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young WA, Fallon EA, Heffernan DS, Efron PA, Cioffi WG, Ayala A. Improved survival after induction of sepsis by cecal slurry in PD-1 knockout murine neonates. Surgery. 2017;161(5):1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock (Augusta, Ga). 2000;13(2):110–6. [DOI] [PubMed] [Google Scholar]
- 30.Mathiak G, Szewczyk D, Abdullah F, Ovadia P, Feuerstein G, Rabinovici R. An improved clinically relevant sepsis model in the conscious rat. Critical care medicine. 2000;28(6):1947–52. [DOI] [PubMed] [Google Scholar]
- 31.Zantl N, Uebe A, Neumann B, Wagner H, Siewert JR, Holzmann B, et al. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infection and immunity. 1998;66(5):2300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier S, Traeger T, Entleutner M, Westerholt A, Kleist B, Huser N, et al. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock (Augusta, Ga). 2004;21(6):505–11. [DOI] [PubMed] [Google Scholar]
- 33.Kinasewitz GT, Chang AC, Peer GT, Hinshaw LB, Taylor FB Jr. Peritonitis in the baboon: a primate model which stimulates human sepsis. Shock (Augusta, Ga). 2000;13(2):100–9. [DOI] [PubMed] [Google Scholar]
- 34.Kazarian KK, Perdue PW, Lynch W, Dziki A, Nevola J, Lee CH, et al. Porcine peritoneal sepsis: modeling for clinical relevance. Shock (Augusta, Ga). 1994;1(3):201–12. [PubMed] [Google Scholar]
- 35.DeSantis DT, Schmaltz LW. The mother-litter relationship in developmental rat studies: cannibalism vs caring. Developmental psychobiology. 1984;17(3):255–62. [DOI] [PubMed] [Google Scholar]
- 36.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock (Augusta, Ga). 2005;24Suppl 1:7–11. [DOI] [PubMed] [Google Scholar]
- 37.Chen P, Stanojcic M, Jeschke MG. Differences between murine and human sepsis. The Surgical clinics of North America. 2014;94(6):1135–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deitch EA. Rodent models of intra-abdominal infection. Shock (Augusta, Ga). 2005;24Suppl 1:19–23. [DOI] [PubMed] [Google Scholar]
- 39.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock (Augusta, Ga). 2003;19(4):310–3. [DOI] [PubMed] [Google Scholar]
- 40.Brown I, Bellevue O, Shawo A, Woldesemayat H, Lyo V, Rayikanti B, et al. Low-dose cyclophosphamide improves survival in a murine treatment model of sepsis. Shock (Augusta, Ga). 2015;43(1):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steele AM, Starr ME, Saito H. Late Therapeutic Intervention with Antibiotics and Fluid Resuscitation Allows for a Prolonged Disease Course with High Survival in a Severe Murine Model of Sepsis. Shock (Augusta, Ga). 2017;47(6):726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denning NL, Yang WL, Hansen L, Prince J, Wang P. C23, an oligopeptide derived from cold-inducible RNA-binding protein, suppresses inflammation and reduces lung injury in neonatal sepsis. Journal of pediatric surgery. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganatra HA, Varisco BM, Harmon K, Lahni P, Opoka A, Wong HR. Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate immunity. 2017;23(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Veterinary pathology. 2012;49(1):32–43. [DOI] [PubMed] [Google Scholar]
- 45.Gentile LF, Cuenca AL, Cuenca AG, Nacionales DC, Ungaro R, Efron PA, et al. Improved emergency myelopoiesis and survival in neonatal sepsis by caspase-1/11 ablation. Immunology. 2015;145(2):300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wynn JL, Wilson CS, Hawiger J, Scumpia PO, Marshall AF, Liu JH, et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuenca AG, Joiner DN, Gentile LF, Cuenca AL, Wynn JL, Kelly-Scumpia KM, et al. TRIF-dependent innate immune activation is critical for survival to neonatal gram-negative sepsis. Journal of immunology (Baltimore, Md : 1950). 2015;194(3):1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brook B, Amenyogbe N, Ben-Othman R, Cai B, Harbeson D, Francis F, et al. A Controlled Mouse Model for Neonatal Polymicrobial Sepsis. Journal of visualized experiments : JoVE. 2019(143). [DOI] [PubMed] [Google Scholar]


