Abstract
Some techniques available at our laboratory were tested for their ability to aid in the morphological diagnosis of hydatid elements (Echinococcus granulosus [“Taenia echinococcus”]) isolated from cysts in humans and sheep. Unstained, methanol-fixed hooklets were fluorescent, most starkly so under violet light (excitation filter wavelength, 405 nm; long-pass filter wavelength, 495 nm). Auramine-rhodamine and Gram procedures failed to stain hooklets. Ziehl-Neelsen stain yielded indifferent results when organisms were viewed under transmitted light but resulted in a surprisingly intense red fluorescence when organisms were viewed under green light (excitation, 546 nm; long pass, 590 nm). Wheatley trichrome stain gave better and more uniform results than fuchsin. Ryan trichrome blue stain was the best under transmitted light; hooklets stained uniformly and intensely and were easily distinguishable from the background. Very satisfactory results were also obtained with a much simpler procedure (modified Baxby technique: no fixation, steaming hot 1% safranin for 2 min, and malachite green for 30 s). Therefore, Ryan and modified Baxby stains are recommended for the examination of E. granulosus under transmitted light. For fluorescence microscopy, Ziehl-Neelsen stain under green excitation light, or violet light with no staining, is also very useful. Epifluorescence microscopy is especially convenient for examining samples concentrated by filtration, as it renders the filter pores inconspicuous.
Hydatid disease, caused by the larval stage of Echinococcus granulosus, is endemic in many countries, including certain areas of Spain.
In the great majority of cases, the diagnosis of hydatid cyst infection is established on clinical grounds. Lesions can be detected by radiological methods, and several serological techniques of variable sensitivities can be used to diagnose the disorder. However, certain cases still present diagnostic dilemmas. Specific diagnoses of hydatid cyst infections are mainly based on microscopic examinations of the cyst fluid and demonstrations of the presence of protoscolices, hooklets, or, even when both are absent, fragments of the laminated membrane (2).
Usually, wet, unstained mounts of hydatid fluid sediment are examined. Several alternative methods to enhance the visibility of hydatid elements have been described. Hooklets are birefringent under polarized light (15). They have been reported to be acid fast with Ziehl-Neelsen and Fite-Faraco stains (4, 10). Trichrome stain has been deemed better than Ziehl-Neelsen stain (8). Filtration instead of centrifugation has proven effective for the processing of high volumes of liquid that is not too viscous (8, 9).
The increasing use of percutaneous puncture and drainage in the diagnosis and treatment of hydatid disease (1, 5, 11) makes the receipt of hydatid fluid samples at our parasitology laboratory more likely. Therefore, we considered it useful to evaluate the effectiveness of several techniques available at our laboratory for visualizing hydatid protoscolices and/or hooklets, especially when the former are absent and the latter are scarce or when a heavy background is present.
MATERIALS AND METHODS
Fluid was obtained from two pulmonary and five hepatic hydatid cysts in humans and from two pulmonary and five hepatic hydatid cysts in sheep. Fluid was either filtered through 5-μm-pore-size polycarbonate filters (Nuclepore) or centrifuged at 500 × g for 10 min. Filters and resuspended pellets were put on microscope slides and allowed to dry.
Each dried slide was submitted to one of the following procedures: (i) methanol fixation (covering the slide and air drying) without staining, (ii) Gram stain (Difco, Detroit, Mich.) (6), (iii) auramine-rhodamine stain (Difco) (13), (iv) Ziehl-Neelsen stain (methanol fixation, hot carbol-fuchsin for 10 min, 3% HCl in 95% ethanol for 30 s, and 1% methylene blue for 30 s) (modified from reference 6), (v) Henriksen and Pohlenz modified Ziehl-Neelsen stain (methanol fixation, carbol-fuchsin for 20 min, 7% sulfuric acid for 30 s, and malachite green for 30 s) (7), (vi) Wheatley modification of Gomori trichrome stain (Para-Pak trichrome stain; Meridian Diagnostics, Cincinnati, Ohio) (16), (vii) Ryan stain (Para-Pak trichrome blue stain; Meridian Diagnostics), with trichrome blue both for 30 min at 37°C, as recommended by the manufacturer, and for 90 min at room temperature, as originally described (12), (viii) Baxby stain (fixation for 3 to 5 min with 3% HCl in methanol, staining with steaming hot 1% safranin for 1 min, and counterstaining with 1% methylene blue for 30 s) (3), and (ix) a modified Baxby stain (no fixation, steaming hot 1% safranin for 2 min, and 5% malachite green in 10% ethanol for 30 s). Each method was tried with at least five human and five ovine samples and at least twice with each sample.
In order to get a dry, permanent mount, dried slides were mounted in Eukitt (O. Kindler GmbH & Co., Freiburg, Germany). All of these slides, as well as wet mounts of cyst fluid sediment, were examined with a Zeiss microscope equipped for phase contrast and epifluorescence. Four filter sets were available for the latter: excitation filter wavelength, 365 nm, and long-pass filter wavelength, 420 nm (F-1); excitation filter wavelength, 405 nm, and long-pass filter wavelength, 495 nm (F-2); excitation filter wavelength, 436 nm, and long-pass filter wavelength, 520 nm (F-3); and excitation filter wavelength, 546 nm, and long-pass filter wavelength, 590 nm (F-4).
RESULTS AND DISCUSSION
Hydatid hooklets failed to stain with the auramine-rhodamine and Gram techniques.
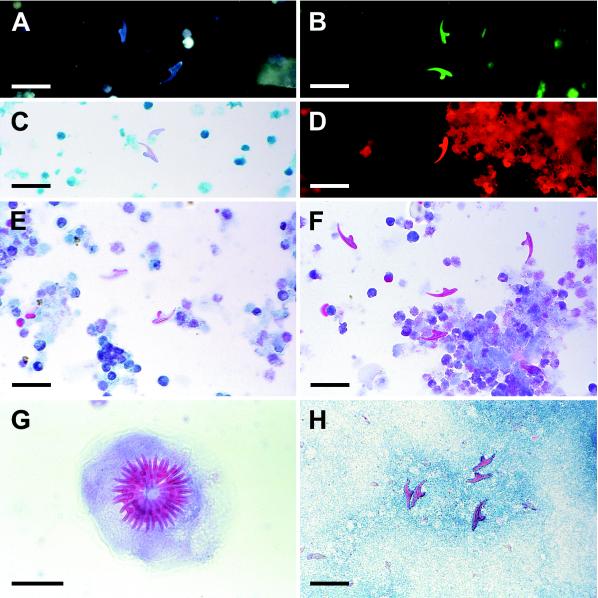
Unstained, methanol-fixed hooklets fluoresced blue under UV (F-1) light and green under violet (F-2) and blue-violet (F-3) light. F-2 proved to be the most useful for ready detection of hooklets under these conditions (Fig. 1A and B). Fluorescence dimmed after intense illumination, such as that provided by a 40× objective lens.
FIG. 1.
(A) Hydatid hooklets, methanol fixed, revealed by epifluorescence microscopy (excitation filter wavelength, 365 nm; long-pass filter wavelength, 420 nm); (B) hydatid hooklets on polycarbonate filter, methanol fixed, examined by epifluorescence microscopy (excitation, 405 nm; long pass, 495 nm); (C) hydatid hooklets revealed by Ziehl-Neelsen stain; (D) hydatid hooklet on polycarbonate filter revealed by Ziehl-Neelsen stain and epifluorescence microscopy (excitation, 546 nm; long pass, 590 nm); (E) hydatid hooklet revealed by trichrome stain; (F) hydatid hooklets revealed by Ryan stain; (G) hydatid protoscolex revealed by Ryan stain; (H) hydatid hooklets revealed by modified Baxby stain. Photomicrographs were shot on negative film, scanned into Kodak Photo CD disks, and processed with Adobe Photoshop version 4.0. Scale bars, 100 μm (G) and 30 μm (all other panels).
Hooklets stained irregularly with carbol-fuchsin (Ziehl-Neelsen and Henriksen-Pohlenz techniques). Most of them showed only a very slight pinkish hue (Fig. 1C) which was occasionally more marked. Heating the carbol-fuchsin and increasing the staining time enhanced the uptake of the dye, but the degrees of staining still differed among samples and among individual hooklets on the same slide.
Hooklets submitted to Ziehl-Neelsen staining showed an orange-yellow fluorescence when observed with the filter set used for fluorescein stains (F-3) and showed a surprisingly bright red fluorescence under green light (F-4) (Fig. 1D). We are not aware of any previous report of this phenomenon in hydatid hooklets, although a similar pattern has been observed in coccidian oocysts (14). Of all tested methods, this one led most quickly to the detection of hooklets, which were much brighter than the background, even in filter slides. Nonetheless, hooklets could be partially or totally obscured where the background was especially heavy.
Trichrome stain was more effective and predictable than Ziehl-Neelsen stain. Hooklets uniformly acquired a light pink color (Fig. 1E).
Ryan stain was the most effective for observation by transmitted-light microscopy. All hooklets showed a marked reddish color which made them readily apparent and distinguishable from other structures in the slides, even when large amounts of debris were present or when hooks lay inside intact protoscolices (Fig. 1F and G).
Baxby technique stained hooklets to a reddish color, almost as intensely as did Ryan stain. However, debris also stained to the same hue, which made this method less effective. Moreover, this technique stained protoscolices too intensely, obscuring their internal structure. Omitting the fixation step and substituting malachite green for methylene blue produced better results, providing a contrasting blue background (Fig. 1H). A thorough heating (until steam was produced) during the safranin step was necessary for a good staining.
Filter slides were difficult to read in transmitted-light microscopy (especially under phase contrast) because of the highly refringent pores; therefore, the most useful techniques were those yielding the most intense shades of color (Ryan and modified Baxby staining). This distracting background did not appear in epifluorescence microscopy, where Ziehl-Neelsen stain and green excitation light (F-4) were the most effective combination (Fig. 1B and D).
Conclusions.
Results are summarized in Table 1. Due to the difficulty of staining and examining filter slides compared to sediment slides, we consider centrifugation preferable to filtration whenever the sample volume to be processed is suitable for the available centrifuge.
TABLE 1.
Comparison of several methods for visualizing hydatid elements in hydatid fluid samplesa
| Method of: | Comment(s) |
|---|---|
| Concentration | |
| Filtration | Practical for high-volume samples. Difficult with viscous samples. Convenient microscopical examination of filters requires special measures for decreasing the relative visibility of the pores (i.e., high-contrast stains or, better, epifluorescence microscopy). |
| Centrifugation | Examination of sediment poses no problems. May be inconvenient for high-volume, clear samples. Recommended whenever possible. |
| Examination | |
| Unstained wet mount | Simplest. Low contrast (enhanced in phase-contrast microscopy, but debris become sharper too). No permanent record possible. |
| Unstained, fixed dry mount | Under violet excitation light (405 nm), hooklets appear bright green and fluorescent. Recommended if available. |
| Gram stain | No staining. |
| Auramine-rhodamine stain | No staining. |
| Ziehl-Neelsen stain | Under transmitted light, staining appears light and irregular. |
| Under green excitation light (546 nm), staining appears very bright red and fluorescent. Recommended if available. | |
| Wheatley trichrome stain | Hooklets stain uniformly but lightly. Laborious. |
| Ryan trichrome blue stain | Excellent results. Laborious. Recommended. |
| Baxby stain | Background stains too intensely. |
| Modified Baxby stain | Good results. Simple, quick, and cheap. Recommended. |
Examination is by transmitted-light microscopy unless otherwise stated.
Ryan stain is the most effective of the tested stains for visualizing hydatid hooklets, either isolated or inside protoscolices, when only transmitted-light microscopy is available. Modified Baxby stain is nearly as useful, and it is much simpler, quicker, and cheaper than Ryan staining.
Where epifluorescence with green exciting light (546 nm) is available, Ziehl-Neelsen stain can be even better, especially for examining filter slides and slides with moderately heavy backgrounds or with scarce hooklets. Epifluorescence with violet exciting light (405 nm) is also very useful for methanol-fixed, unstained samples.
REFERENCES
- 1.Al-Karawi M A, El-Shiekh Mohamed A R, Yasawuy M I. Advances in diagnosis and management of hydatid disease. Hepato-gastroenterology. 1990;37:327–331. [PubMed] [Google Scholar]
- 2.Ascoli V, Teggi A, Gossetti F, Nardi F. Hydatid cyst: primary diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 1990;6:44–48. doi: 10.1002/dc.2840060110. [DOI] [PubMed] [Google Scholar]
- 3.Baxby D, Blundell N. Sensitive, rapid, simple methods for detecting Cryptosporidium in faeces. Lancet. 1983;ii:1149. doi: 10.1016/s0140-6736(83)90669-4. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 4.Brundelet P. Acid-fast staining of hooklets of Taenia echinococcus. Lancet. 1973;i:678. doi: 10.1016/s0140-6736(73)92257-5. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 5.Filice C, Pirola F, Brunetti E, Dughetti S, Strosselli M, Scotti-Foglieni C. A new therapeutic approach for hydatid liver cysts. Gastroenterology. 1990;98:1366–1368. doi: 10.1016/0016-5085(90)90358-8. [DOI] [PubMed] [Google Scholar]
- 6.Hendrickson D A. Reagents and stains. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 1093–1107. [Google Scholar]
- 7.Henriksen S A, Pohlenz J F L. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hira P R, Shweiki H, Lindberg L G, Shaheen Y, Francis I, Leven H, Behbehani K. Diagnosis of cystic hydatid disease: role of aspiration cytology. Lancet. 1988;ii:655–657. doi: 10.1016/s0140-6736(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 9.Ingram E A, Helikson M A. Echinococcosis (hydatid disease) in Missouri: diagnosis by fine-needle aspiration of a lung cyst. Diagn Cytopathol. 1991;7:527–531. doi: 10.1002/dc.2840070518. [DOI] [PubMed] [Google Scholar]
- 10.Ishak K G. Acid-fast staining of hooklets of Taenia echinococcus. Lancet. 1973;i:890–891. doi: 10.1016/s0140-6736(73)91466-9. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 11.Khuroo M S, Wani N A, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997;337:881–887. doi: 10.1056/NEJM199709253371303. [DOI] [PubMed] [Google Scholar]
- 12.Ryan N J, Sutherland G, Coughlan K, et al. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol. 1993;31:3264–3269. doi: 10.1128/jcm.31.12.3264-3269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truant J P, Brett W A, Thomas W., Jr Fluorescence microscopy of tubercle bacilli stained with auramine and rhodamine. Henry Ford Hosp Med Bull. 1962;10:287–296. [PubMed] [Google Scholar]
- 14.Varea M, Clavel A, Doiz O, Castillo F J, Rubio M C, Gómez-Lus R. Fuchsin fluorescence and autofluorescence in Cryptosporidium, Isospora and Cyclospora oocysts. Int J Parasitol. 1998;28:1881–1883. doi: 10.1016/s0020-7519(98)00146-5. [DOI] [PubMed] [Google Scholar]
- 15.Vlachos J D, Zachariadou S. Microscopic structure of Echinococcus granulosus miniature scolices. Birefringence and interference as aids to definition. Arch Pathol. 1972;93:246–247. [PubMed] [Google Scholar]
- 16.Wheatley W B. A rapid staining procedure for intestinal amoebae and flagellates. Am J Clin Pathol. 1951;21:990–991. doi: 10.1093/ajcp/21.10_ts.990. [DOI] [PubMed] [Google Scholar]