Abstract
Background
At interim analysis in a phase 3, observer-blinded, placebo-controlled clinical trial, the mRNA-1273 vaccine showed 94.1% efficacy in preventing coronavirus disease 2019 (Covid-19). After emergency use of the vaccine was authorized, the protocol was amended to include an open-label phase. Final analyses of efficacy and safety data from the blinded phase of the trial are reported.
Methods
We enrolled volunteers who were at high risk for Covid-19 or its complications; participants were randomly assigned in a 1:1 ratio to receive two intramuscular injections of mRNA-1273 (100 μg) or placebo, 28 days apart, at 99 centers across the United States. The primary end point was prevention of Covid-19 illness with onset at least 14 days after the second injection in participants who had not previously been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The data cutoff date was March 26, 2021.
Results
The trial enrolled 30,415 participants; 15,209 were assigned to receive the mRNA-1273 vaccine, and 15,206 to receive placebo. More than 96% of participants received both injections, 2.3% had evidence of SARS-CoV-2 infection at baseline, and the median follow-up was 5.3 months in the blinded phase. Vaccine efficacy in preventing Covid-19 illness was 93.2% (95% confidence interval [CI], 91.0 to 94.8), with 55 confirmed cases in the mRNA-1273 group (9.6 per 1000 person-years; 95% CI, 7.2 to 12.5) and 744 in the placebo group (136.6 per 1000 person-years; 95% CI, 127.0 to 146.8). The efficacy in preventing severe disease was 98.2% (95% CI, 92.8 to 99.6), with 2 cases in the mRNA-1273 group and 106 in the placebo group, and the efficacy in preventing asymptomatic infection starting 14 days after the second injection was 63.0% (95% CI, 56.6 to 68.5), with 214 cases in the mRNA-1273 group and 498 in the placebo group. Vaccine efficacy was consistent across ethnic and racial groups, age groups, and participants with coexisting conditions. No safety concerns were identified.
Conclusions
The mRNA-1273 vaccine continued to be efficacious in preventing Covid-19 illness and severe disease at more than 5 months, with an acceptable safety profile, and protection against asymptomatic infection was observed. (Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases; COVE ClinicalTrials.gov number, NCT04470427.)
The global morbidity, mortality, and societal disruption caused by the coronavirus disease 2019 (Covid-19) pandemic prompted accelerated clinical vaccine development and regulatory interventions to mitigate some of its consequences. Between December 2020 and February 2021, three vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) received Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA) on the basis of data from observer-blinded, randomized, controlled trials demonstrating safety and efficacy against Covid-19 after a median follow-up of 2 months after vaccination.1-4 The short-term efficacy of the vaccines observed in the clinical trials was also observed after vaccine deployment in the general population.5-10 Longer-term safety and efficacy of the vaccines have remained open questions of public health import.
The phase 3 trial of mRNA-1273, a lipid nanoparticle–encapsulated mRNA expressing the prefusion-stabilized spike glycoprotein of SARS-CoV-2,11 showed a 94.1% vaccine efficacy against Covid-19, with an acceptable safety and side-effect profile after a median follow-up of 64 days.1 These early findings supported the issuance of the EUA, after which the protocol was amended to offer participants the option of having the group assignments unblinded and, for those who had received placebo, the option to receive the mRNA-1273 vaccine. Here we report the vaccine efficacy and safety results of the final analysis of the blinded phase of the trial, ending 5.3 months after the second dose, and in additional analyses in important subgroups of interest, as well as findings on the effect of vaccination on asymptomatic infection and on efficacy at various time intervals since vaccination.
Methods
Trial Oversight
In this phase 3, observer-blinded, randomized, placebo-controlled trial, adults in medically stable condition were enrolled at 99 sites in the United States.1 After the FDA issued an EUA for the use of mRNA-1273 in December 2020, the protocol was amended to include two parts (A and B; see Figs. S1 and S2 in the Supplementary Appendix, available together with the protocol with the full text of this article at NEJM.org). Part A, the observer-blinded phase of the trial, concluded when participants were informed of their group assignments; those in the placebo group were offered the opportunity to receive mRNA-1273 (the participant-decision visit). Part B, the open-label phase of the trial, is currently ongoing. Participants will continue to be followed for up to 2 years, as originally planned.
The trial is being conducted in accordance with the Good Clinical Practice guidelines of the International Council Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, and applicable government regulations. The central institutional review board approved the protocol and the consent forms. All participants provided written informed consent.
Participants, Randomization, and Data Blinding
Part A of the trial was a stratified, observer-blinded, randomized, placebo-controlled evaluation of the efficacy, safety, and immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine as compared with placebo in eligible participants who were at least 18 years old and had no known history of SARS-CoV-2 infection and whose locations or circumstances put them at appreciable risk of acquiring SARS-CoV-2 infection or who were at high risk for severe disease (or both).1 Participants were randomly assigned in a 1:1 ratio to receive two doses of the mRNA-1273 vaccine (100 μg) or placebo and were stratified according to age and Covid-19 complications risk criteria (≥18 to <65 years and not at risk, ≥18 to <65 years and at risk, and ≥65 years). The trial design, efficacy assessments, and vaccine have been described previously.1
Safety Assessments
Safety measures included solicited local and systemic adverse events with onset during the 7 days after each injection; unsolicited adverse events with onset during the 28 days after each injection; adverse events leading to discontinuation from receiving injections, participating in the trial, or both; medically attended and serious adverse events occurring during the trial; and severity of the events, which were graded as described in the protocol. Safety data, all Covid-19 cases, and severe Covid-19 cases were continuously monitored by the data and safety monitoring board.
Efficacy Assessments
Participants provided nasopharyngeal swab and blood samples before the first and second injections of vaccine or placebo and before the unblinding of the group assignments at the participant-decision visit. Efficacy assessments included surveillance for Covid-19 symptoms from enrollment throughout the trial. Efficacy end points were adjudicated by an independent adjudication committee whose members were unaware of group assignments.
For the primary end point, mRNA-1273 vaccine efficacy in preventing a first occurrence of Covid-19 with onset at least 14 days after the second injection, Covid-19 cases were defined by at least two systemic symptoms (temperature ≥38°C, chills, myalgia, headache, sore throat, or new olfactory or taste disorders), or at least one respiratory sign or symptom (cough, shortness of breath, or clinical or radiologic evidence of pneumonia), and were confirmed by positive SARS-CoV-2 reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of nasopharyngeal swab, nasal, or saliva samples. Participants were monitored daily for at least 14 days after diagnosis or until symptoms resolved. Severe Covid-19 was defined as confirmed Covid-19 plus one clinical sign of severe systemic illness (Tables S1 and S2). Secondary end points include the efficacy of the mRNA-1273 vaccine in preventing severe Covid-19, Covid-19 after the first dose, Covid-19 regardless of prior SARS-CoV-2 infection, Covid-19 according to a secondary definition (the Centers for Disease Control and Prevention definition, requiring only one symptom), serologically confirmed SARS-CoV-2 infection (positive binding antibody against SARS-CoV-2 nucleocapsid protein in participants who were SARS-CoV-2–negative at baseline), SARS-CoV-2 infection (positive RT-PCR assay) regardless of symptom status, and asymptomatic SARS-CoV-2 infection (absence of symptoms, with infections starting at least 14 days after the second injection, including seroconversion at day 57 or at the participant-decision visit, or a positive RT-PCR assay at the participant-decision visit).
Statistical Analysis
Determination of the sample size (30,000 participants) and aspects of the statistical analysis designed to demonstrate the efficacy of the mRNA-1273 vaccine as compared with placebo for the primary end point, prevention of Covid-19 starting at 14 days after the second dose, were described previously1 and are also provided in the protocol. Analysis populations included the randomization population; the full analysis population, comprising participants who had undergone randomization and received at least one dose of mRNA-1273 or placebo; the modified intention-to-treat population, consisting of participants in the full analysis population who had no immunologic or virologic evidence of SARS-CoV-2 infection before the first dose; the per-protocol population, consisting of participants in the modified intention-to-treat population who received two doses, with no major protocol deviations; and the solicited safety and safety populations (described in Table S3).
The prespecified primary efficacy analysis was performed in the per-protocol data set, starting 14 days after the second dose of vaccine or placebo. The efficacy of the mRNA-1273 vaccine was estimated with a stratified Cox proportional-hazards model. Incidence rates and vaccine efficacy were estimated by 1 minus the hazard ratio (mRNA-1273 vs. placebo), and the corresponding 95% confidence interval was based on the total number of cases adjusted according to total person-time. Additional details of the primary and secondary efficacy analyses are provided in Table S4 and in Supplementary Methods. The final efficacy analysis presented herein is based on cleaned data through the completion of the blinded phase, Part A, with a data cutoff date of March 26, 2021, when 95.0% of the trial participants had completed the participant-decision visit or had discontinued participation in the trial.
Results
Trial Population
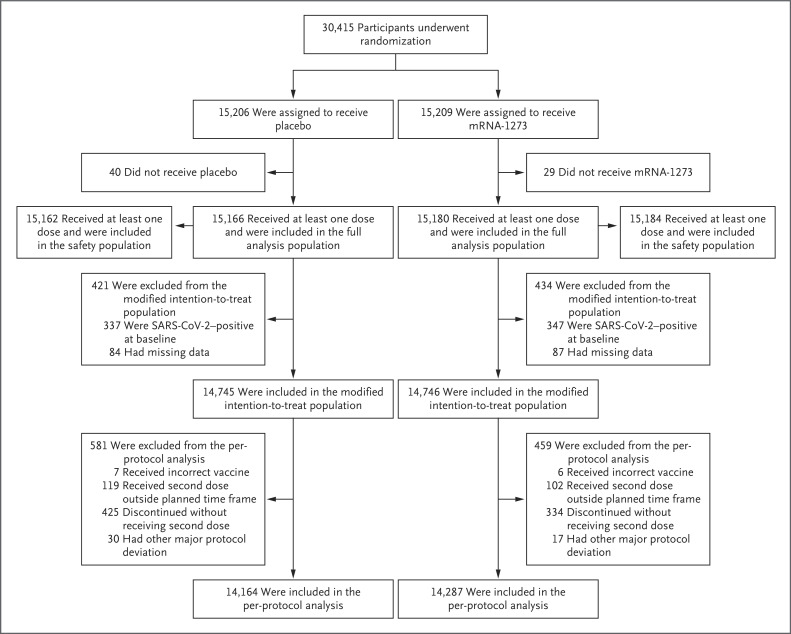
From July 27 to October 23, 2020, a total of 30,415 participants underwent randomization; 15,206 were assigned to the placebo group and 15,209 to the mRNA-1273 group (Figure 1 and Fig. S2).1 More than 96% of participants (14,727 in the placebo group and 14,635 in the mRNA-1273 group) received second injections. A total of 531 participants (3.5%) in the placebo group and 453 (3.0%) in the mRNA-1273 group did not receive the second injection, mainly owing to confirmed SARS-CoV-2 infection or withdrawal of consent. Trial discontinuations in the placebo group (691 participants [4.5%]) and the mRNA-1273 group (440 participants [2.9%]) were most commonly due to protocol deviations, withdrawal of consent, or loss to follow-up. The imbalance of discontinuations between the placebo and mRNA-1273 groups coincided with the FDA issuance of the EUAs for Covid-19 vaccines and reflected the intent of placebo recipients to receive a vaccine under EUA as it became available (Fig. S3). By the data cutoff date (March 26, 2021), 27,109 participants had been informed of their group assignments at a participant-decision visit, and 1855 had been informed before the participant-decision visit because they intended to receive a vaccine under EUA through their provider. A total of 28,964 participants entered the open-label phase of the trial.
Figure 1. Randomization and Analysis Populations.
Eight participants, including six with major protocol deviations and two who erroneously underwent randomization twice, were excluded from the original randomization population (30,423 participants) and from all analysis sets. The full analysis population comprised all participants who had undergone randomization and received at least one injection; the modified intention-to-treat population included participants in the full analysis population who had no immunologic or virologic evidence of previous Covid-19 (i.e., had both a negative nasopharyngeal swab specimen and a negative anti-nucleocapsid antibody test result) at day 1 before the first injection; and the per-protocol population consisted of all participants in the modified intent-to-treat population who received planned injections according to the schedule and had no major protocol deviations that affected key trial data. The safety population included all participants who had undergone randomization and received at least one injection; this population was used for all safety analyses except the analysis for solicited adverse events. For safety analyses, participants were evaluated according to the injection received. Three participants assigned to the mRNA-1273 group received two doses of placebo and were included in the placebo safety population, and seven participants assigned to the placebo group received one or two doses of mRNA-1273 and were included in the mRNA-1273 safety population. The data cutoff date was March 26, 2021.
Vaccine safety was assessed among 30,346 participants in the safety population (Figure 1). The prespecified primary efficacy analysis was performed in the per-protocol population, which included 28,451 participants who were SARS-CoV-2–negative at baseline and had received two doses of vaccine by the final analysis in the blinded phase. The median duration of follow-up from randomization to data cutoff or trial discontinuation was 212 days (interquartile range, 193 to 225), the duration from the second dose to data cutoff or discontinuation was 183 days (interquartile range, 165 to 194), and the duration from randomization to unblinding was 148 days (interquartile range, 131 to 162). Baseline demographic and clinical characteristics were balanced between the placebo group and the mRNA-1273 group (Table S5).1
Safety
At the end of the blinded phase, the frequencies of solicited local and systemic adverse events were consistent with those reported previously,1 with such events occurring less frequently in the placebo group (in 48% and 43% of participants after the first and second injections, respectively) than in the mRNA-1273 group (88% and 92%) (Fig. S4 and Tables S6 through S13). Women were slightly more likely than men to have grade 3 solicited adverse events after the first and second injections (Table S8). Occurrences of solicited adverse events were generally similar with the two injections, regardless of severe Covid-19 risk status (Table S9), and were less common after both doses among participants with previous SARS-CoV-2 infection than among those without previous SARS-CoV-2 infection, with the exception of systemic adverse events after the first dose of mRNA-1273, which occurred more often in participants previously infected with SARS-CoV-2 (62% vs. 55%, respectively) (Tables S11 and S12). The incidence of local adverse events with delayed onset starting on day 8 after an injection was higher after the first injection (80 participants [0.5%]) than after the second injection (10 participants [<0.1%]), and the most common local adverse event reported on or after day 8 was erythema in the mRNA-1273 group after the first (68 participants [0.4%]) and second (6 [<0.1%]) injections (Table S13).
The frequencies of unsolicited, severe, and serious adverse events reported during the 28 days after either injection were generally similar in the two groups in the overall safety population, regardless of age or risk factors for severe Covid-19 (Tables S14 through S18). The frequency of grade 3 and medically attended adverse events that were considered to be related to injection of placebo or vaccine was lower in the placebo group (0.2% and 0.6%, respectively) than in the mRNA-1273 group (0.5% and 1.3%) (Table S14). Overall, 0.6% of placebo recipients and 0.4% of vaccine recipients had adverse events that resulted in their not receiving the second dose, and less than 0.1% in both groups discontinued trial participation because of adverse events after either injection. Adverse events that were considered to be related to the injections were reported by 8.5% of placebo recipients and 13.9% of mRNA-1273 recipients during the observation period of the study and were generally similar to those reported previously regardless of age (Tables S19 through S21). Serious injection-related adverse events occurred in 4 placebo recipients (<0.1%) and in 12 mRNA-1273 recipients (<0.1%).
Hypersensitivity reactions were reported in 1.8% of placebo recipients and in 2.2% of vaccine recipients, with anaphylaxis occurring in 2 participants (<0.1%) in each group (Table S22). Dermal filler reactions were reported in 14 placebo recipients (<0.1%) and in 20 mRNA-1273 recipients (0.1%) with a history of dermal filler injections (Table S23). Three cases of Bell’s palsy (<0.1%) were reported in the placebo group and 8 in the mRNA-1273 group (<0.1%); no case was considered to be related to the placebo or the vaccine (Table S24). Thromboembolic events were observed in 43 placebo recipients (0.3%) and in 47 mRNA-1273 recipients (0.3%) (Table S25). No cases of myocarditis were reported. Pericarditis events occurred in 2 participants each (<0.1%) in the placebo and mRNA-1273 groups (both events >28 days after the second dose) and were considered serious (Tables S20 and S21). A total of 32 deaths had occurred by completion of the blinded phase, with 16 deaths each (0.1%) in the placebo and mRNA-1273 groups; no deaths were considered to be related to injections of placebo or vaccine, and 4 were attributed to Covid-19 (3 in the placebo group and 1 in the mRNA-1273 group) (Tables S19 and S26). The Covid-19 death in the mRNA-1273 group occurred in a participant who had received only one dose; Covid-19 was diagnosed 119 days after the first dose, and the participant died of complications 56 days after diagnosis.
Efficacy Analyses
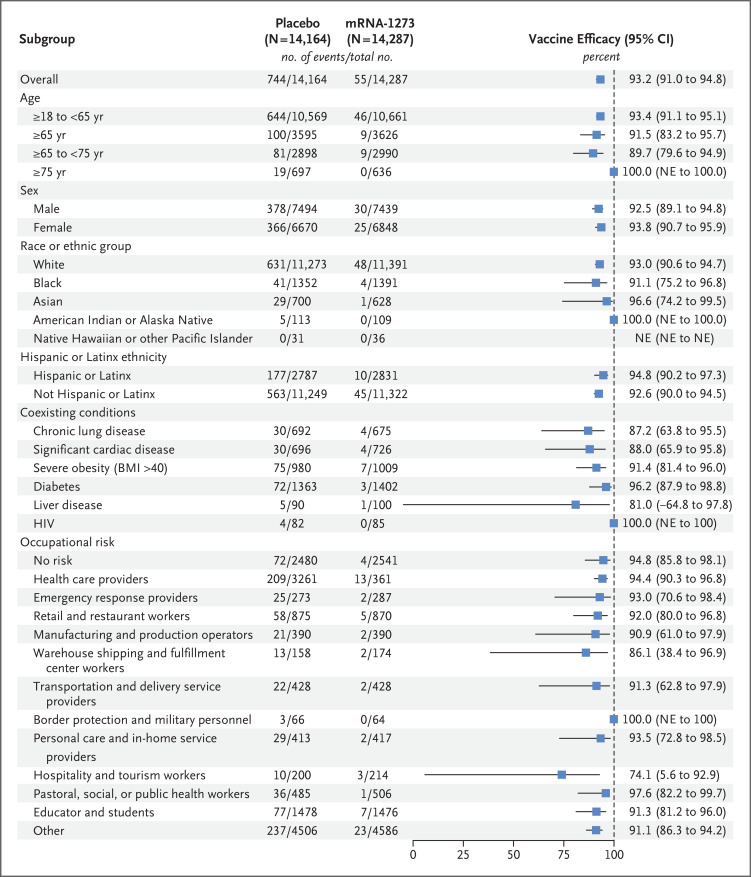
A total of 799 adjudicated cases of Covid-19 in the per-protocol population were included in the primary efficacy analysis; 744 cases (5.3%) were in the placebo group and 55 (0.4%) were in the mRNA-1273 group (Figure 2 and Figure 3 and Tables S27 and S28). The vaccine efficacy was 93.2% for the prevention of Covid-19 starting at least 14 days after the second dose, with incidences of 136.6 cases per 1000 person-years (95% confidence interval [CI], 127.0 to 146.8) in the placebo group and 9.6 cases per 1000 person-years (95% CI, 7.2 to 12.5) in the mRNA-1273 group. The vaccine efficacy for adjudicated cases in the modified intention-to-treat population was 92.3% (95% CI, 90.1 to 93.9). Vaccine efficacy in preventing severe Covid-19, a key secondary end point, was 98.2% (95% CI, 92.8 to 99.6) in the per-protocol population, with 106 severe cases in the placebo group and 2 in the mRNA-1273 group. Vaccine efficacy was consistently high in subgroups, including participants 65 years of age or older and 75 years of age or older, those with coexisting conditions, those belonging to various racial and ethnic groups, and those with various categories of occupational risk exposures (Figure 4 and Table S29). When examined by specific time interval since completion of vaccination over the duration of follow-up, the efficacy of the mRNA-1273 vaccine in preventing Covid-19 remained consistent, with efficacy greater than 90% observed 4 months or more after the second injection (Figure 5, Fig. S5, and Table S30). Symptoms most commonly reported in the adjudicated Covid-19 cases in both groups were cough, fatigue, headaches, and nasal congestion; severe obesity and diabetes were contributing risk factors for severe Covid-19 (Tables S31 and S32).
Figure 2. Efficacy of the mRNA-1273 Vaccine in Preventing Covid-19.
In Panels A and C, the dashed vertical line denotes the adjudicated assessment beginning at day 42 (14 days after the second injection of vaccine or placebo). Tick marks in all three panels indicate censored data. Vaccine efficacy was defined as 1 minus the hazard ratio (mRNA-1273 vs. placebo), and 95% confidence intervals were estimated with the use of a stratified Cox proportional-hazards model with Efron’s method of tie handling and with treatment group as a covariate, adjusted for stratification factor. The data cutoff date was March 26, 2021.
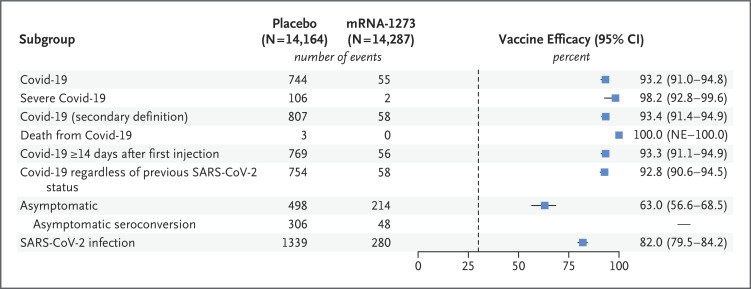
Figure 3. Vaccine Efficacy for Primary and Secondary End Points.
Vaccine efficacy was defined as 1 minus the hazard ratio (mRNA-1273 vs. placebo), and 95% confidence intervals were estimated using a stratified Cox proportional-hazards model with Efron’s method of tie handling and with the treatment group as a covariate, adjusted for stratification factor. The P value for the vaccine efficacy against Covid-19 (upper right corner) is P<0.001. The dashed vertical line represents a vaccine efficacy of 30%, based on the null hypothesis that the primary efficacy of the mRNA-1273 vaccine is 30% or less. In the Covid-19 rows, censoring rules for efficacy analyses (Covid-19 cases based on eligible symptoms and positive reverse-transcriptase–polymerase-chain-reaction [RT-PCR] assay within 14 days before the second injection) were applied, except for deaths from Covid-19. If a participant had a positive RT-PCR assay at the visit before the second dose (day 29) without eligible symptoms within the previous 14 days, or a positive anti-nucleocapsid antibody test at a scheduled visit before Covid-19 was diagnosed, the participant’s data were censored at the date of the positive RT-PCR assay or anti-nucleocapsid antibody test. Covid-19 diagnoses were based on adjudication committee assessments. The data for Covid-19 regardless of previous SARS-CoV-2 status were based on the number of participants in the full analysis population (15,166 participants in the placebo group and 15,180 participants in the mRNA-1273 group). Data for the asymptomatic subgroup include data from the participant-decision visit. Asymptomatic was defined as the absence of symptoms (according to either the primary efficacy end point of Covid-19 or the secondary definition of Covid-19 [the Centers for Disease Control and Prevention definition, requiring only one symptom]) and of infection as detected by RT-PCR assay (at scheduled visits) or seroconversion (anti-nucleocapsid antibody test). In the primary approach, documented asymptomatic infection was counted beginning 14 days after the second injection, which required seroconversion at month 2 (day 57 through the participant-decision visit). Asymptomatic seroconversion excludes infections confirmed by RT-PCR assay only and includes infections confirmed by seroconversion and those confirmed by both RT-PCR and seroconversion (Table S28). Vaccine efficacy and 95% confidence intervals for asymptomatic SARS-CoV-2 infection were estimated with Fine and Gray’s subdistribution hazard model, with disease cases as competing events and with treatment group as a covariate, adjusted for stratification factor. Results for additional end points are summarized in Table S27. The data cutoff date was March 26, 2021. NE indicates that the lower bound of the 95% confidence interval could not be estimated.
Figure 4. Efficacy of the mRNA-1273 Vaccine in Preventing Covid-19 in Subgroups.
Analysis of the vaccine efficacy of mRNA-1273 in the prevention of Covid-19 in various subgroups in the per-protocol population was based on adjudicated assessments starting 14 days after the second injection. Vaccine efficacy, defined as 1 minus the hazard ratio (mRNA-1273 vs. placebo), and 95% confidence intervals were estimated with the use of a stratified Cox proportional-hazards model with Efron’s method of tie-handling and with the treatment group as a covariate, adjusted for stratification factor if applicable. The total number of events for race includes 38 placebo recipients and 3 mRNA-1273 recipients who were in “Multiple,” “Other,” or not reported or unknown categories, and the total number for ethnicity includes 4 placebo recipients and no mRNA-1273 recipients who were in not reported or unknown categories (not shown). Race and ethnic group were reported by the participant. The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. Additional subgroup data are provided in Table S29. The data cutoff date was March 26, 2021. HIV denotes human immunodeficiency virus.
Figure 5. Incidence of Covid-19 According to Time Periods in the Per-Protocol Population.
The incidence rate based on adjudicated Covid-19 cases was defined as the number of participants with an event during the period divided by the number of participants at risk at the beginning of each period and adjusted by person-years (total time at risk) in each treatment group. The dashed vertical line represents a vaccine efficacy of 30% based on the null hypothesis that the primary efficacy of the mRNA-1273 vaccine is 30% or less. The number of person-years was calculated from randomization to the date of onset of Covid-19, the end of each time period, the last date of participation in the trial, or the efficacy data cutoff date, whichever date was the earliest. For the analysis of time intervals starting from 14 days after the first injection, starting from the second injection, and starting 14 days after the second injection, assessed every 2 months, person-years for each time period were defined starting from the beginning of each time interval and truncating at the end of the interval (if there was an ending time). Vaccine efficacy was defined as 1 minus the hazard ratio (mRNA-1273 vs. placebo). The 95% confidence interval for the ratio was calculated with the exact method, conditional on the total number of cases and adjusted for person-years for the time period. The data cutoff date was March 26, 2021.
Secondary end points (Figure 3 and Table S27) also included vaccine efficacy according to the secondary definition of Covid-19 (the Centers for Disease Control and Prevention definition, requiring only one symptom) starting 14 days after the second injection in the per-protocol population; according to the secondary definition, the vaccine efficacy was 93.4% (95% CI, 91.4 to 94.9). Among participants who were SARS-CoV-2–negative at baseline, a total of 712 participants (498 in the placebo group and 214 in the mRNA-1273 group) were found to be SARS-CoV-2–positive by RT-PCR assay or anti-nucleocapsid antibody test in the absence of symptoms starting 14 days after the second injection, through and including the participant-decision visit, and were considered to have asymptomatic infection (Figure 3 and Tables S27 and S28). Vaccine efficacy in preventing asymptomatic SARS-CoV-2 infection, based on the hazard ratio using the competing risk method, was 63.0% (95% CI, 56.6 to 68.5). In an analysis of asymptomatic infections after randomization, with data accrued up to and including the participant-decision visit, 157 participants in the placebo group and 153 in the mRNA-1273 group were RT-PCR–positive only; 306 participants in the placebo group and 48 in the mRNA-1273 group showed seroconversion by anti-nucleocapsid antibodies, and 115 participants in the placebo group and 7 in the mRNA-1273 group tested positive in both anti-nucleocapsid antibody testing and RT-PCR assay in the absence of symptoms. Findings for asymptomatic infection were similar in the modified intention-to-treat population (Table S28). For the secondary end point of prevention of SARS-CoV-2 infection (regardless of symptom or severity), the vaccine efficacy was 82.0% (95% CI, 79.5 to 84.2) beginning 14 days after the second injection in the per-protocol population, with 1339 participants in the placebo group and 280 in the mRNA-1273 group who had documented infection, defined as a positive result on RT-PCR assay at 14 days or more after the second injection or seroconversion at day 57 or later, through the participant-decision visit.
For the secondary end point of Covid-19 with onset at least 14 days after the first injection, the vaccine efficacy, based on adjudicated cases of Covid-19 in the per-protocol population among participants who received both injections (769 in the placebo group and 56 in the mRNA-1273 group), was 93.3% (95% CI, 91.1 to 94.9). In an exploratory analysis performed in a modified intention-to-treat subpopulation of 425 participants in the placebo group and 334 in the mRNA-1273 group who had no evidence of SARS-CoV-2 infection at baseline and who received only one injection, adjudicated Covid-19 cases were observed in 45 participants (10.6%) in the placebo group and in 4 participants (1.2%) in the mRNA-1273 group (Table S33). Six severe Covid-19 cases occurred in recipients of a single injection of placebo (1.4%), and one severe case occurred in a recipient of a single injection of the mRNA-1273 vaccine (0.3%).
Discussion
The data compiled through the completion of the blinded phase of the COVE trial provide further evidence of the safety and efficacy of mRNA-1273 in preventing symptomatic Covid-19 as well as preventing SARS-CoV-2 infection regardless of symptom and severity in adults, including those 65 years of age or older and those with coexisting conditions, and across various ethnic and racial groups. These findings are based on a median follow-up of 148 days in the blinded phase and are similar to those observed previously at a median follow-up of 64 days, indicating that the high efficacy of the mRNA-1273 vaccine is maintained in the medium term. Of importance, the vaccine provided substantial protection against asymptomatic infection (63%; 95% CI, 56.6 to 68.5), though at a lower vaccine efficacy than that for symptomatic infection. The efficacy of the mRNA-1273 vaccine did not wane up to 4 months after the second injection and beyond. It is notable that the efficacies found in phase 3 trials of Covid-19 vaccines have thus far translated into high effectiveness in the general population, including effectiveness against variants of concern that are associated with reductions in neutralization, such as the B.1.351 (beta) and B.1.617.2 (delta) variants.8,12-15 Additional data gathered from regions with current and potential surges in transmission of variants of concern are important toward informing strategies for administering additional doses of vaccine.
No safety concerns were identified in this trial. However, robust safety surveillance systems have identified rare events during the global distribution of Covid-19 vaccines that the phase 3 studies were not powered to detect; thus, continued vigilance is warranted, including monitoring for anaphylactic reactions, especially in persons with allergic phenotypes, and for other potential unexpected reactions, such as myocarditis in adolescents and young adults.16 Given the high efficacies of the vaccines1,3,17 and the burden of the pandemic, the risk–benefit ratio remains strongly in favor of broad deployment of the vaccines. Despite differences in the definitions of disease severity, the vaccine efficacy is supported by real-world data: vaccination has been shown to be highly effective in preventing severe Covid-19, associated hospitalizations, and deaths, as well as mild or asymptomatic infection, regardless of race and age.9,12,18-20
Several important limitations of the trial should be considered. At the trial design stage in early 2020, the efficacy and safety of the mRNA-1273 vaccine were unknown; for that reason, certain key populations such as pregnant women, children, and immunocompromised persons were not included in the trial. Studies in these populations are currently ongoing, and the data that are emerging in adolescents and pregnant women are reassuring.21-24 Although no safety concerns associated with the mRNA vaccines have been identified in immunocompromised persons, these vaccines appear to be less immunogenic in such persons.25,26 Given the period during which the blinded phase of the trial was conducted, assessment of vaccine efficacy in preventing Covid-19 caused by SARS-CoV-2 variants of concern is limited, since circulation of the variants was low. Future exploratory analyses are needed to probe this question. It should also be noted that the sensitivity of detection of asymptomatic infection in this trial was somewhat limited by the assessment of seroconversion at fixed time points, the kinetics of seroconversion of anti-nucleocapsid antibodies (which may take weeks to emerge after an initial infection and then wane relatively quickly), as well as the possible diminished detection of SARS-CoV-2 by RT-PCR, owing to a reduced duration of infection in vaccine recipients and the infrequent collection of samples from asymptomatic participants in the trial.
We found that the efficacy of the mRNA-1273 vaccine against Covid-19 and severe Covid-19 was maintained for more than 5 months after the second dose among all subgroups in the trial, including those at risk for severe complications. Asymptomatic SARS-CoV-2 infections were also reduced. No safety concerns were identified in this trial. The interplay of viral evolution with vaccine distribution in the next months will determine the trajectory of the pandemic, which continues to evade predictions and shape much of the social and economic life in the United States and worldwide.
Acknowledgments
We thank the participants in the trial and the members of the data and safety monitoring board (Richard J. Whitley, chair, University of Alabama School of Medicine; Abdel Babiker, MRC Clinical Trials Unit at University College, London; Lisa Angeline Cooper, Johns Hopkins University School of Medicine and Bloomberg School of Public Health; Susan Smith Ellenberg, University of Pennsylvania; Alan Fix, Vaccine Development Global Program Center for Vaccine Innovation and Access PATH; Marie Griffin, Vanderbilt University Medical Center; Steven Joffe, Perelman School of Medicine, University of Pennsylvania; Jorge Kalil, Clinics Hospital (HC-FMUSP), Universidade de São Paulo, Brazil; Myron M. Levine, University of Maryland School of Medicine; Malegapuru William Makgoba, University of KwaZulu-Natal; Anastasios A. Tsiatis, North Carolina State University; and Renee H. Moore, Emory University) and the adjudication committee (Richard J. Hamill, chair, Baylor College of Medicine; Lewis Lipsitz, Harvard Medical School; Eric S. Rosenberg, Massachusetts General Hospital; and Anthony Faugno, Tufts Medical Center) for their critical and timely review of the trial data. We also acknowledge the contribution from the mRNA-1273 Product Coordination Team from the Biomedical Advanced Research and Development Authority (BARDA) (Robert Bruno, Richard Gorman, Holli Hamilton, Gary Horwith, Chuong Huynh, Nutan Mytle, Corrina Pavetto, Xiaomi Tong, and John Treanor), as well as Frank J. Dutko (Moderna consultant) for editorial support and Katherine Kacena for input on statistics.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on September 22, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract 75A50120C00034), and by the National Institute of Allergy and Infectious Diseases (NIAID). The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI 68614HVTN), the Statistics and Data Management Center (UM1 AI 68635), the HVTN Laboratory Center (UM1 AI 68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI 68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI 68636), and the Infectious Diseases Clinical Research Consortium leadership group 5 (UM1 AI148684-03).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson & Johnson COVID-19 vaccine authorized by U.S. FDA for emergency use — first single-shot vaccine in fight against global pandemic. New Brunswick, NJ: Johnson & Johnson, February 27, 2021. (https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic). [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med 2021;384:1824-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maternal, neonatal, and child health services during COVID-19. Atlanta: Centers for Disease Control and Prevention, November 5, 2020. (https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/pregnancy-services.html). [Google Scholar]
- 7.Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med 2021;385:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers — eight U.S. locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021;70:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years — United States, January–March 2021. MMWR Morb Mortal Wkly Rep 2021;70:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlowski C, Lenehan P, Puranik A, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y) 2021;2(8):979-992.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbett KS, Edwards D, Leist SR, et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. June 11, 2020. (https://www.biorxiv.org/content/10.1101/2020.06.11.145920v1). preprint. [DOI] [PMC free article] [PubMed]
- 12.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med 2021;27:917-924. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021;385:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal JL, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. May 24, 2021. (https://www.medrxiv.org/content/10.1101/2021.05.22.21257658v1). preprint. [DOI] [PubMed]
- 16.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021;148(3):e2021052478-e2021052478. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SJ, Moreira ED, Kitchin N, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. July 28, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.28.21261159v1). preprint. [DOI] [PMC free article] [PubMed]
- 18.Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. Clin Infect Dis 2021. August 6 (Epub ahead of print).34358310 [Google Scholar]
- 19.Jones NK, Rivett L, Seaman S, et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. Elife 2021;10:e68808-e68808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tande AJ, Pollock BD, Shah ND, et al. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin Infect Dis 2021. March 10 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 2021;325:2370-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med 2021;384:2273-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali K, Berman G, Zhou H, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. DOI: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidar G, Agha M, Lukanski A, et al. Immunogenicity of COVID-19 vaccination in immunocompromised patients: an observational, prospective cohort study interim analysis. June 30, 2021. (https://www.medrxiv.org/content/10.1101/2021.06.28.21259576v1). preprint.
- 26.Hadjadj J, Planas D, Ouedrani A, et al. Immunogenicity of BNT162b2 vaccine against the alpha and delta variants in immunocompromised patients. August 9, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.08.21261766v1). preprint. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.