Abstract
Background.
Patients with locally unresectable pancreatic cancer (AJCC stage III) have a median survival of 10–14 months. The objective of this study was to evaluate outcome of initially unresectable patients who respond to multimodality therapy and undergo resection.
Methods.
Using a prospectively collected database, patients were identified who were initially unresectable because of vascular invasion and had sufficient response to nonoperative treatment to undergo resection. Overall survival (OS) was compared with a matched group of patients who were initially resectable. Case matching was performed using a previously validated pancreatic cancer nomogram.
Results.
A total of 36 patients with initial stage III disease were identified who underwent resection after treatment with either systemic therapy or chemoradiation. Initial unresectability was determined by operative exploration (n = 15, 42%) or by cross-sectional imaging (n = 21, 58%). Resection consisted of pancreaticoduodenectomy (n = 31, 86%), distal pancreatectomy (n = 4, 11%), and total pancreatectomy (n = 1, 3%). Pathology revealed T3 lesions in 26 patients (73%), node positivity in 6 patients (16%), and a negative margin in 30 patients (83%). The median OS in this series was 25 months from resection and 30 months since treatment initiation. There was no difference in OS from time of resection between the initial stage III patients and those who presented with resectable disease (P = .35).
Conclusions.
In this study, patients who were able to undergo resection following treatment of initial stage III pancreatic cancer experienced survival similar to those who were initially resectable. Resection is indicated in this highly select group of patients.
Patients who present with locally unresectable, American Joint Committee on Cancer (AJCC) stage III, disease have a median survival of less than a year.1 These patients are defined as being unresectable because vascular invasion precludes resection with negative margins.2 Standard treatment recommendations for patients with locally unresectable disease consist of chemotherapy and external beam radiotherapy. Several studies have suggested potential downstaging of unresectable pancreas cancer through various treatment regimens.3–7 These studies show that the rate of conversion is low, even with the combination of chemotherapy and radiation. Little is known of the long-term survival of these patients because of the small number of patients who are actually resected.
The aims of this study were to identify a cohort of patients who were initially unresectable but converted to resectable disease through treatment with chemotherapy or chemoradiotherapy and to examine the survival of this cohort of patients. This group was then compared with a group of matched patients who presented with initially resectable disease, and survival comparisons then performed.
METHODS
The study design was a matched case-control study. Using a prospectively maintained database, we identified 630 patients who underwent complete resection for pathologically confirmed pancreatic adenocarcinoma between January 2000 and December 2009. Of these, 36 patients (6%) were identified who initially presented with either operative or radiographic stage III pancreatic cancer (locally unresectable), but experienced sufficient response with multimodality therapy to allow complete resection. These 36 patients comprised the cases and were matched in a 1:1 ratio with controls randomly selected from the larger group of patients who underwent curative-intent resection without receiving neoadjuvant treatment. Case matching was performed using nomogram scores from a previously validated pancreatic nomogram for disease-specific survival (DSS).8 For the purposes of case matching, the pathologic T stage after treatment of the initial stage III patients was chosen. This was done to remove an unfair survival advantage for the initial stage III patients.
Patient, pathologic, and treatment-related variables were obtained from the database and confirmed by chart review. Patient factors evaluated included age, gender, medical comorbidities, and symptoms (including weight loss and back pain at presentation). Pathologic variables include tumor, nodes, and metastasis (TNM) stage, margin (positive or negative), tumor location, tumor differentiation (well, moderate, or poor), and the number of nodes harvested. Maximal tumor size was determined and defined as the maximal diameter at pathologic analysis. Margins assessed included the pancreatic resection margin, biliary margin, anterior margin, and posterior margin. Treatment factors included type of pancreas resection, date of operation, estimated blood loss, length of stay (LOS), and adjuvant therapy given. Chart review was performed to define the reason for initial unresectability and the method by which that decision was made. Tumors were defined as locally advanced based on intraoperative assessment or on cross-sectional imaging determining extensive vascular involvement precluding resection. Preoperatively, routine high-quality abdominal and pelvic computed tomography (CT) scans were performed. All patients were reviewed at a weekly Hepatobiliary Disease Management conference attended by surgeons, oncologists, radiologists, and gastroenterologists prior to resection.
Pathologic response was determined using modified criteria from previously published grading schemes to include the variables of (1) viable tumor cell volume and (2) the amount of fibrosis and fibroinflammatory changes around tumor lacunae relative to the portion of predominantly viable tumor.9–11 Viable tumor cells were defined as those with well-defined nuclei with completely intact nuclear membrane, and nonviable cells were those with absent or pyknotic nuclei, and/or those with absent or incomplete nuclear membrane and associated acidophilic or vacuolated cytoplasm. Complete pathologic response was defined as fibrosis or fibroinflammatory changes within an entirely submitted and evaluated gross lesion and without microscopic evidence of viable carcinoma, and histologically negative nodes. Partial response was assessed for its graded histologic response and was determined by the amount of residual viable carcinoma in relation to areas of fibrosis or fibroinflammation within the gross lesion, which was inversely associated with, and expressed as percentage of, a favorable treatment response. Thus, a 100% treatment response indicated fibrosis or fibroinflammation within an entire gross lesion without microscopic evidence of viable carcinoma, and a 0% response represented an entirely viable tumor in the absence of any fibrosis of fibroinflammation, respectively. The presence of any viable tumor cells was suggestive of incomplete response. Acellular mucin was regarded as a form of positive treatment response, not as residual/viable tumor. The pathologic stage of the residual carcinoma was determined by the size of viable tumor and tumor extension within or beyond pancreatic parenchyma. The 7th edition of AJCC staging system for pancreatic carcinoma was applied for posttreatment tumor stage.
OS and DSS were obtained from office records, phone conversations, and the social security death index. Comparisons of baseline characteristics between the groups were conducted using paired Wilcoxon test for continuous and McNemar’s test for categorical variables. OS/DSS were estimated by the Kaplan-Meier method, and the survival curves were compared using the log-rank test. Patients who were alive were censored at time of last follow-up. Multivariable analysis was not performed because of the small sample size. Statistical analysis was done using SAS version 9 (SAS Institute, Cary, NC).
RESULTS
Between January 2000 and December 2009, 630 patients underwent complete gross resection of a pancreatic adenocarcinoma at our institution. Initial systemic treatment for AJCC stage III disease was given to 36 of these patients (6%). The patient demographics are shown in Table 1. The median age of this cohort was 64 years (37–85 years), and 19 patients were female (53%). The lesion was confined to the head in 32 patients (89%), body in 2 patients (6%), and tail in 2 patients (6%). Initial unresectability was determined operatively in 42% of patients, while in the remaining patients it was determined by cross-sectional imaging. Involvement of the superior mesenteric artery (SMA) precluded resection in 12 patients (33%), the superior mesenteric vein and portal vein in 25 patients (69%), and the celiac axis in 3 patients (8%). Some patients had involvement of multiple vessels precluding resection.
TABLE 1.
Patient characteristics
| Characteristic | Number (%) |
|---|---|
| Age | 64 (37–85) |
| Sex | |
| Male | 17 (47.2) |
| Female | 19 (52.8) |
| Location | |
| Head | 32 (88.9) |
| Body | 2 (5.6) |
| Tail | 2 (5.6) |
| Differentiation | |
| Well | 1 (2.8) |
| Moderate | 31 (86.1) |
| Poor | 3 (8.3) |
| Unknown | 1 (2.8) |
| Biliary drainage (n = 32) | |
| No | 5 (15.6) |
| Stent | 14 (43.8) |
| Operative bypass | 13 (40.6) |
| Reason for unresectabilitya | |
| SMA involvement | 12 (33%) |
| SMV/portal involvement | 25 (69%) |
| Celiac involvement | 3 (8%) |
Some patients had multiple reasons
Multimodality therapy was used in 21 patients (58%) and systemic therapy alone in 15 patients (42%). The majority of regimens included gemcitabine (94%). Details of the neoadjuvant therapy are shown in Table 2. The median time from start of treatment to resection was 6 months (range 2.5–13.0 months). Of the 32 patients with pancreatic head lesions, preoperative biliary drainage was required in 27 patients (84%). Biliary drainage consisted of operative bypass in 13 patients and endoscopic placement of internal biliary stents in 14 patients.
TABLE 2.
Neoadjuvant therapy
| Characteristic | Number (%) |
|---|---|
| Neoadjuvant radiation | |
| Yes | 21 (58.3) |
| No | 15 (41.7) |
| Chemotherapy | |
| Gemcitabine | 11 (30.5) |
| Gemcitabine ? taxotere ? xeloda | 11 (30.5) |
| Gemcitabine ? xeloda | 4 (11.1) |
| Gemcitabine ? 5-FU ? leukovorin | 4 (11.1) |
| Gemcitabine ? erlotinib | 3 (8.3) |
| Gemcitabine ? cisplatin | 2 (5.6) |
| 5-FU ? leukovorin | 2 (5.6) |
| Gemcitabine ? oxaliplatin | 1 (2.8) |
| Gemcitabine ? carboplatin | 1 (2.8) |
Clinicopathologic and treatment-related variables are shown in Table 3. The majority of resected patients required a pancreaticoduodenectomy (n = 31, 86%). Portal vein resection was performed in 7 patients (19%). Splenectomy was required in 5 patients (14%). The mean operative time was 312 ± 14 min, and the mean estimated blood loss was 992 ± 136 ml. There was no mortality in this series. The overall morbidity rate was 58%, with the majority of the complications being wound infections (19 patients). There were 4 patients who experienced a pancreatic leak and a single patient with a biliary leak.
TABLE 3.
Clinicopathologic and treatment variables
| Characteristic | Number (%) |
|---|---|
| Procedure | |
| Pancreaticoduodenectomy | 31 (86.1) |
| Distal pancreatectomy | 4 (11.1) |
| Total pancreatectomy | 1 (2.8) |
| Portal vein resection | |
| Yes | 7 (19.4) |
| No | 29 (80.6) |
| Splenectomy | |
| Yes | 5 (13.9) |
| No | 31 (86.1) |
| OR time (minutes)a | 312 (±14) |
| Blood loss (ml)a | 992 (±136) |
| Blood transfused (units)a | 0.49 (±0.17) |
| Pathologic T stage | |
| 0 | 2 (5.6) |
| 1 | 5 (13.9) |
| 2 | 3 (8.3) |
| 3 | 26 (72.2) |
| Pathologic N stage | |
| 0 | 30 (83.3) |
| 1 | 6 (16.7) |
| Nodes examineda | |
| No. positive | 0.44 ±0.18 |
| No. negative | 11.4 ± 0.33 |
| Resection | |
| R0 | 30 (83.3) |
| R1 | 6 (16.7) |
| Margin | |
| Anterior | 0 |
| Posterior | 4 (11.1) |
| Pancreatic | 2 (5.6) |
| Biliary | 1 (2.8) |
| Treatment response | |
| Unknown | 1 (2.8) |
| 0–25% | 11 (30.6) |
| 25–50% | 7 (19.4) |
| 50–99% | 15 (41.7) |
| 100% | 2 (5.6) |
Mean (SEM)
Adjuvant therapy was given to 21 patients (58%), with the majority of regimens containing gemcitabine. Only 2 patients (5.6%) received adjuvant radiotherapy. With a median follow-up of 12 months (range 2–40 months), recurrence was noted in 18 patients (50%). Recurrence data are shown in Table 4. There was no correlation between arterial involvement or vein involvement and the incidence of local recurrence. The median OS for the cohort was 25 months from time of resection and 30 months from time of treatment initiation (Fig. 1a, b). There was no correlation seen between pathologic treatment response and survival.
TABLE 4.
Adjuvant therapy and recurrence data
| Characteristic | Number (%) |
|---|---|
| No. of patients who recurred | 18 (50) |
| Recurrence location | |
| Liver | 6 (33.3) |
| Local | 7 (38.9) |
| Peritoneal | 2 (11.1) |
| Lung | 1 (5.6) |
| Other | 2 (11.1) |
FIG. 1.
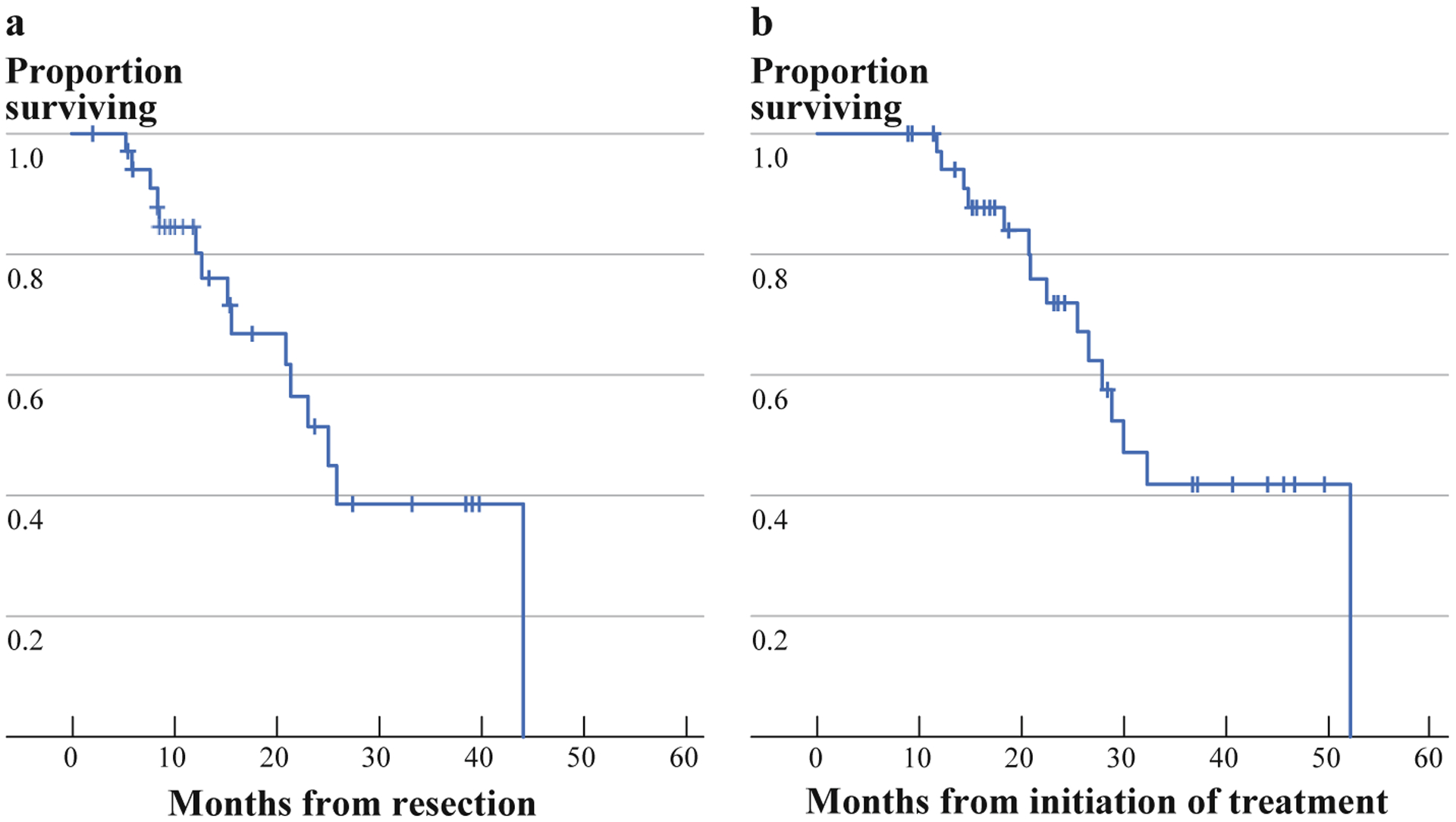
Overall survival. a Since time of resection. b Overall survival since initiation of treatment
We performed a case-matched DSS comparison between patients who were initially AJCC stage III and patients with pancreatic cancer who were initially resectable. Patients were matched based on a previously validated pancreatic nomogram. Characteristics of the 2 cohorts are shown in Table 5. There was no difference in nodal stage, differentiation, margin status, or location. The case-matched cohort was older (62.3 vs. 69.5 years, P = .003). Additionally, the patients who were initially stage III had more pathologic T0 and T1 tumors than the case-matched cohort (P = .028). As shown in Fig. 2, there is no significant difference in DSS between the 2 cohorts (log rank, P = .35).
TABLE 5.
Case matching
| Variable | Number (%) | P value | |
|---|---|---|---|
| Initial stage III (N = 35) | Case matched group (N = 35) | ||
| Age | 62.3 ± 9.9a | 69.5 ± 9.2a | .003 |
| Gender | 1 | ||
| Male | 18 (51) | 18 (51) | |
| Female | 17 (48) | 17 (49) | |
| T stage | .028 | ||
| T0 | 2 (6) | 0 | |
| T1 | 4 (11) | 0 | |
| T2 | 3 (9) | 9 (26) | |
| T3 | 26 (74) | 26 (74) | |
| N stage | 1 | ||
| N0 | 29 (83) | 29 (82.9) | |
| N1 | 6 (17) | 6 (17.1) | |
| Differentiation | .22 | ||
| Well | 1 (3) | 5 (14) | |
| Moderately | 26 (74) | 13 (37) | |
| Poorly | 3 (9) | 12 (34) | |
| Unknown | 5 (14) | 5 (14) | |
| Margin | .312 | ||
| R0 | 29 (83) | 29 (83) | |
| R1 | 6 (17) | 6 (17) | |
| Location | .364 | ||
| Head | 23 (66) | 31 (89) | |
| Neck | 0 | 1 (3) | |
| Body | 2 (6) | 1 (3) | |
| Tail | 10 (29) | 2 (6) | |
FIG. 2.
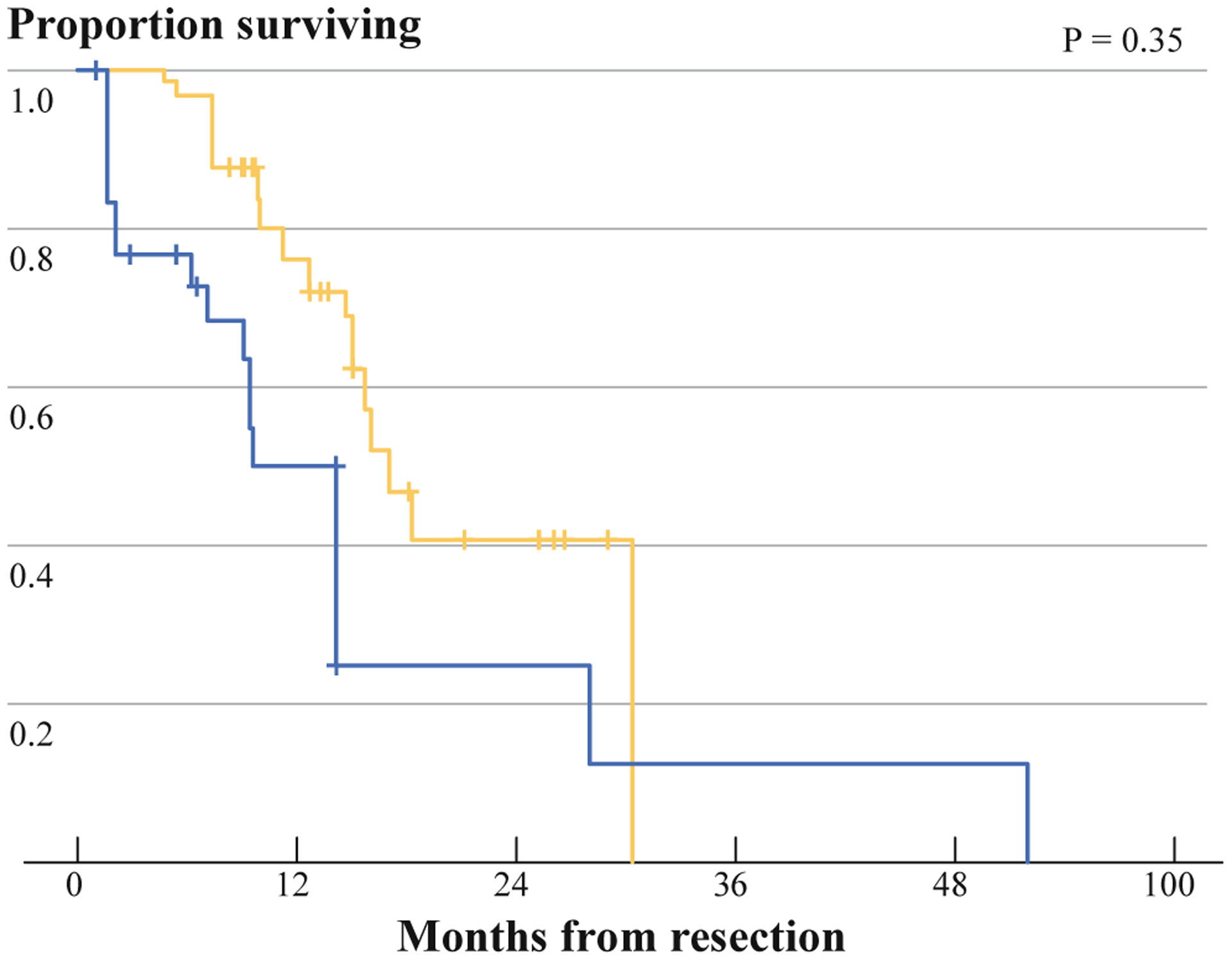
Comparison of disease-specific survival between initial stage III (dashed line) patients and case-matched control group (solid line)
DISCUSSION
Pancreatic adenocarcinoma is a highly aggressive malignancy that is generally lethal. The combination of margin negative resection and adjuvant systemic therapy offers the best chance for long-term survival. The majority of patients, however, are found to have locally unresectable or metastatic disease at the time of diagnosis. Patients who present with locally unresectable disease are generally treated with either chemotherapy or chemoradiotherapy.
Several groups have used neoadjuvant chemotherapy or chemoradiotherapy to downstage locally advanced disease to resectable disease.3–7,12,13 The number of patients who have been successfully resected in previously reported series, however, is small. A recent systematic review concluded that median survival after resection can range from 9 to 23 months (median 13.3 months).14 However, this is difficult to interpret as many series may have included borderline resectable patients. This study demonstrates that patients with initially unresectable pancreatic cancer who can be converted to resectable disease with chemotherapy or radiotherapy have an acceptable survival following resection. The median overall survival in this series of highly selected patients was 25 months since time of resection.
A strength of this study was the case-matched comparison that was performed between the initial stage III patients to patients from our database who were initially resectable (initial stage I and II). Case matching was accomplished using a nomogram for disease-specific survival for pancreas cancer.8 This nomogram has been validated externally in 2 separate studies and has been shown to predict outcome more accurately than AJCC tumor stage.15,16 This nomogram was chosen because of the difficulty in matching TNM stage between a group of patients that initially received chemotherapy or chemoradiotherapy, and one that did not. To prevent an unfair survival advantage in the initial stage III patients, pathologic T stage after treatment was used in the case matching. This case matching demonstrated that patients in this series who were downstaged from initially unresectable disease have a survival postresection that was similar to patients with resectable disease at presentation. Additionally, this series found survival in this group of patients to be similar to other contemporary large series of resections for pancreas cancer.8,12,14,17,18
Significant downstaging occurred in this series as only 19% of patients required a vein resection despite 69% of patients being initially unresectable as a result of involvement of the SMV/portal vein. Additionally, surgical margins were involved in 16% of patients, which is low compared with many series. Treatment responses were variable in this study. There were 2 pathologic complete responses, both of whom remain disease free at 17 and 11 months, respectively. Histologic degree of treatment response was not associated with overall survival. Other series have suggested that treatment response is associated with improved survival.19 However, the small number of patients in this study limits the ability to demonstrate this association. Additionally, since our series consisted only of patients who had enough of a response to therapy to undergo resection, the selection bias introduced by this could dilute the effect of pathological response on survival.
The main limitation of this study is our inability to determine how many patients were evaluated at our institution with stage III disease and did not respond to treatment and therefore did not undergo resection. Given that there were 36 patients who were downstaged in this database of 630 pancreatic adenocarcinomas (6%), it can be reasoned that downstaging is a rare event. A previous study from our institution by Kim et al. prospectively followed 87 patients who were surgically staged with locally advanced disease. Of these patients, 3 had sufficient response to warrant surgical exploration and only 1 was successfully resected (1%). This patient survived 18 months.4 A similar study was conducted by White et al.,7 25 patients with locally advanced pancreatic cancer underwent neoadjuvant chemoradiation. Of these, 8 patients underwent re-exploration, and 5 were ultimately resected (20%). A study by Todd et al. examined 38 patients who were treated with a 4-drug regimen. Of these, 4 patients (11%) were able to be resected, and this led to a median survival of 28 months. Sahora et al.12 reported a study of 33 patients treated with neoadjuvant gemcitabine and oxaliplatin. Only 18 patients in this study had unresectable disease at onset of treatment, while the remaining 15 patients had borderline resectable disease. There were 13 patients who eventually underwent surgery, 6 of whom had initially unresectable disease, indicating a rate of conversion to resectable disease of 33%. The median survival of those resected was 22 months. The study did not report survival rates based on initial respectability. A study from Mt. Sinai Medical Center treated 68 patients with stage III disease with radiation, 5-FU, streptozotocin, and cisplatin.5 In this trial, 30 patients were clinically downstaged and 20 patients were able to be resected. The median survival in this group was 23.6 months. This trial had a high rate of stage III disease that was able to be converted to resectable disease, but this may be due to the approach to staging. The initial staging lacked uniformity, with many patients being staged at multiple centers and using differing diagnostic modalities including CT, angiography, endoscopic ultrasound, and laparotomy. Reni et al. reported a series of 91 patients with stage III disease who were treated with a 4-drug regimen of either cisplatin, epirubicin, 5-FU/capecitabine, and gemcitabine or cisplatin, docetaxel, capecitabine, and gemcitabine followed by chemoradiation.13 Of the 91 patients, 13 patients (14%) had sufficient response to undergo resection with 9 R0 resections, 3 R1 resections, and 1 R2 resection. The median survival for the group was 28.5 months following resection. From several other series it appears that the resection rate for patients with stage III disease who are treated with neoadjuvant therapy ranges from 1 to 33%.3–7,12–14
A second limitation of the study is the lack of formal radiologic review. Many of the patients did not have their complete imaging available in our PACS system for a retrospective review of their radiology. However, these patients did have their initial radiology reviewed by their surgeon and by our Hepatobiliary Disease Management conference prior to resection. Sufficient response was necessary for consideration for re-exploration. Of those patients who had their complete radiology available in the PACS system, there was significant heterogeneity in the type of radiographic response.
In summary, this study identified a group of patients with pancreatic cancer who could be rendered resectable through the use of chemotherapy or chemoradiation. These patients underwent resection with acceptable morbidity and mortality rates. The survival of this highly selected group appears to be similar to a case-matched group of initially resectable patients. Therefore, patients with locally advanced pancreatic cancer who can be rendered resectable by chemotherapy or chemoradiation should be considered for resection.
REFERENCES
- 1.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the National Cancer Database. Cancer. 2007;110:738–44. [DOI] [PubMed] [Google Scholar]
- 2.Varadhachary GR, Tamm EP, Abbruzzese JL, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–46. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman JP, Lipsitz S, Pisansky T, Weese JL, Solin L, Benson AB 3rd. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16:317–23. [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6:763–9. [DOI] [PubMed] [Google Scholar]
- 5.Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89: 314–27. [DOI] [PubMed] [Google Scholar]
- 6.Todd KE, Gloor B, Lane JS, Isacoff WH, Reber HA. Resection of locally advanced pancreatic cancer after downstaging with continuous-infusion 5-fluorouracil, mitomycin-C, leucovorin, and dipyridamole. J Gastrointest Surg. 1998;2:159–66. [DOI] [PubMed] [Google Scholar]
- 7.White R, Lee C, Anscher M, Gottfried M, Wolff R, Keogan M, et al. Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol. 1999;6:38–45. [DOI] [PubMed] [Google Scholar]
- 8.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127: 1335–9. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa O, Ohhigashi H, Teshima T, Chatani M, Inoue T, Tanaka S, et al. Clinical and histopathological appraisal of preoperative irradiation for adenocarcinoma of the pancreatoduodenal region. J Surg Oncol. 1989;40:143–51. [DOI] [PubMed] [Google Scholar]
- 11.Le Scodan R, Mornex F, Partensky C, Mercier C, Valette PJ, Ychou M, et al. Histopathological response to preoperative chemoradiation for resectable pancreatic adenocarcinoma: the French Phase II FFCD 9704-SFRO Trial. Am J Clin Oncol. 2008;31:545–52. [DOI] [PubMed] [Google Scholar]
- 12.Sahora K, Kuehrer I, Eisenhut A, Akan B, Koellblinger C, Goetzinger P, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149:311–20. [DOI] [PubMed] [Google Scholar]
- 13.Reni M, Cereda S, Balzano G, Passoni P, Rognone A, Zerbi A, et al. Outcome of upfront combination chemotherapy followed by chemoradiation for locally advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2009;64:1253–9. [DOI] [PubMed] [Google Scholar]
- 14.Morganti AG, Massaccesi M, La Torre G, Caravatta L, Piscopo A, Tambaro R, et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol. 2010;17:194–205. [DOI] [PubMed] [Google Scholar]
- 15.Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23:7529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Castro SM, Biere SS, Lagarde SM, Busch OR, van Gulik TM, Gouma DJ. Validation of a nomogram for predicting survival after resection for adenocarcinoma of the pancreas. Br J Surg. 2009;96:417–23. [DOI] [PubMed] [Google Scholar]
- 17.Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701–6. [DOI] [PubMed] [Google Scholar]
- 18.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210; discussion 210–1. [DOI] [PubMed] [Google Scholar]
- 19.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–46(discussion 846–8). [DOI] [PMC free article] [PubMed] [Google Scholar]


