Abstract
Membrane protein structures provide fundamental understanding of their molecular actions and are of importance for drug development. Detergents are widely used to solubilize, stabilize and crystallize membrane proteins, but membrane proteins solubilized in conventional detergents are prone to denaturation and aggregation. Thus, developing novel detergents with enhanced efficacy for protein stabilization remains important. We report herein design and synthesis of a class of phenol-derived maltoside detergents. Using two different linkers, we prepared two sets of new detergents, designated maltose-bis(hydroxymethyl)phenol (MBPs) or maltose-tris(hydroxymethyl)phenol (MTPs). The evaluation of these detergents with three transporters and two G-protein coupled receptors allowed us to identify a couple of new detergents (MBP-C9 and MTP-C12) that consistently conferred enhanced stability to all tested proteins compared to a gold standard detergent (DDM). Furthermore, the data analysis based on the detergent structures provides key detergent features responsible for membrane protein stabilization that together will facilitate the future design of novel detergents.
Keywords: Amphiphile design, maltosides, aromatic-aromatic interactions, protein extraction, protein stabilization
Graphical Abstract

Membrane proteins are incredibly important drug targets involved in various human diseases.1,2 A fundamental understanding of the mechanism of action of membrane proteins is essential for both basic biology and drug discovery. However, membrane proteins overall are much less well characterized than their soluble protein counterparts. Much biochemical and biophysical analysis of membrane proteins starts with multiple preparative steps, including protein solubilisation from native membranes and purification. Even though considerable technical progress has been made over the past few years,3 obtaining high quality of membrane proteins necessary for crystallisation, NMR or cryo-EM analysis remains challenging. Even if good quality protein is obtained it is no guarantee of diffraction quality protein crystals.4-7 Protein stability is a key issue when it comes to membrane protein structural study through these cutting-edge technologies. Addition of a tight binding ligand/lipid to the protein sample or introduction of multiple point mutations into a protein sequence are often used to enhance protein stability in aqueous solutions.8
Detergents are mainly used to extract and stabilize membrane proteins in a soluble form required for downstream characterizations.9,10 Over the past decade, various systems have been proposed as alternatives to detergent molecules and are actively being applied to membrane protein study, as exemplified by amphiphilic polymers (acrylamide-based polymers (Apols)11 and styrene-maleic acid copolymers (SMAs)),12 bicelles,13 nanodiscs (NDs)14 and lipidic cubic phase (LCP).15 In addition, peptide-based detergents such as lipopeptide detergents (LPDs), β-peptides (BPs) and Salipro have been invented to tackle the issue of relatively poor membrane protein stability in conventional detergents.16-18 However, most of these systems are poor at protein extraction from the native membranes and are yet to be of wide utility for membrane protein structure study. In addition, detergents remain to date the most widely used tools for extraction, purification, and biophysical analysis of membrane protein including protein crystallography. Indeed, about 80% of the membrane protein structures reported to date have been obtained from the use of detergents, highlighting the importance of detergent tools in membrane protein structural study.19 Carbohydrate-bearing detergents such as n-octyl-β-D-glucoside (OG), n-decyl-β-D-maltoside (DM), n-dodecyl-β-D-maltoside (DDM) and neopentyl glycol (NG)-based amphiphiles20-29 are particularly useful for membrane protein structural characterization. Among these detergents, the two NG class amphiphiles, MNG-3 and GNG-3 (as known as LMNG and OGNG, respectively), are particularly interesting as these novel detergents have contributed to the determination of more than 40 new membrane protein structures including several G protein-coupled receptors (GPCRs) over the past decade.30-46 This remarkable contribution has encouraged chemists to further develop innovative amphiphiles to facilitate membrane protein structural study. Recent examples include glyco-tripod amphiphiles (TPAs),47 fluorinated detergents,48 mannitol/mesitylene-based glucoside amphiphiles (MNAs/MGAs),49,50 rigid core-bearing amphiphiles ((norbornane (NBMs)/resorcinarene (RGAs)/scylloinositol (SIGs)/1,3,5-triazine (TEMs)),51-54 and rigid hydrophobic group-bearing amphiphiles (e.g., glyco-diosgenin (GDN) and penta-phenylene maltoside (PPM)).55,56 Notably, some recent inventions such as penta-saccharide-bearing amphiphiles (PSEs), dendronic trimaltosides (DTMs), vitamin E-based glucosides (VEGs) and terphenyl-cored trimaltosides (TPMs) produced very high quality EM micrographs of human β2-adrenergic receptor (β2AR)-G protein complex.57-60 We recently reported asymmetric MNGs (A-MNGs) and pendant-bearing GNGs (P-GNGs) that are significantly more effective than the original amphiphiles (LMNG and OGNG, respectively) at stabilizing membrane proteins, highlighting the possibility to further improve detergent property via structural modifications.61,62 As a part of our long-term efforts, here we describe two sets of aromatic ring-based maltosides, maltose-bis(hydroxymethyl)phenol (MBPs) and maltose-tris(hydroxymethyl)phenol (MTPs), designated according to the linkers used to connect the head and tail groups. Evaluation of these detergents with several model membrane protein systems revealed that a couple of the new agents conferred enhanced stability to all tested membrane proteins including two GPCRs compared to a gold standard detergent (DDM). In addition, detergent structural features important for protein stabilization identified here provide insight into detergent development for membrane protein research.
Results
Detergent structures and physical characterizations
All new agents share a common architecture of either 2 or 3 maltoside head groups and a branched alkyl chain (Figure 1). These head and tail groups were connected with each other via two different phenol-based linkers containing multiple hydroxymethyl substituents: 3,5-bis(hydroxymethyl)phenol and 2,4,6-tris(hydroxymethyl)phenol. According to the variation in the chemical structure of the linker, these agents were designated maltose-bis(hydroxymethyl)phenol (MBP) or maltose-tris(hydroxymethyl)phenol (MTP) detergents. The maltoside head group and the branched alkyl chain were attached to the hydroxymethyl groups and phenolic alcohol group of the corresponding linker, respectively. Accordingly, the number of the maltoside head groups in each set of new detergents is the same as that of the hydroxymethyl groups of the phenolic linker used for detergent synthesis. The alkyl chain length varied depending on the hydrophilicity of the detergent head group as detergent hydrophile-lipophile balance (HLB) is crucial for membrane protein stability.63 In addition, the hydrophobic length of any detergent needs to be compatible with the hydrophobic dimensions of the protein in order to confer protein stability. The MBPs with two maltoside groups vary in alkyl chain length from C8 to C11, while the alkyl chain length could be further extended to C14 for the MTPs because of the presence of three maltoside units. The variation in the alkyl chain length is indicated in the detergent designation (Figure 1). As a consequence of utilizing two different phenolic linkers, we have prepared the new detergents with alkyl chain lengths ranging from C8 to C14.
Figure 1.
Chemical structures of novel amphiphiles (maltose-bis(hydroxymethyl)phenol (MBPs) and maltose-tris(hydroxymethyl)phenol (MTPs)). The new agents contain two/three maltoside units and branched alkyl chains as the hydrophilic and hydrophobic groups, respectively. The head and tail groups were connected using two different phenolic linkers: 3,5-bis(hydroxymethyl)phenol and 2,4,6-tris(hydroxymethyl)phenol. Two maltose units were conjugated to the 3,5-positions of the phenolic linker for the MBPs, while the 2,4,6-positions of the linker were utilized to attach three maltose units for the MTPs. The alkyl chain length varied depending on the hydrophilicity of the head groups (C8 to C11 for the MBPs and C11 to C14 for the MTPs).
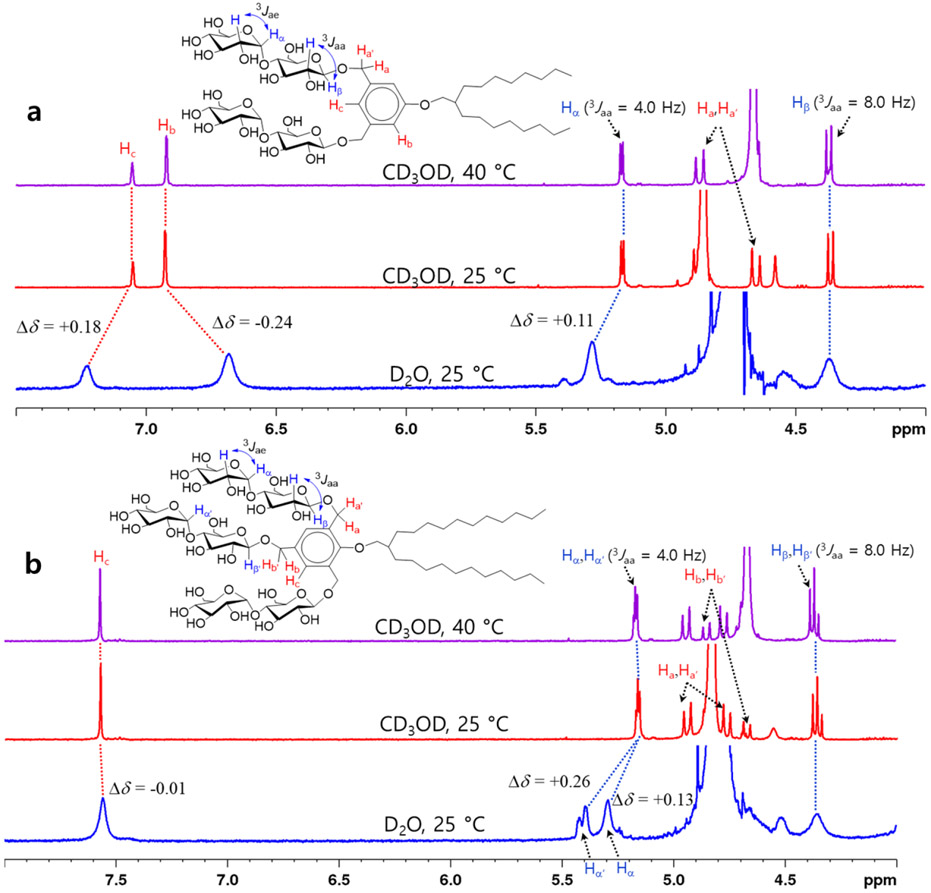
New detergents were prepared using a synthetic protocol comprising four synthetic steps (see Schemes S1 and S2): i) Alkylation of dimethyl 5-hydroxyisophthalate (A)/ethyl 4-hydroxy-3,5-bis(hydroxymethyl)benzoate (B) with the branched alkyl bromide (D/E); ii) ester reduction using LiAlH4; iii) stereo-selective maltosylation; and iv) global deprotection of multiple benzoyl groups (Scheme 1). In order to obtain β-anomeric products, the phenol-based alcohol derivatives (alkylated 3,5-bis(hydroxymethyl)phenol (F)/2,4,6-tris(hydroxymethyl)phenol derivative (G)) and perbenzoylated maltosylbromide were used as glycosyl acceptor and donor, respectively, in the presence of silver triflate (AgOTf) as a promotor. The anomeric purities of the MBPs and MTPs were supported by their 1H NMR spectra taken in CD3OD (Figure 2). For instance, MBP-C8 having the two maltoside head groups at the 3,5-positions of the phenolic linker gave an anomeric signal at 4.36 ppm as a doublet with a coupling constant (3Jaa) of 8.0 Hz, corresponding to the β-anomeric protons (Hβ) (Figure 2a). These values of chemical shift (δ) and coupling constant (3J) reveal successful formation of two β-glycosidic bonds. The same coupling constants were obtained for the β-anomeric signals of MTP-C11, but these signals were separated into two peaks in a 2:1 ratio (Hβ:Hβ) due to the different relative locations of the maltoside groups with respect to the alkyl ether substituent (Figure 2b). Two maltoside groups locate at the ortho-position of the alkyl ether substituent of the benzene ring, while the other maltoside group locates at the para-position of the substituent. We also observed another anomeric signal at 5.16 ppm with a smaller coupling constant (3Jae) of 4.0 Hz in the spectra of both MBP-C8 and MTP-C11. This is due to the presence of the maltoside head groups where two glucose units are connected via an 1,4-α-glycosidic linkage. The benzylic protons (Ha and Ha’) of MBP-C8 generates one set of doublet peaks well separated from each other (4.89 and 4.65 ppm).
Scheme 1.
Synthetic scheme for preparations of MBPs (top) and MTPs (bottom). Two starting materials, dimethyl 5-hydroxyisophthalate (A) and ethyl 4-hydroxy-3,5-bis(hydroxymethyl)benzoate (B), were used to synthesize the MBPs and MTPs, respectively. The branched alkyl bromide (D or E) prepared from the corresponding branched aliphatic alcohol (C) was reacted with the phenol group of the starting material, followed by the ester reduction, to afford 3,5-bis(hydroxymethyl)phenol (F) or 2,4,6-tris(hydroxymethyl)phenol derivatives (G). The final amphiphilic products (MBPs and MTPs) were obtained by stereo-selective glycosylation and global deprotection. The alkyl chain length varied from C8 (n = 1) to C11 (n = 4) for the MBPs or from C11 (n = 4) to C14 (n =7) for the MTP.
Figure 2.
Partial 1H NMR spectra of (a) MBP-C8 and (b) MTP-C11 in CD3OD at 40 (top) and 25 °C (middle), or D2O at 25 °C (bottom). The chemical structures of the amphiphiles (MBP-C8 and MTP-C11) are shown, with the protons of interest (anomeric, benzylic and aromatic protons) labelled. The signals corresponding to these protons are assigned in each spectra. 1H NMR spectrum of MBP-C8 showed a doublet signal at 4.36 ppm, with a coupling constant (3Jaa) of 8.0 Hz, corresponding to β-anomeric protons (Hβ). Similar chemical shifts and the same coupling constants were observed for the β-anomeric protons (Hβ and Hβ’) of MTP-C11, but this signal was divided into two doublets each centered at 4.37 and 4.35 ppm due to their different environments. The α-anomeric protons (Hα or Hα’) present in the maltoside head group yielded a doublet signal at 5.16 ppm with a reduced coupling constant (3Jae = 4.0 Hz). The NMR spectra in CD3OD measured at 40 °C uncovered the peaks corresponding to the benzylic protons (Ha/Ha’ for MBP-C8 and Hb/Hb’ for MTP-C11). The NMR spectra of the detergents were also obtained using D2O at 25 °C. The signals corresponding to the aromatic protons (Hc) directed to the hydrophilic surfaces shifted little or down-field (Δδ = − 0.01 or + 0.24 ppm), whereas the signal corresponding to the aromatic proton directed to the hydrophobic interior (Hb for MBP-C8) shifted substantially up-field (Δδ = − 0.24 ppm). Large downfield shifts were observed for the α-anomeric protons (Hα for MBP-C8 (Δδ = + 0.11 ppm) and Hα/Hα’ for MTP-C11 (Δδ = +0.13/+0.26 ppm) in the micellar environment. Tetramethylsilane (TMS) was used as an internal standard.
At an elevated temperature of 40 °C, the solvent peak corresponding to H2O shifted from 4.85 to 4.67 ppm, uncovering the doublet peak at 4.89 ppm (Figure 2a). In the case of MTP-C11, two sets of doublet signals originating from the two kinds of benzylic protons (Ha and Ha’; Hc and Hc’) were found in a 2:1 ratio. One set appearing at 4.95 and 4.78 ppm corresponds to the benzylic protons (Ha and Ha’) located at the ortho-position of the phenol-based linker, while the other set appearing at 4.87 and 4.68 ppm was assigned to the benzylic proton (Hc and Hc’) located at the para-position. The presence of the NMR signal at 4.86 ppm was clarified in the 1H NMR spectrum obtained at 40 °C (Figure 2b). The integration ratio of these two sets of the benzylic peaks (Ha and Ha’: Hb and Hb’ = 2:1) further supports the peak assignment. The detergent chemical structures were further investigated by 2D NOESY spectra of MBP-C8 and MTP-C11 dissolved in CD3OD (Figure 3). Due to the proximity in space, the ortho-positioned aromatic proton (Hb) of MBP-C8 with respect to the alkoxy substituent strongly correlates with both the benzylic proton (Ha) and the alkyl proton (Hd) (Figure 3a). Another correlation signal was observed between the para-positioned aromatic proton (Hc) and the benzylic proton (Ha) for this detergent. In the case of MTP-C11, the aromatic proton (Hc) also gave strong correlations with two types of benzylic protons (Ha and Hb) (Figure 3b). In addition, the β-anomeric proton (Hβ) in the ortho-positioned substituent and the aromatic proton (Hc) correlate with each other. In contrast, no corresponding signal (Hβ-Hc) was observed for MBP-C8, indicating that the maltoside groups of this detergent are unlikely directed to the opposite side of the alkyl chain in the energy-minimized conformation under the conditions. More correlation signals were found in the NOESY spectra of both MBP-C8 and MTP-C11 between the alkyl chain protons (Hd-He and He-Hf) or between the α-anomeric proton (Hα) and the maltose proton (Hh/Hg) around the α-glycosidic linkage (Figure S3). Thus, the 2D NOESY spectra of the detergents further confirm the regio-chemistry of the phenol-based linkers as well as the stereochemistry of the α/β-glycosidic bond.
Figure 3.
Partial 2D NOESY spectra of MBP-C8 (a) and MTP-C11 (b). Multiple pairs of protons responsible for the main correlation signals are represented in the detergent chemical structures, as indicated by the curved arrows. Due to through-space interactions, strong correlation signals appear between the aromatic protons and the benzylic protons in the linker region. In addition, the alkyl chain proton (Hd) correlates with the ortho-positioned aromatic proton (Hb) in MBP-C8, while the β-anomeric protons (Hβ) correlates with the meta-positioned aromatic proton (Hc) in MTP-C11. Detailed NOESY analysis can be found in the ESI.
High water-solubility is a prerequisite for detergent use in biophysical studies of membrane proteins. All the new agents (MBPs & MTPs) except MBP-C10 (~3%) and MBP-C11 (~1%) were highly soluble in water (> 10 % w/v), indicative of effective self-assembly of these detergents in water. The high water-solubility was well maintained long term; the solutions containing the individual MBPs/MTPs remained transparent over a one-month incubation at room temperature. Aggregation behaviors of the MBPs and MTPs were investigated by measuring critical aggregation concentrations (CACs) and hydrodynamic diameters (Dh) of their self-assemblies in water. Detergent CACs were estimated from the use of a water-insoluble fluorescent dye (i.e., diphenylhexatriene (DPH)),64 while the hydrodynamic diameters (Dh) of detergent self-assemblies were determined through dynamic light scattering (DLS) measurements.
The summarized data for the MBPs and MTPs along with DDM are presented in Table 1. All the new agents introduced here gave lower CACs ranging from ~2 to ~8 μM than DDM (170 μM) indicating that they form more stable self-assemblies than DDM. Interestingly, despite the presence of the long alkyl chain, the CACs of the MBPs were similar to those of the MTPs. As expected, an inverse proportionality was observed between detergent CACs and the alkyl chain lengths. For instance, the CACs of the MBPs were reduced from ~8 to ~3 μM with increasing the alkyl chain length from C8 to C11.
Table 1.
Molecular weights (MWs), critical aggregation concentrations (CACs) of MBP/MTPs along with a conventional detergent (DDM) and hydrodynamic diameters (Dh) (mean ± SD, n = 5) of their self-assemblies in water at 25 °C.
| Detergent | MWa | CAC (mM) |
CAC (wt%) |
Dh (nm)b |
Solubility (wt%) |
|---|---|---|---|---|---|
| MBP-C8 | 1055.2 | ~0.008 | ~0.0008 | 7.4±0.1 | ~10 |
| MBP-C9 | 1083.3 | ~0.004 | ~0.0004 | 8.2±0.1 | ~10 |
| MBP-C10 | 1111.3 | ~0.003 | ~0.0003 | 51.4±0.2 | ~3 |
| MBP-C11 | 1139.4 | ~0.003 | ~0.0003 | 186±27 | ~1 |
| MTP-C11 | 1493.7 | ~0.008 | ~0.0012 | 7.4±0.3 | ~10 |
| MTP-C12 | 1521.7 | ~0.006 | ~0.0009 | 7.8±0.2 | ~10 |
| MTP-C13 | 1549.8 | ~0.004 | ~0.0006 | 8.2±0.1 | ~10 |
| MTP-C14 | 1577.9 | ~0.002 | ~0.0003 | 8.6±0.2 | ~10 |
| DDM | 510.6 | ~0.170 | ~0.0087 | 6.8±0.1 | ~10 |
Molecular weight of detergents.
Hydrodynamic diameter of detergent aggregates determined at 1.0 wt% by dynamic light scattering.
The new agents, particularly MBP-C10 (51 nm) and MBP-C11, tend to form larger aggregates than DDM (7.4 ~186 vs 6.8 nm). The large aggregates formed by MBP-C10 (51.3 nm) and MBP-C11 (186 nm) are likely responsible for their low solubility in water. These large aggregates also indicate that self-assemblies formed by MBP-C10 and MBP-C11 significantly deviate from a spherical micelle in terms of their architecture. When the two sets were compared in terms of aggregate size, the MTPs form self-assemblies significantly smaller than the MBPs. For example, MBP-C11 formed large self-assemblies with a size of 186 nm, while the MTP counterpart (MTP-C11) formed small micelles with a diameter of 7.4 nm. This different behavior can be traced to the large hydrophilic group of the MTPs compared to that of the MBPs. It was a common feature of both MBPs and MTPs that the aggregate size is proportional to the alkyl chain length, but aggregate sizes of the MBPs were more sensitive to the alkyl chain length than the MTPs (Table 1 & Figure S4). This different behavior between the MBPs and MTPs is associated with the effect of detergent alkyl chain length on molecular geometry. DLS profiles obtained from detergent solutions at 1.0 wt% showed that detergent self-assemblies are highly homogeneous, resulting in a single set of size population (Figure S4). Detergent self-assemblies were further investigated by increasing detergent concentration from 0.25 to 2.0 wt% at 25 °C (Figure S5a). Self-assemblies formed by MBP-C9 showed a gradual increase in their size with increasing detergent concentration. In contrast, the self-assembly size formed by MTP-C12 varied little under the same conditions, as observed with DDM. A similar trend was observed when solution temperature varied from 15 to 65 °C, keeping detergent concentration at 1.0 wt% (Figure S5b). The size of self-assemblies formed by MTP-C12 or DDM varied little, while the size of MBP-C9 self-assemblies tended to decrease with increasing temperature under the conditions tested. Thus, self-assemblies formed by MTP-C12 seem more resistant to detergent concentration or solution temperature than those formed by MBP-C9 in terms of size variation.
As the new detergents contain an aromatic group (i.e., benzene) between the head and tail groups, they have potential to form aromatic-aromatic interactions between detergent molecules in a self-assembly environment. In order to investigate this possibility, 1H NMR spectra of MBP-C8 were measured in D2O.56,60 When dissolved in CD3OD at 25 °C, MBP-C8 produced two well-resolved signals at 7.05 and 6.92 ppm, corresponding to aromatic protons of Hb and Hc (Figure 2a). When D2O was used as an NMR solvent, all signals were significantly broadened due to the self-assembly formation in an aqueous environment. More importantly, the two aromatic signals showed different changes in their chemical shifts (Δδ) depending on the directions of the corresponding protons; the signal of Hb directed to the hydrophobic micelle interior shifted upfield by 0.24 ppm (Δδ = − 0.24 ppm), while that of Hc directed to the hydrophilic surface moved downfield by 0.18 ppm (Δδ = + 0.18 ppm). In addition, the NMR signals corresponding to two anomeric protons (Hα and Hβ) located at the hydrophilic region shifted in a way similar to the signal of Hc (Δδ = + 0.11 ppm). Thus, the distinct behavior of the Hb signal (i.e., upfield shift) observed upon solvent change from CD3OD to D2O is likely due to the shielding effect of the benzene ring, suggesting the presence of intermolecular aromatic-aromatic interactions between detergent molecules in the micelle environment. The downfield shifts of the signals corresponding the other aromatic proton (Hc) and two anomeric protons (Hα and Hβ) seem to originate from their interactions with water molecules in the hydrophilic surfaces of the self-assemblies.65 When MTP-C11 was investigated under the same conditions, little change in the chemical shift (Δδ = − 0.01 ppm) was observed for the aromatic proton (Hc) (Figure S5b). Due to the absence of an aromatic proton directed to the hydrophobic interior of the micelles, it is unclear whether micelles formed by this MTP have aromatic-aromatic interactions between detergent molecules under the conditions tested. As for the two anomeric signals corresponding to Hβ/Hβ’ and Hα/Hα’, small and large downfield shifts in the chemical shift (Δδ) were observed, respectively, upon the change in solvent. Notably, the NMR spectrum of MTP-C11 in D2O showed two signals in the anomeric region (5.2 ~ 5.4 ppm) due to the presence of two different α-anomeric protons (Hα and Hα’) in this detergent. The α-anomeric proton (Hα’) at the para-position shifted more downfield (Δδ = + 0.26 ppm) than the ortho-positioned counterpart (Hα; Δδ = + 0.13 ppm.
Detergent evaluation for membrane protein stability
For biological evaluation, the MBPs and MTPs were first tested with the Arabidopsis thaliana boron transporter 1 (AtBOR1).66 AtBOR1 expressed in Saccharomyces cerevisiae was purified in DDM and the resulting DDM-purified AtBOR1 was subjected to detergent exchange into the individual MBPs/MTPs via sample dilution. A thiol-specific maleimide-based fluorophore, N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl]maleimide (CPM), was used to monitor the folded state of the transporter.67 At a detergent concentration of 0.04 wt%, the thermal denaturation profiles showed that all the new detergents were better than DDM at preserving the AtBOR1 folded state (Figure 4a and S6). Based on the CPM results, we selected MBP-C9 for a fluorescence size exclusion chromatography (FSEC) study with the transporter. This methodology allows us to monitor both integrity and homogeneity of the detergent-solubilized transporter. S. cerevisiae membranes containing AtBOR1-GFP fusion protein were incubated with DDM and MBP-C9 at 1.0 wt% for one hour. DDM or MBP-C9-extracted AtBOR1 was subjected to a thermal treatment at 46 °C for 10 min before loading onto a SEC column. The DDM-extracted transporter gave rise to a monodisperse peak at about fraction 35 corresponding to the intact fusion protein (Figure 4b). After the heat treatment, however, the height of the main peak was substantially reduced, along with appearance of a large aggregation peak at fraction 7. This result indicates that the DDM-solubilized transporter is relatively unstable. When MPB-C9 was used for transporter extraction and solubilization under the same conditions, the amount of solubilized fusion protein (i.e., AtBOR1-GFP) was slightly smaller than that observed with DDM (unheated control). However, very little change was observed in terms of either the monodispersed peak or the aggregation peak upon heating, leading to the conclusion that this new detergent is clearly superior to DDM at maintaining AtBOR1 integrity.
Figure 4.
(a) Thermal denaturation profiles of AtBOR1 in individual MBPs and (b) fluorescence size exclusion chromatography (FSEC) profiles of AtBOR1-GFP fusion protein in MBP-C9. DDM was used as a control. Thermal stability of the transporter was monitored using a detergent concentration of 0.04 wt% via CPM assay performed at 40 °C for 120 min. The amounts of folded transporter were normalized relative to that observed for the DDM-solubilized protein. The FSEC traces were obtained before or after heating the detergent-solubilized AtBOR1 at 46 °C for 10 min. The data shown is a representative of two independent experiments. ‘h’ on the detergent label denotes the heat-treated sample.
The promising results with AtBOR1 encouraged us to further evaluate the MBPs and MTPs with another transporter, melibiose permease from Salmonella typhimurium (MelBSt).68,69 E. coli membranes containing MelBSt were solubilized on ice by the MBPs/MTPs or DDM at a concentration of 1.5 wt% for 90 min. The amounts of solubilized MelBSt in each detergent were assessed using Western blot and are represented as percentages (%) of initial transporter present in the untreated membranes. At 0 °C, most of the detergents tested here efficiently extracted MelBSt from the membranes, with the best performance observed for MTP-C12. This C12 alkyl-chained detergent extracted the transporter as effectively as DDM (~100%), while the other detergents yielded reasonable amounts of soluble MelBSt (70~85%) under the conditions tested (Figure 5). To further investigate the ability of a detergent to maintain MelBSt stability, the detergent-extracted MelBSt was treated at an elevated temperature (45, 55, or 65 °C) for another 90 min. For all the new detergents tested, the additional incubation at 45 °C substantially increased the amounts of the soluble MelBSt compared to those obtained from the initial incubation at 0 °C. This could be due to the extended incubation time and/or the enhanced detergent solubility/increased membrane dynamics at the elevated temperature. A further increase in incubation temperature to 55 °C led to a large differentiation in preserving MelBSt stability. At this elevated temperature, the DDM-extracted MelBSt mostly aggregated, while all the tested new detergents (MBP-C8, MBP-C9, MTP-C12, MTP-C13 and MTP-C14) retained significant amounts of soluble MelBSt (75~95%), indicating the superior capability of the new detergents to retain MelBSt in a soluble state.
Figure 5.
Thermo-stability of MelBSt in individual MBPs (MBP-C8/C9) or MTPs (MTP-C12/C13/C14). DDM was used as a reference. E. coli membranes containing MelBSt were treated with the designated detergents at 0 °C for 90 min and the resulting detergent extracts were subjected to an additional incubation at an elevated temperature (45, 55, or 65 °C) for 90 min. The supernatant obtained from ultracentrifugation was analysed by SDS-PAGE and Western blot (top). The amounts of soluble MelBSt under the conditions were expressed as percentage yields (%) in histogram (bottom). The first two lanes represent a total amount of MelBSt present in the untreated membranes (‘Memb.’). Error bars, SEM, n = 3.
The MBPs and MTPs were further evaluated with the bacterial LeuT from Aquifex aeolicus.70 The transporter purified in 0.05% DDM was diluted into buffer solutions including the individual MBPs, MTPs, or DDM to give the final detergent concentration of 0.2 wt%. The ability of the transporter to bind the radio-active substrate ([3H]-Leucine (Leu)) was monitored via scintillation proximity assay at regular intervals during a 14-day incubation at room temperature.71 The DDM-solubilized transporter gave high initial activity (t = 0 day), but a rather rapid loss in protein activity was found over time (Figure 6a). On the other hand, the transporter in the individual MBPs/MTPs initially gave a rather low Leu binding ability compared to the protein in DDM. The Leu binding ability was particularly low for transporter in MBP-C11, MTP-C13, or MTP-C14. When it comes to time course retention in protein activity, however, all the new agents were superior to DDM. It is interesting to note that the three poorly-behaving detergents (MBP-C11, MTP-C13 and MTP-C14) have the longest alkyl chains in each set of new detergents (MBPs/MTPs), suggesting that a relatively hydrophobic detergent or a long alkyl-chained detergent is unsuitable for LeuT stability. Collectively, the results reveal that the short alkyl-chained MBPs/MTPs (MBP-C8/C9/C10 and MTP-C11/C12) were more effective than DDM at preserving LeuT stability over time.
Figure 6.
Time course stability of LeuT (a), β2AR (b) and MOR (c) solubilized in individual MBPs/MTPs, using DDM as a control. MBP-C11 was not tested due to the solubility issue in water and selected detergents were tested for MOR stability. The detergents were used at 0.2 wt% (LeuT and β2AR) or 0.1 wt% (MOR). LeuT stability was assessed by measuring the ability of the transporter to bind the radio-active substrate (3[H]-leucine (Leu)) at regular intervals during a 14-day incubation at room temperature via scintillation proximity assay. β2AR and MOR stability was assessed by measuring the receptor ability to bind the radio-active ligand ([3H]-dihydroalprenolol (DHA) and [3H]-diprenorphine (DPN), respectively) during a 6-day incubation at room temperature. Error bars: SEM, n = 2-3 (LeuT); n = 3 (β2AR and MOR).
In order to investigate the efficacy of the new detergents at stabilizing a G protein-coupled receptor (GPCR), the MBPs and MTPs were further evaluated with the human β2 adrenergic receptor (β2AR).72 The receptor purified in DDM was subjected to detergent exchange conducted by a 30-min sample dilution into the individual detergent-containing buffers. A ligand binding assay was conducted using a radio-active antagonist ([3H]-dihydroalprenolol (DHA)) to assess receptor stability at a detergent concentration of 0.2 wt%.73,74 Following detergent exchange, the receptor solubilized in MBP-C11 or MTP-C11 exhibited poor DHA-binding, while the other new detergents were more or less comparable to each other and DDM in terms of ligand binding (Figure S7). Thus, we selected the two MBPs (MBP-C9/C10) and three MTPs (MTP-C12/C13/C14) to further investigate their effects on receptor stability over time. Receptor stability was monitored at the designated time points during a 6-day incubation at room temperature (Figure 6b). When solubilized in DDM, β2AR rapidly lost ligand binding activity, resulting in only ~10% residual activity following one-day incubation. A similar result was observed for MBP-C10. In contrast, the other tested detergents were significantly more effective than DDM, with the best performance detected with MBP-C9, MTP-C12, or MTP-C13.
Because of the notably enhanced detergent efficacy for stabilizing β2AR, we selected four detergents (MBP-C9, MTP-C12, MTP-C13 and MTP-C14) for further evaluation with another GPCR, the mouse μ-opioid receptor (MOR).75 Protein stability was assessed via a similar method used for β2AR analysis, utilizing the receptor-specific radio-active ligand ([3H]-diprenorphine (DPN)). Conventional DDM completely failed to stabilize this GPCR (Figure 6b). Use of the new detergents substantially enhanced MOR stability compared to DDM. The most promising result was obtained with MBP-C9 that retained almost half of the starting receptor function over the course of 6-day incubation. Taken together, along with β2AR result, these results suggest the utility of MBP-C9 for GPCR structural study.
The selected new detergents were further compared with recently developed detergents (GNG-3,14, TEM-E10 and LMNG).54,62 Based on the protein stability results, two new detergents (MBP-C9 and MTP-C12/MTP-C13) were included in this assessment of LeuT/MOR stability. The optimized NG class detergent (i.e., LMNG) was superior to DDM for both LeuT and MOR stability, consistent with its generally favorable behavior for membrane protein stability (Figure 7). In contrast, another NG class detergent, GNG-3,14, was effective at maintaining LeuT stability under the same conditions, but this pendant-bearing detergent was rather poor at stabilizing MOR. The triazine-based amphiphile, TEM-E10, was more effective than DDM for LeuT and MOR stability, giving overall similar results to LMNG. When the new detergents (MBP-C9 and MTP-C12/MTP-C13) were used, these detergents were superior to both LMNG and TEM-E10 for LeuT and/or MOR stability. MBP-C9 was notable as this agent was significantly better than LMNG and the other recently developed detergents at stabilizing both membrane proteins long term.
Figure 7.
Time course stability of LeuT (a) and MOR (b) solubilized in recently reported (GNG-3,14 and TEM-E10) or new detergents (MBP-C9 and MTP-C12/C13). DDM and LMNG were used as control agents. The detergents were used at 0.2 wt% (LeuT) or 0.1 wt% (MOR). Protein stability was assessed using the same methods as described above. Error bars: SEM, n = 2 (LeuT); n = 3 (MOR).
Discussion
Here we describe the design and synthesis of a new class of maltoside detergents with 3,5-bis(hydroxylmethyl)phenol and 2,4,6-tris(hydroxymethyl)phenol linkers, designated MBPs and MTPs, respectively. These novel detergents were evaluated with several model membrane proteins (three transporters (AtBOR1, LeuT, and MelBSt)) and two GPCRs (β2AR and MOR)) using various methodologies for assessment of protein stability including thermal denaturation assays and ligand binding assays. Our evaluation focused on detergent efficacy for protein stabilization as protein stability is a prerequisite for structural determination of membrane proteins. Protein stabilization efficacy of the MBPs/MTPs varied from one protein to another. For instance, MTP-C14 was among the best agents for MelBSt, while this long alkyl-chained detergent failed to stabilize LeuT. In the case of MTP-C13, this detergent was markedly effective at stabilizing AtBOR, MelBSt, and β2AR, but was unfavourable for stabilizing LeuT. The variation in detergent efficacy depending on the model membrane protein observed here is consistent with the widely accepted notion that there is no single solution to effective maintenance of membrane protein stability in aqueous solution. This is due to large variations in the structures and functions of individual membrane proteins, strongly associated with protein tendency to denature or aggregate. Despite the protein-dependent variation in detergent efficacy, however, two new detergents (MBP-C9 and MTP-C12) were notably more effective than a gold standard classical detergent (DDM) at stabilizing all the tested membrane proteins. When compared with LMNG and recently developed detergents (GNG-3,14 and TEM-E10), MBP-C9 and MTP-C12/MTP-C13 conferred enhanced stability onto LeuT/MOR, with the best performance observed with MBP-C9. This result indicates that MBP-C9 can be used more favourably than other classes of detergents for membrane protein research.
Detergent efficacy for protein stabilization is not solely dependent on a single feature of detergent molecules or micelles. Rather, multiple detergent properties individually or in combination affect detergent behaviors for protein stability and thus it is challenging to pinpoint which specific features of MBP-C9 and MTP-C12 are responsible for the enhanced protein stabilization efficacy observed here. By extracting common structural features of these detergents, however, it is possible to find detergent features favorable for protein stabilization. Two detergents, MBP-C9 and MTP-C12, have the different alkyl chain lengths (C9 vs C12), but their hydrophobic lengths cannot be simply estimated from the alkyl chain lengths. The two sets of new detergents (MBPs and MTPs) have variation in the substitution patterns of the phenolic linkers that results in different polarity behavior of these linking units in the environment of the micelle (Figure 1). Due to the presence of the maltoside head groups at the 2,4,6-positions, the phenolic linker of the MTPs is unlikely to strongly interact with the hydrophobic group/surface of other detergent molecules in micelle environments. Despite its hydrophobic nature, consequently, the phenolic linker of this set would behave like the hydrophilic rather than hydrophobic group in aqueous environment. In the case of the MBP set, in contrast, the maltoside groups are conjugated to the 3,5-positions of the phenolic linker and thus the linking unit of this set behaves like the hydrophobic group under the same conditions. Thus, it is reasonable that the hydrophobic lengths of the MBPs is estimated by adding the length of the phenolic linker to their alkyl chain lengths. This is in contrast with the MTPs where detergent hydrophobic length is estimated from the respective alkyl chain length, with a minor contribution from the phenolic linker. As a result, MBP-C9 and MTP-C12 would differ little in their hydrophobic lengths from each other, as supported by the comparable hydrophobic length calculated from their energy-minimized conformations obtained from DFT calculations (Figure S7). Detergent hydrophobic length matters as it needs to be compatible with the hydrophobic dimensions of the protein surfaces for optimal protein stability. It is also important to note that these detergents have intermediate alkyl chain lengths (C9 and C12) within the individual sets of MBPs (C8~C11) and MTPs (C11~C14), respectively. The short (e.g., MBP-C8 and MTP-C11) or long alkyl chain versions (e.g., MBP-C11 and MTP-C14) in each set failed to give the generally favourable effect on protein stability. This result indicates the importance of HLB for protein stabilization. The short alkyl chain detergents are too hydrophilic to strongly interact with membrane proteins, whereas the hydrophobicity of the long alkyl chain detergents may be too high to prevent protein denaturation. Another interesting feature of the current MBPs is the potential aromatic-aromatic interactions between the phenol-based linking units under micelle or proteomicelle environments, as supported by the 1H NMR study. The relative low CMCs of these detergents (3~8 μM) is likely to be associated with these favorable detergent-detergent interactions in the micelle environment, resulting in enhanced thermodynamic stability of micelles/ proteomicelles. Therefore, the current results showed that the detergent hydrophobic length and HLB play key roles in enhanced protein stabilization efficacy observed for MBP-C9 and MTP-C12, with a potential contribution of detergent aromatic-aromatic interactions.
Conclusions
In summary, we designed and prepared two sets of phenol-based detergents (MBPs and MTPs) with variations in the number of maltoside head groups and alkyl chain length by implementing two different phenolic linkers into the detergent core region. In the evaluation of these detergents with a set of model membrane proteins including two GPCRs, we identified two detergents (MBP-C9 and MTP-C12) that were markedly superior to a gold standard detergent (DDM) at protein stabilization. The multiple favorable attributes described here such as synthetic convenience, reasonable protein extraction efficiency and notable protein stabilization efficacy mean that these detergents hold significant potential for membrane protein study. Furthermore, the favorable detergent features identified here for protein stabilization facilitates rational detergent design and selection for membrane protein research.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) (2021R1A2C2006067 and 2018R1A6A1A03024231 to P.S.C.). This study was also supported by the National Institutes of Health (Grants R01GM122759 and R21NS105863 to L.G.). This work was also supported by Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/N016467/1 awarded to B.B. as well as the European Union's Horizon 2020 research and innovation programme, RAMP-ITN: Rationalising Membrane Protein Crystallisation Innovative Training Network, under the Marie Sklodowska-Curie grant agreement No 722687 (C.C.).
Footnotes
ASSOCIATED CONTENT
The supporting information is available free of charge via the internet at http://pubs.acs.org, including Figures S1 through S8, supplementary methods on detergent evaluation with membrane proteins, and synthetic protocols and characterizations of the new detergents.
The authors declare the following competing financial interest(s): P.S.C. and M.E. are inventors on a patent application that covers the MBPs/MTPs.
REFERENCES
- (1).Rask-Andersen M, Almén MS, and Schiöth HB (2011) Trends in the exploitation of novel drug targets. Nature reviews Drug discovery, 10, 579–590. [DOI] [PubMed] [Google Scholar]
- (2).Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, and Gloriam DE (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nature reviews Drug discovery, 16, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Zhou HX, and Cross TA (2013) Influences of membrane mimetic environments on membrane protein structures. Annual review of biophysics, 42, 361–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kim HJ, Howell SC, Van Horn WD, Jeon YH, and Sanders CR (2009) Recent advances in the application of solution NMR spectroscopy to multi-span integral membrane proteins. Progress in nuclear magnetic resonance spectroscopy, 55, 335–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Raschle T, Hiller S, Etzkorn M, and Wagner G (2010) Nonmicellar systems for solution NMR spectroscopy of membrane proteins. Current opinion in structural biology, 20, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Daum B, Vonck J, Bellack A, Chaudhury P, Reichelt R, Albers SV, Rachel R, and Kühlbrandt W (2017) Structure and in situ organisation of the Pyrococcus furiosus archaellum machinery. Elife, 6, p.e27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Goldie KN, Abeyrathne P, Kebbel F, Chami M, Ringler P, and Stahlberg H (2014) Cryo-electron microscopy of membrane proteins. In Electron Microscopy Humana Press, Totowa, NJ: 325–341. [DOI] [PubMed] [Google Scholar]
- (8).Celej MS, Montich GG, and Fidelio GD (2003) Protein stability induced by ligand binding correlates with changes in protein flexibility. Protein science, 12,1496–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Privé GG (2007) Detergents for the stabilization and crystallization of membrane proteins. Methods, 41, 388–397. [DOI] [PubMed] [Google Scholar]
- (10).Garavito RM, and Ferguson-Miller S (2001) Detergents as tools in membrane biochemistry. Journal of Biological Chemistry, 276, 32403–32406. [DOI] [PubMed] [Google Scholar]
- (11).Tribet C, Audebert R, and Popot JL (1996) Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proceedings of the National Academy of Sciences, 93, 15047–15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Dörr JM, Scheidelaar S, Koorengevel MC, Dominguez JJ, Schäfer M, van Walree CA, and Killian JA (2016) The styrene–maleic acid copolymer: a versatile tool in membrane research. European Biophysics Journal, 45, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ujwal R, and Bowie JU (2011). Crystallizing membrane proteins using lipidic bicelles. Methods, 55, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Denisov IG, and Sligar SG (2016) Nanodiscs for structural and functional studies of membrane proteins. Nature structural & molecular biology, 23, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Caffrey M (2015) A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallographica Section F: Structural Biology Communications, 71, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).McGregor CL, Chen L, Pomroy NC, Hwang P, Go S, Chakrabartty A, and Privé GG (2003) Lipopeptide detergents designed for the structural study of membrane proteins. Nature biotechnology, 21, 171–176. [DOI] [PubMed] [Google Scholar]
- (17).Tao H, Lee SC, Moeller A, Roy RS, Siu FY, Zimmermann J, Stevens RC, Potter CS, Carragher B, and Zhang Q (2013) Engineered nanostructured β-sheet peptides protect membrane proteins. Nature methods, 10, 759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Frauenfeld J, Löving R, Armache JP, Sonnen AF, Guettou F, Moberg P, Zhu L, Jegerschöld C, Flayhan A, Briggs JA, and Garoff H (2016) A saposin-lipoprotein nanoparticle system for membrane proteins. Nature methods, 13, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).White S (2017) Membrane proteins of known 3D structure, http://blanco.biomol.uci.edu/mpstruc/. [Google Scholar]
- (20).Chae PS, Rana RR, Gotfryd K, Rasmussen SGF, Kruse AC, Cho KH, Capaldi S, Carlsso E, Kobilka B, Loland CJ, Gether U, Banerjee S, Byrne B, Lee JK, and Gellman SH (2013) Glucose-neopentyl glycol (GNG) amphiphiles for membrane protein study. Chem. Commun, 49, 2287–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kellosalo J, Kajander T, Kogan K, Pokharel K, and Goldman A (2012) The structure and catalytic cycle of a sodium-pumping pyrophosphatase. Science, 337, 473–476. [DOI] [PubMed] [Google Scholar]
- (22).Frick A, Eriksson UK, de Mattia F, Öberg F, Hedfalk K, Neutze R, Willem J, Deen PM, and Törnroth-Horsefield S (2014) X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. U. S. A, 111, 6305–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hauer F, Gerle C, Fischer N, Oshima A, Shinzawa-Itoh K, Shimada S, and Stark H (2015) GraDeR: membrane protein complex preparation for single-particle cryo-EM. Structure, 23, 1769–1775. [DOI] [PubMed] [Google Scholar]
- (24).Yin J, Mobarec JC, Kolb P, and Rosenbaum DM (2015) Crystal structure of the human OX 2 orexin receptor bound to the insomnia drug suvorexant. Nature, 519, 247–250. [DOI] [PubMed] [Google Scholar]
- (25).Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, and Xu Q (2015) Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature, 523, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Perez C, Gerber S, Boilevin J, Bucher M, Darbre T, Aebi M, and Locher KP (2015) Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature, 524, 433–438. [DOI] [PubMed] [Google Scholar]
- (27).Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, and Ruda GF (2015) K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science, 347, 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Paulsen CE, Armache JP, Gao Y, Cheng Y, and Julius D (2015) Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature, 520, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Schmidt HR, Zheng S, Gurpinar E, Koehl A, Manglik A, and Kruse AC (2016) Crystal structure of the human σ 1 receptor. Nature, 532, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi H-J, DeVree BT, and Sunahara RK (2011) Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature, 469, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Haga K, Kruse AC, Asada H, Yurugi-Kobayashi T, Shiroishi M, Zhang C, and Kobayashi T (2012) Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature, 482, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, and Christopoulos A (2013) Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature, 504, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, and Fujiyoshi Y (2014) Crystal structure of a claudin provides insight into the architecture of tight junctions. Science, 344, 304–307. [DOI] [PubMed] [Google Scholar]
- (34).Dickson VK, Pedi L, and Long SB (2014) Structure and insights into the function of a Ca2+-activated Cl− channel. Nature, 516, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hauer F, Gerle C, Fischer N, Oshima A, Shinzawa-Itoh K, Shimada S, and Stark H (2015) GraDeR: membrane protein complex preparation for single-particle cryo-EM. Structure, 23, 1769–1775. [DOI] [PubMed] [Google Scholar]
- (36).Yin J, Mobarec JC, Kolb P, and Rosenbaum DM (2015) Crystal structure of the human OX 2 orexin receptor bound to the insomnia drug suvorexant. Nature, 519, 247–250. [DOI] [PubMed] [Google Scholar]
- (37).Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, and Xu Q (2015) Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature, 523, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Perez C, Gerber S, Boilevin J, Bucher M, Darbre T, Aebi M, and Locher KP (2015) Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature, 524, 433–438. [DOI] [PubMed] [Google Scholar]
- (39).Taniguchi R, Kato HE, Font J, Deshpande CN, Wada M, Ito K, and Nureki O (2015) Outward-and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat. Commun, 6, 8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, and Ruda GF (2015) K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science, 347, 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Paulsen CE, Armache JP, Gao Y, Cheng Y, and Julius D (2015) Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature, 520, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Schmidt HR, Zheng S, Gurpinar E, Koehl A, Manglik A, and Kruse AC (2016) Crystal structure of the human σ 1 receptor. Nature, 532, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SG, Christopoulos G, Coudrat T, and Danev R (2017) Phase-plate cryo-EM structure of a class B GPCR–G-protein complex. Nature, 546, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).James ZM, Borst AJ, Haitin Y, Frenz B, DiMaio F, Zagotta WN, and Veesler D (2017) CryoEM structure of a prokaryotic cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. U. S. A, 114, 4430–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jörg M, Scammells PJ, May LT, Sexton PM, and Christopoulos A (2017) Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell, 168, 867–877. [DOI] [PubMed] [Google Scholar]
- (46).Tanaka Y, Iwaki S, and Tsukazaki T (2017) Crystal structure of a plant multidrug and toxic compound extrusion family protein. Structure, 25, 1455–1460. [DOI] [PubMed] [Google Scholar]
- (47).Chae PS, Cho KH, Wander MJ, Bae HE, Gellman SH, and Laible PD (2014) Hydrophobic variants of ganglio-tripod amphiphiles for membrane protein manipulation. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1838, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Frotscher E, Danielczak B, Vargas C, Meister A, Durand G, and Keller S (2015) A Fluorinated Detergent for Membrane-Protein Applications. Angewandte Chemie International Edition, 54, 5069–5073. [DOI] [PubMed] [Google Scholar]
- (49).Hussain H, Du Y, Scull NJ, Mortensen JS, Tarrasch J, Bae HE, Loland CJ, Byrne B, Kobilka BK, and Chae PS (2016) Accessible mannitol-based amphiphiles (MNAs) for membrane protein solubilisation and stabilisation. Chemistry.–Eur. J, 22, 7068–7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Cho KH, Ribeiro O, Du Y, Tikhonova E, Mortensen JS, Markham K, Hariharan P, Loland CJ, Guan L, Kobilka BK, Byrne B, and Chae PS (2016) Mesitylene-cored glucoside amphiphiles (MGAs) for membrane protein studies: importance of alkyl chain density in detergent efficacy. Chem.–Eur. J, 22, 18833–18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Das M, Du Y, Ribeiro O, Hariharan P, Mortensen JS, Patra D, Skiniotis G, Loland CJ, Guan L, Kobilka BK, Byrne B, and Chae PS (2017) Conformationally preorganized diastereomeric norbornane-based maltosides for membrane protein study: Implications of detergent kink for micellar properties. J. Am. Chem. Soc, 139, 3072–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Hussain H, Du Y, Tikhonova E, Mortensen JS, Ribeiro O, Santillan C, Das M, Ehsan M, Loland CJ, Guan L, Kobilka BK, Byrne B, and Chae PS (2017) Resorcinarene-Based Facial Glycosides: Implication of Detergent Flexibility on Membrane-Protein Stability. Chem.–Eur. J, 23, 6724–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Sadaf A, Ramos M, Mortensen JS, Du Y, Bae HE, Munk CF, Hariharan P, Byrne B, Kobilka BK, Loland CJ, Guan L, and Chae PS (2019) Conformationally Restricted Monosaccharide-Cored Glycoside Amphiphiles: The Effect of Detergent Headgroup Variation on Membrane Protein Stability. ACS chemical biology, 14, 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Ghani L, Munk CF, Zhang X, Katsube S, Du Y, Cecchetti C, Huang W, Bae HE, Saouros S, Ehsan M, Guan L, Liu X, Loland CJ, Kobilka BK, Byrne B, and Chae PS (2019) 1,3,5-triazine–cored maltoside amphiphiles for membrane protein extraction and stabilization. J. Am. Chem. Soc 141, 19677–49687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Kruse AC, Manglik A, Cho KH, Nurva S, Gether U, Guan L, Loland CJ, Byrne B, Kobilka BK, and Gellman SH (2012) A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins. Chem.–Eur. J, 18, 9485–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ehsan M, Kumar A, Mortensen JS, Du Y, Hariharan P, Kumar KK, Ha B, Byrne B, Guan L, Kobilka BK, Loland CJ, and Chae PS (2019) Self-Assembly Behaviors of a Penta-Phenylene Maltoside and Its Application for Membrane Protein Study. Chem. –Asian J, 14, 1926–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ehsan M, Du Y, Scull NJ, Tikhonova E, Tarrasch J, Mortensen JS, Loland CJ, Skiniotis G, Guan L, Byrne B Kobilka BK, and Chae PS (2016) Highly branched pentasaccharide-bearing amphiphiles for membrane protein studies. J. Am. Chem. Soc, 138, 3789–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Sadaf A, Du Y, Santillan C, Mortensen JS, Molist I, Seven AB, Hariharan P, Skiniotis G and Loland CJ, Kobilka BK, Guan L, Byrne B, and Chae PS (2017) Dendronic trimaltoside amphiphiles (DTMs) for membrane protein study. Chem. Sci 8, 8315–8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Ehsan M, Du Y, Molist I, Seven AB, Hariharan P, Mortensen JS, Ghani L, Loland CJ, Skiniotis G, Guan L and Byrne B Kobilka BK, and Chae PS (2018) Vitamin E-based glycoside amphiphiles for membrane protein structural studies. Org. Biomol. Chem, 16, 2489–2498. [DOI] [PubMed] [Google Scholar]
- (60).Ehsan M, Du Y, Mortensen JS, Hariharan P, Qu Q, Ghani L, Das M, Grethen A, Byrne B, Skiniotis G and Keller S, Loland CJ, Guan L, Kobilka BK, and Chae PS (2019) Self-assembly behaviors and application of terphenyl-cored trimaltosides for membrane protein study: Impact of detergent hydrophobic group geometry on protein stability. Chem.–Eur. J, 25, 11545–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Bae HE, Du Y, Hariharan P, Mortensen JS, Kumar KK, Ha B, Das M, Lee HS, Loland CJ, Guan L, Kobilka BK and Chae PS (2019) Asymmetric maltose neopentyl glycol amphiphiles for a membrane protein study: effect of detergent asymmetricity on protein stability. Chem. Sci, 10, 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Bae HE, Cecchetti C, Du Y, Katsube S, Mortensen JS, Huang W, Rehan S, Lee HJ, Loland CJ, Guan L and Kobilka BK, Byrne B, and Chae PS (2020) Pendant-bearing glucose-neopentyl glycol (P-GNG) amphiphiles for membrane protein manipulation: Importance of detergent pendant chain for protein stabilization. Acta Biomaterialia, 112, 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Chae PS, Kruse AC, Gotfryd K, Rana RR, Cho KH, Rasmussen SG, Bae HE, Chandra R, Gether U, Guan L, Kobilka BK, Loland CJ, Byrne B, and Gellman SH (2013) Novel tripod amphiphiles for membrane protein analysis. Chem.–Eur. J, 19, 15645–15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Chattopadhyay A, and London E (1984) Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal. Biochem 139, 408–412. [DOI] [PubMed] [Google Scholar]
- (65).Wu S, Shi F, Zhang Q, and Bubeck C (2009) Stable hydrogen-bonding complexes of poly (4-vinylpyridine) and polydiacetylenes for photolithography and sensing. Macromolecules, 42, 4110–4117. [Google Scholar]
- (66).Thurtle-Schmidt BH, and Stroud RM (2016) Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proc. Natl. Acad. Sci. U. S. A, 113, 10542–10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, and Stevens RC (2008) A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure, 16, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Guan L, Nurva S, and Ankeshwarapu SP (2011) Mechanism of melibiose/cation symport of the melibiose permease of Salmonella typhimurium. J. Biol. Chem, 286, 6367–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Ethayathulla AS, Yousef MS, Amin A, Leblanc G, Kaback HR, and Guan L (2014) Structure-based mechanism for Na+/melibiose symport by MelB. Nat. Commun, 5, 3009–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL, Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, Huber R, Feldman RA, Short JM, Olsen GJ, and Swanson RV (1998) The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature, 392, 353–358. [DOI] [PubMed] [Google Scholar]
- (71).Quick M, and Javitch JA (2007) Monitoring the function of membrane transport proteins in detergent-solubilized form. Proc. Natl. Acad. Sci. U. S. A, 104, 3603–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, and Kobilka BK (2007) GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science, 318, 1266–1273. [DOI] [PubMed] [Google Scholar]
- (73).Yao X, Parnot C, Deupi X, Ratnala VR, Swaminath G, Farrens D, and Kobilka B (2006) Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat. Chem. Biol 2, 417–422. [DOI] [PubMed] [Google Scholar]
- (74).Swaminath G, Steenhuis J, Kobilka B, and Lee TW (2002) Allosteric modulation of β2-adrenergic receptor by Zn2+. Molecular pharmacology, 61, 65–72. [DOI] [PubMed] [Google Scholar]
- (75).Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, and Granier S (2012) Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature, 485, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.