Abstract
Apolipoprotein A-I (ApoA-I) mimetic peptides are potential therapeutic agents for promoting the efflux of excess cellular cholesterol, which is dependent upon the presence of an amphipathic helix. Since α-methylated Ala enhances peptide helicity, we hypothesized that incorporating other types of α-methylated amino acids into ApoA-I mimetic peptides may also increase their helicity and cholesterol efflux potential. The last helix of apoA-I, peptide ‘A’ (VLESFKVSFLSALEEYTKKLNT), was used to design peptides containing a single type of α-methylated amino acid substitution (Ala/Aα, Asp/Dα, Lys/Kα, Leu/Lα), as well as a peptide containing both α-methylated Lys and Leu (6α). Depending on the specific residue, the α-helical content as measured by CD-spectroscopy and calculated hydrophobic moments were sometimes higher for peptides containing other types of α-methylated amino acids than those with α-methylated Ala. In ABCA1-transfected cells, cholesterol efflux to the peptides showed the following order of potency: 6α>Ka≈Lα≈Aα>>Dα≈A. In general, α-methylated peptides were resistant to proteolysis, but this varied depending on the type of protease and specific amino acid substitution. In summary, increased helicity and amphilicity due to α-methylated amino acid substitutions in ApoA-I mimetic peptides resulted in improved cholesterol efflux capacity and resistance to proteolysis, indicating that this modification may be useful in the future design of therapeutic ApoA-I mimetic peptides.
Keywords: peptides, cholesterol, atherosclerosis, alpha-helix, alpha-methyl amino acids
Introduction
Apolipoprotein A-I (ApoA-I) mimetics are short synthetic peptides that are being developed as a therapy for atherosclerotic cardiovascular disease (ASCVD) and other disorders [1,2]. They are designed to mimic ApoA-I, the main protein component of High-Density Lipoproteins (HDL) [3]. HDL is typically measured by its cholesterol content (HDL-C), which when low is inversely related to the incidence of ASCVD [3]. HDL has several potential anti-atherogenic functions, but the best understood is its ability to promote the efflux of excess cellular cholesterol by the ABCA1 transporter [4]. Several drugs, however, that raise HDL-C did not reduce ASCVD events in clinical trials [5], but HDL-C may be an unreliable metric for the anti-atherogenic function of HDL [3]. A phase III trial of reconstituted HDL made with purified full-length apoA-I and phospholipids (CSL112) is now being tested as an intravenous infusion therapy in acute coronary syndrome patients [6–8]. ApoA-I mimetic peptides have several potential advantages over the use of full-length ApoA-I protein in terms of drug development [1,2] and have also been tested in early stage clinical trials [9,10].
ApoA-I contains a tandem array of amphipathic helices, the structural motif that is necessary for ApoA-I to remove excess cholesterol from cells [11,12]. By forming an amphipathic helix, these peptides can act like selective detergents that solubilize both phospholipids and cholesterol from lipid microdomains formed by the ABCA1 transporter on the plasma membrane [4,13]. In the current study, we took a novel approach in the design of ApoA-I mimetic peptides by incorporating α-methylated amino acids [14]. In these modified amino acids, the hydrogen atom attached to the α-carbon is replaced with a methyl group. In the case of α-methyl Ala (amino-isobutyric acid), this structural change is known to restrict the conformational flexibility of the peptide bond that it can form by favoring helix formation [15]. Natural and synthetic helical peptides containing α-methyl Ala have been well described [14, 16–18], but the impact of other types of α-methylated amino acid substitutions on protein structure has not been systematically investigated. Using the last helical domain of ApoA-I, which is inactive in cholesterol efflux [19], we designed a series of peptide derivatives containing several different types of α-methyl amino acids substitutions and tested their effect on the structure and function of ApoA-I mimetic peptides. Results from this study have important implications not only in the design of ApoA-I mimetics but also for other therapeutic peptides that depend on the presence of a stable α-helix.
Materials and Methods
Peptide Synthesis.
Peptides were synthesized by solid-phase FMOC chemistry on a Biosearch 9600 synthesizer (Milligen, Bedford, MA) and HPLC purified by more than 95% on an Aquapore RP-300 column (Perkin Elmer, Waltham, MA). α-methylated amino acids were obtained from Iris Biotech GmbH (Germany). The N and C-terminus of the peptides were blocked with acetyl and amino groups, respectively.
Hydrophobic moment calculation.
The BOLBOACĂ value [20] for α-methyl Ala was used for assigning its hydrophobicity score. The difference between the hydrophobic score of Ala and α-methyl Ala was then added to the hydrophobicity score of the other amino acids, using the Fauchere and Pliska hydrophobicity scale [21], to estimate the increased hydrophobicity of the other α-methylated amino acids. A Python script was used to generate the hydrophobic moments of the peptides and were cross-referenced with MPEx tool [22] and Peptides package [23] in R.
Circular Dichroism (CD) spectroscopy.
CD spectra were obtained on a Chirascan™ Q100 spectropolarimeter (Applied Photophysics, UK) at 24° C in 1% acetonitrile (ACN) plus/minus 10% trifluoroethanol (TFE). Stock peptides were diluted to 0.1 mg/ml and loaded into flow cells with 1 mm pathlength and CD spectra from 190 to 250 nm were recorded. Helical estimates were determined from the mean residual ellipticity with online software (http://bestsel.elte.hu/). Coefficient of variation for % helicity estimation was <1%.
Solubilization of lipid vesicles by peptides.
Dimyristoylphosphatidylcholine (DMPC) vesicles (1 mg/mL) were prepared by resuspending dried DMPC in PBS and vortexing for 5 min. Changes in light scattering at 24 °C upon addition of peptides (final concentration 0.1 mg/mL) were recorded every 5 sec for 40 min at 660 nm, with shaking (fast-setting) in a Victor3-microplate reader (Perkin Elmer, Waltham, MA).
Peptide proteolysis.
Peptides (20 mg/mL) were diluted 80-fold with 100 mM NH4HC03 (pH 8), containing either trypsin (1 μg/mL) or Asp-N (5 μg/ml). After incubation at 37 °C for 2 h, samples were added to tubes containing MALDI standards (Bombesin, ACTH 1-17, and ACTH 18-39) in 50% ACN, 2.5% TFA to quench protease activity. Samples were dried on a MALDI AnchorChip target at 45 °C and matrix (saturated CHCA in 50% ACN, 2.5% TFA) was overlaid. Samples were analyzed on a Bruker AutoFlex III (Bruker Daltonics) in positive ion reflectron mode. Relative protein concentrations were determined from areas under the curve, using MALDI standards as calibrators.
Cholesterol efflux assay.
A radiolabeled cholesterol efflux study from BHK cells expressing ABCA1 was performed as previously described [11,12,24]. Cholesterol efflux was expressed as the percent of total [3H]-cholesterol transferred from cells to serum free medium containing peptides over 18 h.
Plasma HDL remodeling.
Remodeling of HDL in plasma was assessed after addition of peptides (final concentration 0.5 mg/mL) to pooled human plasma and incubated at 37°C for 1 h. Samples (10 ul) were mixed with 10 ul of 2X Native Tris-Glycine Sample Buffer (Novex/Thermo-Fisher, Carlsbad, CA) and electrophoresed on a native Tris-Glycine 10-20% acrylamide Novex WedgeWell gel and blotted onto PVDF as described earlier [25]. After blocking, the membrane was incubated with anti-human ApoA-I antibody preconjugated to HRP (Meridian Life Sciences, Memphis, TN), washed, and detected with Western Lightning (Perkin-Elmer, Waltham, MA).
Results
Structure and physical properties of α-methylated peptides.
A helical wheel plot of the A (control) peptide, which is based on the last helix of human ApoA-I (residues 227-266) is shown in Figure 1A. It is predicted to form a prototypical Type-A amphipathic helix, with hydrophobic residues on one side and charged or polar residues on the other side of the helix [26]. The hydrophobic face of this peptide is relatively small with a calculated hydrophobic moment of only 0.418, which accounts for its poor cholesterol efflux capacity [19].
Figure 1. Peptide Structure and Helicity.
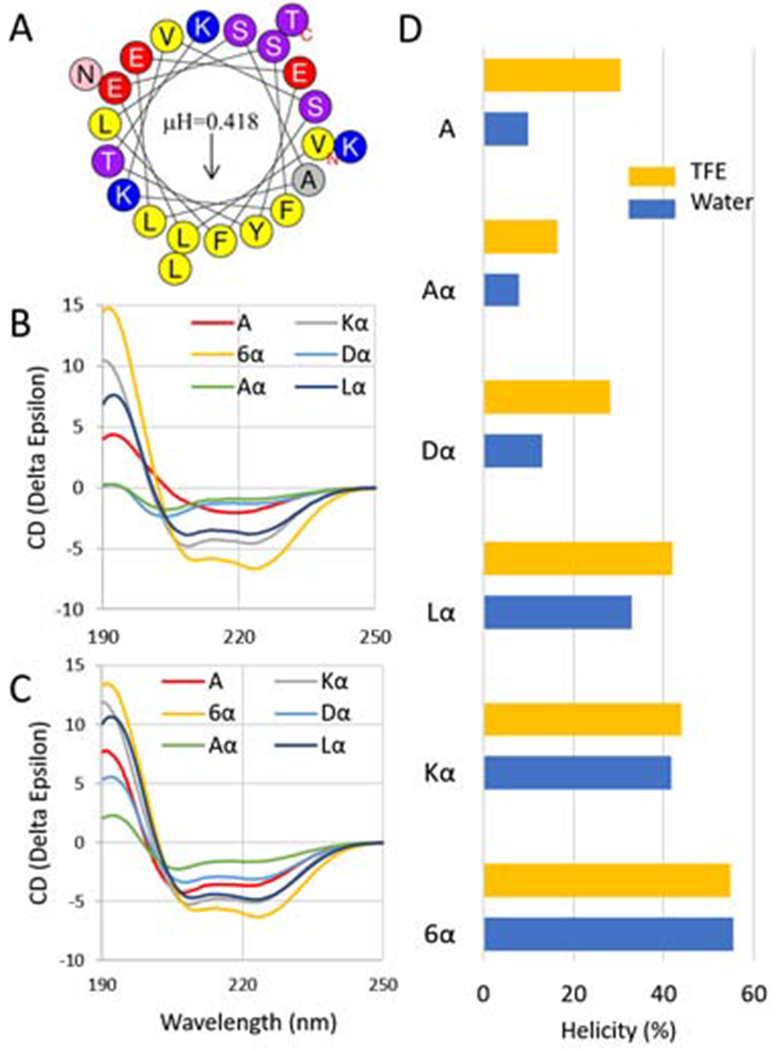
(A) Helical wheel plot of A peptide. Arrow indicates direction of hydrophobic moment (μH). (B) CD spectra of peptides in aqueous buffer. (C) CD spectra of peptides in aqueous buffer with 10% TFE. (D) Quantitation of % α-helix content of peptides in aqueous buffer (blue bars) or in aqueous buffer with 10% TFE (yellow bars).
Table 1 lists the sequences of the α-methylated derivatives of peptide A. Each peptide is named based on the type of α-methylated amino acid substitution except for the 6α peptide, in which case the designation refers to the total number of α-methylated amino acid substitutions (three Lys and three Leu residues). α-methyl Asp was used to replace Glu because they are isomers and because α-methyl Glu is not commercially available. In cases of identical adjacent amino acids, only one α-methylated amino substitution was made because of poor yield when peptides with both substitutions were made. Although their predicted charges were identical and they all had similar molecular weights, the calculated hydrophobic moments of the peptide derivatives differed (Table 1). The Aα peptide containing α-methylated Ala was calculated to have a lower hydrophobic moment than the original A peptide. This occurs because α-methylated Ala, which has two hydrophobic methyl groups that are both attached to its α-carbon, was used to replace Leu (Table 1), which has 4 aliphatic carbon groups in its side chain. Because the Leu residues in the A peptide are situated in the center of the hydrophobic face (Fig. 1A), replacement of Leu with the less hydrophobic α-methylated Ala reduces the hydrophobic moment of the peptide. Similarly, the Dα peptide has a slightly lower hydrophobic moment than the original A peptide (Table 1), because α-methylated Asp is more hydrophobic than Glu it was used to replace in the polar face of the helix (Fig. 1A). In contrast, the other α-methylated amino acid substitutions increased the hydrophobic moment, because they occurred in the hydrophobic face and the α-methylated amino acids were all more hydrophobic than the original residues. A similar rank order for peptide hydrophobic moments was obtained when we used several other different amino acid hydrophobicity scales that were also adjusted for the α-methylation modification (Supplement Table 1).
Table 1:
Amino acid sequence of peptides and biophysical characteristics.
| Name | Amino Acid Position | Number of substitutions | MW, Da | Hydrophobic moment, μH | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | ||||
| A | V | L | E | S | F | K | V | S | F | L | S | A | L | E | E | Y | T | K | K | L | N | T | 0 | 2739 | 0.42 |
| Aα | V | Aα | E | S | F | K | V | S | F | Aα | S | A | Aα | E | E | Y | T | K | K | Aα | N | T | 4 | 2478 | 0.31 |
| Dα | V | L | Dα | S | F | K | V | S | F | L | S | A | L | E | Dα | Y | T | K | K | L | N | T | 2 | 2618 | 0.41 |
| Kα | V | L | E | S | F | Kα | V | S | F | L | S | A | L | E | E | Y | T | K | Kα | L | N | T | 2 | 2645 | 0.43 |
| Lα | V | Lα | E | S | F | K | V | S | F | Lα | S | A | Lα | E | E | Y | T | K | K | Lα | N | T | 4 | 2590 | 0.46 |
| 6α | V | Lα | E | S | F | Kα | V | S | F | Lα | S | A | Lα | E | E | Y | T | Kα | K | Lα | N | T | 6 | 2674 | 0.45 |
Peptides were analyzed for helicity by CD spectroscopy (Figure 1B–D) in either an aqueous buffer or in the same buffer that also contained 10% TFE, to mimic a membrane environment [27]. The Aα peptide, which had the lowest hydrophobic moment (Table 1), also had the lowest α-helical content in aqueous buffer, but its helicity significantly increased in the presence of 10% TFE. The A and Dα peptides also had relatively low α-helical contents that also increased with TFE. The α-methylated peptides that contained either a Leu or Lys α-methyl amino acid substitutions or both (6α) were more helical than A, and these peptides did not show as much of an increase in helicity with TFE. All the peptides show very high α-helical content (56-60%) in a buffer containing 30% TFE, except Aα, which had an α-helical content of 40.5%.
α-methylated peptides show better detergent-like properties.
The detergent-like solubilization ability of the peptides were determined by monitoring the effects of peptide addition on the turbidity of large multilamellar vesicles made with DMPC phospholipids (Figure 2). This property of ApoA-I mimetic peptides to act like detergents can sometimes correlate with their cholesterol efflux capacity [24,28]. All of the peptides except Aα, which had both the lowest predicted hydrophobic moment (Table 1) and measured helicity (Fig. 1D), rapidly reduced turbidity within 15 min. The control A peptide was less effective than the other active peptides, which again is consistent with its comparatively lower hydrophobic moment and helicity. The Dα peptide, however, has a similarly low hydrophobic moment and helicity than the A peptide but was better in solubilizing DMPC vesicles than the A peptide.
Figure 2. Lipid solubilization assay.

Turbidity values of DMPC vesicles are shown over time after the addition of indicated peptides. Results for negative control (PBS addition) and positive control (1% TX-100 addition) are also shown.
α-methylated peptides show increase protease resistance.
To assess the ability of α-methylated peptides to resist proteolytic digestion, an important feature in the design of therapeutic peptides, we incubated each peptide with either trypsin or Asp-N (Figure 3). After incubation with trypsin, only the Kα and 6α peptides survived the digestion almost fully intact. A total of 3 Lys residues and no Arg residues, both of which are sensitive to trypsin, are present in the A peptide and two of them were replaced with α-methyl Lys for both Kα and 6α peptides (Table 1). Two of the Lys residues in the A peptide are adjacent to each other, which may account for why substitution of only one of the two adjacent Lys residues still resulted in almost complete protection from proteolysis. Asp-N, which is specific for Asp but has some reactivity toward Glu [29], almost fully digested A, but all of the α-methylated peptides showed some partial resistance. The Dα peptide was fully resistant to proteolysis, most likely due to the fact that the original Glu residues were replaced with α-methylated Asp. The partial resistance for the other α-methylated peptides may be due to their increased helicity, because most proteases only digest unfolded proteins in a random coil configuration [30]. It may have also occurred, because in many cases, the α-methylated substitution occurred next to a Glu residue (Table 1).
Figure 3. Peptide digestion by proteases.

Peptide susceptibility to proteolysis is shown after treatment with either trypsin (A) or Asp-N (B) for 2h at 37°C. Results are expressed as mean±1SD of triplicates. Statistical significance was calculated for all peptides individually against control peptide A, using two-tailed t-test where *** indicates p<0.0001 and ** indicates p<0.001.
α-methylated peptides stimulate cholesterol efflux.
The A and Dα peptides, which have one of lowest helical contents and lowest hydrophobic moments, were almost completely inactive in ABCAI-mediated cholesterol efflux (Figure 4A). The most effective peptide was 6α, which had the greatest number of α-methyl amino acid substitutions, the highest helicity and nearly the highest hydrophobic moment (Table 1). All of the α-methylated peptides were significantly better than A, including Aα, despite the fact that it also had a relatively low helical content and hydrophobic moment. When tested on control BHK cells not expressing ABCA1, all of the peptides showed less than 5% cholesterol efflux, indicating their ABCA1 transporter dependence (Supplemental Figure 1).
Figure 4. Cholesterol efflux by peptides.

(A) Cholesterol efflux from ABCA1-BHK cells as a function of peptide concentration. Results are expressed as mean ± 1SD of triplicates. (B) pre-β HDL formation by peptides. Lane 1 (left) contains plasma alone, whereas subsequent lanes contain a mixture of plasma with either Kα, Lα, Aα, Dα, 6α, or A (0.5 mg/mL) and the last two lanes (right) contain only either 6α or A peptides (0.5 mg/mL). Migration position of HDL subfractions is shown on the right.
Next, we investigated whether the α-methylated peptides can remodel plasma HDL (Figure 4B). Previously, we showed that the remodeling of HDL by ApoA-I mimetic peptides results in the formation of small pre-β HDL particles [24], which are potent stimulators of ABCA1-dependent cholesterol efflux [31]. Only treatment, however, with those modified peptides with the highest hydrophobic moments and helicity, namely Kα, and Lα and 6α peptides, showed any increase in pre-β HDL levels.
Discussion
One of the challenges in developing short synthetic peptides into therapeutics is that they often lack the required type of secondary structure for biological activity they would have when in the context of a complete protein, because of the absence of long range interactions and the other types of cooperative forces that promote overall protein folding. In case of ApoA-I mimetic peptides, it is necessary for them to form amphipathic helices in order to be competent for effluxing cholesterol by the ABCA1 transporter [11,12]. Various strategies for stabilizing helices in ApoA-I mimetic peptides with salt bridges [32], hydrocarbon staples [19], or the use of conformationally restrained amino acids like proline [33] have all been shown to increase their cholesterol efflux potential.
In this study, we used a different type of conformationally restrained amino acid, namely α-methylated amino acids, to promote helix formation. α-methylated residues are strong helix inducers as they contain geminal methyl substituents on their α-carbons. This structural change limits the conformational freedom of their peptide bonds and favors helix formation by promoting hydrogen bonding between the amino and carbonyl groups in the peptide backbone [14,16–18]. α-methyl Ala has been utilized for many years in peptide synthesis [14], but other type of α-methylated amino acids have only recently become commercially available. In general, compared to most other amino acids, Ala is already more helicogenic, because its short methyl side chain does not sterically interfere with helix formation [26,34]. Despite these two favorable factors, one of the major findings from this study is that other types of α-methylated amino acids may be better suited than α-methyl Ala in the design of ApoA-I mimetic peptides.
One likely reason why α-methyl Ala was not the best α-methylated amino acid substitution for ApoA-I mimetic peptides is that they require not just a helix but an amphipathic helix in order to efflux cholesterol by the ABCA1 transporter [11,12]. The amphipathic helix not only facilitates the initial interaction of these peptides with the lipid microdomains created by ABCA1, but it also helps stabilize the discoidal pre-β HDL-like particles formed after the lipid efflux process by the orderly covering of the hydrophobic acyl chains of phospholipids on the side of these nanodisc-shaped structures [24]. In the Aα peptide, we replaced the more hydrophobic Leu residue in the center of the hydrophobic face with the less hydrophobic α-methyl Ala, which decreases its hydrophobic moment and makes it less amphipathic. Depending on the primary sequence of a peptide and the position of the substitution, α-methyl Ala could still be a preferred substitution for at least stabilizing an α-helix. At this time more research is needed to better understand the general effects of the use of the different possible α-methylated amino acids on peptide structure and function. It is also important to note that although the 6α peptide, which had the most substitutions and one of the highest predicted hydrophobic moment and helicity, was the most effective peptide for cholesterol efflux, the rank order of some of the other α-methylated peptides for cholesterol efflux was not always predictable based on hydrophobic moment and helicity. It is known that amphipathic peptides with large hydrophobic moments tend to oligomerize, which then further strengthens their hydrophobic moment, which can make it difficult to use a calculated hydrophobic moment for predicting the function of these peptides[28,35]. In addition, there is a nonmonotonic relationship between hydrophobic moments and cholesterol efflux potential, because those peptides with the highest hydrophobic moments form very stable oligomers, which are inactive in the DMPC solubilization assay and in cholesterol efflux by cells [28,35]. The estimation of α-helical content by CD spectroscopy can also be unreliable in the presence of other types of secondary structures [36]. Until more is understood about the specific effects of α-methylated peptides on the structure and function of any given class of peptides, the optimization of such peptides will still likely depend on empirical substitution experiments.
Another interesting feature of α-methylated peptides in terms of drug development is their proteolysis resistance, which has been previously shown for α-methyl Ala substituted peptides [14, 16–18]. As we found in our study, this feature of α-methylated peptides is likely due to two factors. First, the specific type of amino acid substitution may lead to a protease no longer recognizing the peptide sequence containing α-methylated amino acid as a substrate. The second factor is likely due to the global effect of α-methylated amino acid substitutions in increasing overall helicity of a peptide, which by steric hindrance decreases the entry of a structured peptide into the active site of a protease [30,37]. The apolipoprotein mimetic peptide, D4F, which is entirely made of D-amino acids and is completely resistant to proteolysis, is active in cholesterol efflux. It has been investigated as a possible therapy because of its partial oral availability, but there is concern that it may accumulate in some organs like the kidney and cause toxicity [10,38]. Thus, the use of α-methylated peptide substitutions to confer only partial protease resistance could, therefore, be advantageous over the use of D-amino acids in the design of ApoA-I mimetic peptides.
In summary, we describe a new modification of apoA-I mimetic peptides, involving the use of α-methylated amino acids, which offer several advantages in stabilizing peptide structure and in conferring protease resistance that should also be applicable to the design of other types of therapeutic peptides.
Supplementary Material
ApoA-I mimetics are short synthetic peptides with an amphipathic helix
ApoA-I mimetic peptides stimulate the efflux of excess cholesterol from cells
α-methylated amino acids promote peptide helix formation
Incorporation of α-methylated peptides into ApoA-I mimetics enhances their biological properties
Acknowledgements
This research was supported by Intramural National Institutes of Health Funds from the National Heart Lung and Blood Institute. Authors thank NHLBI-Biophysics Core Facility for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
No competing interests.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Sethi AA, Amar M, Shamburek RD, Remaley AT, Apolipoprotein AI mimetic peptides: possible new agents for the treatment of atherosclerosis, Curr Opin Investig Drugs 8 (2007) 201–212. [PubMed] [Google Scholar]
- [2].Stoekenbroek RM, Stroes ES, Hovingh GK, ApoA-I mimetics, Handb Exp Pharmacol 224 (2015) 631–648. 10.1007/978-3-319-09665-0_21. [DOI] [PubMed] [Google Scholar]
- [3].Karathanasis SK, Freeman LA, Gordon SM, Remaley AT, The Changing Face of HDL and the Best Way to Measure It, Clin Chem 63 (2017) 196–210. 10.1373/clinchem.2016.257725. [DOI] [PubMed] [Google Scholar]
- [4].Rosenson RS, Brewer HB Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L, Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport, Circulation 125 (2012) 1905–1919. 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xiang AS, Kingwell BA, Rethinking good cholesterol: a clinicians’ guide to understanding HDL, Lancet Diabetes Endocrinol 7 (2019) 575–582. 10.1016/S2213-8587(19)30003-8. [DOI] [PubMed] [Google Scholar]
- [6].Chen N, Frishman WH, High-Density Lipoprotein Infusion Therapy and Atherosclerosis: Current Research and Future Directions, Cardiol Rev 24 (2016) 298–302. 10.1097/CRD.0000000000000111. [DOI] [PubMed] [Google Scholar]
- [7].Sposito AC, Carmo HR, Barreto J, Sun L, Carvalho LSF, Feinstein SB, Zanotti I, Kontush A, Remaley A, HDL-Targeted Therapies During Myocardial Infarction, Cardiovasc Drugs Ther 33 (2019) 371–381. 10.1007/s10557-019-06865-1. [DOI] [PubMed] [Google Scholar]
- [8].Capodanno D, Mehran R, Gibson CM, Angiolillo DJ, CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I: safety and tolerability profiles and implications for management in patients with myocardial infarction, Expert Opin Investig Drugs 27 (2018) 997–1005. 10.1080/13543784.2018.1543399. [DOI] [PubMed] [Google Scholar]
- [9].Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ, Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients, J Lipid Res 49 (2008) 1344–1352. 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy ST, Soffer D, Rader DJ, Fogelman AM, Schecter A, Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function, J Lipid Res 52 (2011) 361–373. 10.n94/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL, Santamarina-Fojo S, Brewer HB, Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway, J Lipid Res 44 (2003) 828–836. 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- [12].D’Souza W, Stonik JA, Murphy A, Demosky SJ, Sethi AA, Moore XL, Chin-Dusting J, Remaley AT, Sviridov D, Structure/function relationships of apolipoprotein a-I mimetic peptides: implications for antiatherogenic activities of high-density lipoprotein, Circ Res 107 (2010) 217–227. 10.1161/CIRCRESAHA.110.216507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rothblat GH, Phillips MC, High-density lipoprotein heterogeneity and function in reverse cholesterol transport, Curr Opin Lipidol 21 (2010) 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aravinda S, Shamala N, Balaram P, Aib residues in peptaibiotics and synthetic sequences: analysis of nonhelical conformations, Chem Biodivers 5 (2008) 1238–1262. 10.1002/cbdv.200890112. [DOI] [PubMed] [Google Scholar]
- [15].Ceccacci F, Mancini G, Rossi P, Scrimin P, Sorrenti A, Tecilla P, Deracemization and the first CD spectrum of a 3(10)-helical peptide made of achiral alpha-amino-isobutyric acid residues in a chiral membrane mimetic environment, Chem Commun (Camb) 49 (2013) 10133–10135. 10.1039/c3cc44713h. [DOI] [PubMed] [Google Scholar]
- [16].Tavenor NA, Reinert ZE, Lengyel GA, Griffith BD, Horne WS, Comparison of design strategies for alpha-helix backbone modification in a protein tertiary fold, Chem Commun (Camb) 52 (2016) 3789–3792. 10.1039/c6cc00273k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Altmann E, Altmann KH, Nebel K, Mutter M, Conformational studies on host-guest peptides containing chiral alpha-methyl-alpha-amino acids. Comparison of the helix-inducing potential of alpha-aminoisobutyric acid, (S)-2-ethylalanine and (S)-2-methylserine, Int J Pept Protein Res 32 (1988) 344–351. [PubMed] [Google Scholar]
- [18].Formaggio F, Moretto V, Crisma M, Toniolo C, Kaptein B, Broxterman QB, New tools for the control of peptide conformation: the helicogenic Calpha-methyl, Calpha-cyclohexylglycine, J Pept Res 63 (2004) 161–170. 10.1111/j.1399-3011.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- [19].Sviridov DO, Ikpot IZ, Stonik J, Drake SK, Amar M, Osei-Hwedieh DO, Piszczek G, Turner S, Remaley AT, Helix stabilization of amphipathic peptides by hydrocarbon stapling increases cholesterol efflux by the ABCA1 transporter, Biochem Biophys Res Commun 410 (2011) 446–451. 10.1016/j.bbrc.2011.05.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].BOLBOACA SD, Modelling analysis of amino acids hydrophobicity, MATCH-communications in mathematical and in computer chemistry (2008).
- [21].Fauchere J-L, and Pliska V, Hydrophobic parameters pi of amino-acid side chains from the partitioning of N-acetyl-amino-acid amides., Eur. J. Med. Chem 18 (1983) 369–375. [Google Scholar]
- [22].Snider C, Jayasinghe S, Hristova K, White SH, MPEx: a tool for exploring membrane proteins, Protein Sci 18 (2009) 2624–2628. 10.1002/pro.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Osorio D, Rondon-Villarreal P & Torres R , Peptides: A package for data mining of antimicrobial peptides., The R Journal 7 (2015) 4–14. [Google Scholar]
- [24].Islam RM, Pourmousa M, Sviridov D, Gordon SM, Neufeld EB, Freeman LA, Perrin BS Jr., Pastor RW, Remaley AT, Structural properties of apolipoprotein A-I mimetic peptides that promote ABCA1-dependent cholesterol efflux, Sci Rep 8 (2018) 2956. 10.1038/s41598-018-20965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Freeman LA, Lipoproteins and Cardiovascular Disease, Humana Press, Totowa, NJ, 2013. [Google Scholar]
- [26].Segrest JP, De Loof H, Dohlman JG, Brouillette CG, Anantharamaiah GM, Amphipathic helix motif: classes and properties, Proteins 8 (1990) 103–117. 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- [27].Gast K, Zirwer D, Muller-Frohne M, Damaschun G, Trifluoroethanol-induced conformational transitions of proteins: insights gained from the differences between alpha-lactalbumin and ribonuclease A, Protein Sci 8 (1999) 625–634. 10.1110/ps.8.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D’Souza W, Sviridov D, Remaley AT, Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides, J Biol Chem 283 (2008) 32273–32282. 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Szecsi PB, The aspartic proteases, Scand J Clin Lab Invest Suppl 210 (1992) 5–22. [PubMed] [Google Scholar]
- [30].Bhattacharya S, Zhang H, Debnath AK, Cowburn D, Solution structure of a hydrocarbon stapled peptide inhibitor in complex with monomeric C-terminal domain of HIV-1 capsid, J Biol Chem 283 (2008) 16274–16278. 10.1074/jbc.C800048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Didichenko SA, Navdaev AV, Cukier AM, Gille A, Schuetz P, Spycher MO, Therond P, Chapman MJ, Kontush A, Wright SD, Enhanced HDL Functionality in Small HDL Species Produced Upon Remodeling of HDL by Reconstituted HDL, CSL112: Effects on Cholesterol Efflux, Anti-Inflammatory and Antioxidative Activity, Circ Res 119 (2016) 751–763. 10.1161/aRCRESAHA.116.308685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, Johansson J, Azhar S, A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice, J Lipid Res 51 (2010) 1496–1503. 10.1194/jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sviridov DO, Drake SK, Freeman LA, Remaley AT, Amphipathic polyproline peptides stimulate cholesterol efflux by the ABCA1 transporter, Biochem Biophys Res Commun 471 (2016) 560–565. 10.1016/j.bbrc.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Myers JK, Pace CN, Scholtz JM, A direct comparison of helix propensity in proteins and peptides, Proc Natl Acad Sci U S A 94 (1997) 2833–2837. 10.1073/pnas.94.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wolska A, Lo L, Sviridov DO, Pourmousa M, Pryor M, Ghosh SS, Kakkar R, Davidson M, Wilson S, Pastor RW, Goldberg IJ, Basu D, Drake SK, Cougnoux A, Wu MJ, Neher SB, Freeman LA, Tang J, Amar M, Devalaraja M, Remaley AT, A dual apolipoprotein C-II mimetic-apolipoprotein C-III antagonist peptide lowers plasma triglycerides, Sci Transl Med 12 (2020). 10.1126/scitranslmed.aaw7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wallace BA, The role of circular dichroism spectroscopy in the era of integrative structural biology, Curr Opin Struct Biol 58 (2019) 191–196. 10.1016/j.sbi.2019.04.001. [DOI] [PubMed] [Google Scholar]
- [37].Lv Z, Chu Y, Wang Y, HIV protease inhibitors: a review of molecular selectivity and toxicity, HIV AIDS (Auckl) 7 (2015) 95–104. 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Navab M, Reddy ST, Van Lenten BJ, Fogelman AM, HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms, Nat Rev Cardiol 8 (2011) 222–232. 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


